Development of a γ-Al2O3-Based Heterogeneous Fenton-like Catalyst and Its Application in the Advanced Treatment of Maotai-Flavored Baijiu Wastewater
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterisation
2.2. Effect of Catalyst Type on the Removal of COD from Maotai-Flavored Baijiu Wastewater
2.3. Effect of Reaction Time on the Removal of COD from Maotai-Flavored Baijiu Wastewater
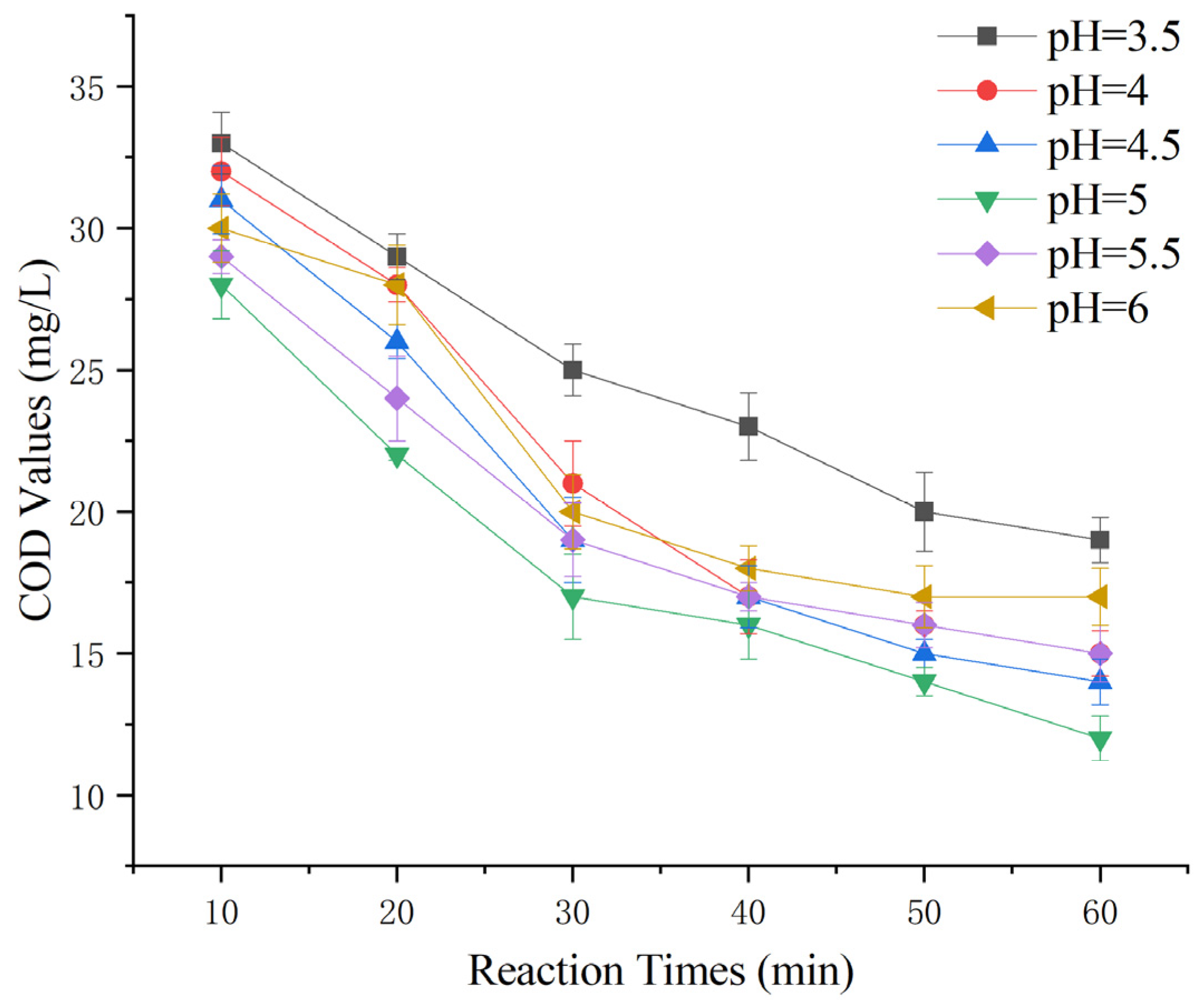
2.4. Effect of Initial pH on the Removal of COD from Maotai-Flavored Baijiu Wastewater
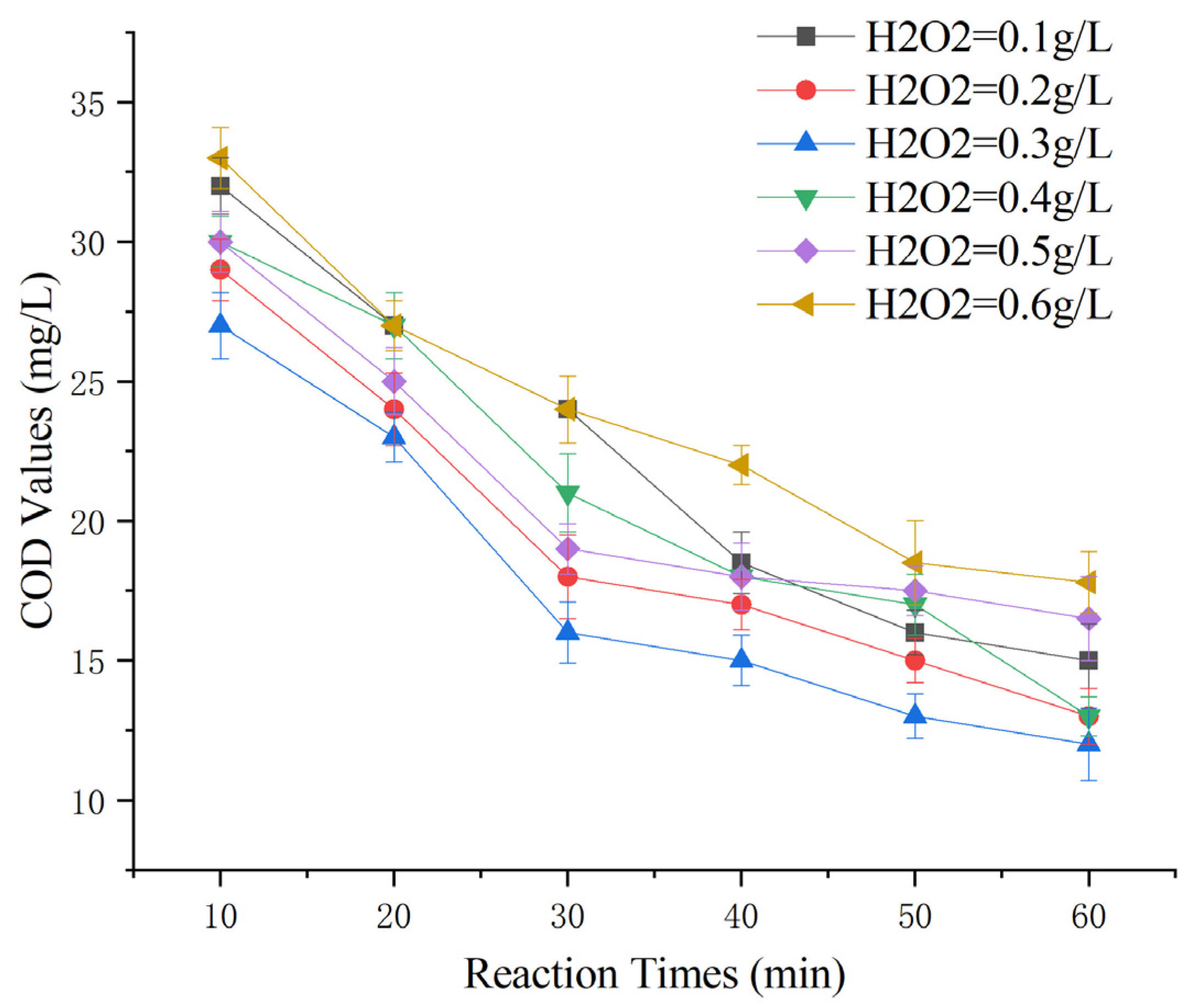
2.5. Effect of H2O2 Dosage on the Removal of COD from Maotai-Flavored Baijiu Wastewater
2.6. Effect of Catalyst Dosage on the Removal of COD from Maotai-Flavored Baijiu Wastewater
2.7. Catalyst Stability Analysis
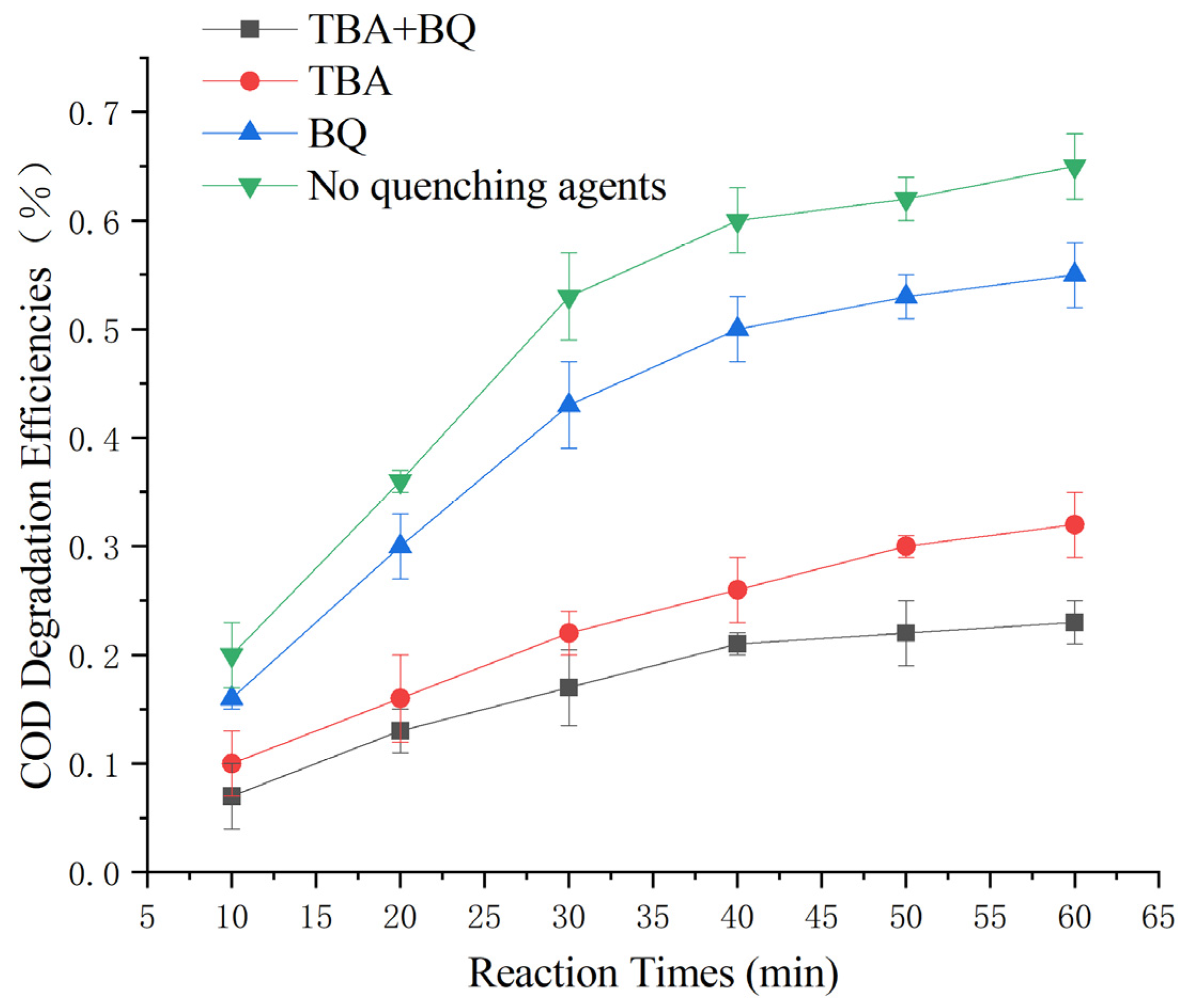
2.8. Reaction Kinetics Studies
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of Catalysts
3.3. Characterization of the Catalysts
3.4. Heterogeneous Fenton-like Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latessa, S.H.; Hanley, L.; Tao, W. Characteristics and Practical Treatment Technologies of Winery Wastewater: A Review for Wastewater Management at Small Wineries. J. Environ. Manag. 2023, 342, 118343. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, X.Q.; Zhang, L.; Yuan, H.; Liu, C. The Law of the Residual COD in Distilled Spirit Wastewater after Two Stages of Biochemical Treatment. Appl. Mech. Mater. 2014, 675–677, 596–601. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.Q.; Xiong, C.; Cai, Z.L.; Xiang, K. Comparative Study on the Anaerobically Methanogenic Results of Sauce Sweet Model Liquor and Fen-Flavor Liquor Wastewater. Adv. Mater. Res. 2013, 726–731, 2636–2640. [Google Scholar] [CrossRef]
- Fu, J.X.; Li, H.M.; Yu, P.F.; Zhao, K. Research on Waste Materials with Decentralized White Spirit Wastewater Pretreatment by Fe-C Micro-Electrolysis. In Proceedings of the International Conference on Machine Tool Technology and Mechatronics Engineering, Guilin, China, 22–23 June 2014. [Google Scholar]
- Moletta, R. Winery and Distillery Wastewater Treatment by Anaerobic Digestion. Water Sci. Technol. 2005, 51, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Sinha, S.; Ashokan, H.; Paul, M.V.; Hariharan, S.P.; Arun, J.; Gopinath, K.; Le, Q.H.; Pugazhendhi, A. Advanced Oxidation Process (AOP) Combined Biological Process for Wastewater Treatment: A Review on Advancements, Feasibility and Practicability of Combined Techniques. Environ. Res. 2023, 237, 116944. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.; Teixeira, A.; Ruotolo, L. Critical Review of Fenton and Photo-Fenton Wastewater Treatment Processes over the Last Two Decades. Int. J. Environ. Sci. Technol. 2023, 20, 13995–14032. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2 Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Wang, X.; Wang, P.; Ma, J.; Liu, H.; Ning, P. Synthesis, Characterization, and Reactivity of Cellulose Modified Nano Zero-Valent Iron for Dye Discoloration. Appl. Surf. Sci. 2015, 345, 57–66. [Google Scholar] [CrossRef]
- Wang, J.; Chang, F.; Zheng, M. Advanced Treatment of Coking Wastewater by Polyaluminum Silicate Sulfate for Organic Compounds Removal. Int. J. Environ. Res. Public Health 2023, 20, 6342. [Google Scholar] [CrossRef]
- Qian, W.; Huang, H.; Diao, Z.; Li, H.; Liu, H.; Ye, M.; Deng, Y.; Xu, Z. Advanced Treatment of Dye Wastewater Using a Novel Integrative Fenton-like/MnO2-Filled Upward Flow Biological Filter Bed System Equipped with Modified Ceramsite. Environ. Res. 2021, 194, 110641. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J. Fenton Oxidation of Municipal Secondary Effluent: Comparison of Fe/Ce-RGO (Reduced Graphene Oxide) and Fe2+ as Catalysts. Environ. Sci. Pollut Res. 2018, 25, 31358–31367. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, G.; Zhang, N.; Ye, J.; Lu, P. Fe0-H2O2 for Advanced Treatment of Citric Acid Wastewater: Detailed Study of Catalyst after Several Times Use. Chem. Eng. J. 2018, 336, 233–240. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, M.; Peng, J. Fenton-like Degradation of Bisphenol A by Fe3O4 Rhombic Dodecahedrons. New J. Chem. 2023, 47, 10857–10865. [Google Scholar] [CrossRef]
- Tolba, A.; Alalm, M.G.; Elsamadony, M.; Mostafa, A.; Afify, H.; Dionysiou, D.D. Modeling and Optimization of Heterogeneous Fenton-like and Photo-Fenton Processes Using Reusable Fe3O4-MWCNTs. Process Saf. Environ. Prot. 2019, 128, 273–283. [Google Scholar] [CrossRef]
- Guo, M.; Lu, M.; Zhao, H.; Lin, F.; He, F.; Zhang, J.; Wang, S.; Dong, P.; Zhao, C. Efficient Electro-Fenton Catalysis by Self-Supported CFP@CoFe2O4 Electrode. J. Hazard. Mater. 2022, 423, 127033. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, Y. Ascorbic Acid Enhanced CuFe2O4-Catalyzed Heterogeneous Photo-Fenton-like Degradation of Phenol. J. Environ. Chem. Eng. 2023, 11, 111009. [Google Scholar] [CrossRef]
- Leichtweis, J.; Carissimi, E.; Hagemann, U.; Ulbricht, M.; Fischer, L. NiFe2O4/Biochar Decorated Porous Polymer Membranes for the Flow-through Photo-Fenton Degradation of Tetracycline. Chem. Eng. J. 2023, 477, 147203. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, S.; Li, X.; Zhang, S.; Nguyen, T.T.; Guo, M.; Gao, X. Ultrasound-Assisted Heterogeneous Fenton-like Process for Methylene Blue Removal Using Magnetic MnFe2O4/Biochar Nanocomposite. Appl. Surf. Sci. 2021, 566, 150654. [Google Scholar] [CrossRef]
- Bautista, P.; Mohedano, A.F.; Menendez, N. Catalytic Wet Peroxide Oxidation of Cosmetic Wastewaters with Fe-Bearing Catalysts. Catal. Today 2010, 151, 148–152. [Google Scholar] [CrossRef]
- Liu, P.; He, S.; Wei, H.; Wang, J.; Sun, C. Chenglin Characterizationof α-Fe2O3/γ-Al2O3 Catalysts for CatalyticWet Peroxide Oxidation of m-Cresol. Ind. Eng. Chem. Res. 2015, 54, 130–136. [Google Scholar] [CrossRef]
- Lim, H.; Lee, J.; Jin, S.; Kim, J.; Yoon, J.; Hyeon, T. Highly Active Heterogeneous Fenton Catalyst Using Iron Oxide Nanoparticles Immobilized in Alumina Coated Mesoporous Silica. Chem. Commun. 2006, 4, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Prins, R. On the Structure of γ-Al2O3. J. Catal. 2020, 392, 336–346. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Yin, J.; Luo, J. Characteristic Reflection Peak and Its Origin of Nanostructured Material Containing Small Metal Nanoparticles: Two Case Studies. Plasmonics 2023, 9, 551–559. [Google Scholar] [CrossRef]
- Liu, J. Characterizing Industrial Catalysts: Challenges and Opportunities. Microsc. Microanal. 2001, 7, 1098–1099. [Google Scholar] [CrossRef]
- Martins, A.R.; Carvalho, L.S.; Reyes, P.; Grau, J.M.; Rangel, M.D.C. Hydrogen Production on Alumina-Supported Platinum Catalysts. Mol. Catal. 2016, 429, 1–9. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Wei, G.; He, H.; Stucki, J.W.; Ma, L.; Pentrakova, L.; Pentrák, M.; Zhu, J. Heterogeneous Reduction of 2-Chloronitrobenzene by Co-Substituted Magnetite Coupled with Aqueous Fe2+: Performance, Factors, and Mechanism. ACS Earth Space Chem. 2019, 3, 728–737. [Google Scholar] [CrossRef]
- Li, Y.; Wei, G.; Liang, X.; Zhang, C.; Arai, Y. Metal Substitution-Induced Reducing Capacity of Magnetite Coupled with Aqueous Fe(II). ACS Earth Space Chem. 2020, 4, 905–911. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Chai, S.; Zhao, H.; Wang, Y. Three-Dimensional Homogeneous Ferrite-Carbon Aerogel: One Pot Fabrication and Enhanced Electro-Fenton Reactivity. ACS Appl. Mater. Interfaces 2013, 5, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Long, M.; Hu, P.; Chen, Y.; Huang, J. Magnetically Separable Core-Shell Structural γ-Fe2O3@Cu/Al-MCM-41 Nanocomposite and Its Performance in Heterogeneous Fenton Catalysis. J. Hazard. Mater. 2014, 264, 195–202. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Yang, X.; Shao, E.; Deng, X.; Liu, N.; Wu, M. Facile Synthesis of Three-Dimensional Mn3O4 Hierarchical Microstructures and Their Application in the Degradation of Methylene Blue. J. Mater. Chem. A 2015, 3, 2934–2941. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, G.; Liu, C.; Lan, H.; Liu, H.; Qu, J. Interface Stabilization of Undercoordinated Iron Centers on Manganese Oxides for Nature-Inspired Peroxide Activation. ACS Catal. 2018, 8, 1090–1096. [Google Scholar] [CrossRef]
- Gu, L.; Nie, J.-Y.; Zhu, N.; Wang, L.; Yuan, H.-P.; Shou, Z. Enhanced Fenton’s Degradation of Real Naphthalene Dye Intermediate Wastewater Containing 6-Nitro-1-Diazo-2-Naphthol-4-Sulfonic Acid: A Pilot Scale Study. Chem. Eng. J. 2012, 189–190, 108–116. [Google Scholar] [CrossRef]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.-C. Kinetics of 2,6-Dimethylaniline Oxidation by Various Fenton Processes. J. Hazard. Mater. 2011, 192, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, C.; Fegeant, B.; Svensson, E.; Wiklund, L.; Jonsson, M. On the Selectivity of Radical Scavengers Used To Probe Hydroxyl Radical Formation in Heterogeneous Systems. J. Phys. Chem. C Nanomater. Interfaces 2022, 126, 12435–12440. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, Y.; Li, B.; Feng, L.; Du, Z.; Zhang, L. Reaction Kinetics of Dissolved Black Carbon with Hydroxyl Radical, Sulfate Radical and Reactive Chlorine Radicals. Sci. Total Environ. 2022, 828, 153984. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, K.; Xu, Y. Tartaric Acid Enhanced CuFe2O4-Catalyzed Heterogeneous Photo-Fenton-like Degradation of Methylene Blue. Mater. Sci. Eng. B 2019, 245, 75–84. [Google Scholar] [CrossRef]
- Huanyan, X.U.; Wang, Y.; Shi, T.N.; Zhao, H.; Tan, Q.; Zhao, B.C.; Xiulan, H.E.; Shuyan, Q.I. Heterogeneous Fenton-like Discoloration of Methyl Orange Using Fe3O4/MWCNTs as Catalyst: Kinetics and Fenton-like Mechanism. Front. Mater. Sci. 2018, 12, 34–44. [Google Scholar]
- Chengjie, Z.; Kaifeng, Y.; Shiyu, H.; Feng, C. Adsorption-Depended Fenton-like Reaction Kinetics in CeO2-H2O2 System for Salicylic Acid Degradation. Colloids Amp. Surf. A Physicochem. Amp. Eng. Asp. 2018, 553, 456–463. [Google Scholar]
- Audino, F.; Conte, L.O.; Schenone, A.V.; Pérez-Moya, M.; Graells, M.; Alfano, O.M. A Kinetic Study for the Fenton and Photo-Fenton Paracetamol Degradation in an Annular Photoreactor. Environ. Sci. Pollut. Res. 2019, 26, 4312–4323. [Google Scholar] [CrossRef]
- Xi, Z.; Jin, B.X.; Shan, Z. Reaction Mechanisms Involving Peroxy Radical in the Low-Temperature Oxidation of Coal. Fuel 2021, 300, 120943. [Google Scholar] [CrossRef]
- Pochon, A.; Vaughan, P.P.; Gan, D.; Vath, P.; Blough, N.V.; Falvey, D.E. Photochemical Oxidation of Water by 2-Methyl-1,4-Benzoquinone: Evidence against the Formation of Free Hydroxyl Radical. J. Phys. Chem. A 2002, 106, 2889–2894. [Google Scholar] [CrossRef]
- Bakhoda, A.G.; Wiese, S.; Greene, C.; Figula, B.C.; Bertke, J.A.; Warren, T.H. Radical Capture at Nickel(II) Complexes: C–C, C–N, and C–O Bond Formation. Organometallics 2020, 39, 1710–1718. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Li, J.; Fan, A.; Zhang, Y.; Xu, C.; Qin, H.; Mu, F.; Xu, T. Research on COD Measurement Method Based on UV-Vis Absorption Spectra of Transmissive and Reflective Detection Systems. Front. Environ. Sci. 2023, 11, 1175363. [Google Scholar] [CrossRef]
- Ma, Y.; Tie, Z.; Zhou, M.; Wang, N.; Cao, X.; Xie, Y. Accurate Determination of Low-Level Chemical Oxygen Demand Using a Multistep Chemical Oxidation Digestion Process for Treating Drinking Water Samples. Anal. Methods 2016, 8, 3839–3846. [Google Scholar] [CrossRef]
- Korenaga, T. The Continuous Determination of Filtered Chemical-Oxygen Demand with Potassium Dichromate by Means of Flow-Injection Analysis. Bull. Chem. Soc. Jpn. 1982, 55, 1033–1038. [Google Scholar] [CrossRef]











| Catalyst Performance | BET Specific Surface Area (m²/g) | Pore Volume (cm³/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| γ-Al2O3 | 201.50 | 0.40 | 8.30 |
| γ-Al2O3 (600 °C, 4 h) | 236.10 | 0.68 | 12.80 |
| Mn-Fe/Al (Mn:Fe = 2:1) | 151.50 | 0.45 | 11.84 |
| Mn-Fe/Al (Mn:Fe = 2:2) | 151.70 | 0.43 | 11.46 |
| Catalysts and Reagents | Wastewater Type | Results |
|---|---|---|
| CuFe2O4 + TA + H2O2 [37] | Methylene blue | The results showed that introducing TA enhanced MB decolorization rate from 52.0% to 92.1% within 80 min [37]. |
| MnFe2O4/biochar + H2O2 [19] | Methylene blue | When using the MnFe2O4/BC composite as an ultrasound-assisted heterogeneous Fenton-like catalyst, 95% of MB (20 mg/L) was degraded at pH = 5 in the presence of 15 mmol/L H2O2 in 20 min [19]. |
| Polyaluminum silicate sulfate [10] | Coking wastewater | A dosage of 7 mmol/L PASS, flocculation velocity of 75 r/min, flocculation time of 30 min, pH of 7, and temperature of 20 °C could decrease the COD concentration from 196.67 mg/L to 59.94 mg/L [10]. |
| Fe/Ce-RGO + H2O2 [12] | Municipal secondary effluent | The removal efficiencies of DOC, SCOD, and SMT were 36.30%, 63.10%, and 35.00% using Fe/CeRGO as catalyst [12]. |
| Fe0 + H2O2 [13] | Citric acid wastewater | When the initial pH was 3.0, H2O2/COD = 0.7 × Fe0/H2O2 = 3.29 (weight ratio), coagulation pH = 9.0, and the COD removal reached to over 35% in 2 h [13]. |
| Modified ceramsite + H2O2 + MBFB (Fenton-like/MBFB) [11] | Synthetic dye wastewater | The optimal conditions were H2O2 concentration of 600 mg/L and HRT of 6 h, while the influent COD concentration was 90 mg/L, achieving COD-removal efficiency of 64.8%. |
| Mn-Fe/γ-Al2O3 + H2O2 | Maotai-flavored Baijiu wastewater | The results revealed that the most effective removal of organic matter was achieved with a Mn-Fe/γ-Al2O3 catalyst dosage of 30 g/100 g water, pH of 5.0, H2O2 dosage of 0.3 g/L, and reaction time of 60 min, while the influent COD concentration is 35 mg/L; the effluent COD value was 12 ± 1 mg/L, and the degradation rate was 65.7 ± 3%. |
| Organic Substance | Match Score | Percentage (%) | Estimated Concentration (mg/L) | Molecular Formula | C | H | O | Cl | S | N | P | I | Si | Br | Molecular Mass | COD Conversion Factor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxime-methoxyphenyl | 82.8 | 0.88 | 0.130358919 | C8H9NO2 | 8 | 9 | 2 | 1 | 151.2 | 1.957671958 | ||||||
| Cycloheptasiloxane, tetramethyl | 91.7 | 1.53 | 0.342745045 | C14H42O7Si7 | 14 | 42 | 7 | 7 | 519.1 | 1.294548257 | ||||||
| o-Hydroxybiphenyl | 96.7 | 5.12 | 0.564091429 | C12H10O | 12 | 10 | 1 | 170.2 | 2.632197415 | |||||||
| Hexadecamethylcyclooctasiloxane | 96.6 | 0.52 | 0.116477292 | C16H48O8Si8 | 16 | 48 | 8 | 8 | 593.2 | 1.29467296 | ||||||
| 1,2,4-Triazine-3,5(2H.) | 51.2 | 0.98 | 0.365261591 | C3H3N3O2 | 3 | 3 | 2 | 2 | 113.1 | 0.778072502 | ||||||
| 2-([1,1′-Biphenyl]-2-yloxy) | 93.8 | 44.15 | 5.196572064 | C14H14O2 | 14 | 14 | 2 | 214.3 | 2.463835744 | |||||||
| 3-Methyl-1-(trimethylsiloxy) | 50.2 | 0.94 | 0.108335 | C10H20OSi | 10 | 20 | 1 | 1 | 184.4 | 2.51626898 | ||||||
| 2-(3-Iodopropyl)-1,3-dioxolane | 50.5 | 1.01 | 0.285931815 | C6H11IO2 | 6 | 11 | 2 | 1 | 242.1 | 1.024370095 | ||||||
| N-(Methoxymethyl)-1,1-diphenyl-N-[(trimethylsilyl)methyl]methylamine | 62.3 | 1.15 | 0.129396349 | C19H27NOSi | 19 | 27 | 1 | 1 | 1 | 313.5 | 2.577352472 | |||||
| o-chlorobromobenzene | 50.6 | 1.12 | 0.353404545 | C4H6BrC1 | 4 | 6 | 1 | 1 | 191.5 | 0.919060052 | ||||||
| 1-Acenaphthenol | 60.4 | 35.01 | 3.857195491 | C12H10O | 12 | 10 | 1 | 170.2 | 2.632197415 | |||||||
| Triphenylphosphine oxide | 95.9 | 0.85 | 0.10088375 | C18H15OP | 18 | 15 | 1 | 1 | 278.3 | 2.443406396 | ||||||
| 3,5-Dimethylphenyl terephthalate | 51.1 | 1.47 | 0.193849527 | C18H18O4 | 18 | 18 | 4 | 298.3 | 2.199128394 | |||||||
| Ethyl 1,3-dithiane-2-carboxylate | 62.5 | 0.76 | 0.147162917 | C7H12O2S2 | 7 | 12 | 2 | 2 | 192.3 | 1.497659906 | ||||||
| look for a draw (chess) | 95.49 | 11.89166573 |
| Dynamics (Math.) | Kinetic Model | R2 | K Value/min−1 |
|---|---|---|---|
| First order | y = −0.0008x + 0.0113 | 0.9837 | 0.0008 |
| Second order | y = −2 × 10−5x + 0.0003 | 0.9705 | 0.00002 |
| Third order | y = 8 × 10−5x + 0.0004 | 0.9935 | 0.00008 |
| Active Ingredients | Proportional |
|---|---|
| Mn-Fe | 1:1 wt%; 2:1 wt%; 3:1 wt%; 2:2 wt%; 3:3 wt% |
| Mn-Cu | 1:1 wt%; 2:1 wt%; 3:1 wt%; 2:2 wt%; 3:3 wt% |
| Mn-Fe-Cu | 1:1:1 wt%; 2:2:1 wt%; 3:2:1 wt% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Yan, Y.; Li, J.; Guo, F.; Huang, W.; Yang, X.; Ning, H.; Kang, Q.; He, H.; Zhou, X.; et al. Development of a γ-Al2O3-Based Heterogeneous Fenton-like Catalyst and Its Application in the Advanced Treatment of Maotai-Flavored Baijiu Wastewater. Catalysts 2024, 14, 422. https://doi.org/10.3390/catal14070422
Luo B, Yan Y, Li J, Guo F, Huang W, Yang X, Ning H, Kang Q, He H, Zhou X, et al. Development of a γ-Al2O3-Based Heterogeneous Fenton-like Catalyst and Its Application in the Advanced Treatment of Maotai-Flavored Baijiu Wastewater. Catalysts. 2024; 14(7):422. https://doi.org/10.3390/catal14070422
Chicago/Turabian StyleLuo, Benfu, Yujing Yan, Jinyin Li, Fei Guo, Weiwei Huang, Xi Yang, Haiyan Ning, Qicheng Kang, Haixing He, Xuanyu Zhou, and et al. 2024. "Development of a γ-Al2O3-Based Heterogeneous Fenton-like Catalyst and Its Application in the Advanced Treatment of Maotai-Flavored Baijiu Wastewater" Catalysts 14, no. 7: 422. https://doi.org/10.3390/catal14070422
APA StyleLuo, B., Yan, Y., Li, J., Guo, F., Huang, W., Yang, X., Ning, H., Kang, Q., He, H., Zhou, X., Zhou, X., Wang, S., & Liu, Y. (2024). Development of a γ-Al2O3-Based Heterogeneous Fenton-like Catalyst and Its Application in the Advanced Treatment of Maotai-Flavored Baijiu Wastewater. Catalysts, 14(7), 422. https://doi.org/10.3390/catal14070422







