Study on Novel SCR Catalysts for Denitration of High Concentrated Nitrogen Oxides and Their Reaction Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Activity Testing
- (1)
- Within the temperature range of 200–400 °C, with increasing vanadium loading, there is little variation observed in the catalytic performance of the catalysts, the NOx conversion rates of the five catalysts are similar.
- (2)
- As the temperature rises, the catalytic denitrification performance improves, indicating that higher temperatures lead to enhanced catalyst activity.
- (3)
- The catalytic effect of all five catalysts was above 80% at 200 °C, showing good low-temperature catalytic activity and the catalytic effect was close to 100% at 400 °C, and the high temperature did not affect the catalytic activity.

2.2. Catalyst Modification
- (1)
- The increase in MoO3 loading significantly enhances the denitrification performance of vanadium-based catalysts. Within the temperature range of 200–350 °C, the NOx conversion rates of the V3Mo5/TiO2 catalyst and the V3Mo10/TiO2 catalyst are higher overall compared to the V3Mo3/TiO2 catalyst. The V3Mo5/TiO2 catalyst exhibits the optimal low-temperature denitrification performance, achieving a NOx conversion rate of 93.4% at 200 °C, while the V3Mo10/TiO2 catalyst and the V3Mo3/TiO2 catalyst achieve rates of 91.7% and 86.5%, respectively. The order of catalytic activity among different catalysts is as follows: V3Mo5/TiO2 > V3Mo10/TiO2 > V3Mo3/TiO2. It can be observed that when the MoO3 loading is further increased to 10 wt%, the catalytic activity of the catalyst decreases instead, indicating an optimal loading ratio for the promoter MoO3.
- (2)
- When V2O5 loading is 3 wt%, MoO3 loading is 3 wt%, and WO3 loading is 3 wt%, the V3W3Mo3/TiO2 catalyst exhibits superior denitrification performance. It achieves slightly higher NOx conversion rates at 200 °C and 225 °C compared to the optimal V-Mo ratio V3Mo5/TiO2 catalyst. Moreover, at 250 °C, the denitrification conversion rate reaches 98.8%, whereas the V3Mo5/TiO2 catalyst without WO3 only achieves 94.5%.
- (3)
- When both MoO3 and WO3 loading are increased to 5 wt%, the denitrification performance of the V3W5Mo5/TiO2 catalyst decreases within the temperature range of 200–300 °C, indicating an optimal loading amount for MoO3 and WO3 when co-doped. Considering the NOx conversion rates of catalysts with different compositions.
2.3. Catalyst Reaction Mechanism Investigation
2.3.1. Catalyst Physical Characterization
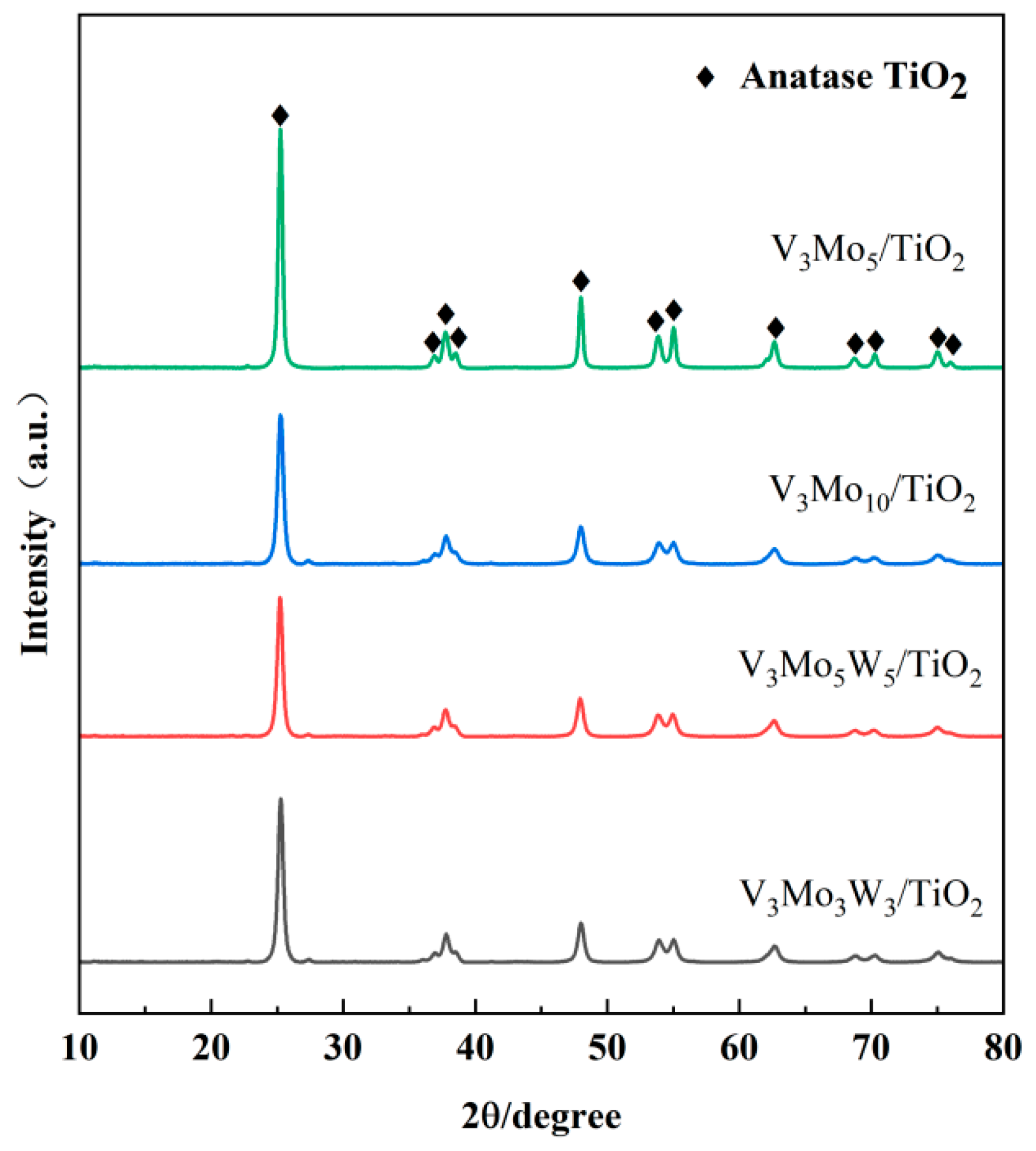
2.3.2. Catalyst Crystal Structure Analysis
2.3.3. Catalyst Surface Acidity Analysis
2.3.4. Catalyst Reductivity Analysis
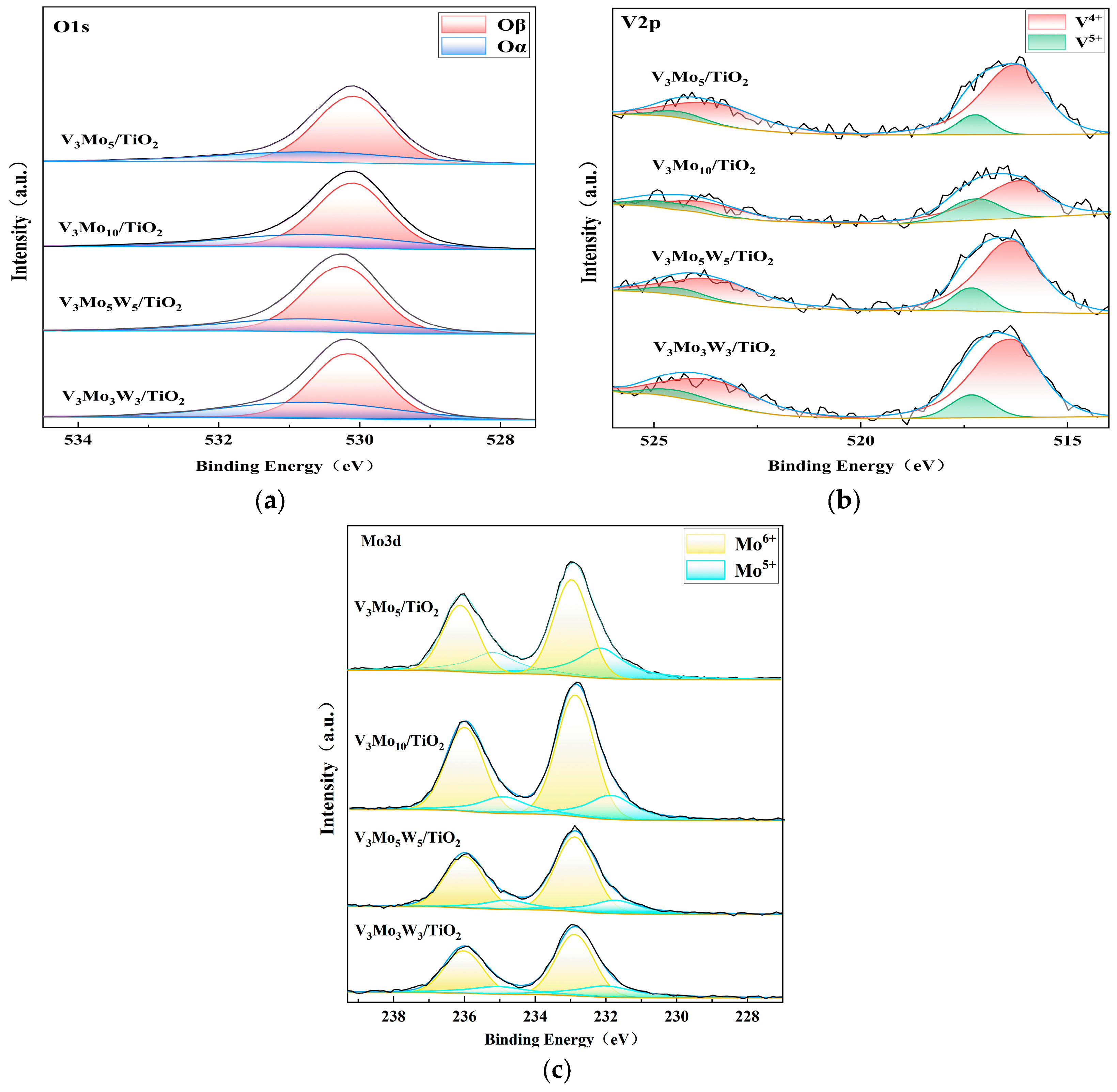
2.3.5. Catalyst Surface Elemental Analysis
3. Experiment
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Activity Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, V.; Shvab, A.; Brendakov, V. Mathematical Modeling of the Process of Decomposition of Ammonium Polyuranates. In Proceedings of the All-Russian Scientific Conference on Thermophysical Basis of Energy Technologies (TBET), Tomsk, Russia, 9–11 October 2019; National Research Tomsk Polytechnic University: Tomsk, Russia, 2019; Volume 2212. [Google Scholar]
- Dussoubs, B.; Jourde, J.; Patisson, F.; Houzelot, J.; Ablitzer, D. Mathematical modelling of uranium dioxide conversion in a moving bed furnace. Powder Technol. 2002, 128, 168–177. [Google Scholar] [CrossRef]
- Xie, W. Treatment technology and production practice of high concentrations nitrogen oxide. China Nonferrous Metall. 2010, 39, 47–49. [Google Scholar]
- Gao, F.Y.; Tang, X.L.; Yi, H.H.; Li, J.Y.; Zhao, S.Z.; Wang, J.G.; Chu, C.; Li, C.L. Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, Y.Q.; Chang, Z.F.; Zong, Y.H.; Wang, H.; Zhang, S.L.; Yu, Y. Promotional effect of phosphorus modification on improving the Na resistance of V2O5-MoO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3. Mol. Catal. 2021, 506, 111565. [Google Scholar] [CrossRef]
- Lee, G.M.Y.; Ye, B.R.; Kim, W.G.; Jung, J.I.; Park, K.Y.; Jeong, B.; Kim, H.D.; Kim, T. V2O5-WO3 catalysts treated with titanium isopropoxide using a one-step co-precipitation method for selective catalytic reduction with NH3. Catal. Today 2023, 411–412, 113924. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, K.; Seo, S.H.; Kim, T.H.; Yu, S.; Kim, T.H.; Son, Y.S. A study on additives to improve electron beam technology for NOx and SO2 reduction. Radiat. Phys. Chem. 2021, 183, 109397. [Google Scholar] [CrossRef]
- Jang, H.S.; Xing, S.L. A model to predict ammonia emission using a modified genetic artificial neural network: Analyzing cement mixed with fly ash from a coal-fired power plant. Constr. Build. Mater. 2020, 230, 117025. [Google Scholar] [CrossRef]
- Ye, B.; Jeong, B.; Lee, M.J.; Kim, T.H.; Park, S.S.; Jung, J.; Lee, S.; Kim, H.D. Recent trends in vanadium-based SCR catalysts for NOx reduction in industrial applications: Stationary sources. Nano Converg. 2022, 9, 51. [Google Scholar] [CrossRef]
- Kwon, D.W.; Park, K.H.; Hong, S.C. Enhancement of SCR activity and SO2 resistance on VOx/TiO2 catalyst by addition of molybdenum. Chem. Eng. J. 2016, 284, 315–324. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.Q.; Dong, Y.; Ma, C.Y. NO2-NH3 SCR over Activated Carbon: A Combination of NH4NO3 Formation and Consumption. Energy Fuels 2021, 35, 6167–6178. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, L.Q.; Chen, G.F.; Wang, T.; Dong, Y.; Ma, C.Y. Investigation of low-temperature NO2 reduction by NH3 over activated carbon: Effect of pore structure and flue gas conditions. J. Energy Inst. 2022, 101, 243–253. [Google Scholar] [CrossRef]
- Colombo, M.; Nova, I.; Tronconi, E.; Schmeisser, V.; Bandl-Konrad, B.; Zimmermann, L. NO/NO2/N2O-NH3 SCR reactions over a commercial Fe-zeolite catalyst for diesel exhaust aftertreatment: Intrinsic kinetics and monolith converter modelling. Appl. Catal. B-Environ. 2012, 111, 106–118. [Google Scholar] [CrossRef]
- Selleri, T.; Gramigni, F.; Nova, I.; Tronconi, E. NO oxidation on Fe- and Cu-zeolites mixed with BaO/Al2O3: Free oxidation regime and relevance for the NH3-SCR chemistry at low temperature. Appl. Catal. B-Environ. 2018, 225, 324–331. [Google Scholar] [CrossRef]
- Gao, X.; Liu, S.J.; Zhang, Y.; Du, X.S.; Luo, Z.Y.; Cen, K.F. Low temperature selective catalytic reduction of NO and NO2 with NH3 over activated carbon-supported vanadium oxide catalyst. Catal. Today 2011, 175, 164–170. [Google Scholar] [CrossRef]
- Chen, C.M.; Cao, Y.; Liu, S.T.; Chen, J.M.; Jia, W.B. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Zhang, T.T.; Shi, T.; Wang, Y.; Hao, Y.H.; Gao, Y.H.; Li, H.R.; Jia, L.; Liu, F.R.; Zeng, S.H. Orchestrating dual adsorption sites and unravelling Ce-Mn interaction and reaction mechanisms for efficient NH3-SCR. J. Catal. 2024, 429, 115260. [Google Scholar] [CrossRef]
- Geng, Y.; Shan, W.P.; Yang, S.J.; Liu, F.D. W-Modified Mn-Ti Mixed Oxide Catalyst for the Selective Catalytic Reduction of NO with NH3. Ind. Eng. Chem. Res. 2018, 57, 9112–9119. [Google Scholar] [CrossRef]
- Zong, L.Y.; Dong, F.; Zhang, G.D.; Han, W.L.; Tang, Z.C.; Zhang, J.Y. Highly Efficient Mesoporous V2O5/WO3-TiO2 Catalyst for Selective Catalytic Reduction of NOx: Effect of the Valence of V on the Catalytic Performance. Catal. Surv. Asia 2017, 21, 103–113. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, G.D.; Tang, Z.C.; Zhang, J.Y. Controlled Synthesis of Mesoporous CeO2-WO3/TiO2 Microspheres Catalysts for the Selective Catalytic Reduction of NOx with NH3. Catal. Surv. Asia 2019, 23, 311–321. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, B.; Liu, Z.Q.; Wu, X.Z.; Guo, J.F.; Zheng, C.Z.; Zhou, J.F.; Su, T.X.; Han, P.C.; Yang, C.Z.; et al. Review on advances in structure-activity relationship, reaction & deactivation mechanism and rational improving design of selective catalytic reduction deNOx catalysts: Challenges and opportunities. Fuel 2023, 343, 127924. [Google Scholar]
- Qi, G.S.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B-Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Gao, C.R.; Li, J.W.; Zhang, J.; Sun, X.L. DFT Study on the Combined Catalytic Removal of N2O, NO, and NO2 over Binuclear Cu-ZSM-5. Catalysts 2022, 12, 438. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef]
- Fu, Y.; Zhong, X.; Chang, H.; Li, J. Mechanism study on CO-SCR over Ce-Co-Ox mixed oxides catalysts. China Environ. Sci. 2018, 38, 2934–2940. [Google Scholar]
- Seo, C.K. Improvement of NOx and CO Reduction Performance at Low Temperature of H2-SCR Catalyst. Int. J. Automot. Technol. 2022, 23, 1509–1515. [Google Scholar] [CrossRef]
- Liu, Z.M.; Jia, B.; Zhang, Y.Y.; Haneda, M. Engineering the Metal-Support Interaction on Pt/TiO2 Catalyst to Boost the H2-SCR of NOx. Ind. Eng. Chem. Res. 2020, 59, 13916–13922. [Google Scholar] [CrossRef]
- Liu, Z.M.; Li, Y.; Zhu, T.L.; Su, H.; Zhu, J.Z. Selective Catalytic Reduction of NOx by NH3 over Mn-Promoted V2O5/TiO2 Catalyst. Ind. Eng. Chem. Res. 2014, 53, 12964–12970. [Google Scholar] [CrossRef]
- Arfaoui, J.; Ghorbel, A.; Petitto, C.; Delahay, G. Novel V2O5-CeO2-TiO2-SO42- nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2. Appl. Catal. B-Environ. 2018, 224, 264–275. [Google Scholar] [CrossRef]
- Jung, M.G.; Shin, J.H.; Kwon, D.W.; Hong, S.C. Promotional effects of Me (Sb, La, Ce, Mo) additives on the NH3-SCR activity and SO2 durability of V2O5-WO3/TiO2 catalysts. Process Saf. Environ. Prot. 2024, 183, 911–924. [Google Scholar] [CrossRef]
- Su, C.X.; Zhu, L.; Xu, M.T.; Zhong, Z.P.; Wang, X.Y.; Gao, Y.; Zhu, Y.Z. Influences analysis of Sb/Si dopant in TiO2 on NH3-SCR activity and low temperature SO2 resistance of V2O5/TiO2 catalysts. Appl. Surf. Sci. 2023, 637, 157996. [Google Scholar] [CrossRef]
- Kim, D.H.; Kwon, D.W.; Hong, S.C. Structural characteristics of V-based catalyst with Sb on selective catalytic NOx reduction with NH3. Appl. Surf. Sci. 2021, 538, 148088. [Google Scholar] [CrossRef]
- Wang, P.; Guo, R.T. The promotion effect of copper doping on the potassium resistance of V/TiO2 catalyst for selective catalytic reduction of NO with NH3. Chem. Pap. 2017, 71, 2253–2259. [Google Scholar] [CrossRef]
- Wang, R.A.; Zhang, Y.L.; Fan, X.; Li, J. Cobalt-/pH-Modified V2O5-MoO3/TiO2 Catalyst with Enhanced Activity for the Low-Temperature Selective Catalytic Reduction Process. Catalysts 2023, 13, 844. [Google Scholar] [CrossRef]
- Kim, J.; Won, J.M.; Jeong, S.K.; Yu, K.S.; Shin, K.; Hwang, S.M. Fe-promoted V/W/TiO2 catalysts for enhanced low-temperature denitrification efficiency. Appl. Surf. Sci. 2022, 601, 154290. [Google Scholar] [CrossRef]
- Kang, T.H.; Kim, H.S.; Lee, H.; Kim, D.H. Synergistic effect of V2O5-WO3/TiO2 and H-ZSM-5 catalysts prepared by physical mixing on the selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2023, 614, 156159. [Google Scholar] [CrossRef]
- Yuan, P.; Cui, C.S.; Han, W.; Bao, X.J. The preparation of Mo/γ-Al2O3 catalysts with controllable size and morphology via adjusting the metal-support interaction and their hydrodesulfurization performance. Appl. Catal. A-Gen. 2016, 524, 115–125. [Google Scholar] [CrossRef]
- Zhang, H.; Han, J.; Niu, X.W.; Han, X.; Wei, G.D.; Han, W. Study of synthesis and catalytic property of WO3/TiO2 catalysts for NO reduction at high temperatures. J. Mol. Catal. A-Chem. 2011, 350, 35–39. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.D.; Ran, R.; Si, Z.C.; Ma, Z.R.; Wang, B.D.; Weng, D. Effects of MoOx on dispersion of vanadia and low-temperature NH3-SCR activity of titania supported catalysts: Liquid acidity and steric hindrance. Appl. Surf. Sci. 2022, 585, 152710. [Google Scholar] [CrossRef]
- Lázaro, M.J.; Boyano, A.; Herrera, C.; Larrubia, M.A.; Alemany, L.J.; Moliner, R. Vanadium loaded carbon-based monoliths for the on-board No reduction: Influence of vanadia and tungsten loadings. Chem. Eng. J. 2009, 155, 68–75. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Chang, H.; Li, X.; Crittenden, J.C.; Hao, J. Chemical poison and regeneration of SCR catalysts for NOx removal from stationary sources. Front. Environ. Sci. Eng. 2016, 10, 413–427. [Google Scholar] [CrossRef]





| Sample | BET (m2/g) |
|---|---|
| V3Mo3W3/TiO2 | 56.7 |
| V3W5Mo5/TiO2 | 56.2 |
| V3Mo10/TiO2 | 66.7 |
| V3Mo5/TiO2 | 54.4 |
| Sample | Peak Area | Atomic Ratio % | |||
|---|---|---|---|---|---|
| V | Mo | V4+/(V5+ + V4+) | Mo6+/(Mo6+ + Mo5+) | Oα/(Oα + Oβ) | |
| V3W3Mo3/TiO2 | 6242.5 | 12,949.0 | 85.8 | 71.0 | 35.2 |
| V3W5Mo5/TiO2 | 5412.5 | 15,172.2 | 85.1 | 76.1 | 29.1 |
| V3Mo5/TiO2 | 5180.2 | 20,918.6 | 87.9 | 63.3 | 31.2 |
| V3Mo10/TiO2 | 3684.6 | 24,661.4 | 72.9 | 74.7 | 29.4 |
| Number | Chemical Formula | Specifications | Manufacturer |
|---|---|---|---|
| 1 | TiO2 | Nano grade | Macklin (Shanghai, China) |
| 2 | (NH4)10H2(W2O7)6 | Analytical grade | Macklin (Shanghai, China) |
| 3 | NH4VO3 | Analytical grade | Macklin (Shanghai, China) |
| 4 | (NH4)6Mo7O24 | Analytical grade | Macklin (Shanghai, China) |
| 5 | C2H2O4·2H2O | Analytical grade | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| Chemical Composition | Named |
|---|---|
| V: 1%, Mo: 3%, TiO2:96% | V1Mo3/TiO2 |
| V: 2%, Mo: 3%, TiO2:95% | V2Mo3/TiO2 |
| V: 3%, Mo: 3%, TiO2:94% | V3Mo3/TiO2 |
| V: 4%, Mo: 3%, TiO2:93% | V4Mo3/TiO2 |
| V: 5%, Mo: 3%, TiO2:92% | V5Mo3/TiO2 |
| V: 3%, Mo: 5%, TiO2:92% | V3Mo5/TiO2 |
| V: 3%, Mo: 10%, TiO2:87% | V3Mo10/TiO2 |
| V: 3%, W: 3%, Mo: 3%, TiO2:91% | V3W3Mo3/TiO2 |
| V: 3%, W: 5%, Mo: 5%, TiO2:87% | V3W5Mo5/TiO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Liu, X.; Wu, S.; Yang, H.; Zhou, S.; Yang, L.; Liu, F. Study on Novel SCR Catalysts for Denitration of High Concentrated Nitrogen Oxides and Their Reaction Mechanisms. Catalysts 2024, 14, 406. https://doi.org/10.3390/catal14070406
Yu B, Liu X, Wu S, Yang H, Zhou S, Yang L, Liu F. Study on Novel SCR Catalysts for Denitration of High Concentrated Nitrogen Oxides and Their Reaction Mechanisms. Catalysts. 2024; 14(7):406. https://doi.org/10.3390/catal14070406
Chicago/Turabian StyleYu, Bo, Xingyu Liu, Shufeng Wu, Heng Yang, Shuran Zhou, Li Yang, and Fang Liu. 2024. "Study on Novel SCR Catalysts for Denitration of High Concentrated Nitrogen Oxides and Their Reaction Mechanisms" Catalysts 14, no. 7: 406. https://doi.org/10.3390/catal14070406






