Abstract
Catalytic dechlorination of organic chlorides by palladium (Pd) with HCOOH represents one of the most effective and promising techniques for environmental remediation. In this study, we adopted alkaline-modified porous natural sponge as support of a Pd nanocatalyst (Pd@M-Sponge) and HCOOH as a hydrogen source for the hydrodechlorination of florfenicol (FF), o-chlorophenol (o-CP), and p-chlorophenol (p-CP). Favorable conversion efficiency of FF, o-CP, and p-CP was achieved at 25 °C and atmospheric pressure attributed to the small diameter and high catalytic reactivity of the prepared Pd NPs, in addition to the slight internal mass transfer limitation of the prepared Pd@M-Sponge. High reaction rate constants were obtained even in the conditions of a low molar ratio of HCOOH to p-CP (10:1) and a high concentration of p-CP (500 mg/L). The prepared catalyst also demonstrated superior recyclability without any obvious decrease in catalytic reactivity in 20 successive p-CP dechlorination cycles. This work provides an ideal recyclable and cost-effective catalyst based on renewable and biocompatible natural material for the catalytic hydrodechlorination of chlorinated organic pollutants with formic acid and a new view for the exploration and designing of highly reactive and stable catalysts for hydrodechlorination.
1. Introduction
Organic chlorides have been classified as persistent organic pollutants owing to their high toxicity, carcinogenic, bio-refractory, and bio-accumulative characteristics [1,2,3]. Although some of these chemicals have been banned, the existing contaminated environmental compartments can serve as “secondary sources” for the diffusion and migration of chlorinated organics, continuously posing serious toxicological risks to both human beings and the environment [4,5]. Hence, the development of practical methods for the elimination of chlorinated organics in contaminated environmental compartments is an important and urgent environmental topic.
Many techniques have been proposed for the remediation of chlorinated organics, such as adsorption, advanced oxidation processes, photocatalytic degradation, biological degradation, and chemical reduction [6,7]. Among these strategies, catalytic hydrodechlorination can significantly diminish or even eliminate the toxicity of the chlorinated organics and generate less or even no harmful/toxic species, which has been regarded as a cost-effective and environmentally friendly technology, giving promise for non-destructive and high-efficient remediation of organic chlorides [8,9].
Generally, hydrogen gas (H2) and nano-zero-valent iron (nZVI) is most commonly employed as hydrogen source for the hydrodechlorination [10,11,12]. Direct catalytic hydrodechlorination with pressurized H2 gas catalyzed by Pd or Pt-based catalysts has been extensively studied in the dechlorination of trichloroethane, dichloroethane, chlorophenol, and polyvinyl chloride, etc. [13,14,15,16]. The application of H2 in hydrodechlorination is subject to its low utilization efficiency and the potential risks during its production, transportation, and utilization [17,18]. nZVI and its composites have been widely adopted in the chemical dechlorination of chlorinated organics, while the reactivity of nZVI inevitably suffers from oxidation by water and dissolved oxygen [19,20]. Notably, catalytic dechlorination via transfer hydrogenation has attracted growing research interests, particularly the employing of liquid hydrogen donors, such as organic acids and alcohols [4,21,22]. Among these liquid hydrogen donors, formic acid (HCOOH) seems to be a superior hydrogen carrier owing to its good stability in transportation and storage and can be cheaply and abundantly produced via the oxidation of methanol. Besides that, the emerging green technology of electrocatalytic CO2 conversion for the production of formic acid may provide a realistic expectation for the supply of sustainable formic acid in the future [23,24]. Therefore, formic acid is considered as an ideal hydrogen carrier for dechlorination via catalytic transfer hydrogenation, in consideration of its nontoxic nature, high storage capacity (21.5 mol/L), and capability of releasing H2 via dehydrogenation (Equation (1)) [25,26,27]. Catalytic transfer hydrogenation with formic acid plays an increasing role in the conversion of various organics by hydrogenation of unsaturated groups (C=N, C=C, N=O, C=O, C≡N, C≡C, etc.), hydrogenolysis of C–O, and hydrodechlorination of organic chlorides, etc. [28,29].
Dechlorination via transfer hydrogenation with formic acid is a method that deprives H2 from HCOOH for the hydrodechlorination of chlorinated organics [22]. To continuously produce H2 from HCOOH supplied for hydrodechlorination, noble metals, including Pd, Ru, Pt, etc., are usually essential in dehydrogenation [30,31]. Among these metals, Pd-based nanomaterials are considered the most effective catalysts for the dehydrogenation of HCOOH [32,33,34]. Meanwhile, Pd is an effective catalyst for the activation of H2 and its subsequent hydrodechlorination, making it a unique candidate for the catalytic hydrodechlorination of chlorinated organics by formic acid [9,35,36]. Catalytic-transfer hydrodechlorination with HCOOH by a Pd-based catalyst has been applied in the dechlorination of lindane, diclofenac, chlorophenols, and chloroethylene [7,25,28,37]. Despite that, a large number of supported Pd catalysts (SiO2, ZrO2, activated carbon, etc.) have been developed in the dehydrogenation of HCOOH for hydrogen [38], Pd-based catalysts with high reactivity, stability, and recyclability remain in need of exploration in catalytic-transfer hydrodechlorination [39].
The catalytic activities of homogeneous nanocatalysts are generally dependent on the size of the nanoparticles (NPs) and the dispersion state of the nanocatalysts. Small-diameter NPs can be synthesized via regulation by stabilizers during growth, although they inevitably tend to aggregate in solutions, resulting from their high surface energy and van der Waals interactions [40]. Therefore, despite the fact that homogeneous catalysts usually have low diffusion resistance for reactants due to their uniform dispersion in the reaction system, their catalytic reactivities always suffer from an aggregation of NPs. Heterogeneous catalysts that support NPs on/in solid supports can significantly suppress the aggregation of NPs and have advantages in the separation and recycling of catalysts [32,40]. While various applicated supports, such as activated carbon, insoluble polymeric resins, silica, zeolites, and so on, may make the prepared heterogeneous catalysts susceptible to diffusional limitations, attributed to the lack of well-connected pores for the mass transfer of reactants or the insufficient utilization of internal nanocatalysts. In addition, special efforts are required for the separation and recycling of nanocatalysts supported in these powders or monodisperse particles. Accordingly, a monobloc support has (i) abundant functional groups serving as stabilizers for the growth of high-reactivity NPs and their stabilization; (ii) interconnected porous for the unlimited diffusion of reactants from solutions to catalytic active sites; and (iii) convenience in catalyst recovery and recycling, which is even more appealing [7,41].
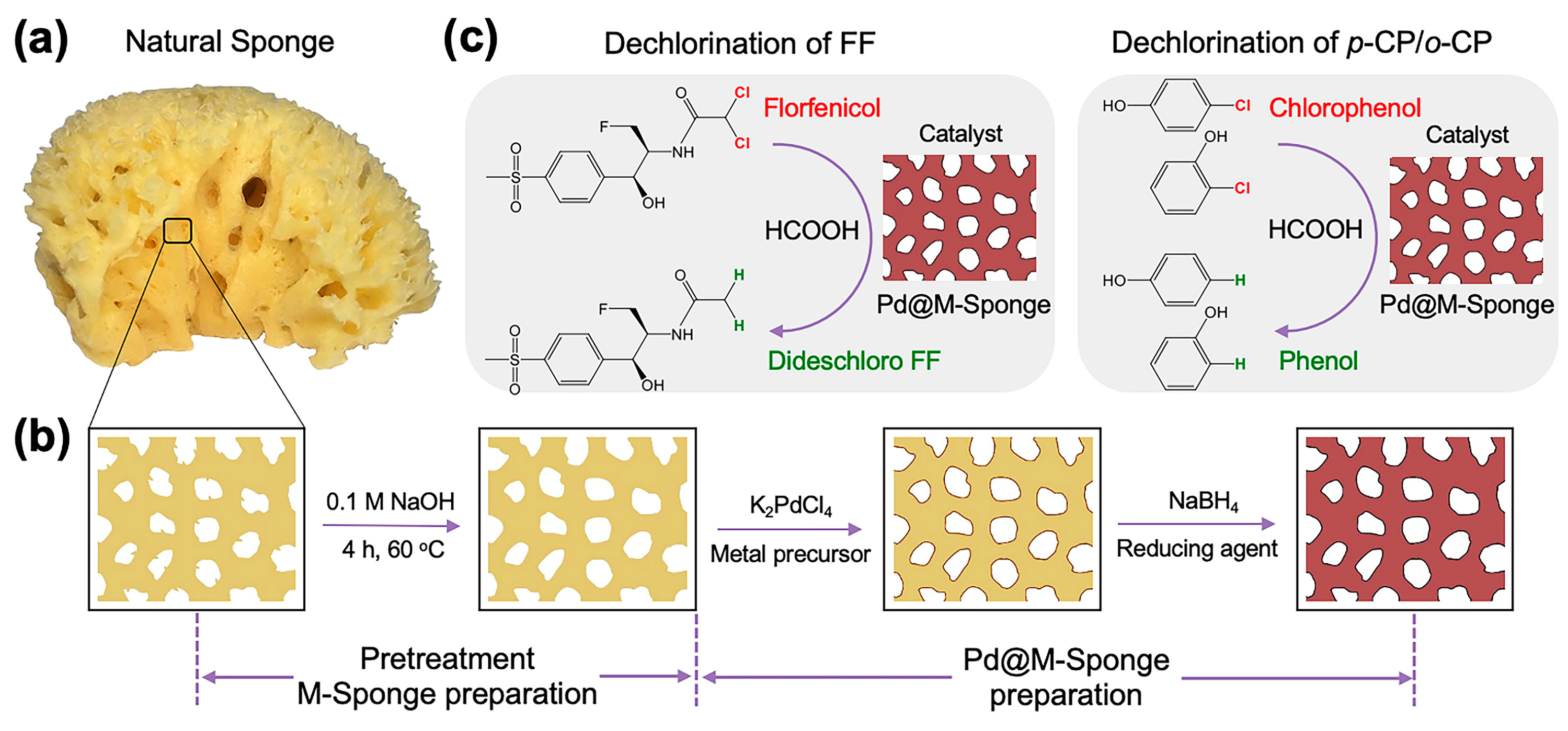
Natural sponge is an old cellulose-based marine organism and thus consists of an abundance of dehydrated tissues of multicellular animals, which provide abundant functional groups for the regulable formation and stabilization of NPs [42]. Typically, a monobloc natural sponge is split into interconnected open-cell pores by its three-dimensional interpenetrating microfibers, which has an apparent advantage in mass transfer and has inspired numerous studies on artificial materials by imitating its fiber-pore structure [43]. Consequently, this abundant and renewable material is an ideal support for the designing of heterogeneous catalysts with high catalytic reactivity and excellent recyclability. Our previous work found that sponge was a preferable support for the loading of Au and Ag nanocatalysts, which have high reactivity and stability in batch-wise, push-in-out, and continuous-flow reaction systems for the catalytic reduction of pollutants, owing to its compressible properties [44]. Herein, we adopted alkaline-modified natural sponge as support for the loading of Pd nanocatalysts for the hydrodechlorination of polychlorinated aliphatic hydrocarbon pollutants (florfenicol, FF) and two chlorinated aromatic pollutants (o-chlorophenol, o-CP; p-chlorophenol, p-CP) with HCOOH as a H2 donor (Scheme 1).
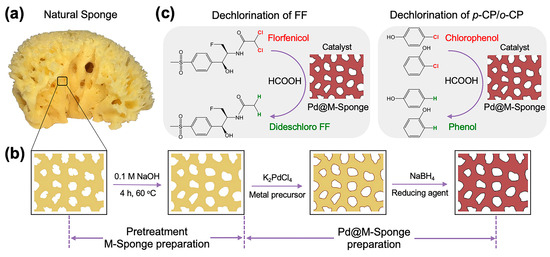
Scheme 1.
Schematic illustration of the preparation of sponge-supported a Pd-based catalyst and its applications in the dechlorination of florfenicol and chlorophenol. (a) Photo of a typical natural sponge; (b) Modification of natural sponge by NaOH and the subsequent preparation of Pd@M-Sponge [44]. The change in the color from yellow to dark red indicates the formation of Pd NPs in the sponge. Application of the prepared catalyst in the hydrodechlorination of (c) florfenicol and p-chlorophenol and o-chlorophenol.
2. Results
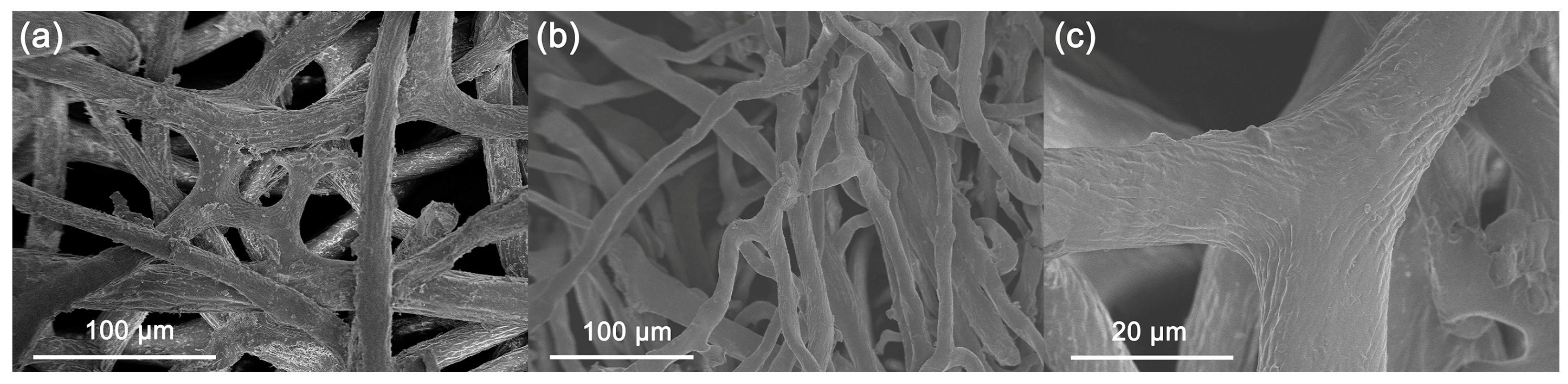
The surfaces of the fibers of natural sponge always attach with tissue flakes (Figure 1), the desquamation of which during catalytic application may lead to the loss of nanocatalyst and thus be adverse to the recycling of the catalyst [44]. Therefore, the natural sponge was first treated with alkaline. The pretreatment of soaking sponge in alkaline conditions (M-Sponge) can effectively remove these flakes on the fiber surfaces of the sponge and is beneficial for the recycling of the nano-engineered sponge (Figure 1c).

Figure 1.
SEM images of the natural sponge (a) before and after (b,c) NaOH pretreatment.
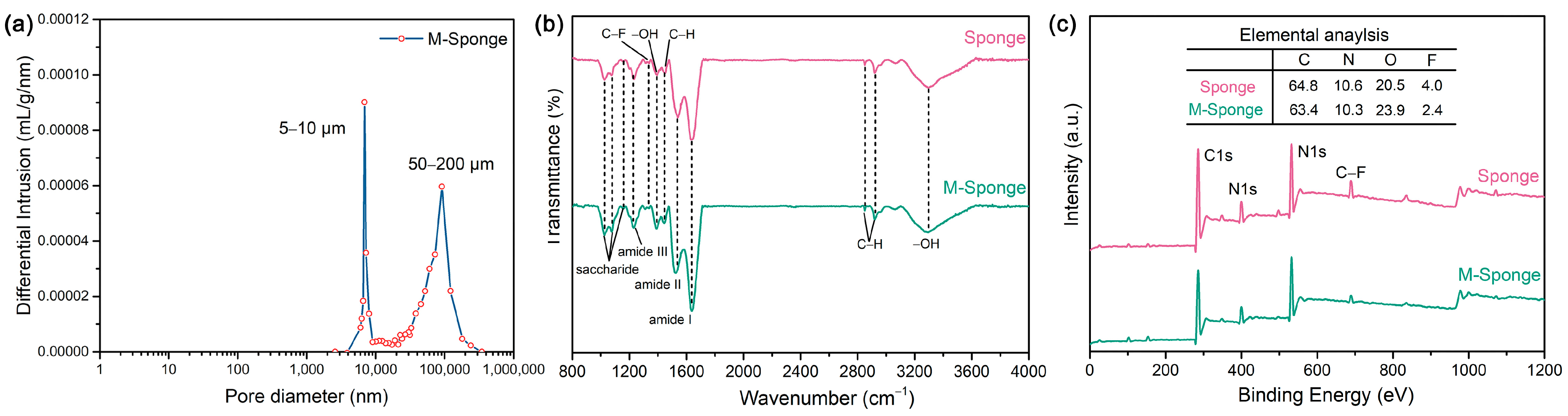
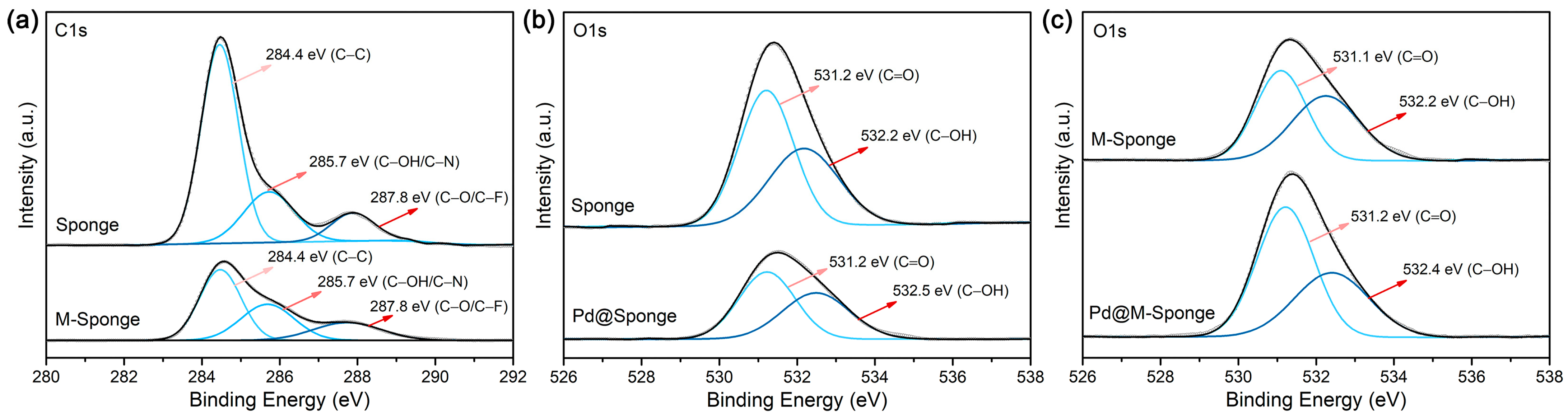
After being hydrolyzed by alkaline, the surface functional groups, which are not only beneficial for the complexation of metal precursors but also effectively reinforce the interaction between the support and Pd NPs, can be improved while preserving the porous structure of the sponge. Figure 2a shows that the pretreated sponge has an abundance of micropores with size distributions of 5–10 μm and 50–200 μm. The FTIR and XPS spectra of sponge indicate the existence of hydroxyl groups (Figure 2b,c), the content of which was further increased after pretreatment (Table 1), attributed to the presumable hydrolysis of C–F groups and the formation of hydroxyl groups during the pretreatment [45]. The high-resolution spectra of C1s and O1s of sponge and M-Sponge (Figure 3) also suggest the presence of C–OH (285.7 eV, 532.2 eV) [46]. Compared with sponge, the peak of C–OH of Pd NP-loaded sponge slightly shifted to high binding energy (~0.3 eV, Figure 3), implying the interaction of NPs with the hydroxyl groups [47,48].
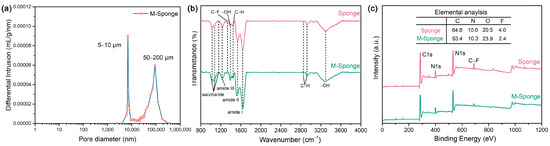
Figure 2.
(a) Pore size distribution plots obtained by mercury intrusion porosimetry for M-Sponge. (b) FTIR spectra and (c) XPS survey spectra of Sponge and M-Sponge.

Table 1.
Elemental analysis of sponge and M-Sponge from XPS.
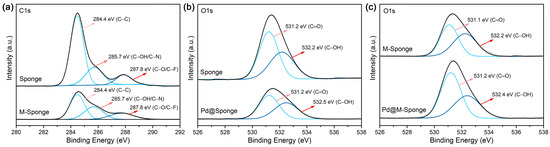
Figure 3.
XPS spectra of sponge and sponge composites. (a) C1s of Sponge and M-Sponge. (b) O1s of Sponge and Pd@Sponge. (c) O1s of M-Sponge and Pd@M-Sponge. The grey lines represent the experimental XPS curve, the blue lines represent the fitting curves, and the black lines represent the sum of the fitting curves.
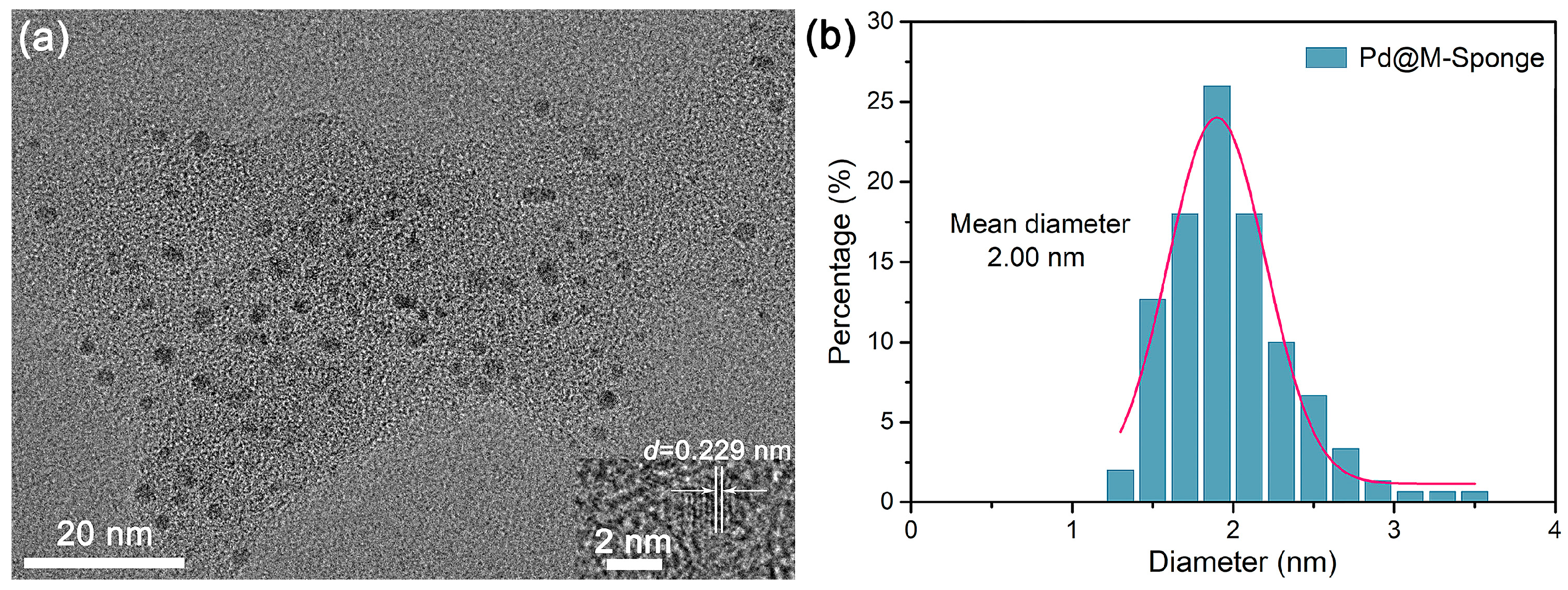
The composite of pretreated sponge decorated with Pd NPs (Pd@M-Sponge) was synthesized by the common NaBH4 reduction method. Attributing to that, the abundant –OH groups and proteins in sponge fibers [44,49] can serve as stabilizers for the binding of metal precursors and the subsequent formation and stabilization of Pd NPs [50,51], the synthesized Pd NPs have a narrow size distribution and a small mean diameter (Figure 4). The d spacing value of the Pd NP was 0.229 nm, corresponding to its [111] crystal surface [52,53]. While Pd@Sponge possessed a broad size distribution and a mean size of 2.97 nm (Figure S1). The variation in the size distribution of Pd NPs between Pd@Sponge and Pd@M-Sponge may be attributed to the enriched functional groups in M-Sponge after pretreatment, such as –OH and C=O. These oxygenated functional groups are believed to be crucial in the formation and stabilization of Pd NPs by acting as stabilizing agents [54,55], which promotes the generation of well-dispersed Pd NPs and prevents them from agglomerating, leading to a small particle size and uniform dispersion of Pd NPs [56]. Moreover, benefiting from the stabilization of Pd NPs by oxygenated functional groups, the well-dispersed Pd NPs in Pd@M-Sponge has almost no change after the dechlorination reaction (Figure S1).
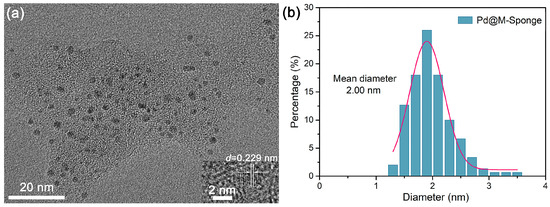
Figure 4.
Typical TEM image of (a) Pd@M-Sponge and (b) the corresponding size distribution of Pd NPs. The insert is the HRTEM image of Pd NP. The pink line is the fitting curve of the size distribution of Pd NPs.
The catalytic dechlorination performance of Pd@M-Sponge was first conducted in the dechlorination of a typical polychlorinated aliphatic hydrocarbon compound, FF [57]. FF is a broad-spectrum antibiotic that is effective for many Gram-positive and Gram-negative bacteria and thus has been used worldwide as one of the permitted antibiotics in aquaculture and cannot be effectively remediated by traditional water treatment technologies [58,59]. As it is difficult to be degraded and may cause deleterious effects on wild aquatic organisms even in low concentrations, the removal of florfenicol from aquatic systems is a significant issue [60,61]. Yet, there is seldom relevant research reporting on the dechlorination of FF by Pd NPs with HCOOH currently.
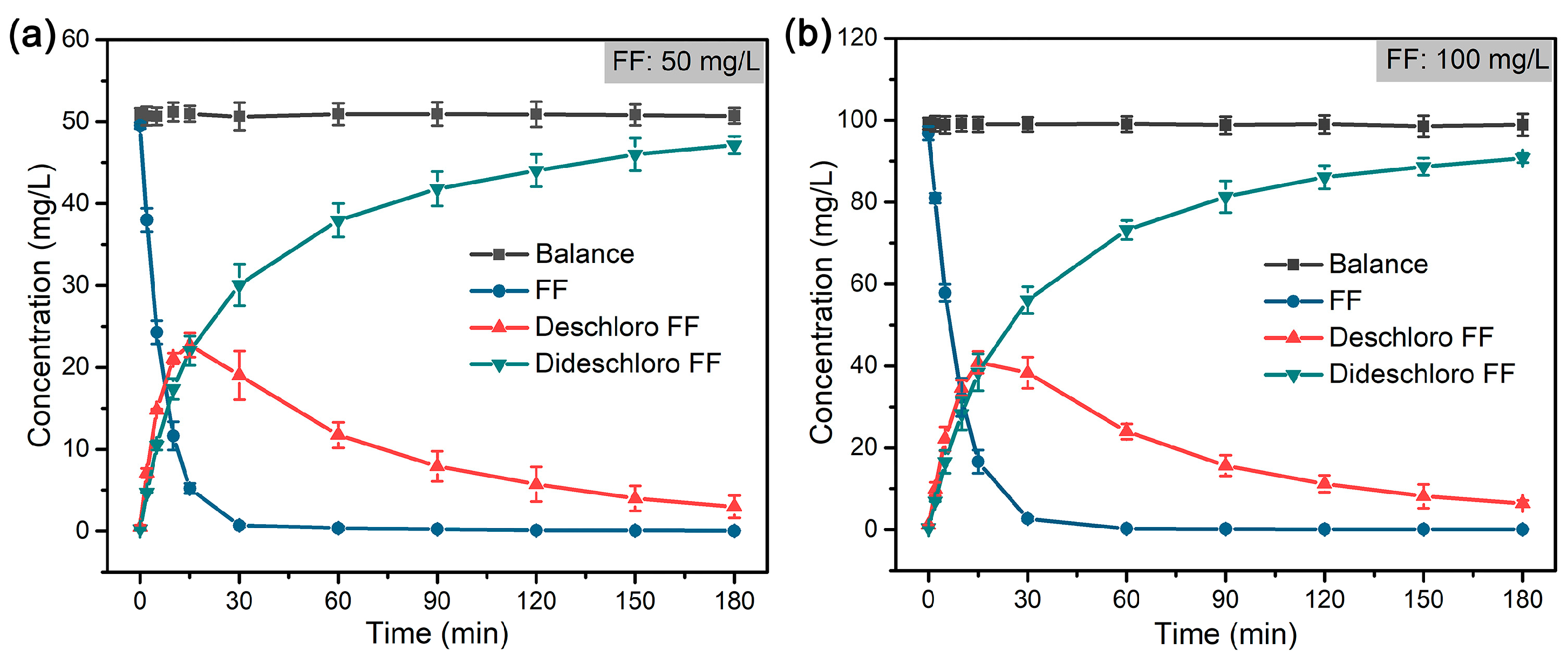
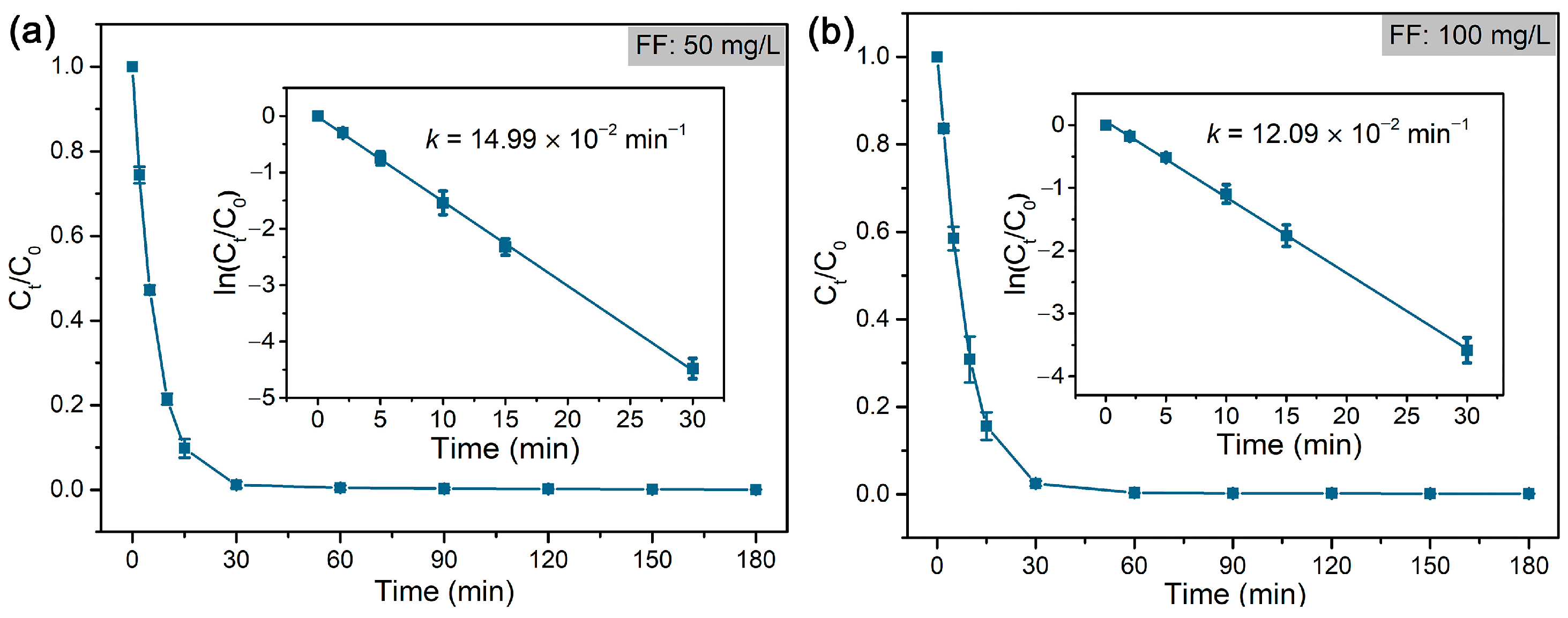
As shown in Figure 5, FF was rapidly dechlorinated and formed deschloro FF (with one Cl atom) in 30 min, which was almost completely reduced to dideschloro FF (with no Cl atom) in 120 min. The dechlorination of FF follows a pseudo-first-order reaction, and the rate constant (k) was calculated accordingly (Figure 6). The results indicated that a low concentration of FF (50 mg/L) displayed a higher rate constant (14.99 × 10−2 min−1) compared to that (12.09 × 10−2 min−1) of high concentration of FF (100 mg/L) at the same concentration of formic acid (~39 mM). Compared with the reported literature on the dechlorination of FF that mostly adopts the electrocatalytic method or reductive dechlorination by nZVI materials [58,62,63,64], the hydrodechlorination of FF with formic acid by Pd@M-Sponge may be more advantageous for future potential practical applications, owing to the high reaction rate constant, cheap and stable character of the hydrogen carrier, and convenient recycling of the catalyst.
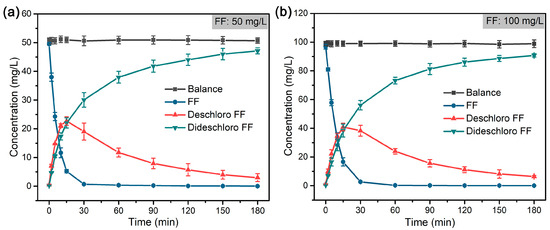
Figure 5.
Mass balance of FF and dechlorinated products during the dechlorination of FF by Pd@M-Sponge at FF concentration of (a) 50 mg/L and (b) 100 mg/L. The concentration of formic acid is ~39 mM, and the reaction temperature is 25 °C.
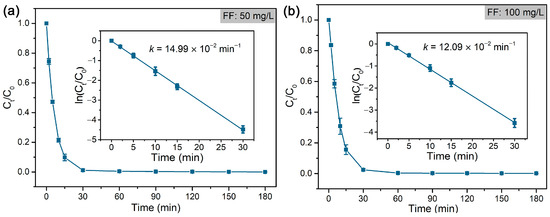
Figure 6.
Rate constants of the dechlorination of FF by Pd@M-Sponge at FF concentration of (a) 50 mg/L and (b) 100 mg/L. The reaction temperature is 25 °C. The insert figures are the corresponding linear fittings.
We further investigated the catalytic reactivity of Pd@M-Sponge in the dechlorination of two typical chlorinated aromatic pollutants, o-CP and p-CP. Chlorophenols have been widely used as chemical raw materials, intermediates, and solvents in the production of antiseptics, disinfectants, herbicides, and pesticides and thus have attracted great attention in the areas of chemistry, pharmaceutical science, and toxicology [65,66]. However, the abuse of chlorophenols has led to their ubiquitous distribution in the environment, and chlorophenols have become highly hazardous pollutants, as they have limited biodegradability and are carcinogenic, teratogenic, and cytotoxic to aquatic and terrestrial life [67,68]. Therefore, they have been listed as priority pollutants by the United States Environmental Protection Agency [69].
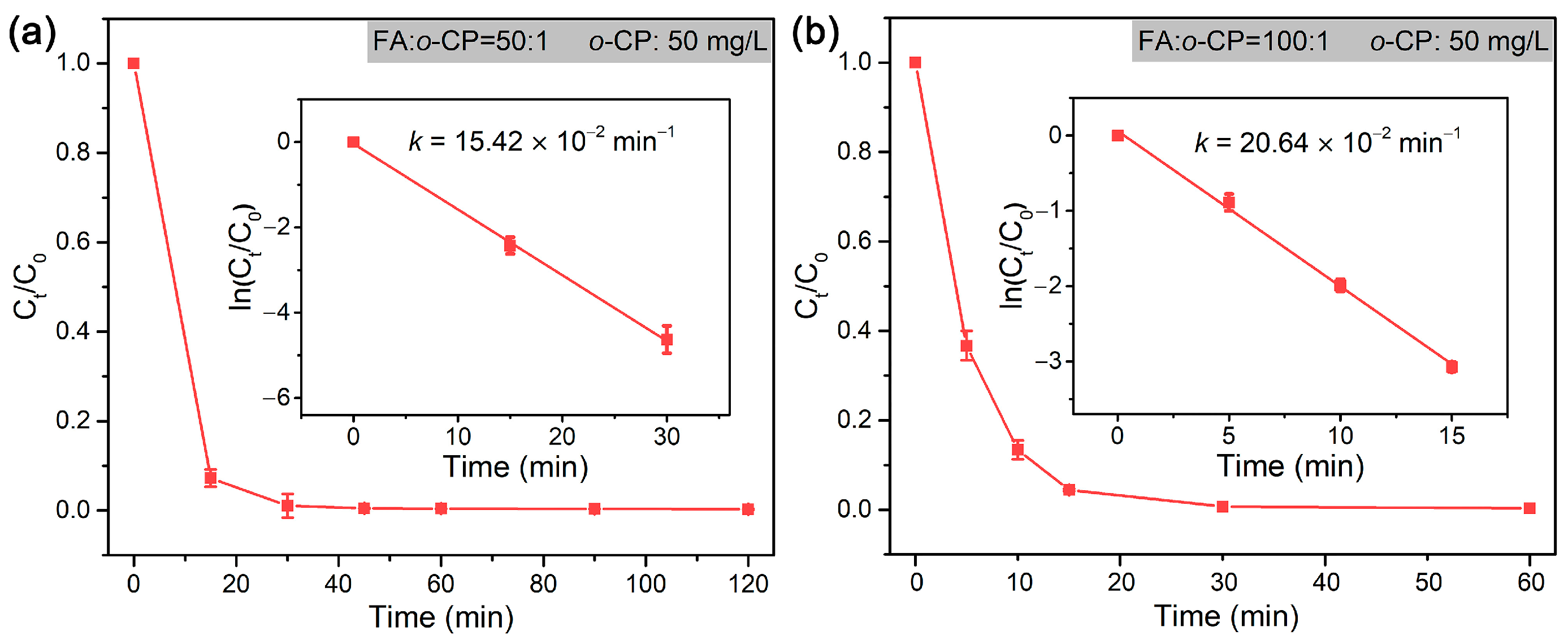
The dechlorination of o-CP follows a pseudo-first-order model, and the rate constant at RFA/o-CP of 50:1 and 100:1 was 15.42 × 10−2 min−1 and 20.64 × 10−2 min−1, respectively (Figure 7). As formic acid is actually the source of H2, the formation of which is highly dependent on the catalytic decomposition of formic acid by Pd NPs [70,71], a higher concentration (high RFA/o-CP) of formic acid potentially gives rise to higher reaction rate constant. The present work demonstrated superior efficiency in the dechlorination of o-CP compared to the dechlorination systems employing micro-sized (MCM-48 mesoporous microspheres (M48N)) or even nano-sized (metal—organic framework of UiO-66, multiwall carbon nanotubes (MWCNTs), and porous carbon nanosheets (PCN), and mesoporous silica–carbon nano-composite (MSCN)) support for the loading of Pd NPs [35,55,72,73,74].
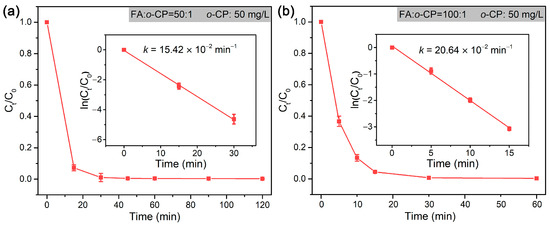
Figure 7.
Rate constants of the dechlorination of o-CP by Pd@M-Sponge at RFA/o-CP of (a) 50:1 and (b) 100:1. The concentration of o-CP is 50 mg/L. The reaction temperature is 25 °C. The insert figures are the corresponding linear fittings.
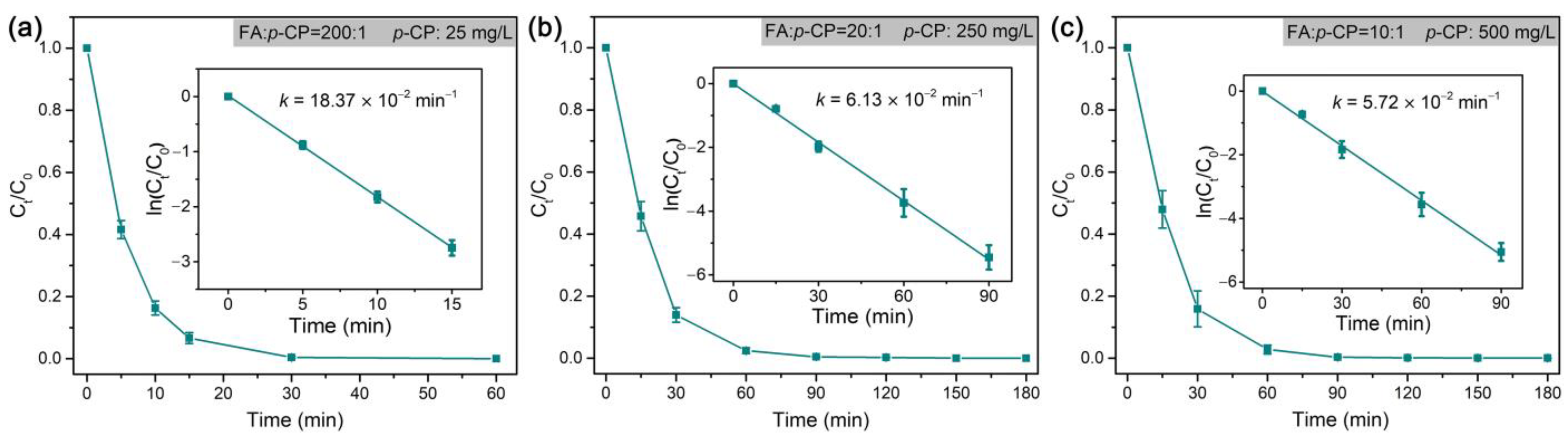
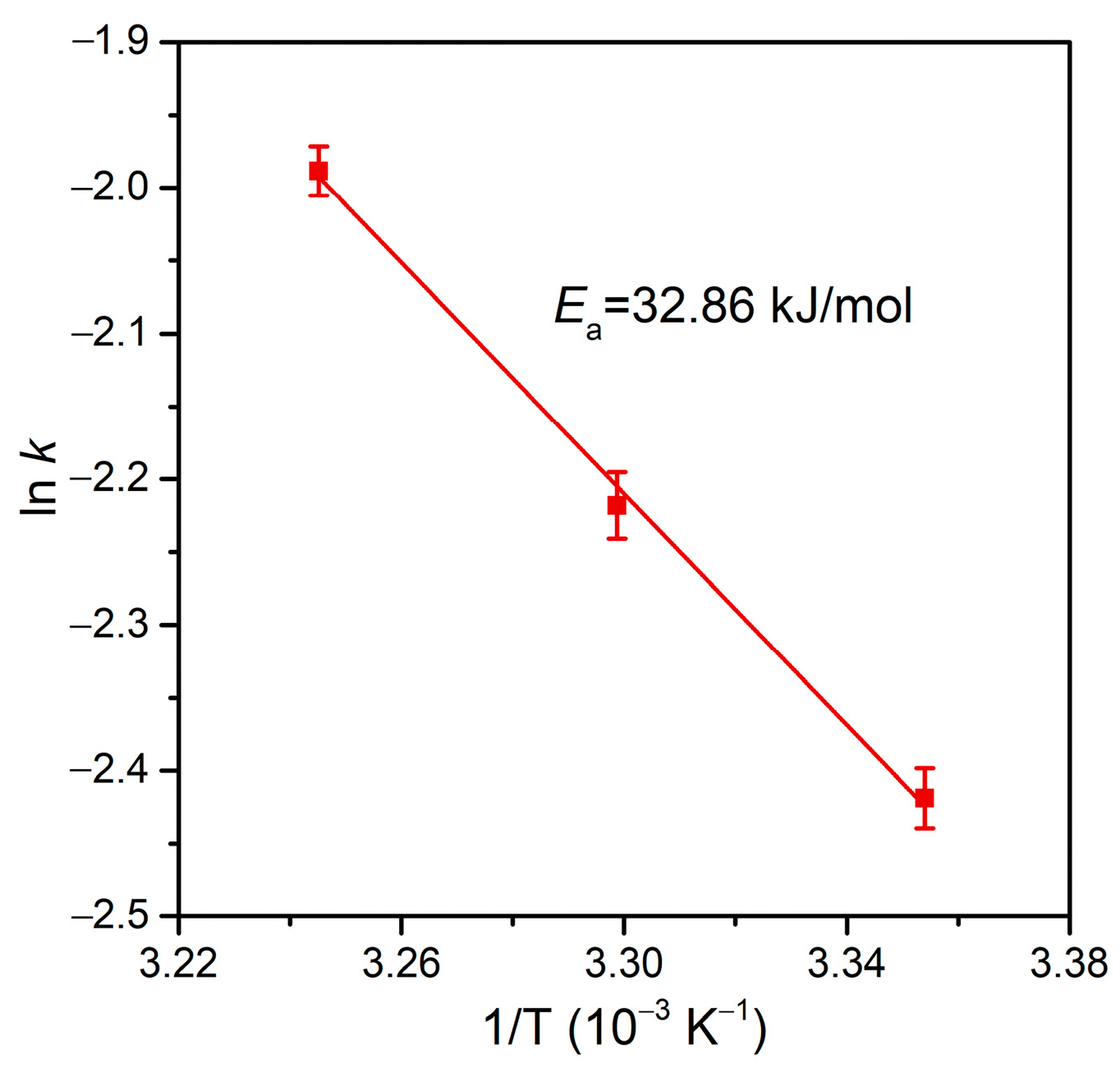
As the dechlorination of p-CP is generally more difficult than that of o-CP [75], the rate constant at an even higher RFA/p-CP (200:1) is still lower than that at an RFA/o-CP of 100:1 (Figure 8). Therefore, we conducted tests of the dechlorination of this refractory organochloride at a high target concentration in the validation of the high catalytic dechlorination reactivity of Pd@Spone4h. As shown in Figure 8b,c, high concentrations of p-CP (250 mg/L and 500 mg/L) with RFA/p-CP of 20:1 and 10:1 were completely dechlorinated in 90 min and exhibited pseudo-first-order rate constants of 6.13 × 10−2 min−1 and 5.72 × 10−2 min−1, respectively. These results demonstrated that formic acid is efficiently transformed and utilized in dechlorination in the catalysis of Pd@M-Sponge. The Arrhenius plots in Figure 9 reveal that the apparent activation energy (Ea) of Pd@Spone4h is 32.86 kJ/mol, which is lower than the reported 34.38, 41.7, or 78.6 kJ/mol of Pd NPs supported on micron-sized supports [8,76,77], revealing the high catalytic dechlorination reactivity of the prepared composite.
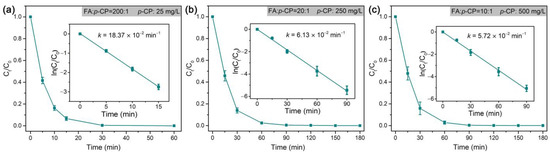
Figure 8.
Rate constants of the dechlorination of p-CP by Pd@M-Sponge at different concentrations of p-CP and different RFA/p-CP: (a) 25 mg/L, RFA/p-CP is 200:1, (b) 250 mg/L, RFA/p-CP is 20:1, (c) 500 mg/L, RFA/p-CP is 10:1. The reaction temperature is 25 °C. The insert figures are the corresponding linear fittings.
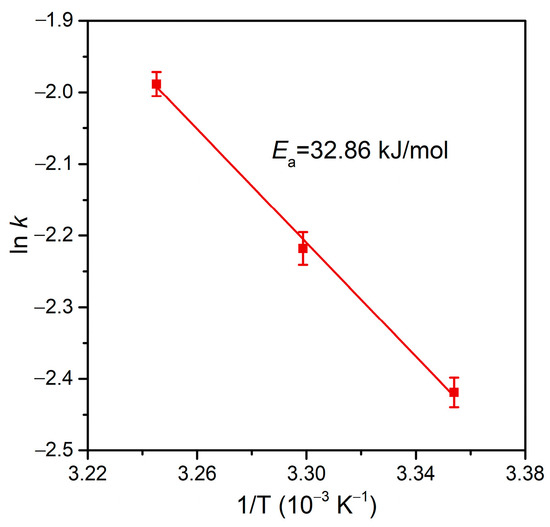
Figure 9.
Arrhenius plot for the dechlorination of p-CP by Pd@M-Sponge at 25, 30, and 35 °C. The concentration of p-CP is 50 mg/L, and the RFA/p-CP is 100:1. The red line represents the linear fitting.
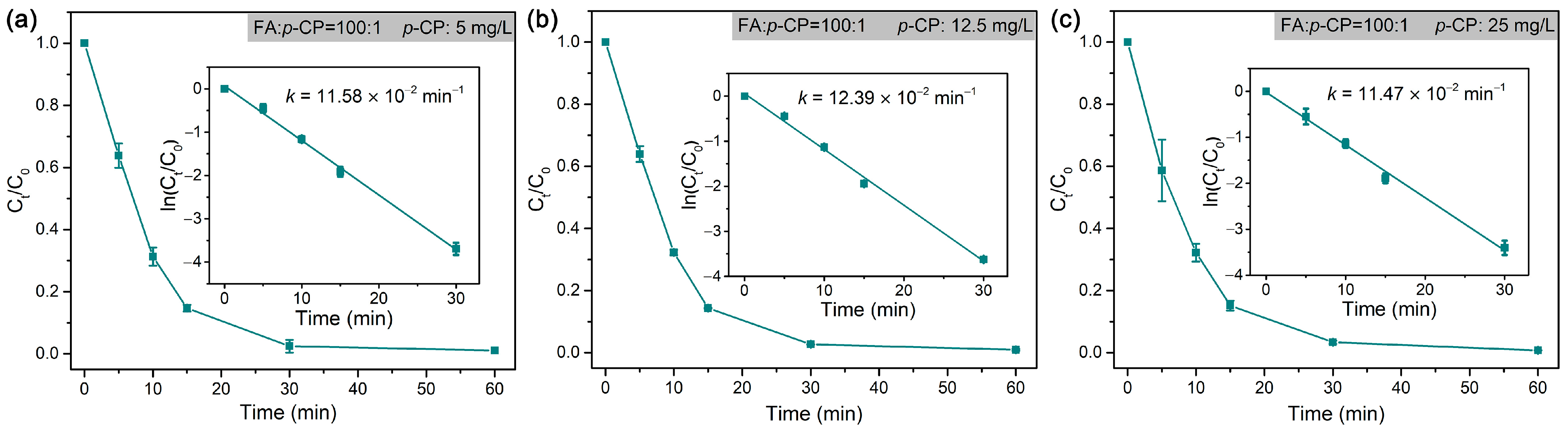
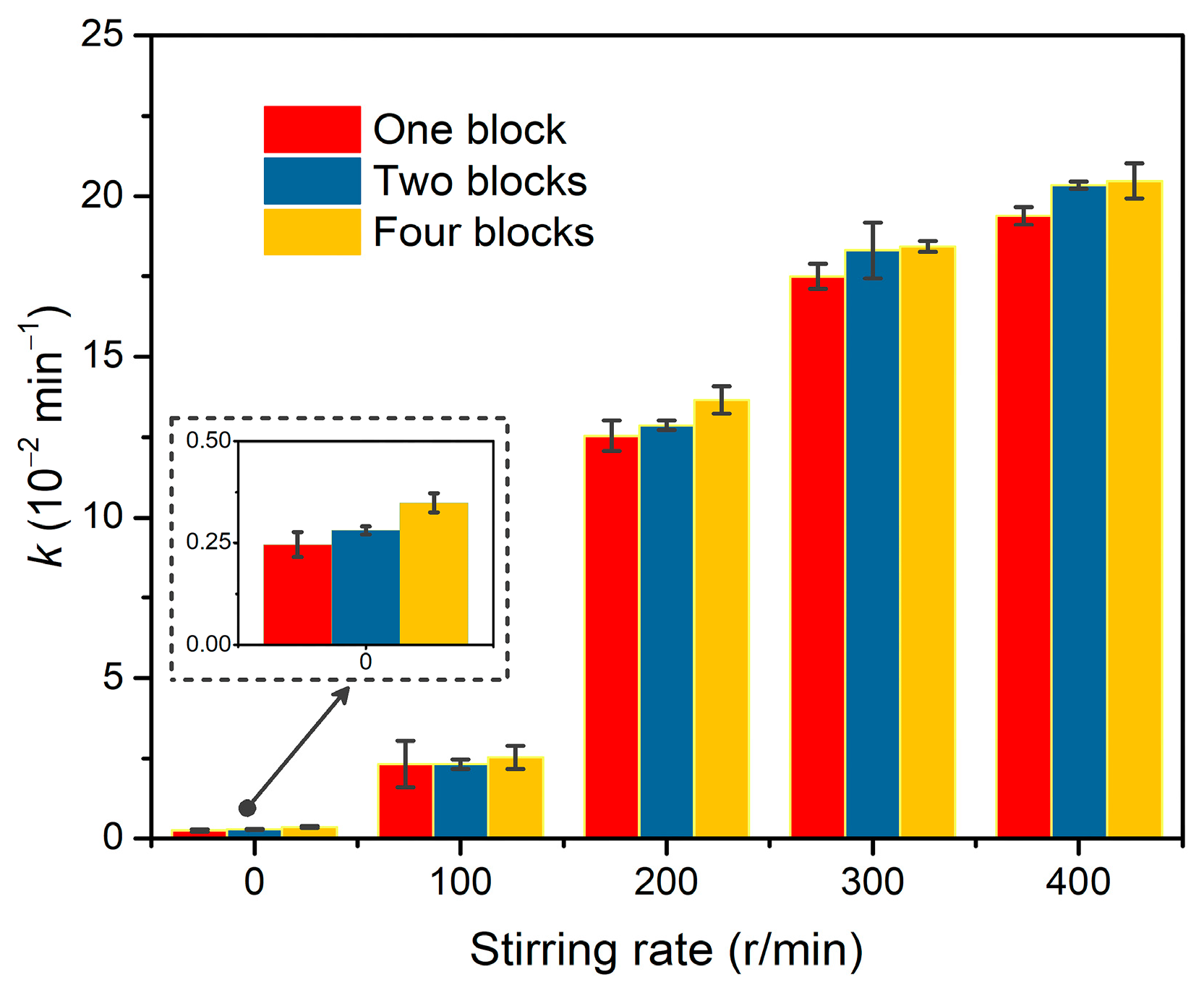
In the dechlorination of p-CP by Pd@M-Sponge, the same RFA/p-CP (100:1) with different concentrations of p-CP give roughly similar rate constants (Figure 10). Specifically, the catalytic dechlorination of p-CP at low concentrations (5 mg/L) exhibited a relatively high reaction rate constant, suggesting that internal diffusion may be negligible. To further investigate the effects of internal and external diffusion on the catalytic performance, 50 mg Pd@M-Sponge of one, two, and four blocks were adopted in the common batch experiment with various stirring rates. As evident from Figure 11, the differences in k values of the same amount Pd@M-Sponge (50 mg) with different blocks (one, two, and four) measured under different stirring rates were obvious, indicating a significant external diffusion in the catalytic reaction, especially when the stirring rate is less than 200 r/min. Despite this, the differences in the k values of Pd@M-Sponge with different blocks measured under stirring rate were significantly small, demonstrating that the internal diffusion of the sponge-based composite can be negligible. The low internal diffusion resistance of the prepared catalyst ensures a high utilization of Pd NPs in Pd@M-Sponge and further enhances the catalytic reactivity of the catalyst. Therefore, Pd@M-Sponge demonstrated superior catalytic activity and recyclability to other Pd-based catalysts (PdNi NPs and Pd NPs supported on UiO-66, foam Ni, active carbon, reduced graphene oxide, or PCN) in the electrocatalytic dechlorination and hydrodechlorination of p-CP with formic acid or H2 as a hydrogen source (Table 2) [55,72,78,79,80,81,82]. Moreover, Pd@M-Sponge demonstrated almost no delay in the initial reaction stage, which is considered to be caused by internal diffusion limitations [80].
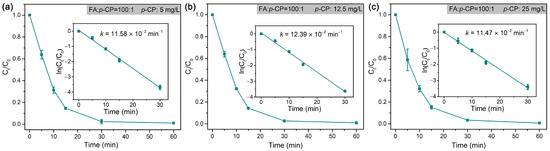
Figure 10.
Rate constants of the dechlorination of p-CP by Pd@M-Sponge at different concentrations of p-CP: (a) 5 mg/L, (b) 12.5 mg/L, (c) 25 mg/L, RFA/p-CP is 100:1. The reaction temperature is 25 °C. The insert figures are the corresponding linear fittings.
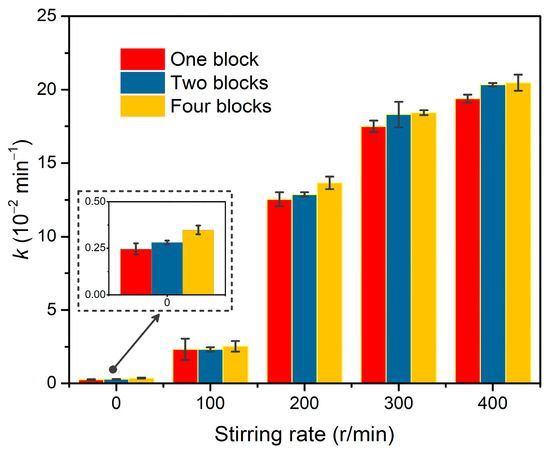
Figure 11.
Comparison of the apparent rate constant k values of Pd@M-Sponge with the same amounts (50 mg) but under various conditions including the number of blocks (one, two, or four blocks) and stirring rates (0, 100, 200, 300, or 400 r/min). The concentration of p-CP is 50 mg/L, RFA/p-CP is 100:1, and the reaction temperature is 25 °C.

Table 2.
Evaluation of catalytic performances of the prepared catalysts (Pd@M-Sponge). The reaction temperature is 25 °C. “–” indicates that the related data were deficient. “H*” indicates the active hydrogen atom generated in electrocatalytic process.
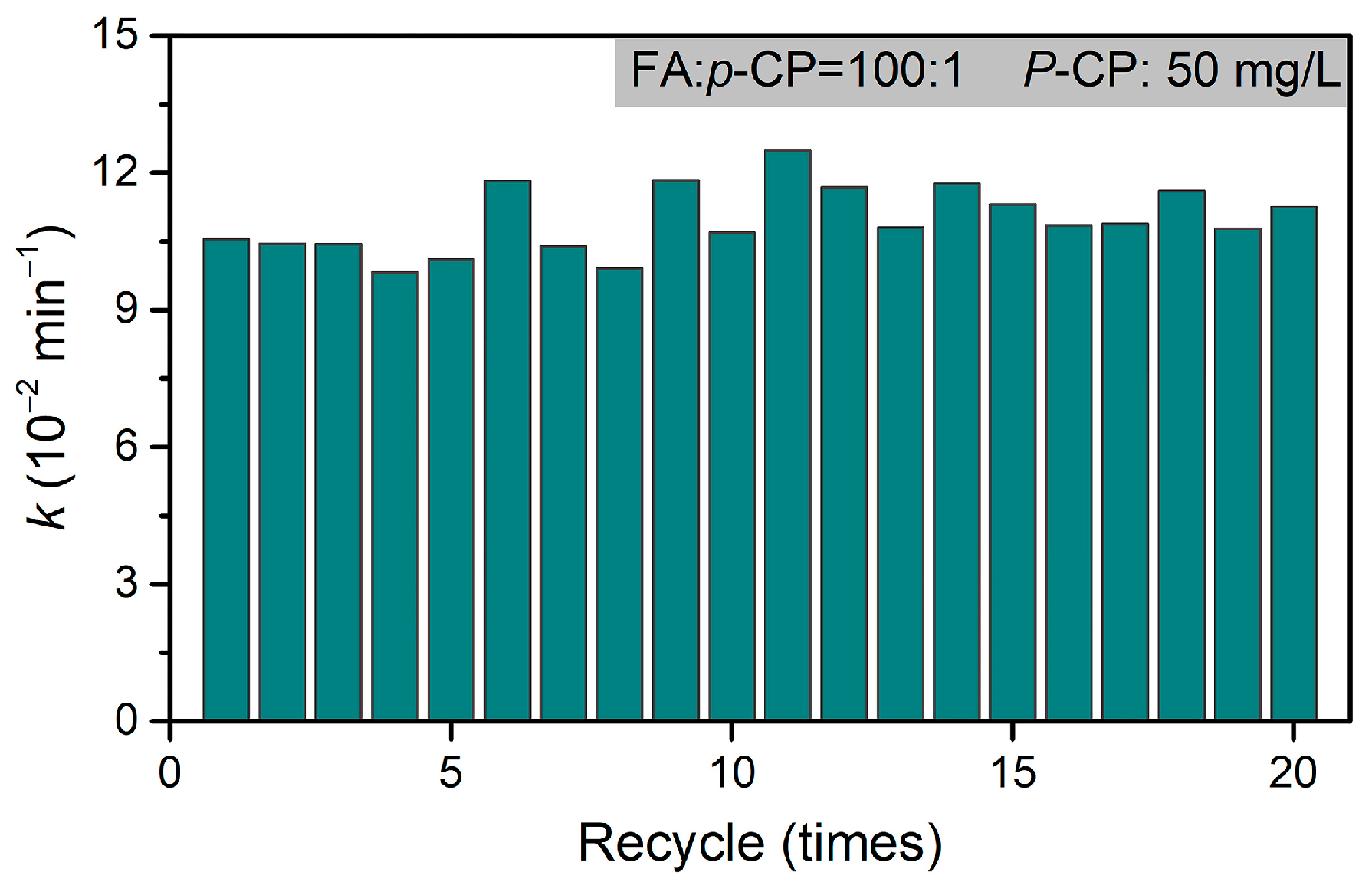
Moreover, the excellent recyclability of Pd@M-Sponge is further confirmed in the 20 successive dechlorination cycles, during which p-CP was converted into phenol and the reaction rate constant was maintained at a high level (~11 × 10−2 min−1, Figure 12), demonstrating that the catalytic acidity of Pd@M-Sponge was well maintained during dechlorination and not affected by Cl or other by-products [72]. Compared with the reported powdery or monodispersed catalyst, this monobloc catalyst not only has significantly surpassing recyclability (Table 2) [8], but also has outstanding advantages in separation and recycling.
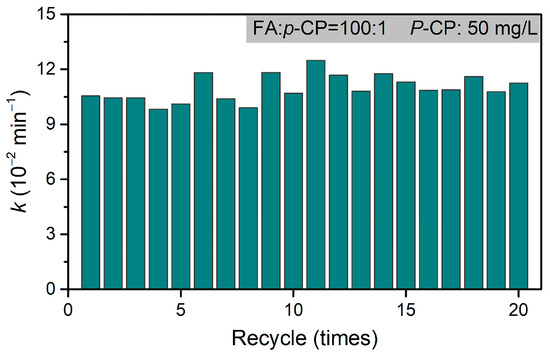
Figure 12.
The recycling dechlorination of p-CP in 20 successive reaction cycles. The concentration of p-CP is 50 mg/L, RFA/p-CP is 100:1, and reaction temperature is 25 °C.
Notably, compared with Pd@M-Sponge, Pd@Sponge demonstrated poor dechlorination efficiency (Figure S2) despite it having a superior diffusivity equal to that of Pd@M-Sponge. The results of the control experiments indicated that no obvious conversion of the reactant was observed in the presence of M-Sponge without Pd loading (Figure S3), indicating that M-Sponge has no catalytic effect and that the notable difference in terms of catalytic reactivity of Pd@Sponge and Pd@M-Sponge cannot simply be ascribed to the variation in support. In consideration of the indispensable role of Pd NPs in these reactions, the inferior catalytic dechlorination performance of Pd@Sponge in comparison with that of Pd@M-Sponge may not only be attributed to the low Pd loading capacity of un-pretreated sponge but also to the easy loss of Pd NPs from sponge during dechlorination. This deduction is further verified by the variations of Pd loading in Pd@Sponge and Pd@M-Sponge before and after dechlorination application (Table 3). The higher content of Pd in Pd@M-Sponge than Pd@Sponge may be attributed to the higher density of oxygenated functional groups in M-Sponge than that of natural sponge, which promotes the complexation of Pd precursor [84,85], causing more Pd precursors to be adsorbed and reduced in the M-Sponge.

Table 3.
Pd loading of the catalysts before and after application in the dechlorination reaction.
3. Materials and Methods
3.1. Materials and Reagents
Potassium chloropalladite (K2PdCl4, 98%), florfenicol (FF, C12H14Cl2FNO4S, 98%), o-chlorophenol (o-CP, 99%), p-chlorophenol (p-CP, 99%), and sodium borohydride (NaBH4, 98%) were purchased from Aladdin Reagent Company (Shanghai, China). Formic acid (HCOOH, 88%) was purchased from Kemiou Chemical Reagent Company (Tianjin, China). Sodium hydroxide (NaOH, 98%) was purchased from Solarbio Science and Technology (Beijing, China). Natural sponge (Mediterranean bath sponge, Family: Spongiidae, Genus: Spongia) was purchased from Jingdong Century Trade. Ltd. (Beijing, China). All water was purified by the Sartorius arium pro VF water purification system (18.2 MΩ resistivity, Göttingen, Germany).
3.2. Preparation of Pd@Sponge, Pd@M-Sponge
M-Sponge was first prepared by soaking 500 mg of natural sponge in 0.1 M NaOH for 4 h at 60 °C. Then, the pretreated sponge (M-Sponge) was washed with water four times. To obtain Pd@Sponge and Pd@M-Sponge, 500 mg of sponge and the prepared M-Sponge was soaked in a solution of 200 mL K2PdCl4 (1 mM) for 1 h at 30 °C, respectively. After soaking, the samples were washed with water to remove the unbound Pd precursors. Subsequently, the samples were soaked in a solution of 0.1 M NaBH4 with stirring for 10 min. During this process, the color of the sponge composites changed from yellow to dark brown, attributed to the binding metal precursors in sponge fibers being reduced by NaBH4 and generating Pd NPs. All the prepared samples were washed with water five times and dried in a vacuum drying oven at 60 °C for 24 h before use. Pd NPs supported by untreated sponge and alkaline-modified sponge were marked as Pd@Sponge and Pd@M-Sponge, respectively.
3.3. Characterization
The morphology of sponge and the Pd NPs were characterized by scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan) and high-resolution transmission electron microscopy (HRTEM, JEM2100F, JEOL, Akishima, Japan). To prepare HRTEM samples, Pd@Sponge and Pd@M-Sponge were first subjected to ultrasonication, after which a drop of the suspension containing Pd NPs was deposited onto carbon-coated copper grids for HRTEM analysis. Inductively coupled plasma optical emission spectrometry (ICP-OES) (Thermo iCAP 7400, Waltham, MA, USA) was used to determine the Pd loading of the samples (Pd@Sponge and Pd@M-Sponge before and after application in the dechlorination reaction). Specifically, the samples of sponge-/M-Sponge-supported Pd NPs (40 mg) were cut into pieces and dissolved by 2 mL HNO3 (65%). An amount of 1 mL of the digestive solution was then diluted to 250 mL in a volumetric flask for the ICP-OES measurements. X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi) of the samples were carried out using an Al Ka source. The mercury intrusion measurements were carried out on AutoPore IV 9500, Micromeritics Instrument Corporation, Norcross, GA, USA.
3.4. Evaluation of Catalytic Activity of Catalysts in Dechlorination
As with typical FF dechlorination experiments, 2.5 g/L of stock solution of FF was first prepared by dissolving 2.5 g FF in a mixture of 2 mL water and 3 mL ethanol, which was finally diluted to 1 L with water. Next, 100 mL of FF solution with concentrations of 100 mg/L and 50 mg/L were prepared by spiking 4 mL and 2 mL of stock solution into water, respectively. Then, 50 mg of catalyst (each one with the size of ~1.2 cm × 1.2 cm × 1.2 cm) and the desired amount of formic acid were added to the prepared FF solution to initiate the dechlorination reaction at 25 °C. To measure the concentration of FF, aliquots of liquid samples were taken out at the desired sampling time and kept in centrifuge tubes. The samples were further subjected to high-performance liquid chromatography analysis. Detector: UV at 210 nm; column: XDB-C18 (Agilent, Santa Clara, CA, USA), 250 × 4.6 mm; flow rate: 0.6 mL/min; mobile phase: methanol/water = 40/60 (v/v); injection volume: 10 μL. All the experiments were conducted in triplicate, and the standard deviations of the data were calculated.
In the dechlorination experiment of p-CP and o-CP, 3 g/L stock solutions of p-CP and o-CP were first prepared by dissolving desired amounts of p-CP and o-CP in water, which was finally diluted to 1 L with water. Next, p-CP and o-CP solutions with specific concentrations were prepared by spiking stock solution into water. Then, 50 mg catalyst and the desired amount of formic acid were added to the prepared p-CP or o-CP solution to initiate the dechlorination reaction at 25 °C. To measure the concentration of p-CP or o-CP, aliquots of liquid samples were taken out at the desired sampling time and kept in centrifuge tubes. The samples were further subjected to high-performance liquid chromatography analysis. Detector: UV at 280 nm; column: XDB-C18, 150 × 4.6 mm; flow rate: 0.6 mL/min; mobile phase: methanol/water = 70/30 (v/v); injection volume: 20 μL. All the experiments were conducted in triplicate, and the standard deviations of the data were calculated.
In the repeating catalytic performance tests of Pd@M-Sponge, the concentration of p-CP was 50 mg/L, the mass of catalyst was 50 mg, the molar ratio of formic acid and p-CP (RFA/p-CP) was 100:1, and the reaction temperature was 25 °C. After each dechlorination experiment was finished, the catalyst was carefully washed five times with water and then repeatedly employed in the next dechlorination experiment. The reaction rate constant (k) was calculated from the pseudo-first-order equation ln(Ct/C0) = −kt, where Ct is the concentration of pollutant at time t and C0 is the initial concentration of pollutant.
To examine the effect of diffusion on the catalytic dechlorination performance of Pd@M-Sponge, three 50 mg blocks of Pd@M-Sponge were uniformly cut into one, two, or four blocks and adopted in the common batch experiment with various stirring rates (0/100/200/300/400 r/min) at 25 °C. The shape and size of the magnetons employed in the experiments were all the same. The concentration of p-CP was 25 mg/L, and that of RFA/p-CP was 100:1.
4. Conclusions
In this work, we developed an excellent catalyst for hydrodechlorination with formic acid via transfer hydrogenation, which showed high catalytic reactivity and no reaction delay in the dechlorination of polychlorinated aliphatic hydrocarbon (FF) and chlorinated aromatics (o-CP and p-CP). The results of the test on 20 successive dechlorination cycles showed that there was almost no catalytic reactivity loss, indicating the outstanding recyclability of the prepared catalyst. Compared to natural sponge, the alkaline-modified sponge possesses a higher density of oxygenated functional groups for the binding of Pd precursors and the stabilization of Pd NPs, resulting in a small size and narrow size distribution of Pd NPs, and enhanced catalytic reactivity and stability. Additionally, the excellent catalytic performance is also attributed to the negligible internal mass transfer limitation of the catalyst promoted by the porous structure of the sponge. After being subjected to a hydrolysis process, the connected microcells separated by the three-dimensional network were maintained, endowing the catalyst with high diffusivity and allowing it to be free from the issues of mass transfer and reaction delay which are otherwise common in the reported supported catalysts. This work not only offers a novel alternative based on biocompatible natural material for the development of recyclable and cost-effective catalysts for hydrodechlorination but also provides a new perspective for the exploration and designing of highly reactive and stable catalysts for hydrodechlorination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14070424/s1, Figure S1: Typical TEM image of (a) Pd@Sponge and (b) the corresponding size distribution of Pd NPs. Typical TEM image of (c) Pd@M-Sponge after reaction and (d) the corresponding size distribution of Pd NPs. Figure S2. Dechlorination of p-CP in 5 successive reaction cycles by (a) Pd@Sponge and (b) Pd@M-Sponge for a reaction time of 60 min at 25 °C. The concentration of p-CP is 50 mg/L, RFA/p-CP is 100:1. Figure S3: (a) HPLC chromatogram of the dechlorination process of p-CP catalyzed by M-Sponge in 2 h; (b) HPLC chromatogram of the dechlorination process of p-CP catalyzed by Pd@M-Sponge without formic acid in 2 h. Figure S4: HPLC chromatogram of the dechlorination process of FF of (a) 50 mg/L and (b) 100 mg/L by Pd@M-Sponge. Figure S5: HPLC chromatogram of the dechlorination process of o-CP at RFA/o-CP of (a) 100:1 and (b) 50:1 by Pd@M-Sponge. Figure S6: HPLC chromatogram of the dechlorination process of o-CP at different concentrations of p-CP and different RFA/p-CP by Pd@M-Sponge: (a) 25 mg/L, RFA/p-CP is 200:1, (b) 250 mg/L, RFA/p-CP is 20:1, (c) 500 mg/L, RFA/p-CP is 10:1. Figure S7: HPLC chromatogram of the dechlorination process of p-CP at different concentrations of p-CP by Pd@M-Sponge: (a) 5 mg/L, (b) 12.5 mg/L, (c) 25 mg/L, RFA/p-CP was 100:1.
Author Contributions
Conceptualization, M.L.; Investigation and data analysis, M.L. and Z.H.; writing—original draft preparation, M.L.; writing—review and editing, G.C., Z.S., A.Z. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, S.; Tian, F.; Dai, J.; Tian, X.; Li, G.; Liu, Y.; Chen, Z.; Chen, R. Highly efficient degradation of chlorophenol over bismuth oxides upon near-infrared irradiation: Unraveling the effect of Bi-O-Bi-O defects cluster and 1O2 involved process. Appl. Catal. B 2021, 298, 120576. [Google Scholar] [CrossRef]
- Choi, C.; Wang, X.; Kwon, S.; Hart, J.L.; Rooney, C.L.; Harmon, N.J.; Sam, Q.P.; Cha, J.J.; Goddard III, W.A.; Elimelech, M. Efficient electrocatalytic valorization of chlorinated organic water pollutant to ethylene. Nat. Nanotechnol. 2023, 18, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, X.; Jiang, J.; Li, W. How much of the total organic halogen and developmental toxicity of chlorinated drinking water might be attributed to aromatic halogenated DBPs? Environ. Sci. Technol. 2021, 55, 5906–5916. [Google Scholar] [CrossRef]
- Yang, J.; Qi, X.; Shen, F.; Qiu, M.; Smith, R.L. Complete dechlorination of lindane over N-doped porous carbon supported Pd catalyst at room temperature and atmospheric pressure. Sci. Total Environ. 2020, 719, 137534. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, F.; Koziol, K.; Frankowski, M.; Nowicki, Ł.; Marlin, C.; Sulej-Suchomska, A.M.; Polkowska, Ż. Sea spray as a secondary source of chlorinated persistent organic pollutants? Conclusions from a comparison of seven fresh snowfall events in 2019 and 2021. Sci. Total Environ. 2023, 891, 164357. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wan, Y.; Wang, Y.; Xu, J.; Li, X. Robust photoelectrocatalytic degradation of antibiotics by organic-inorganic PDISA/Bi2WO6 S-scheme heterojunction membrane. J. Environ. Chem. Eng. 2024, 12, 112328. [Google Scholar] [CrossRef]
- Shao, F.; Gao, Y.; Xu, W.; Sun, F.; Chen, L.; Li, F.; Liu, W. Catalytic activation of formic acid using Pd nanocluster decorated graphitic carbon nitride for diclofenac reductive hydrodechlorination. J. Hazard. Mater. 2023, 446, 130677. [Google Scholar] [CrossRef]
- Long, X.; Chen, W.; Lei, C.; Xie, Q.; Zhang, F.; Huang, B. Ultrafine Pd nanoparticles@g-C3N4 for highly efficient dehalogenation of chlorinated environmental pollutant: Structure, efficacy and mechanisms. Sci. Total Environ. 2021, 775, 145178. [Google Scholar] [CrossRef]
- Guo, B.; Niu, X.; Yang, J.; Li, L.; Chen, Q.; Zhou, J. Ultra-fast catalytic hydrodechlorination of chloroacetic acids over Pd catalyst supported on CeO2 with exposed (1 1 0) plane. Chem. Eng. J. 2023, 472, 145126. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Y.; Su, H.; Liu, S.; Liu, Y.; Li, Q.; Xia, C. Novel insights into the mechanism for protic solvent promoting Pd/C-catalyzed hydrodechlorination of chlorinated organic compounds. Chem. Eng. J. 2022, 431, 133729. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, X.; Chen, Z. Zeolite imidazolate framework-8 metal-organic frameworks embedded with bimetallic Fe/Pd nanoparticles for reductive dechlorination. ACS Appl. Nano Mater. 2020, 3, 8088–8095. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Xu, Y.; Yu, Z.; Saravanamurugan, S.; Wu, Z.; Yang, S.; Luque, R. Heterogeneous (de)chlorination-enabled control of reactivity in the liquid-phase synthesis of furanic biofuel from cellulosic feedstock. Green Chem. 2020, 22, 637–645. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Zhou, C.; Bi, Y.; Long, X.; Wang, B.; Tang, Y.; Krajmalnik-Brown, R.; Rittmann, B.E. Long-term continuous co-reduction of 1,1,1-trichloroethane and trichloroethene over palladium nanoparticles spontaneously deposited on H2-transfer membranes. Environ. Sci. Technol. 2020, 55, 2057–2066. [Google Scholar] [CrossRef]
- Xu, L.; Stangland, E.E.; Dumesic, J.A.; Mavrikakis, M. Hydrodechlorination of 1,2-dichloroethane on platinum catalysts: Insights from reaction kinetics experiments, density functional theory, and microkinetic modeling. ACS Catal. 2021, 11, 7890–7905. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Li, J.; Wang, S.; Li, G.; Liu, X. Hydrodechlorination and deep hydrogenation on single-palladium-atom-based heterogeneous catalysts. Appl. Catal. B 2021, 282, 119518. [Google Scholar] [CrossRef]
- Svadlenak, S.; Wojcik, S.; Ogunlalu, O.; Vu, M.; Dor, M.; Boudouris, B.W.; Wildenschild, D.; Goulas, K.A. Upcycling of polyvinyl chloride to hydrocarbon waxes via dechlorination and catalytic hydrogenation. Appl. Catal. B 2023, 338, 123065. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, L.; Yang, H.B.; Zhang, Y.; Yao, Z.; Wu, H.; Huang, Y.; Xu, Y.; Liu, B. Atomically dispersed Pd electrocatalyst for efficient aqueous phase dechlorination reaction. Electrochim. Acta 2021, 391, 138886. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Gbadamosi, A.O.; Epelle, E.I.; Abdulrasheed, A.A.; Haq, B.; Patil, S.; Al-Shehri, D.; Kamal, M.S. Hydrogen production, transportation, utilization, and storage: Recent advances towards sustainable energy. J. Energy Storage 2023, 73, 109207. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Xu, L.; Gao, T.; Zhong, A.; Song, Z. Recent advances in nanoscale zero-valent iron (nZVI)-based advanced oxidation processes (AOPs): Applications, mechanisms, and future prospects. Nanomaterials 2023, 13, 2830. [Google Scholar] [CrossRef]
- Chi, H.Y.; Zhou, X.X.; Wu, M.R.; Shan, W.Y.; Liu, J.F.; Wan, J.Q.; Yan, B.; Liu, R. Regulating the reaction pathway of nZVI to improve the decontamination performance through magnetic spatial confinement effect. J. Hazard. Mater. 2023, 447, 130799. [Google Scholar] [CrossRef]
- Lei, M.; Tang, Y.; Wang, H.; Zhu, L.; Zhang, G.; Zhou, Y.; Tang, H. A catalytic strategy for rapid cleavage of C-Cl bond under mild conditions: Effects of active hydrogen induced by Pd nanoparticles on the complete dechlorination of chlorobenzenes. Chem. Eng. J. 2021, 419, 129510. [Google Scholar] [CrossRef]
- Naik, P.J.; Kunal, P.; Liu, D.-J.; Evans, J.W.; Slowing, I.I. Efficient transfer hydrodehalogenation of halophenols catalyzed by Pd supported on ceria. Appl. Catal. A Gen. 2023, 650, 119007. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, M.; Chen, X.; Wang, B.; Fan, W.; Yang, C.; Yang, X.; Zhang, Z.; Yang, X.; Li, C. A bismuth-based zeolitic organic framework with coordination-linked metal cages for efficient electrocatalytic CO2 reduction to HCOOH. Angew. Chem. Int. Ed. 2023, 62, e202311223. [Google Scholar] [CrossRef]
- Yang, S.; An, H.; Arnouts, S.; Wang, H.; Yu, X.; de Ruiter, J.; Bals, S.; Altantzis, T.; Weckhuysen, B.M.; van der Stam, W. Halide-guided active site exposure in bismuth electrocatalysts for selective CO2 conversion into formic acid. Nat. Catal. 2023, 6, 796–806. [Google Scholar] [CrossRef]
- Li, Z.; Xu, J.; Meng, F.; Yang, K.; Lin, D. Modification of Pd nanoparticles with lower work function elements for enhanced formic acid dehydrogenation and trichloroethylene dechlorination. ACS Appl. Mater. Interfaces 2022, 14, 30735–30745. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Wu, X.; Jiang, X.; Li, H.; Xu, J.; Yang, K.; Lin, D. Few-atomic zero-valent palladium ensembles for efficient reductive dehydrogenation and dehalogenation catalysis. ACS Nano 2023, 17, 22859–22871. [Google Scholar] [CrossRef] [PubMed]
- Al-Nayili, A.; Majdi, H.S.; Albayati, T.M.; Saady, N.M.C. Formic acid dehydrogenation using noble-metal nanoheterogeneous catalysts: Towards sustainable hydrogen-based energy. Catalysts 2022, 12, 324. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent advances in catalytic transfer hydrogenation with formic acid over heterogeneous transition metal catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Taleb, B.; Jahjah, R.; Cornu, D.; Bechelany, M.; Al Ajami, M.; Kataya, G.; Hijazi, A.; El-Dakdouki, M.H. Exploring hydrogen sources in catalytic transfer hydrogenation: A review of unsaturated compound reduction. Molecules 2023, 28, 7541. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, X.; Cui, M.; Wang, W.; Wang, P.; Johnson, G.; Nie, Y.; Lv, X.; Zhang, X.; Dong, F.; et al. Surface ligand environment boosts the electrocatalytic hydrodechlorination reaction on palladium nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 4072–4083. [Google Scholar] [CrossRef]
- Ribota Peláez, M.; Ruiz-López, E.; Domínguez, M.; Ivanova, S.; Centeno, M. Formic acid dehydrogenation over a monometallic Pd and bimetallic Pd: Co catalyst supported on activated carbon. Catalysts 2023, 13, 977. [Google Scholar] [CrossRef]
- Hafeez, S.; Harkou, E.; Adamou, P.; Barlocco, I.; Zanella, E.; Manos, G.; Al-Salem, S.M.; Chen, X.; Delgado, J.J.; Dimitratos, N.; et al. Formic acid decomposition using palladium-zinc preformed colloidal nanoparticles supported on carbon nanofibre in batch and continuous flow reactors: Experimental and computational fluid dynamics modelling studies. Nanomaterials 2023, 13, 2993. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Z.; Zhan, X.; Chen, W.; Li, C.; Zhu, L.; Dai, Y.; Deng, J. Catalytic dechlorination of carbon tetrachloride to combustible hydrocarbons by Pd-Fe hydroxides through atomic hydrogen attack and direct electron transfer. Sep. Purif. Technol. 2024, 346, 127449. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Prosvirin, I.P.; Gerasimov, E.Y. Effect of platinum precursor on the properties of Pt/N-graphene catalysts in formic acid decomposition. Catalysts 2022, 12, 1022. [Google Scholar] [CrossRef]
- Mu, D.; Li, Z.; Yu, S.; Liu, S. Hydrodechlorination of chlorophenols with methanol as hydrogen donor over carbon nanotube supported Pd-catalysts. Catal. Today 2022, 405–406, 47–56. [Google Scholar] [CrossRef]
- Ran, W.; Zhao, H.; Zhang, X.; Li, S.; Sun, J.-F.; Liu, J.; Liu, R.; Jiang, G. Critical review of Pd-catalyzed reduction process for treatment of waterborne pollutants. Environ. Sci. Technol. 2024, 58, 3079–3097. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.A.; Savina, I.N. Cryogels with noble metal nanoparticles as catalyst for “green” decomposition of chlorophenols. Inorganics 2023, 11, 23. [Google Scholar] [CrossRef]
- Qi, X.; Obata, K.; Yui, Y.; Honma, T.; Lu, X.; Ibe, M.; Takanabe, K. Potential-rate correlations of supported palladium-based catalysts for aqueous formic acid dehydrogenation. J. Am. Chem. Soc. 2024, 146, 9191–9204. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Li, Z.; Yu, S.; Liu, S. Wastewater treatment via hydro-de-heteroatoms using hydrogen donors. Catal. Today 2022, 402, 67–78. [Google Scholar] [CrossRef]
- Liu, M.; Yu, T.; Huang, R.; Qi, W.; He, Z.; Su, R. Fabrication of nanohybrids assisted by protein-based materials for catalytic applications. Catal. Sci. Technol. 2020, 10, 3515–3531. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent advances of magnetite (Fe3O4)-based magnetic materials in catalytic applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Heidari, M.K.; Fouladi, M.; Sooreh, H.A.; Tavakoli, O. Superhydrophobic and super-oleophilic natural sponge sorbent for crude oil/water separation. J. Water Process Eng. 2022, 48, 102783. [Google Scholar] [CrossRef]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shan, C.; Chang, H.; Zhang, Z.; Huang, R.; Lee, D.W.; Qi, W.; He, Z.; Su, R. Nano-engineered natural sponge as a recyclable and deformable reactor for ultrafast conversion of pollutants from water. Chem. Eng. Sci. 2022, 247, 117049. [Google Scholar] [CrossRef]
- Pranzetti, A.; Mieszkin, S.; Iqbal, P.; Rawson, F.J.; Callow, M.E.; Callow, J.A.; Koelsch, P.; Preece, J.A.; Mendes, P.M. An electrically reversible switchable surface to control and study early bacterial adhesion dynamics in real-time. Adv. Mater. 2013, 25, 2181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.-D.; Wang, Y.; Zou, X.-H.; Yin, W.-M.; Wang, X.-Y.; Guo, Y.-R.; Pan, Q.-J. Fabrication of cellulose@ Mg(OH)2 composite filter via interfacial bonding and its trapping effect for heavy metal ions. Chem. Eng. J. 2021, 426, 130812. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Xie, Z.; Peng, X.; Guo, L.; Yu, X.; Yang, X.; Lu, Z.; Zhang, X.; Li, L. Polar bonds induced strong Pd-support electronic interaction drives remarkably enhanced oxygen reduction activity and stability. Appl. Catal. B 2022, 305, 121020. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, Q.; Ma, Y.; Guan, Q.; Jin, R.; Wang, H.; Yang, B.; Lu, J. Support-induced unusual size dependence of Pd catalysts in chemoselective hydrogenation of para-chloronitrobenzene. J. Catal. 2021, 400, 173–183. [Google Scholar] [CrossRef]
- Inbakandan, D.; Venkatesan, R.; Khan, S.A. Biosynthesis of gold nanoparticles utilizing marine sponge Acanthella elongata (Dendy, 1905). Colloids Surf. B Biointerfaces 2010, 81, 634–639. [Google Scholar] [CrossRef]
- Peng, W.; Yan, Y.; Zhang, D.; Zhou, Y.; Na, D.; Xiao, C.; Yang, C.; Wen, G.; Zhang, J. Preparation of thermal stable supported metal (Cu, Au, Pd) nanoparticles via cross-linking cellulose gel confinement strategy. Colloids Surf. Physicochem. Eng. Asp. 2021, 624, 126809. [Google Scholar] [CrossRef]
- Bekdemir, A.; Stellacci, F. A centrifugation-based physicochemical characterization method for the interaction between proteins and nanoparticles. Nat. Commun. 2016, 7, 13121. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Y.-H.; Long, X.; Roldan, M.A.; Yang, S.; Zhou, C.; Zhou, D.; Rittmann, B.E. Reductive dehalogenation of herbicides catalyzed by Pd0 NPs in a H2-based membrane catalyst-film reactor. Environ. Sci. Technol. 2022, 56, 18030–18040. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Han, J.; Kang, Y.; Mi, Y.; Wang, W. A novel N and Se codoped-carbon support anchoring Pd nanoparticles as an efficient electrocatalyst towards ethylene glycol electrooxidation. Mater. Sci. Eng. B 2020, 252, 114467. [Google Scholar] [CrossRef]
- Guo, M.; Jayakumar, S.; Luo, M.; Kong, X.; Li, C.; Li, H.; Chen, J.; Yang, Q. The promotion effect of π-π interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 2022, 13, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, L.; Chen, Q.; Mao, M.; Jiang, W.; Long, Y.; Fan, G. Oxygenated functional group-driven spontaneous fabrication of Pd nanoparticles decorated porous carbon nanosheets for electrocatalytic hydrodechlorination of 4-chlorophenol. J. Hazard. Mater. 2021, 408, 124456. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Liu, M.; Huang, R.; Che, M.; Su, R.; Qi, W.; He, Z. Tannic acid-assisted fabrication of Fe-Pd nanoparticles for stable rapid dechlorination of two organochlorides. Chem. Eng. J. 2018, 352, 716–721. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.; Xu, X.; Lowry, G.V. Removal of antibiotic florfenicol by sulfide-modified nanoscale zero-valent iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef]
- Song, C.; Wei, Y.; Qiu, Y.; Qi, Y.; Li, Y.; Kitamura, Y. Biodegradability and mechanism of florfenicol via Chlorella sp. UTEX1602 and L38: Experimental study. Bioresour. Technol. 2019, 272, 529–534. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, A.; Yu-ming, M.H.; Zhao, Y.; He, Y.; Xu, J.; Lu, Z. Elucidating degradation mechanisms of florfenicol in soil by stable-isotope assisted nontarget screening. J. Hazard. Mater. 2021, 403, 123974. [Google Scholar] [CrossRef]
- Qian, Z.; Na, L.; Bao-Long, W.; Tao, Z.; Peng-Fei, M.; Wei-Xiao, Z.; Sraboni, N.Z.; Zheng, M.; Ying-Qi, Z.; Liu, Y. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J. Environ. Manag. 2022, 320, 115673. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fu, X.; Liu, Y.; Zhang, Y.; Chen, S.; Li, D.; Zhang, C.; Gao, J.; Fu, Y. Electrocatalytic dechlorination of florfenicol using a Pd-loaded on blue TiO2 nanotube arrays cathode. Sep. Purif. Technol. 2023, 323, 124460. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Cui, D.; Luo, X.; Liang, B.; Yang, L.; Liu, T.; Wang, A.; Luo, S. Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol. Chem. Eng. J. 2019, 359, 894–901. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Xu, W.; Shi, H.; Hu, X.; Xu, J.; Lou, L. Adsorption-reduction coupling mechanism and reductive species during efficient florfenicol removal by modified biochar supported sulfidized nanoscale zerovalent iron. Environ. Res. 2023, 216, 114782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, G.; Liu, S.; Qin, L.; Lin, B.; Wang, M.; Yang, L.; Zheng, M. Free radical mechanism of toxic organic compound formations from o-chlorophenol. J. Hazard. Mater. 2023, 443, 130367. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, J.; Yang, Y.; Li, H.; Zheng, X.; Liu, G.; Jiang, Y. Synergistic degradation of chlorophenol pollutants by a photo-enzyme integrated catalyst. J. Environ. Chem. Eng. 2022, 10, 107909. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Sun, J.; Wu, X.; Liang, H.; Qu, Y.; Jing, L. BiFeO3/Bi2Fe4O9 S-scheme heterojunction hollow nanospheres for high-efficiency photocatalytic o-chlorophenol degradation. Appl. Catal. B 2022, 319, 121893. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Khan, M.A.; Khan, Q.; Habibi-Yangjeh, A.; Dang, A.; Maqbool, M. Review on the hazardous applications and photodegradation mechanisms of chlorophenols over different photocatalysts. Environ. Res. 2021, 195, 110742. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Cai, Y.; Long, X.; Zhou, D.; Zhou, C.; Rittmann, B.E. Palladium (Pd0) loading-controlled catalytic activity and selectivity for chlorophenol hydrodechlorination and hydrosaturation. Environ. Sci. Technol. 2022, 56, 4447–4456. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Jin, X.; Liu, X. Selective and controlled H2 generation upon additive-free HCOOH dehydrogenation over a Pd/NCS nanocatalyst. Nanoscale 2023, 15, 15975–15981. [Google Scholar] [CrossRef]
- Song, J.; Bai, S.; Sun, Q. Strong metal-support interaction of Pd/CeO2 enhances hydrogen production from formic acid decomposition. Colloids Surf. Physicochem. Eng. Asp. 2023, 658, 130645. [Google Scholar] [CrossRef]
- Tong, L.; Song, X.; Jiang, Y.; Zhao, B.; Li, Y. Efficiently catalytic transfer hydrogenation of aryl and heteroaryl halides by ultrafine palladium nanoparticles confined into UiO-66. Int. J. Hydrogen Energy 2022, 47, 15753–15763. [Google Scholar] [CrossRef]
- Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G. Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 2011, 186, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Le, X.; Zhang, W.; Gu, H.; Xue, R.; Ma, J. Catalysis of the hydro-dechlorination of 4-chlorophenol by Pd(0)-modified MCM-48 mesoporous microspheres with an ultra-high surface area. New J. Chem. 2015, 39, 4519–4525. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yue, P.L.; Chen, G. Catalytic dechlorination of chlorophenols in water by palladium/iron. Water Res. 2001, 35, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, R.; Li, C.; Che, M.; Su, R.; Li, S.; Yu, J.; Qi, W.; He, Z. Continuous rapid dechlorination of p-chlorophenol by Fe-Pd nanoparticles promoted by procyanidin. Chem. Eng. Sci. 2019, 201, 121–131. [Google Scholar] [CrossRef]
- Xie, Q.; Lei, C.; Chen, W.; Huang, B. Mesoporous ferrihydrite-supported Pd nanoparticles for enhanced catalytic dehalogenation of chlorinated environmental pollutant. J. Colloid Interface Sci. 2022, 608, 2907–2920. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Zhang, T.; Yu, G.; Deng, S.; Huang, J. Catalytic hydrodechlorination of 4-chlorophenol in an aqueous solution with Pd/Ni catalyst and formic acid. Ind. Eng. Chem. Res. 2010, 49, 4561–4565. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Zhang, J.; Tang, T.; Xu, Q.Q.; Zhao, B.; Lu, J.; Li, R.; Han, D. Stabilizing Pd nanoparticles in supported-ionic-liquid-phase (SILP) catalyst using polydimethylsiloxane via hydrophobic structure for boosting hydrodechlorination of 4-chlorophenol. ChemistrySelect 2020, 5, 14626–14631. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Xu, L.; Pei, Y.; Niu, J. Efficient hydrogenation of p-chlorophenol and Cr(VI) driven by hydrogen rich balls over Pd/C catalysts. J. Hazard. Mater. 2022, 437, 129434. [Google Scholar] [CrossRef]
- Ruiz-Garcia, C.; Lei, Y.; Heras, F.; Elías, A.L.; Terrones, M.; Gilarranz, M.A. Functional Pd/reduced graphene oxide nanocomposites: Effect of reduction degree and doping in hydrodechlorination catalytic activity. J. Nanopart. Res. 2019, 21, 276. [Google Scholar] [CrossRef]
- Mao, M.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Active site and adsorption behavior engineering of subsize PdNi nanoparticles for boosting electrocatalytic hydrodechlorination of 4-chlorophenol. Appl. Surf. Sci. 2022, 600, 153988. [Google Scholar] [CrossRef]
- Escobedo, E.; Kim, J.; Oh, D.; Cho, K.; Chang, Y.-S. Electrocatalytic dehalogenation of aqueous pollutants by dealloyed nanoporous Pd/Ti cathode. Catal. Today 2021, 361, 63–68. [Google Scholar] [CrossRef]
- Feng, S.; Huang, K.; Huang, Z.; Liu, G.; Zhang, G.; Gou, G. Highly selective extraction of Pd(II) using functionalized molecule of 2-[(2-ethylhexyl)thio]benzoxazole and its Pd(II) extraction mechanism. J. Mol. Struct. 2021, 1230, 129639. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, S.; Pei, Y.; Luo, X. Growing Pd NPs on cellulose microspheres via in-situ reduction for catalytic decolorization of methylene blue. Int. J. Biol. Macromol. 2021, 166, 1419–1428. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).