Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid
Abstract
:1. Introduction
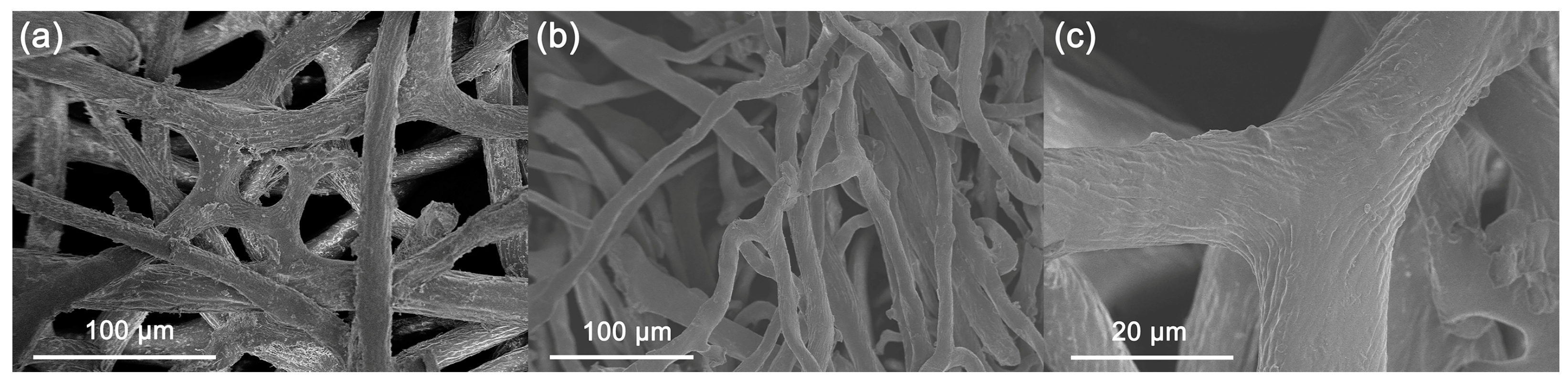
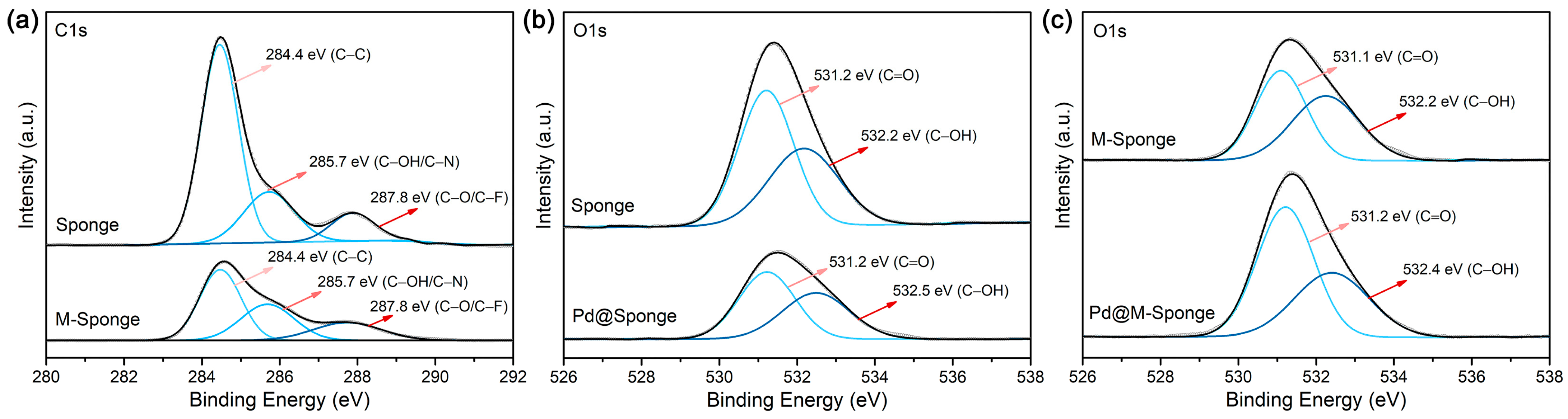
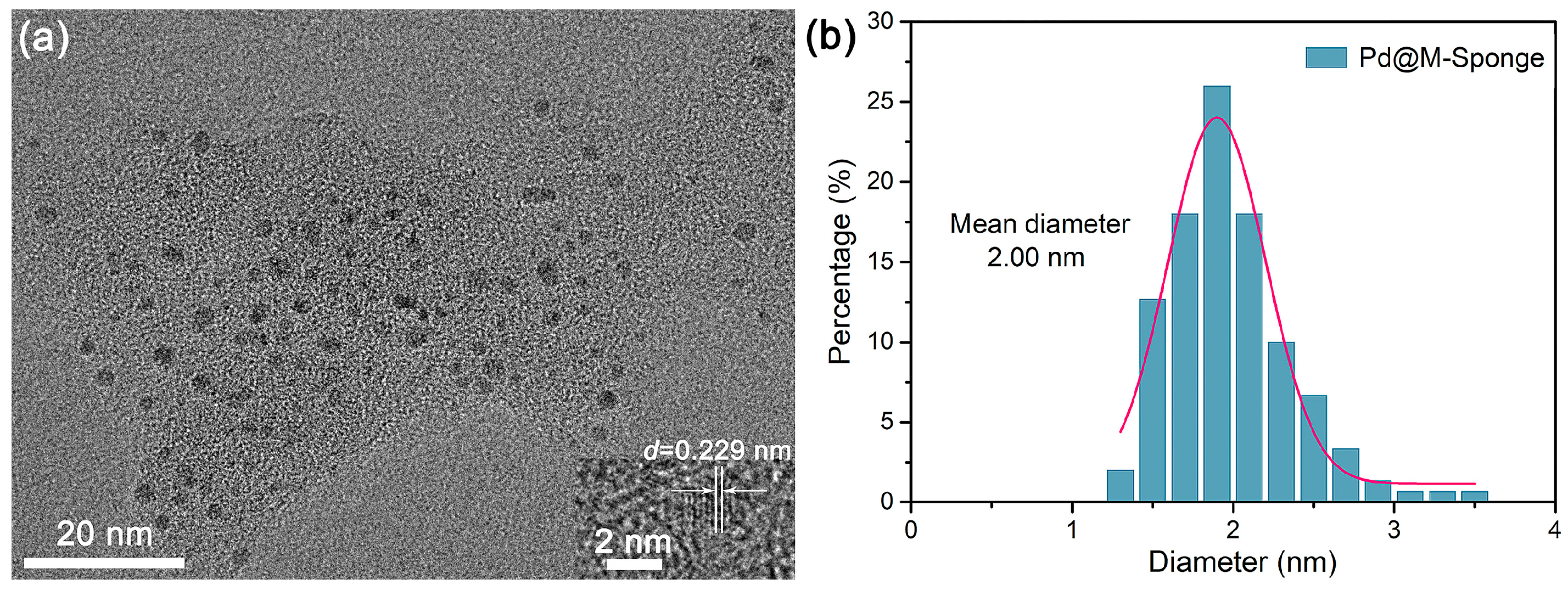
2. Results
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Pd@Sponge, Pd@M-Sponge
3.3. Characterization
3.4. Evaluation of Catalytic Activity of Catalysts in Dechlorination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Tian, F.; Dai, J.; Tian, X.; Li, G.; Liu, Y.; Chen, Z.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and 1O2 involved process. Appl. Catal. B 2021, 298, 120576. [Google Scholar] [CrossRef]
- Choi, C.; Wang, X.; Kwon, S.; Hart, J.L.; Rooney, C.L.; Harmon, N.J.; Sam, Q.P.; Cha, J.J.; Goddard III, W.A.; Elimelech, M. Efficient electrocatalytic valorization of chlorinated organic water pollutant to ethylene. Nat. Nanotechnol. 2023, 18, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, X.; Jiang, J.; Li, W. How much of the total organic halogen and developmental toxicity of chlorinated drinking water might be attributed to aromatic halogenated DBPs? Environ. Sci. Technol. 2021, 55, 5906–5916. [Google Scholar] [CrossRef]
- Yang, J.; Qi, X.; Shen, F.; Qiu, M.; Smith, R.L. Complete dechlorination of lindane over N-doped porous carbon supported Pd catalyst at room temperature and atmospheric pressure. Sci. Total Environ. 2020, 719, 137534. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, F.; Koziol, K.; Frankowski, M.; Nowicki, Ł.; Marlin, C.; Sulej-Suchomska, A.M.; Polkowska, Ż. Sea spray as a secondary source of chlorinated persistent organic pollutants? Conclusions from a comparison of seven fresh snowfall events in 2019 and 2021. Sci. Total Environ. 2023, 891, 164357. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wan, Y.; Wang, Y.; Xu, J.; Li, X. Robust photoelectrocatalytic degradation of antibiotics by organic-inorganic PDISA/Bi2WO6 S-scheme heterojunction membrane. J. Environ. Chem. Eng. 2024, 12, 112328. [Google Scholar] [CrossRef]
- Shao, F.; Gao, Y.; Xu, W.; Sun, F.; Chen, L.; Li, F.; Liu, W. Catalytic activation of formic acid using Pd nanocluster decorated graphitic carbon nitride for diclofenac reductive hydrodechlorination. J. Hazard. Mater. 2023, 446, 130677. [Google Scholar] [CrossRef]
- Long, X.; Chen, W.; Lei, C.; Xie, Q.; Zhang, F.; Huang, B. Ultrafine Pd nanoparticles@g-C3N4 for highly efficient dehalogenation of chlorinated environmental pollutant: Structure, efficacy and mechanisms. Sci. Total Environ. 2021, 775, 145178. [Google Scholar] [CrossRef]
- Guo, B.; Niu, X.; Yang, J.; Li, L.; Chen, Q.; Zhou, J. Ultra-fast catalytic hydrodechlorination of chloroacetic acids over Pd catalyst supported on CeO2 with exposed (1 1 0) plane. Chem. Eng. J. 2023, 472, 145126. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Y.; Su, H.; Liu, S.; Liu, Y.; Li, Q.; Xia, C. Novel insights into the mechanism for protic solvent promoting Pd/C-catalyzed hydrodechlorination of chlorinated organic compounds. Chem. Eng. J. 2022, 431, 133729. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, X.; Chen, Z. Zeolite imidazolate framework-8 metal-organic frameworks embedded with bimetallic Fe/Pd nanoparticles for reductive dechlorination. ACS Appl. Nano Mater. 2020, 3, 8088–8095. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Xu, Y.; Yu, Z.; Saravanamurugan, S.; Wu, Z.; Yang, S.; Luque, R. Heterogeneous (de)chlorination-enabled control of reactivity in the liquid-phase synthesis of furanic biofuel from cellulosic feedstock. Green Chem. 2020, 22, 637–645. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Zhou, C.; Bi, Y.; Long, X.; Wang, B.; Tang, Y.; Krajmalnik-Brown, R.; Rittmann, B.E. Long-term continuous co-reduction of 1,1,1-trichloroethane and trichloroethene over palladium nanoparticles spontaneously deposited on H2-transfer membranes. Environ. Sci. Technol. 2020, 55, 2057–2066. [Google Scholar] [CrossRef]
- Xu, L.; Stangland, E.E.; Dumesic, J.A.; Mavrikakis, M. Hydrodechlorination of 1,2-dichloroethane on platinum catalysts: Insights from reaction kinetics experiments, density functional theory, and microkinetic modeling. ACS Catal. 2021, 11, 7890–7905. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Li, J.; Wang, S.; Li, G.; Liu, X. Hydrodechlorination and deep hydrogenation on single-palladium-atom-based heterogeneous catalysts. Appl. Catal. B 2021, 282, 119518. [Google Scholar] [CrossRef]
- Svadlenak, S.; Wojcik, S.; Ogunlalu, O.; Vu, M.; Dor, M.; Boudouris, B.W.; Wildenschild, D.; Goulas, K.A. Upcycling of polyvinyl chloride to hydrocarbon waxes via dechlorination and catalytic hydrogenation. Appl. Catal. B 2023, 338, 123065. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, L.; Yang, H.B.; Zhang, Y.; Yao, Z.; Wu, H.; Huang, Y.; Xu, Y.; Liu, B. Atomically dispersed Pd electrocatalyst for efficient aqueous phase dechlorination reaction. Electrochim. Acta 2021, 391, 138886. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Gbadamosi, A.O.; Epelle, E.I.; Abdulrasheed, A.A.; Haq, B.; Patil, S.; Al-Shehri, D.; Kamal, M.S. Hydrogen production, transportation, utilization, and storage: Recent advances towards sustainable energy. J. Energy Storage 2023, 73, 109207. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Xu, L.; Gao, T.; Zhong, A.; Song, Z. Recent advances in nanoscale zero-valent iron (nZVI)-based advanced oxidation processes (AOPs): Applications, mechanisms, and future prospects. Nanomaterials 2023, 13, 2830. [Google Scholar] [CrossRef]
- Chi, H.Y.; Zhou, X.X.; Wu, M.R.; Shan, W.Y.; Liu, J.F.; Wan, J.Q.; Yan, B.; Liu, R. Regulating the reaction pathway of nZVI to improve the decontamination performance through magnetic spatial confinement effect. J. Hazard. Mater. 2023, 447, 130799. [Google Scholar] [CrossRef]
- Lei, M.; Tang, Y.; Wang, H.; Zhu, L.; Zhang, G.; Zhou, Y.; Tang, H. A catalytic strategy for rapid cleavage of C-Cl bond under mild conditions: Effects of active hydrogen induced by Pd nanoparticles on the complete dechlorination of chlorobenzenes. Chem. Eng. J. 2021, 419, 129510. [Google Scholar] [CrossRef]
- Naik, P.J.; Kunal, P.; Liu, D.-J.; Evans, J.W.; Slowing, I.I. Efficient transfer hydrodehalogenation of halophenols catalyzed by Pd supported on ceria. Appl. Catal. A Gen. 2023, 650, 119007. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, M.; Chen, X.; Wang, B.; Fan, W.; Yang, C.; Yang, X.; Zhang, Z.; Yang, X.; Li, C. A bismuth-based zeolitic organic framework with coordination-linked metal cages for efficient electrocatalytic CO2 reduction to HCOOH. Angew. Chem. Int. Ed. 2023, 62, e202311223. [Google Scholar] [CrossRef]
- Yang, S.; An, H.; Arnouts, S.; Wang, H.; Yu, X.; de Ruiter, J.; Bals, S.; Altantzis, T.; Weckhuysen, B.M.; van der Stam, W. Halide-guided active site exposure in bismuth electrocatalysts for selective CO2 conversion into formic acid. Nat. Catal. 2023, 6, 796–806. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Meng, F.; Yang, K.; Lin, D. Modification of Pd nanoparticles with lower work function elements for enhanced formic acid dehydrogenation and trichloroethylene dechlorination. ACS Appl. Mater. Interfaces 2022, 14, 30735–30745. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Wu, X.; Jiang, X.; Li, H.; Xu, J.; Yang, K.; Lin, D. Few-atomic zero-valent palladium ensembles for efficient reductive dehydrogenation and dehalogenation catalysis. ACS Nano 2023, 17, 22859–22871. [Google Scholar] [CrossRef] [PubMed]
- Al-Nayili, A.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Formic acid dehydrogenation using noble-metal nanoheterogeneous catalysts: Towards sustainable hydrogen-based energy. Catalysts 2022, 12, 324. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent advances in catalytic transfer hydrogenation with formic acid over heterogeneous transition metal catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Taleb, B.; Jahjah, R.; Cornu, D.; Bechelany, M.; Al Ajami, M.; Kataya, G.; Hijazi, A.; El-Dakdouki, M.H. Exploring hydrogen sources in catalytic transfer hydrogenation: A review of unsaturated compound reduction. Molecules 2023, 28, 7541. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, X.; Cui, M.; Wang, W.; Wang, P.; Johnson, G.; Nie, Y.; Lv, X.; Zhang, X.; Dong, F.; et al. Surface ligand environment boosts the electrocatalytic hydrodechlorination reaction on palladium nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 4072–4083. [Google Scholar] [CrossRef]
- Ribota Peláez, M.; Ruiz-López, E.; Domínguez, M.; Ivanova, S.; Centeno, M. Formic acid dehydrogenation over a monometallic Pd and bimetallic Pd: Co catalyst supported on activated carbon. Catalysts 2023, 13, 977. [Google Scholar] [CrossRef]
- Hafeez, S.; Harkou, E.; Adamou, P.; Barlocco, I.; Zanella, E.; Manos, G.; Al-Salem, S.M.; Chen, X.; Delgado, J.J.; Dimitratos, N.; et al. Formic acid decomposition using palladium-zinc preformed colloidal nanoparticles supported on carbon nanofibre in batch and continuous flow reactors: Experimental and computational fluid dynamics modelling studies. Nanomaterials 2023, 13, 2993. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Zhan, X.; Chen, W.; Li, C.; Zhu, L.; Dai, Y.; Deng, J. Catalytic dechlorination of carbon tetrachloride to combustible hydrocarbons by Pd-Fe hydroxides through atomic hydrogen attack and direct electron transfer. Sep. Purif. Technol. 2024, 346, 127449. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Prosvirin, I.P.; Gerasimov, E.Y. Effect of platinum precursor on the properties of Pt/N-graphene catalysts in formic acid decomposition. Catalysts 2022, 12, 1022. [Google Scholar] [CrossRef]
- Mu, D.; Li, Z.; Yu, S.; Liu, S. Hydrodechlorination of chlorophenols with methanol as hydrogen donor over carbon nanotube supported Pd-catalysts. Catal. Today 2022, 405–406, 47–56. [Google Scholar] [CrossRef]
- Ran, W.; Zhao, H.; Zhang, X.; Li, S.; Sun, J.-F.; Liu, J.; Liu, R.; Jiang, G. Critical review of Pd-catalyzed reduction process for treatment of waterborne pollutants. Environ. Sci. Technol. 2024, 58, 3079–3097. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.A.; Savina, I.N. Cryogels with noble metal nanoparticles as catalyst for “green” decomposition of chlorophenols. Inorganics 2023, 11, 23. [Google Scholar] [CrossRef]
- Qi, X.; Obata, K.; Yui, Y.; Honma, T.; Lu, X.; Ibe, M.; Takanabe, K. Potential-rate correlations of supported palladium-based catalysts for aqueous formic acid dehydrogenation. J. Am. Chem. Soc. 2024, 146, 9191–9204. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Li, Z.; Yu, S.; Liu, S. Wastewater treatment via hydro-de-heteroatoms using hydrogen donors. Catal. Today 2022, 402, 67–78. [Google Scholar] [CrossRef]
- Liu, M.; Yu, T.; Huang, R.; Qi, W.; He, Z.; Su, R. Fabrication of nanohybrids assisted by protein-based materials for catalytic applications. Catal. Sci. Technol. 2020, 10, 3515–3531. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent advances of magnetite (Fe3O4)-based magnetic materials in catalytic applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Heidari, M.K.; Fouladi, M.; Sooreh, H.A.; Tavakoli, O. Superhydrophobic and super-oleophilic natural sponge sorbent for crude oil/water separation. J. Water Process Eng. 2022, 48, 102783. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shan, C.; Chang, H.; Zhang, Z.; Huang, R.; Lee, D.W.; Qi, W.; He, Z.; Su, R. Nano-engineered natural sponge as a recyclable and deformable reactor for ultrafast conversion of pollutants from water. Chem. Eng. Sci. 2022, 247, 117049. [Google Scholar] [CrossRef]
- Pranzetti, A.; Mieszkin, S.; Iqbal, P.; Rawson, F.J.; Callow, M.E.; Callow, J.A.; Koelsch, P.; Preece, J.A.; Mendes, P.M. An electrically reversible switchable surface to control and study early bacterial adhesion dynamics in real-time. Adv. Mater. 2013, 25, 2181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.-D.; Wang, Y.; Zou, X.-H.; Yin, W.-M.; Wang, X.-Y.; Guo, Y.-R.; Pan, Q.-J. Fabrication of cellulose@ Mg(OH)2 composite filter via interfacial bonding and its trapping effect for heavy metal ions. Chem. Eng. J. 2021, 426, 130812. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Xie, Z.; Peng, X.; Guo, L.; Yu, X.; Yang, X.; Lu, Z.; Zhang, X.; Li, L. Polar bonds induced strong Pd-support electronic interaction drives remarkably enhanced oxygen reduction activity and stability. Appl. Catal. B 2022, 305, 121020. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, Q.; Ma, Y.; Guan, Q.; Jin, R.; Wang, H.; Yang, B.; Lu, J. Support-induced unusual size dependence of Pd catalysts in chemoselective hydrogenation of para-chloronitrobenzene. J. Catal. 2021, 400, 173–183. [Google Scholar] [CrossRef]
- Inbakandan, D.; Venkatesan, R.; Khan, S.A. Biosynthesis of gold nanoparticles utilizing marine sponge Acanthella elongata (Dendy, 1905). Colloids Surf. B Biointerfaces 2010, 81, 634–639. [Google Scholar] [CrossRef]
- Peng, W.; Yan, Y.; Zhang, D.; Zhou, Y.; Na, D.; Xiao, C.; Yang, C.; Wen, G.; Zhang, J. Preparation of thermal stable supported metal (Cu, Au, Pd) nanoparticles via cross-linking cellulose gel confinement strategy. Colloids Surf. Physicochem. Eng. Asp. 2021, 624, 126809. [Google Scholar] [CrossRef]
- Bekdemir, A.; Stellacci, F. A centrifugation-based physicochemical characterization method for the interaction between proteins and nanoparticles. Nat. Commun. 2016, 7, 13121. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Y.-H.; Long, X.; Roldan, M.A.; Yang, S.; Zhou, C.; Zhou, D.; Rittmann, B.E. Reductive dehalogenation of herbicides catalyzed by Pd0 NPs in a H2-based membrane catalyst-film reactor. Environ. Sci. Technol. 2022, 56, 18030–18040. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Han, J.; Kang, Y.; Mi, Y.; Wang, W. A novel N and Se codoped-carbon support anchoring Pd nanoparticles as an efficient electrocatalyst towards ethylene glycol electrooxidation. Mater. Sci. Eng. B 2020, 252, 114467. [Google Scholar] [CrossRef]
- Guo, M.; Jayakumar, S.; Luo, M.; Kong, X.; Li, C.; Li, H.; Chen, J.; Yang, Q. The promotion effect of π-π interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 2022, 13, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, L.; Chen, Q.; Mao, M.; Jiang, W.; Long, Y.; Fan, G. Oxygenated functional group-driven spontaneous fabrication of Pd nanoparticles decorated porous carbon nanosheets for electrocatalytic hydrodechlorination of 4-chlorophenol. J. Hazard. Mater. 2021, 408, 124456. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Liu, M.; Huang, R.; Che, M.; Su, R.; Qi, W.; He, Z. Tannic acid-assisted fabrication of Fe-Pd nanoparticles for stable rapid dechlorination of two organochlorides. Chem. Eng. J. 2018, 352, 716–721. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef]
- Song, C.; Wei, Y.; Qiu, Y.; Qi, Y.; Li, Y.; Kitamura, Y. Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: Experimental study. Bioresour. Technol. 2019, 272, 529–534. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, A.; Yu-ming, M.H.; Zhao, Y.; He, Y.; Xu, J.; Lu, Z. Elucidating degradation mechanisms of florfenicol in soil by stable-isotope assisted nontarget screening. J. Hazard. Mater. 2021, 403, 123974. [Google Scholar] [CrossRef]
- Qian, Z.; Na, L.; Bao-Long, W.; Tao, Z.; Peng-Fei, M.; Wei-Xiao, Z.; Sraboni, N.Z.; Zheng, M.; Ying-Qi, Z.; Liu, Y. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J. Environ. Manag. 2022, 320, 115673. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fu, X.; Liu, Y.; Zhang, Y.; Chen, S.; Li, D.; Zhang, C.; Gao, J.; Fu, Y. Electrocatalytic dechlorination of florfenicol using a Pd-loaded on blue TiO2 nanotube arrays cathode. Sep. Purif. Technol. 2023, 323, 124460. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Cui, D.; Luo, X.; Liang, B.; Yang, L.; Liu, T.; Wang, A.; Luo, S. Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol. Chem. Eng. J. 2019, 359, 894–901. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Xu, W.; Shi, H.; Hu, X.; Xu, J.; Lou, L. Adsorption-reduction coupling mechanism and reductive species during efficient florfenicol removal by modified biochar supported sulfidized nanoscale zerovalent iron. Environ. Res. 2023, 216, 114782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, G.; Liu, S.; Qin, L.; Lin, B.; Wang, M.; Yang, L.; Zheng, M. Free radical mechanism of toxic organic compound formations from o-chlorophenol. J. Hazard. Mater. 2023, 443, 130367. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, J.; Yang, Y.; Li, H.; Zheng, X.; Liu, G.; Jiang, Y. Synergistic degradation of chlorophenol pollutants by a photo-enzyme integrated catalyst. J. Environ. Chem. Eng. 2022, 10, 107909. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Sun, J.; Wu, X.; Liang, H.; Qu, Y.; Jing, L. BiFeO3/Bi2Fe4O9 S-scheme heterojunction hollow nanospheres for high-efficiency photocatalytic o-chlorophenol degradation. Appl. Catal. B 2022, 319, 121893. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Cai, Y.; Long, X.; Zhou, D.; Zhou, C.; Rittmann, B.E. Palladium (Pd0) loading-controlled catalytic activity and selectivity for chlorophenol hydrodechlorination and hydrosaturation. Environ. Sci. Technol. 2022, 56, 4447–4456. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Jin, X.; Liu, X. Selective and controlled H2 generation upon additive-free HCOOH dehydrogenation over a Pd/NCS nanocatalyst. Nanoscale 2023, 15, 15975–15981. [Google Scholar] [CrossRef]
- Song, J.; Bai, S.; Sun, Q. Strong metal-support interaction of Pd/CeO2 enhances hydrogen production from formic acid decomposition. Colloids Surf. Physicochem. Eng. Asp. 2023, 658, 130645. [Google Scholar] [CrossRef]
- Tong, L.; Song, X.; Jiang, Y.; Zhao, B.; Li, Y. Efficiently catalytic transfer hydrogenation of aryl and heteroaryl halides by ultrafine palladium nanoparticles confined into UiO-66. Int. J. Hydrogen Energy 2022, 47, 15753–15763. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G. Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 2011, 186, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Le, X.; Zhang, W.; Gu, H.; Xue, R.; Ma, J. Catalysis of the hydro-dechlorination of 4-chlorophenol by Pd(0)-modified MCM-48 mesoporous microspheres with an ultra-high surface area. New J. Chem. 2015, 39, 4519–4525. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yue, P.L.; Chen, G. Catalytic dechlorination of chlorophenols in water by palladium/iron. Water Res. 2001, 35, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, R.; Li, C.; Che, M.; Su, R.; Li, S.; Yu, J.; Qi, W.; He, Z. Continuous rapid dechlorination of p-chlorophenol by Fe-Pd nanoparticles promoted by procyanidin. Chem. Eng. Sci. 2019, 201, 121–131. [Google Scholar] [CrossRef]
- Xie, Q.; Lei, C.; Chen, W.; Huang, B. Mesoporous ferrihydrite-supported Pd nanoparticles for enhanced catalytic dehalogenation of chlorinated environmental pollutant. J. Colloid Interface Sci. 2022, 608, 2907–2920. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Zhang, T.; Yu, G.; Deng, S.; Huang, J. Catalytic hydrodechlorination of 4-chlorophenol in an aqueous solution with Pd/Ni catalyst and formic acid. Ind. Eng. Chem. Res. 2010, 49, 4561–4565. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhang, J.; Tang, T.; Xu, Q.Q.; Zhao, B.; Lu, J.; Li, R.; Han, D. Stabilizing Pd nanoparticles in supported-ionic-liquid-phase (SILP) catalyst using polydimethylsiloxane via hydrophobic structure for boosting hydrodechlorination of 4-chlorophenol. ChemistrySelect 2020, 5, 14626–14631. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Xu, L.; Pei, Y.; Niu, J. Efficient hydrogenation of p-chlorophenol and Cr(VI) driven by hydrogen rich balls over Pd/C catalysts. J. Hazard. Mater. 2022, 437, 129434. [Google Scholar] [CrossRef]
- Ruiz-Garcia, C.; Lei, Y.; Heras, F.; Elías, A.L.; Terrones, M.; Gilarranz, M.A. Functional Pd/reduced graphene oxide nanocomposites: Effect of reduction degree and doping in hydrodechlorination catalytic activity. J. Nanopart. Res. 2019, 21, 276. [Google Scholar] [CrossRef]
- Mao, M.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Active site and adsorption behavior engineering of subsize PdNi nanoparticles for boosting electrocatalytic hydrodechlorination of 4-chlorophenol. Appl. Surf. Sci. 2022, 600, 153988. [Google Scholar] [CrossRef]
- Escobedo, E.; Kim, J.; Oh, D.; Cho, K.; Chang, Y.-S. Electrocatalytic dehalogenation of aqueous pollutants by dealloyed nanoporous Pd/Ti cathode. Catal. Today 2021, 361, 63–68. [Google Scholar] [CrossRef]
- Feng, S.; Huang, K.; Huang, Z.; Liu, G.; Zhang, G.; Gou, G. Highly selective extraction of Pd(II) using functionalized molecule of 2-[(2-ethylhexyl)thio]benzoxazole and its Pd(II) extraction mechanism. J. Mol. Struct. 2021, 1230, 129639. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Pei, Y.; Luo, X. Growing Pd NPs on cellulose microspheres via in-situ reduction for catalytic decolorization of methylene blue. Int. J. Biol. Macromol. 2021, 166, 1419–1428. [Google Scholar] [CrossRef]

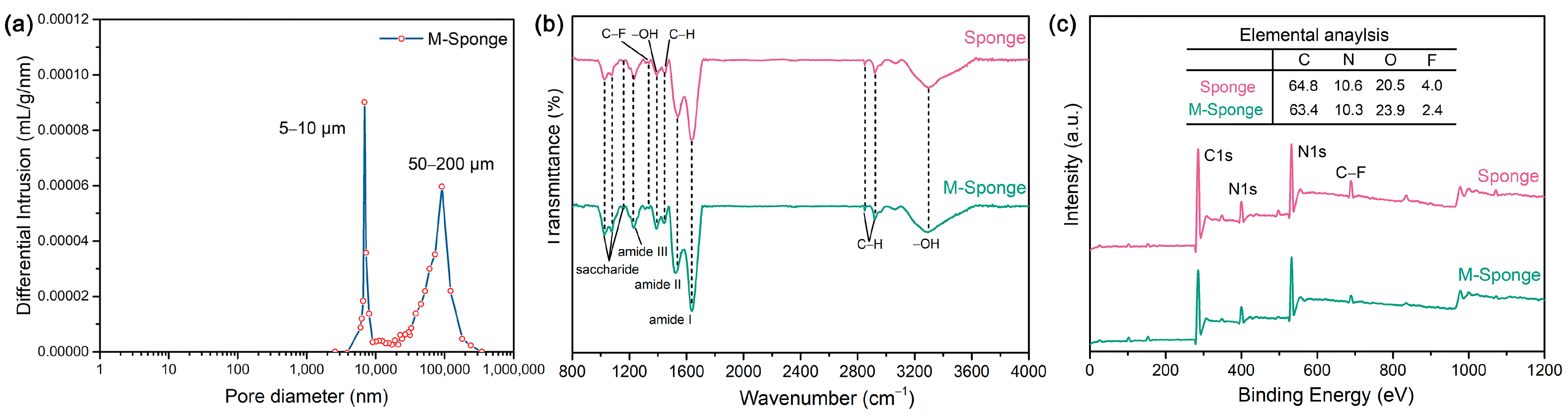

| Elemental Composition by XPS | ||||
|---|---|---|---|---|
| Sample | Elements (at.%) | |||
| C | N | O | F | |
| Sponge | 64.8 | 10.6 | 20.5 | 4.0 |
| M-Sponge | 63.4 | 10.3 | 23.9 | 2.4 |
| Target | Method | Catalyst | Temperature (°C) | Concentration (mg/L) | Reductant | Molar Ratio of Target to Reductant | k (10−2 min−1) | Recycle Times | Reactivity Retention Rate | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
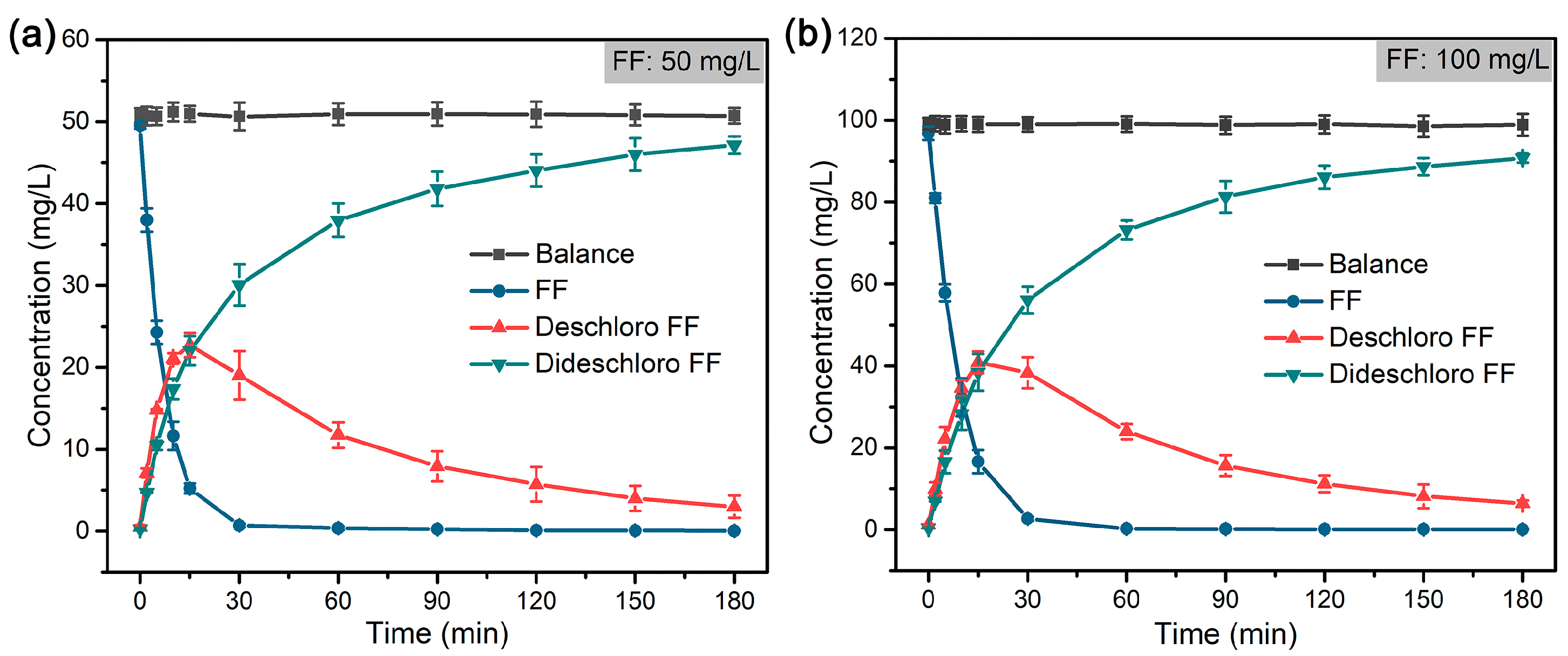
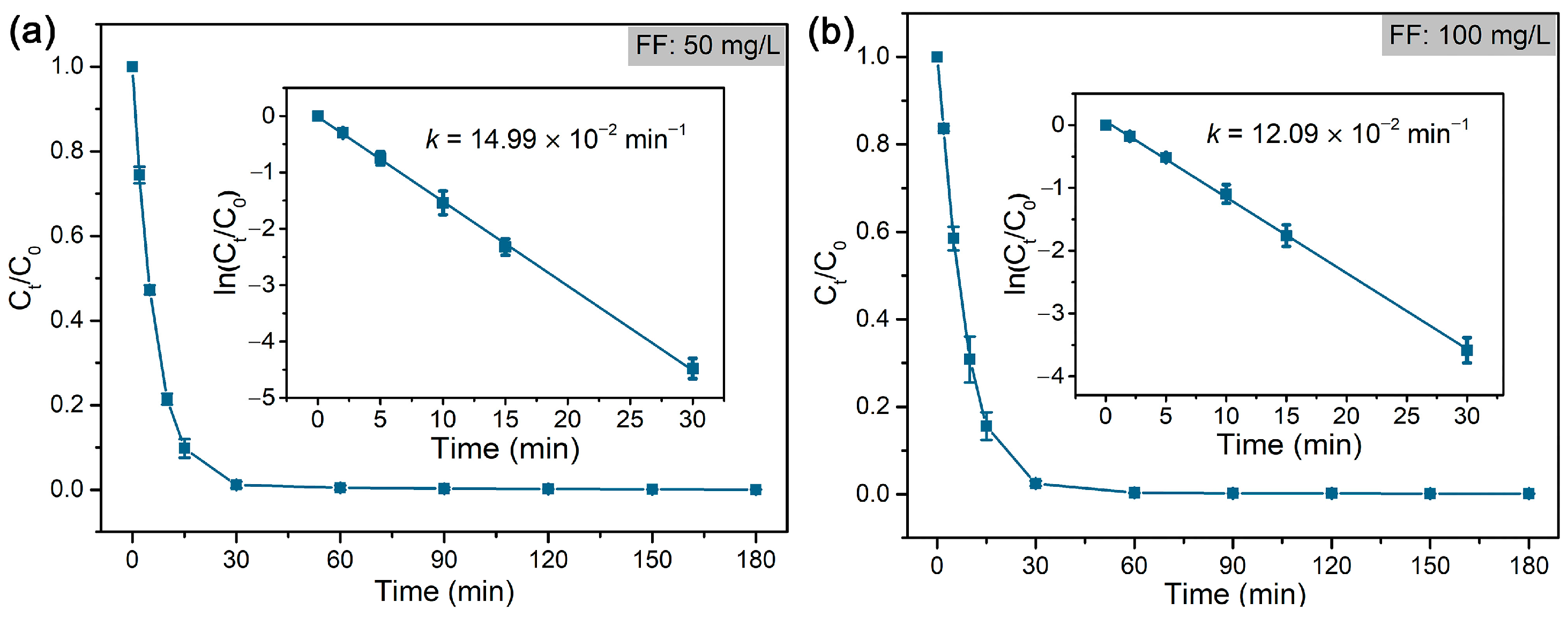
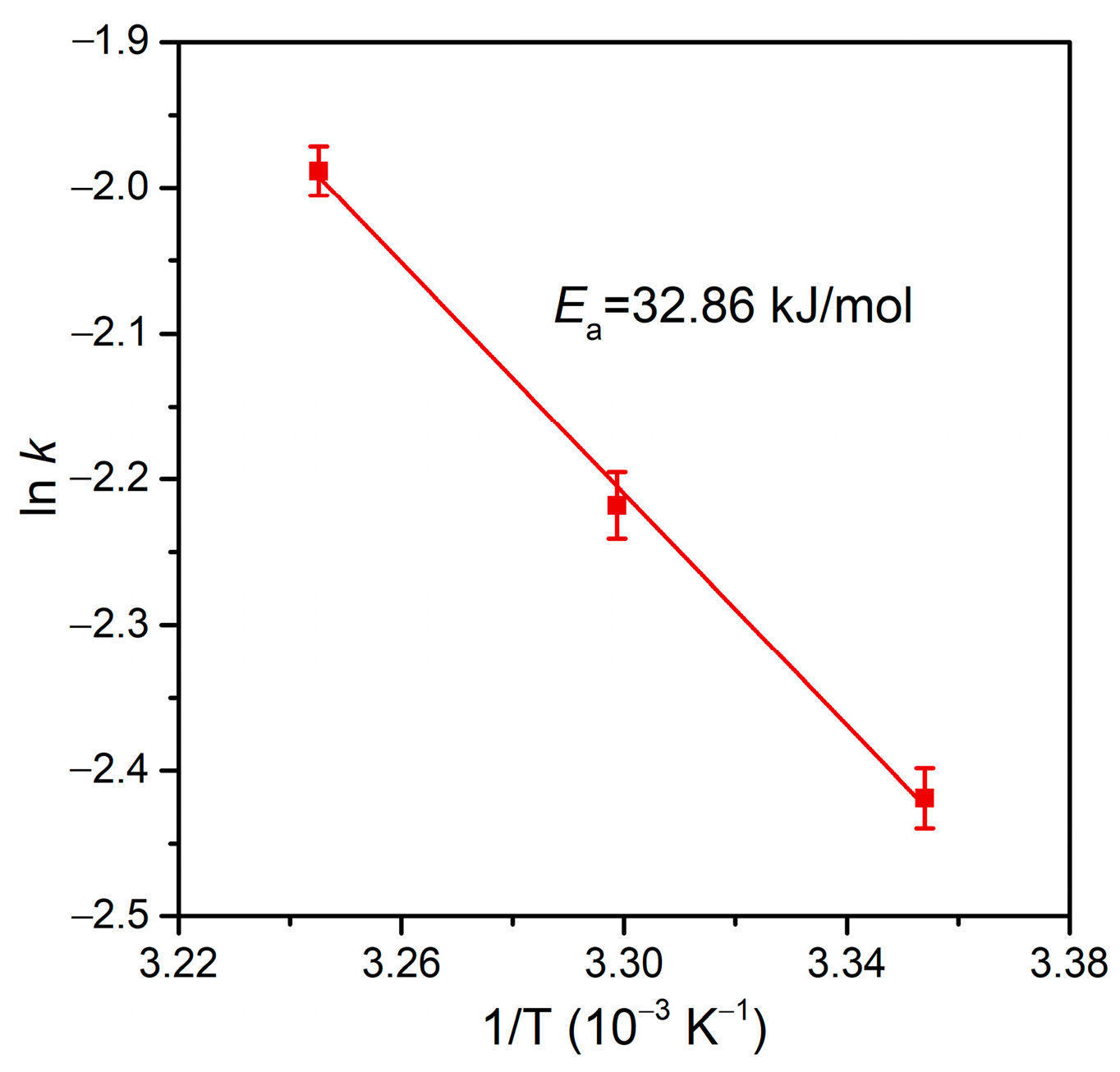
| FF | Hydrodechlorination | Pd@M-Sponge | 25 | 50 | HCOOH | 1:280 | 14.99 | – | – | This work |
| 100 | 1:140 | 12.09 | ||||||||
| Electrocatalytic dechlorination | Pd-TiO2 | 25 ± 5 | 20 | H* | – | 0.035 | 5 | 97% | [62] | |
| Pd@Ni-foam | 25 ± 1 | 20 | H* | – | 0.044 | 20 | 95% | [63] | ||
| Reductive dechlorination | S-nZVI | 25 | 100 | nZVI | 1:60 | 1.9 | – | – | [58] | |
| S-nZVI | 25 | 50 | nZVI | 1:190 | 5.7 | 5 | 21% | [64] | ||
| o-CP | Hydrodechlorination | Pd@M-Sponge | 25 | 50 | HCOOH | 1:100 | 20.64 | – | – | This work |
| 50 | 1:50 | 15.42 | ||||||||
| Pd@UiO-66 | 35 | 128 | NH4COOH | 1:10 | ~1.54 | – | – | [72] | ||
| Pd/N-MWCNTs | 25 | 180 | CH3OH | 1:1764 | 0.06 | – | – | [35] | ||
| 100 | 0.39 | |||||||||
| Pd/MSCN | −15 | 643 | H2 | – | 0.23 | 5 | ~98% | [73] | ||
| Pd/M48N | – | 128 | H2 | – | 0.34 | 5 | ~98% | [74] | ||
| Electrocatalytic dechlorination | Pd/Ti | 30 | 25 | H* | – | 0.85 | 10 | 94.5% | [83] | |
| Pd/PCN | 25 | 100 | H* | – | 2.7 | – | – | [55] | ||
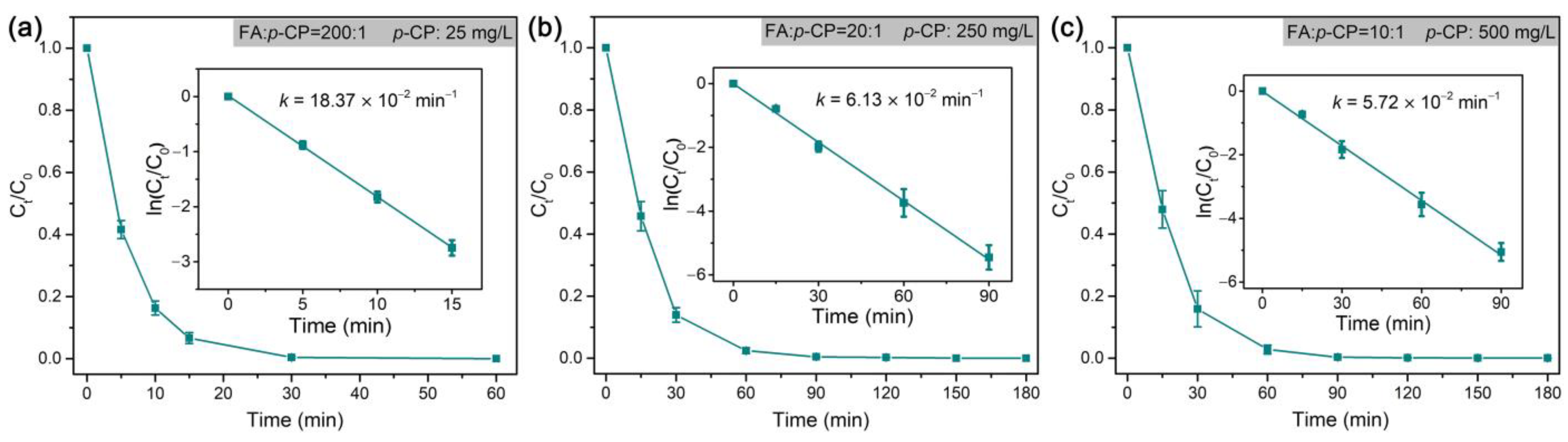
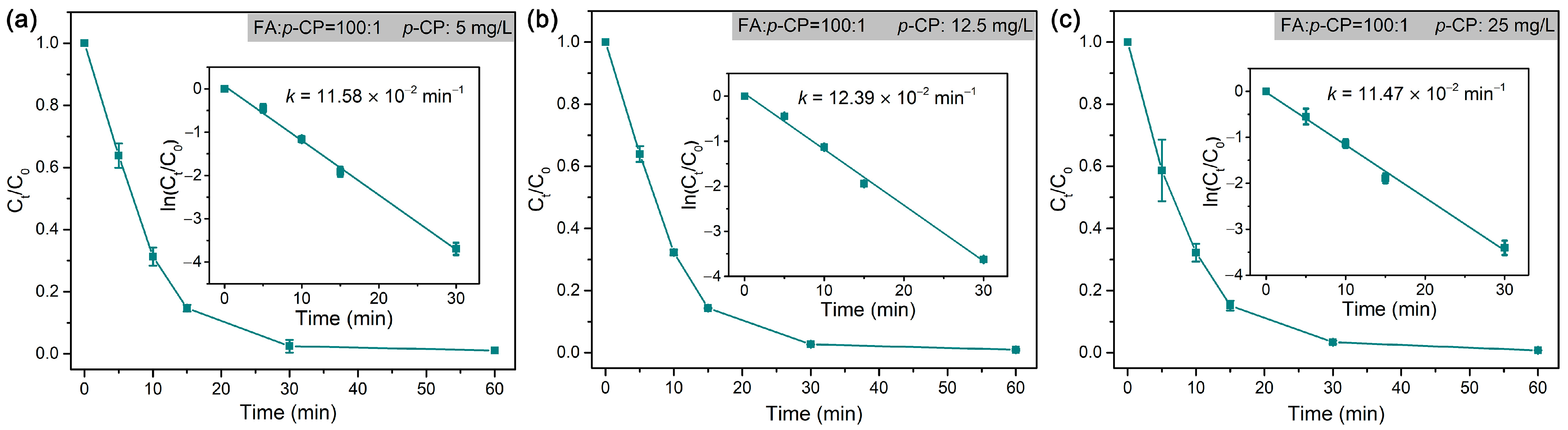
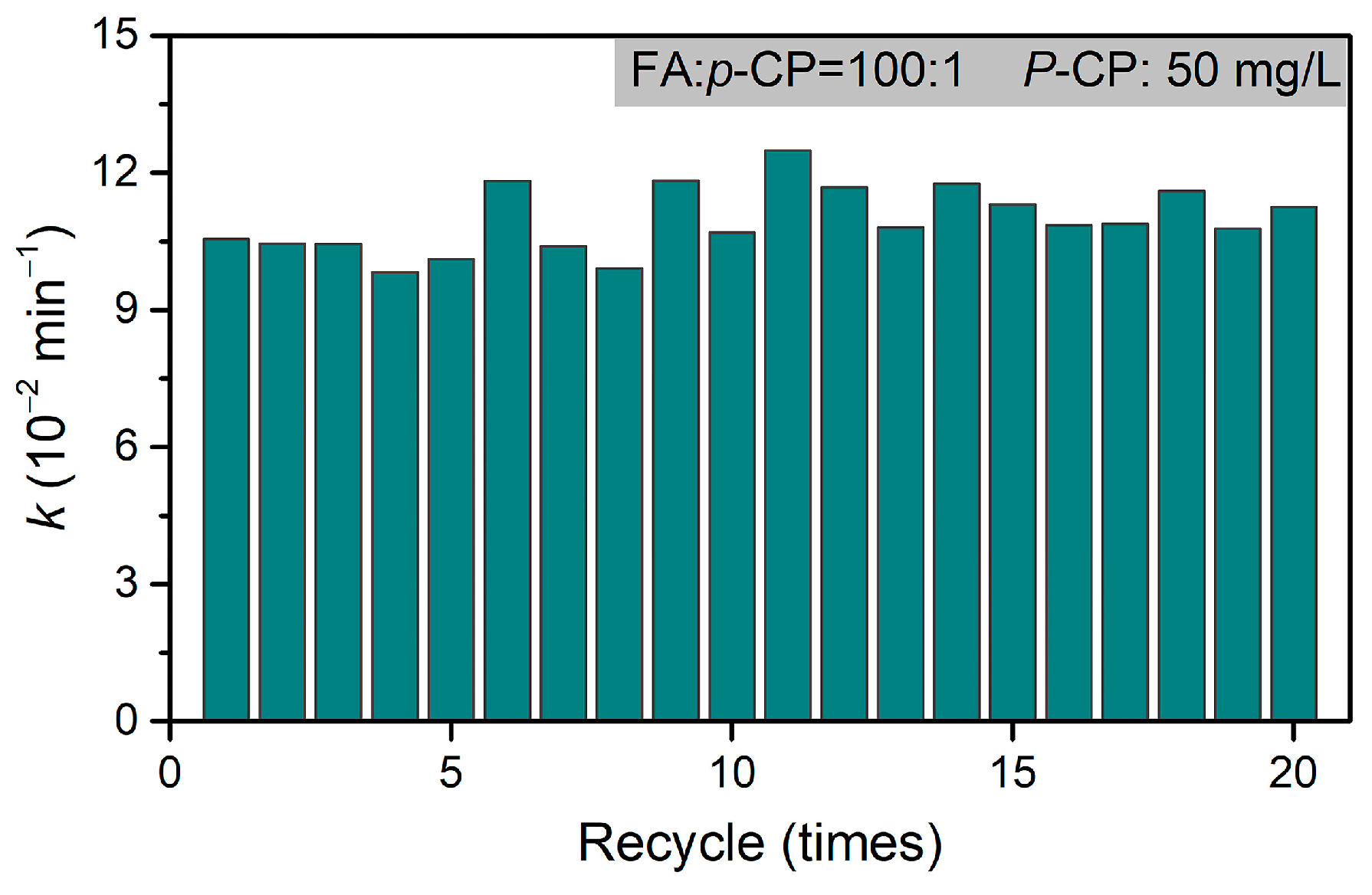
| p-CP | Hydrodechlorination | Pd@M-Sponge | 25 | 5 | HCOOH | 1:100 | 11.58 | 20 | ~100% | This work |
| 12.5 | 1:100 | 12.39 | ||||||||
| 25 | 1:100 | 11.47 | ||||||||
| 25 | 1:200 | 18.37 | ||||||||
| 50 | 1:100 | ~11 | ||||||||
| 250 | 1:20 | 6.13 | ||||||||
| 500 | 1:10 | 5.72 | ||||||||
| Pd@UiO-66 | 35 | 128 | HCOOH | 1:10 | 0.33 | 5 | 95.29% | [72] | ||
| 128 | NH4COOH | 1:10 | 1.79 | |||||||
| Pd/Ni | 25 ± 3 | 50 | HCOOH | 1:51 | 2.7 | 3 | 97% | [78] | ||
| Pd/AC-P | 30 | 4000 | HCOOH + HCOONa | 1:29 | 5.3 | 5 | 94% | [79] | ||
| Pd/C | 25 | 30 | H2 | – | 2.9 | 5 | – | [80] | ||
| Pd/rGOEt | 30 | 100 | H2 | – | 5.64 | – | – | [81] | ||
| Electrocatalytic dechlorination | PdNi NPs | – | 100 | H* | – | 2.3 | 5 | 85% | [82] | |
| Pd/PCN | 25 | 100 | H* | – | 3.9 | 5 | 94.5% | [55] |
| Pd@Sponge | Pd@M-Sponge | |
|---|---|---|
| Before application (wt%) | 3.5 | 3.9 |
| After application (wt%) | 2.9 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Chen, G.; Song, Z.; He, Z.; Zhong, A.; Cui, M. Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid. Catalysts 2024, 14, 424. https://doi.org/10.3390/catal14070424
Liu M, Chen G, Song Z, He Z, Zhong A, Cui M. Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid. Catalysts. 2024; 14(7):424. https://doi.org/10.3390/catal14070424
Chicago/Turabian StyleLiu, Mingyue, Gang Chen, Zhenjun Song, Zhicai He, Aiguo Zhong, and Mei Cui. 2024. "Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid" Catalysts 14, no. 7: 424. https://doi.org/10.3390/catal14070424
APA StyleLiu, M., Chen, G., Song, Z., He, Z., Zhong, A., & Cui, M. (2024). Catalytic Dechlorination of Three Organochlorides by Recyclable Nano-Palladium-Engineered Natural Sponge with Formic Acid. Catalysts, 14(7), 424. https://doi.org/10.3390/catal14070424






