Application of Platinum Nanoparticles Decorating Mesoporous Carbon Derived from Sustainable Source for Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Materials
2.2. Characterization
3. Catalytic Tests
3.1. Catalyst Characterization
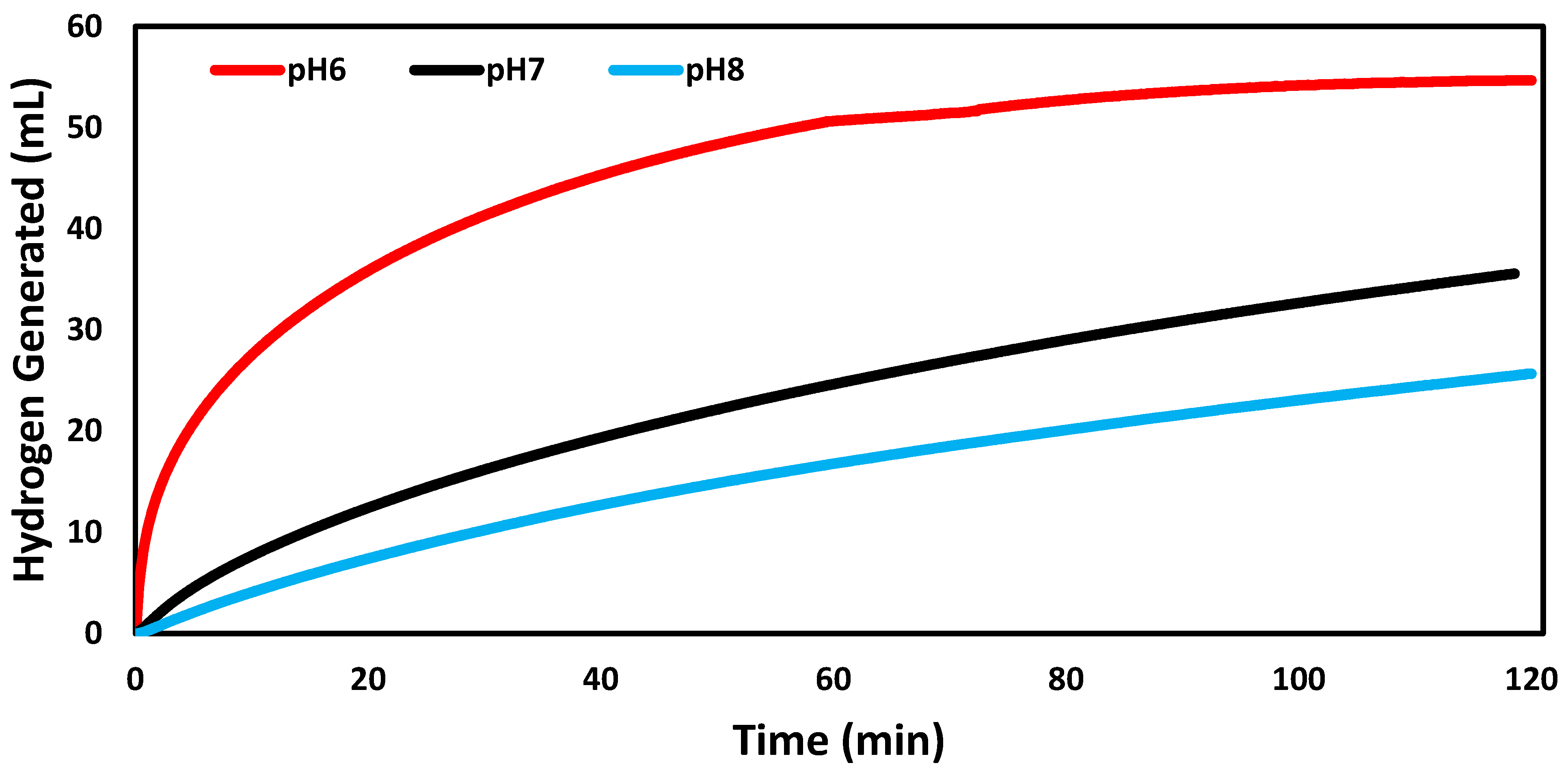
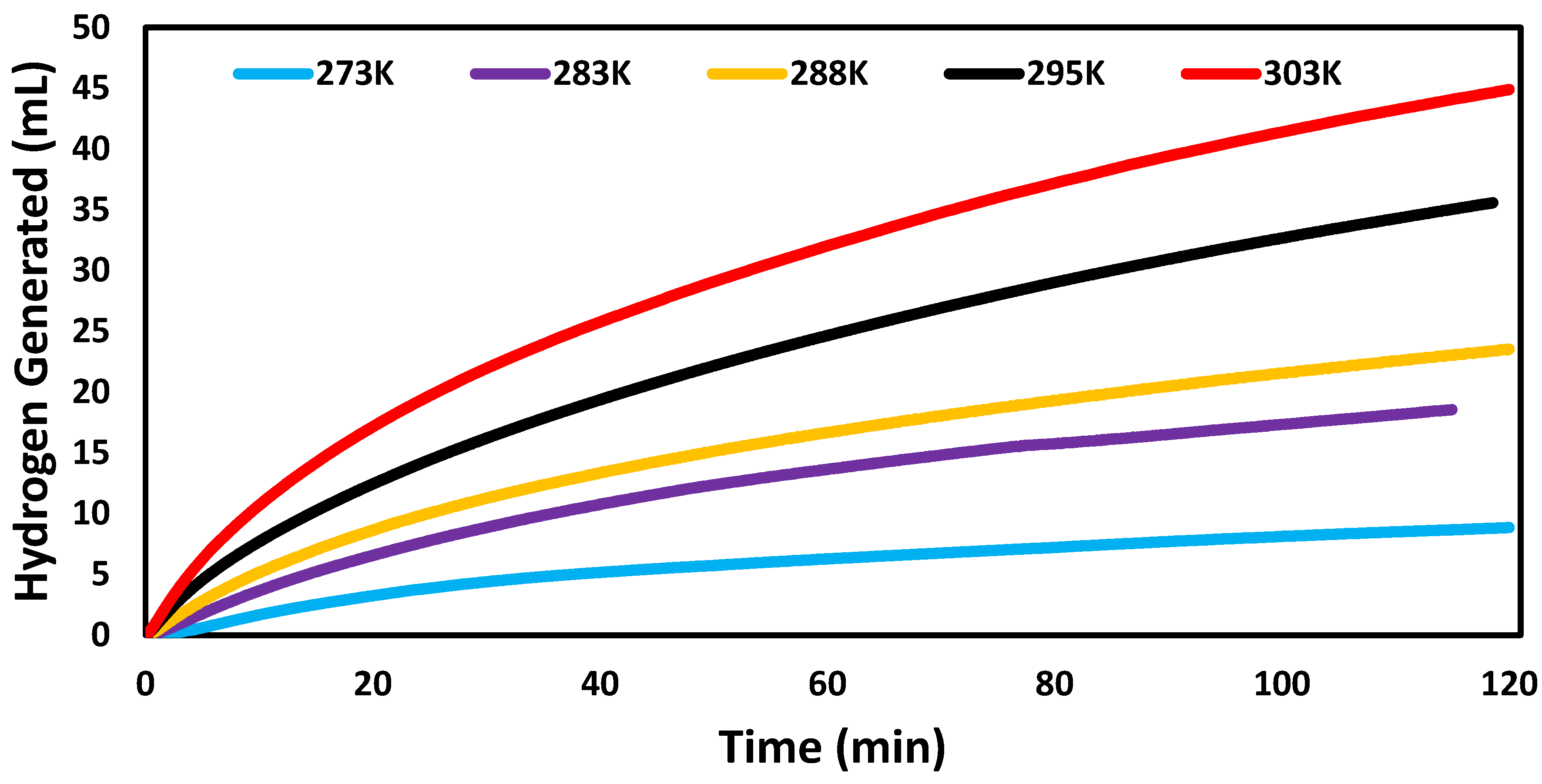
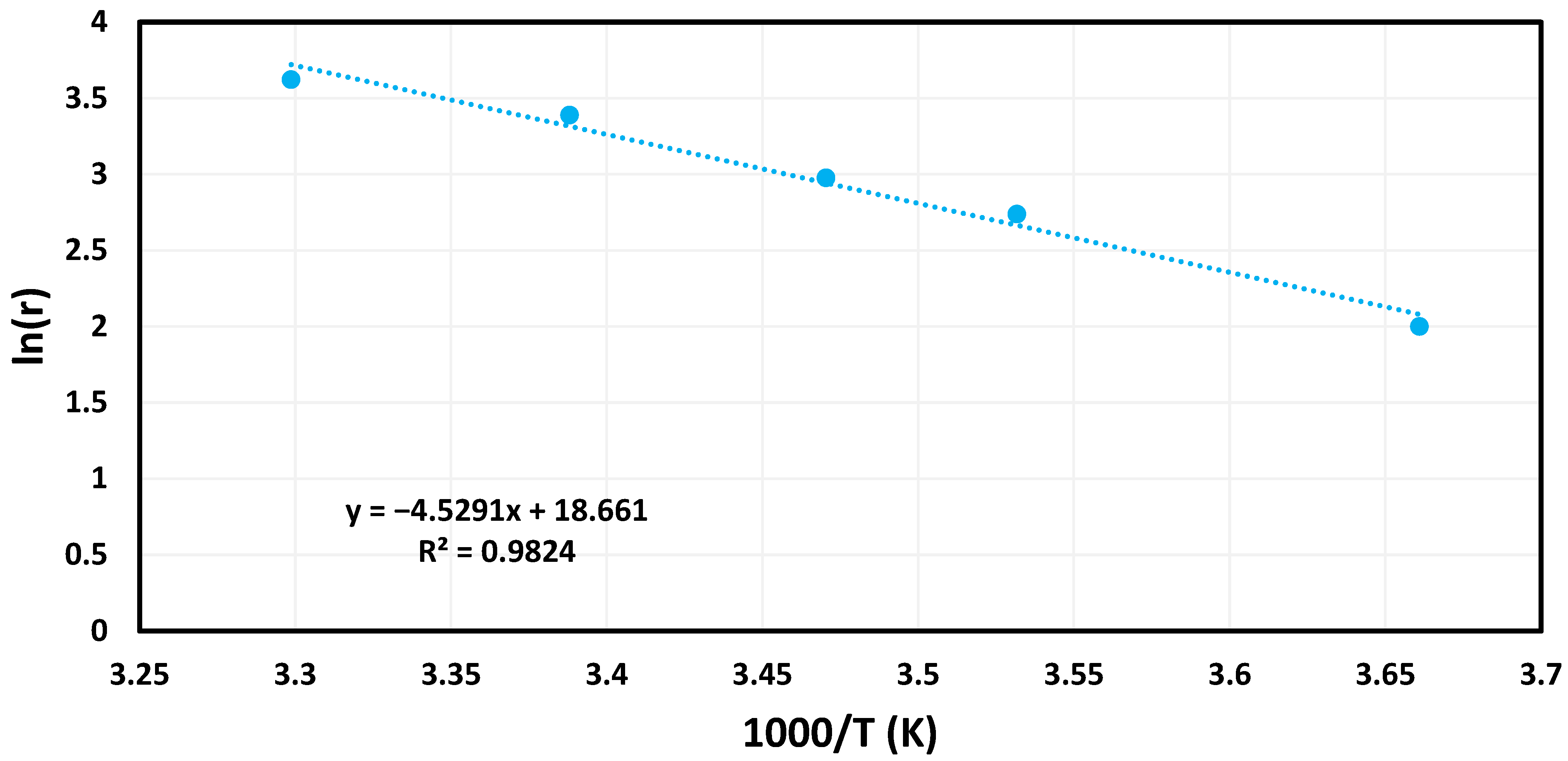
3.2. Catalytic Trials
3.3. Reusability Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allister, D. The Heat Is On: Taking Stock of Global Climate Ambition; NDC Global Outlook Report 2019; UNDP: New York, NY, USA, 2019; p. 13. Available online: https://unfccc.int/sites/default/files/resource/NDC%20Outlook.pdf (accessed on 19 June 2024).
- Veziroglu, T.N. 21st Century’s Energy: Hydrogen Energy System. In Assessment of Hydrogen Energy for Sustainable Development; Springer: Dordrecht, The Netherlands, 2007; pp. 9–31. [Google Scholar] [CrossRef]
- Rivkin, C.; Burgess, R.; Buttner, W. Hydrogen Technologies Safety Guide; National Renewable Energy Lab.: Golden, CO, USA, 2015. [Google Scholar] [CrossRef]
- Yang, W.; Chen, S. Recent progress in electrode fabrication for electrocatalytic hydrogen evolution reaction: A mini review. Chem. Eng. J. 2020, 393, 124726. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Jin, C.; Huo, L.; Tang, J.; Li, S.; Jiang, K.; He, Q.; Dong, H.; Gong, Y.; Hu, Z. Precise Atomic Structure Regulation of Single-Atom Platinum Catalysts toward Highly Efficient Hydrogen Evolution Reaction. Small 2023, 20, 2309509. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Z.; Li, J.; Gang, H.; Mei, D.; Yin, D.; Deng, R.; Zhu, Y.; Li, X.; Wang, N.; et al. Engineering a hollow bowl-like porous carbon-confined Ru–MgO hetero-structured nanopair as a high-performance catalyst for ammonia borane hydrolysis. Mater. Horiz. 2024, 11, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, H.; He, P.; Ren, Z.; Shen, G.; Liu, R.; Wang, L.; Luo, G. The performance and corrosion resistance of an electrodeposited Ni-Mo-Cu HER catalyst. Surf. Coat. Technol. 2023, 465, 129596. [Google Scholar] [CrossRef]
- Demirci, U.B.; Miele, P. Sodium borohydride versus ammonia borane, in hydrogen storage and direct fuel cell applications. Energy Environ. Sci. 2009, 2, 627. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Horváth, E.; Puskás, R.; Rémiás, R.; Mohl, M.; Kukovecz, Á.; Kónya, Z.; Kiriesi, I. A Novel Catalyst Type Containing Noble Metal Nanoparticles Supported on Mesoporous Carbon: Synthesis, Characterization and Catalytic properties. Top. Catal. 2009, 52, 1242–1250. [Google Scholar] [CrossRef]
- Xia, Y.; Mokaya, R. Generalized and Facile Synthesis Approach to N-Doped Highly Graphitic Mesoporous Carbon Materials. Chem. Mater. 2005, 17, 1553–1560. [Google Scholar] [CrossRef]
- Su, F.; Zeng, J.; Bao, X.; Yu, Y.; Lee, J.Y.; Zhao, X.S. Preparation and Characterization of Highly Ordered Graphitic Mesoporous Carbon as a Pt Catalyst Support for Direct Methanol Fuel Cells. Chem. Mater. 2005, 17, 3960–3967. [Google Scholar] [CrossRef]
- Chang, H.; Joo, S.H.; Pak, C. Synthesis and characterization of mesoporous carbon for fuel cell applications. J. Mater. Chem. 2007, 17, 3078–3088. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.; Wixtrom, A. Catalytic Reduction of 4-Nitrophenol Using Gold Nanoparticles Supported on Carbon Nanotubes. ECS J. Solid State Sci. Technol. 2014, 3, M18–M20. [Google Scholar] [CrossRef]
- Huff, C.; Dushatinski, T.; Barzanji, A.; Abdel-Fattah, N.; Barzanji, K.; Abdel-Fattah, T.M. Pretreatment of Gold Nanoparticle Multi-Walled Carbon Nanotube Composites for Catalytic Activity toward Hydrogen Generation Reaction. ECS J. Solid State Sci. Technol. 2017, 6, 69–71. [Google Scholar] [CrossRef]
- Dushatinski, T.; Huff, C.; Abdel-Fattah, T.M. Characterization of electrochemically deposited films from aqueous and ionic liquid cobalt precursors toward hydrogen evolution reactions. Appl. Surf. Sci. 2016, 385, 282–288. [Google Scholar] [CrossRef]
- Quach, Q.; Biehler, E.; Elzamzami, A.; Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Catalytic Activity of Beta-Cyclodextrin-Gold Nanoparticles Network in Hydrogen Evolution Reaction. Catalysts 2021, 11, 118. [Google Scholar] [CrossRef]
- Huff, C.; Biehler, E.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Synthesis of highly dispersive platinum nanoparticles and their application in a hydrogen generation reaction. Colloid Surf. A 2021, 610, 125734. [Google Scholar] [CrossRef]
- Antolini, E. Formation, microstructural characteristics and stability of carbon supported platinum catalysts for low temperature fuel cells. J. Mater. Sci. 2003, 38, 2995–3005. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Zhao, X.; Xin, Q.; Sun, G.; Yi, B. Studies on performance degradation of a direct methanol fuel cell (DMFC) in life test. Phys. Chem. Chem. Phys. 2004, 6, 134. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909. [Google Scholar] [CrossRef]
- Periana, R.A.; Taube, D.J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Platinum Catalysts for the High-Yield Oxidation of Methane to a Methanol Derivative. Science 1998, 24, 560–564. [Google Scholar] [CrossRef]
- Sabourault, N.; Mignani, G.; Wagner, A.; Mioskowski, C. Platinum Oxide (PtO2): A Potent Hydrosilylation Catalyst. Org. Lett. 2002, 4, 2117–2119. [Google Scholar] [CrossRef]
- Ely, J.C.; Neal, C.R.; Kulpa, C.F.; Schneegurt, M.A.; Seidler, J.A.; Jain, J.C. Implications of Platinum-Group Element Accumulation along U.S. Roads from Catalytic-Converter Attrition. Environ. Sci. Technol. 2001, 35, 3816–3822. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reziq, R.; Wang, D.; Post, M.; Alper, H. Platinum Nanoparticles Supported on Ionic Liquid-Modified Magnetic Nanoparticles: Selective Hydrogenation Catalysts. Adv. Synth. Catal. 2007, 349, 2145–2150. [Google Scholar] [CrossRef]
- Fang, B.; Chaudhari, N.K.; Kim, M.-S.; Kim, J.H.; Yu, J.-S. Homogeneous Deposition of Platinum Nanoparticles on Carbon Black for Proton Exchange Membrane Fuel Cell. J. Am. Chem. Soc. 2009, 131, 15330–15338. [Google Scholar] [CrossRef]
- Su, C.; Yang, T.; Zhou, W.; Wang, W.; Xuc, X.; Shao, Z. Pt/C–LiCoO2 composites with ultralow Pt loadings as synergistic bifunctional electrocatalysts for oxygen reduction and evolution reactions J. Mater. Chem. A 2016, 4, 4516–4524. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T. Screening study of Different Carbon Based Materials for Hydrogen. ECS J. Solid State Sci. Technol. 2023, 12, 081002. [Google Scholar] [CrossRef]
- Antolini, E.; Cardellini, F. Formation of carbon supported PtRu alloys: An XRD analysis. J. Alloys Compd. 2001, 315, 118–122. [Google Scholar] [CrossRef]
- Karthik, R.; Sasikumar, R.; Chen, S.-M.; Govindasamy, M.; Vinoth Kumar, J.; Muthuraj, V. Green Synthesis of Platinum Nanoparticles Using Quercus Glauca Extract and Its Electrochemical Oxidation of Hydrazine in Water Samples. Int. J. Electrochem. Sci. 2016, 11, 8245–8255. [Google Scholar] [CrossRef]
- Grzeschik, R.; Schäfer, D.; Holtum, T.; Küpper, S.; Hoffmann, A.; Schlücker, S. On the Overlooked Critical Role of the pH Value on the Kinetics of the 4-Nitrophenol NaBH4-Reduction Catalyzed by Noble Metal Nanoparticles (Pt, Pd, Au). J. Phys. Chem. C 2020, 124, 2939–2944. [Google Scholar] [CrossRef]
- Kaufman, C.M.; Sen, B. Hydrogen Generation by Hydrolysis of Sodium Tetrahydroborate: Effects of Acids and Transition Metals and Their Salts. J. Chem. Soc. Dalton Trans. 1985, 2, 307–313. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium borohydride, its hydrolysis and its use as a reducing agent and in the generation of hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Patel, N.; Patton, B.; Zanchetta, C.; Fernandes, R.; Guella, G.; Kale, A.; Miotello, A. Pd-C power and thin film catalysts for hydrogen production by hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2008, 33, 287–292. [Google Scholar] [CrossRef]
- Peña-Alonso, R.; Sicurelli, A.; Callone, E.; Carturan, G.; Raj, R. A picoscale catalyst for hydrogen generation from NaBH4 for fuel cells. J. Power Sources 2007, 165, 315–323. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Aboulatta, A.; Heyman, A.; Abdel-Fattah, T.M. Silver Nanoparticle/Multi-Walled Carbon Nanotube Composite as Catalyst for Hydrogen Production. ECS J. Solid State Sci. Technol. 2017, 6, 115–118. [Google Scholar] [CrossRef]
- Huff, C.; Long, J.M.; Heyman, A.; Abdel-Fattah, T.M. Palladium Nanoparticle Multiwalled Carbon Nanotube Composite as Catalyst for Hydrogen Production by the Hydrolysis of Sodium Borohydride. ACS Appl. Energy Mater. 2018, 1, 4635–4640. [Google Scholar] [CrossRef]
- Huff, C.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Nanocomposite Catalyst Derived from Ultrafine Platinum Nanoparticles and Carbon Nanotubes for Hydrogen Generation. ECS J. Solid State Sci. Technol. 2020, 9, 101008. [Google Scholar] [CrossRef]
- Quach, Q.; Biehler, E.; Abdel-Fattah, T.M. Synthesis of Copper Nanoparticles Supported over Graphene-like Material Composite as a Catalyst for Hydrogen Evolution. J. Compos. Sci. 2023, 7, 279. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Gold Nanoparticles AuNP Decorated on Fused Graphene-like Materials for Application in a Hydrogen Generation. Materials 2023, 16, 4779. [Google Scholar] [CrossRef]
- Quach, Q.; Biehler, E.; Abdel-Fattah, T.M. Synthesis of Palladium Nanoparticles Supported over Fused Graphene-like Material for Hydrogen Evolution Reaction. Catalysts 2023, 13, 1117. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Synthesis of Platinum Nanoparticles Supported on Fused Nanosized Carbon Spheres Derived from Sustainable Source for Application in a Hydrogen Generation Reaction. Nanomaterials 2023, 13, 1994. [Google Scholar] [CrossRef]
- Bilen, M.; Guru, M.; Cakanyildirim, C. Role of NaCl in NaBH4 production and its hydrolysis. Energy Convers. Manag. 2013, 72, 134–140. [Google Scholar] [CrossRef]
- Abdel-Fattah, T.M.; Biehler, E. Carbon Based Supports for Metal Nanoparticles for Hydrogen Generation Reactions Review. Adv. Carbon J. 2024, 1, 1–19. [Google Scholar] [CrossRef]






| Catalyst | Activation Energy (kJ mol−1) | Reaction Conditions (K) | Ref. |
|---|---|---|---|
| BCD-AuNP | 54.7 | 283–303 | [18] |
| PtNPs | 39.2 | 283–303 | [19] |
| Pd/C | 28 | 298–328 | [35] |
| Pt–Pd/CNTs | 19 | 302–332 | [36] |
| AuMWCNTs | 21.1 | 273–303 | [16] |
| Ag/MWCNTs | 44.5 | 273–303 | [37] |
| Pd/MWCNTs | 62.7 | 273–303 | [38] |
| PtMWCNTs | 46.2 | 283–303 | [39] |
| CuGLM | 46.8 | 283–303 | [40] |
| AuFGLM | 45.5 | 283–303 | [41] |
| PdFGLM | 45.1 | 283–303 | [42] |
| PtFCS | 53.0 | 283–303 | [43] |
| Pt-MCM | 37.7 | 273–303 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biehler, E.; Quach, Q.; Abdel-Fattah, T.M. Application of Platinum Nanoparticles Decorating Mesoporous Carbon Derived from Sustainable Source for Hydrogen Evolution Reaction. Catalysts 2024, 14, 423. https://doi.org/10.3390/catal14070423
Biehler E, Quach Q, Abdel-Fattah TM. Application of Platinum Nanoparticles Decorating Mesoporous Carbon Derived from Sustainable Source for Hydrogen Evolution Reaction. Catalysts. 2024; 14(7):423. https://doi.org/10.3390/catal14070423
Chicago/Turabian StyleBiehler, Erik, Qui Quach, and Tarek M. Abdel-Fattah. 2024. "Application of Platinum Nanoparticles Decorating Mesoporous Carbon Derived from Sustainable Source for Hydrogen Evolution Reaction" Catalysts 14, no. 7: 423. https://doi.org/10.3390/catal14070423
APA StyleBiehler, E., Quach, Q., & Abdel-Fattah, T. M. (2024). Application of Platinum Nanoparticles Decorating Mesoporous Carbon Derived from Sustainable Source for Hydrogen Evolution Reaction. Catalysts, 14(7), 423. https://doi.org/10.3390/catal14070423









