Abstract
A novel alkaline serine protease, derived from the Staphylococcus aureus strain ALA1 previously isolated from dromedary milk, was subjected to purification and characterization. Optimal protease production occurred under specific culture conditions. The purified protease, designated S. aureus Pr with a molecular mass of 23,662 Da and an N-terminal sequence, showed an approximately 89% similar identity with those of other Staphylococcus strains. It exhibited its highest enzymatic activity at a pH of 10.0 and 60 °C in the presence of 3 mM Ca2+. Remarkable thermostability was observed at temperatures up to 70 °C and within a pH range of 6.0 to 10.0 for 2 h. The presence of Ca2+ or Mg2+ and Zn2+ significantly enhanced both enzymatic activity and thermal stability. Additionally, notable stability was demonstrated in the presence of reducing and chaotropic agents as well as in surfactants, oxidizing agents, and organic solvents commonly found in detergent compositions. This highlights the enzyme’s potential as a versatile biocatalyst, especially in detergents. Its stability and compatibility with laundry detergents matched Alcalase 2.5 L, type Dx, and the Stearothermophilus protease, used as controls. Collectively, this study investigated the potential utilization of S. aureus Pr in industrial detergents as an excellent candidate for incorporation as an additive in detergent formulations.
1. Introduction
Proteases or peptidases (EC 3.4) represent a significant category of catalytic enzymes renowned for their ability to hydrolyze peptide bonds within proteins [1,2]. They represent the most important and adaptable group within the hydrolase category, fulfilling crucial roles in all aspects of a living organism’s operations [3,4]. Proteases are mainly characterized by their catalytic active site, substrate specificity, and optimal pH and temperature [3]. Based on their functions and the site of peptide bond cleavage, protease enzymes are categorized into two groups: exopeptidases that cleave peptide bonds located close to either the N-terminal or the C-terminal of the substrate, and endopeptidases that hydrolyze peptide bonds within the polypeptide chains [3]. Additionally, they can be classified based on the character of their catalytic active site into four groups: serine proteases [5], cysteine proteases [6], aspartic proteases, and metalloproteases [7]. Moreover, neutral (pH range 6.0–8.0), acidic (pH range 2.0–6.5) or alkaline (pH range 8.0–14.0) proteases are defined according to their optimal pH [7].
Various organisms, such as plants, animals, and microorganisms, extensively synthesize proteases. Notably, bacterial proteases have gained significant attention for several commercial applications owing to their large-scale production because of the rapid growth of bacteria, enabling the generation of new recombinant enzymes with desired properties [3]. Indeed, microbial proteases contribute to approximately two-thirds of the global market for commercial proteases [8,9]. Their significance lies not only in their crucial role in metabolic processes but also in their extensive utilization across various industries such as detergent, pharmaceutical, leather, food, and agricultural sectors [8]. Proteolytic enzymes contribute to nearly 65% of the total global industrial enzyme sales, surpassing USD 3 billion [3,10].
Enzymes best suited for the detergent industry should exhibit high activity and stability under extremely high temperatures and alkaline conditions [3]. Due to their robust production capacity and catalytic efficiency, neutral and alkaline bacterial proteases show promising potential for utilization in the detergent and leather tanning sectors, driven by the growing emphasis on developing environmentally friendly technologies. These proteases exhibit notable activity within an alkaline pH range of 8.0 to 12.0 and display optimal temperature performance between 50 °C and 70 °C. They necessitate bivalent cations like Ca2+, Mg2+, Zn2+, Cu2+, Co2+, Fe2+, or Mn2+ to shield the enzyme from thermal denaturation and uphold the integrity of the enzyme’s active site, particularly at high temperatures [11]. These distinctive characteristics make bacterial alkaline proteases highly suitable for use in the detergent industry [2,10].
Domestic detergents comprise various harmful chemicals like polycarboxylates and chlorine phosphonates, detrimental to the environment and capable of causing skin ailments. Moreover, the primary constituents of detergents also include ionic surfactants like sodium dodecyl sulfate (SDS), nonionic surfactants such as Triton X-100, Tween 20, and Tween-80, as well as oxidizing agents like hydrogen peroxide (H2O2) and sodium perborate (NaBO3), along with bleaching agents such as sodium hypochlorite [11]. The effectiveness of enzymes in detergents hinges on factors like the washing temperature, stain type, water hardness, and washing method. Therefore, proteases to be used in the detergent preparations must show high activity and stability at alkaline pH levels, wide-ranging temperature stability, broad substrate specificity, resilience in the presence of surfactants, oxidizing agents, and bleaching agents, as well as high storage stability and compatibility with detergents for long periods [11]. They must also ensure stability in the presence of chelating agents such as Ethylenediaminetetraacetic Acid (EDTA) which is essential for any detergent enzyme since it serves as a water softener in detergent formulations [12]. Indeed, it has been proposed that the protease within the detergent solution should remain active and compatible for a duration ranging from 60 to 90 min, corresponding to the time required for washing clothes either through machine washing or manual hand washing [13]. Actually, proteases represent around 60.0% of total enzyme sales within the detergent sector [14]. The utilization of proteases in the detergent sector constitutes 20% of worldwide enzyme sales, contributing to 30–40% of total enzyme revenues globally. Moreover, it is anticipated to experience continuous growth, projecting a compound annual growth rate of approximately 15.5% from 2020 to 2025 [15]. The proteases suitable for detergent use are mainly synthesized by Bacillus and Aspergillus species since these species can thrive on inexpensive media, produce significant enzyme quantities rapidly, and are easily amenable to genetic manipulation, resulting in enhanced proteases with favorable characteristics [11].
Staphylococci, notably Staphylococcus aureus (S. aureus), are acknowledged for their synthesis of diverse extracellular proteases, including those of the serine, cysteine, and metalloenzyme types [16]. Several recent investigations have revealed that proteases originating from staphylococci have the capability to engage with host defense mechanisms and tissue components, and even modify virulence factors derived from other pathogens [10]. Limited attention has been devoted to characterizing this protease for biotechnological applications, as has been documented for Bacillus, Aeromonas, Arthrobacter, Halomonas, Pseudomonas, and Serratia [10].
The demand for alkaline proteases continues to rise steadily and it becomes imperative to investigate techniques for enhancing commercial production from recently discovered strains [17]. Given the high demand for detergent-compatible proteases, ongoing advancements drive the constant screening of new bacteria capable of producing proteases with enhanced stability features. This study focused on optimizing culture conditions, purifying, and biochemically characterizing a new thermostable and alkaline protease derived from the S. aureus strain ALA1, initially isolated from dromedary milk. Demonstrating potential for industrial use, especially in detergent formulations, this protease showcases promising applicability.
2. Results and Discussion
2.1. Optimization of Medium Compounds for Protease Production
2.1.1. Incubation Time
The S. aureus strain ALA1 (GenBank no. KF 678862) was previously isolated and identified by Ben Bacha et al. [18].
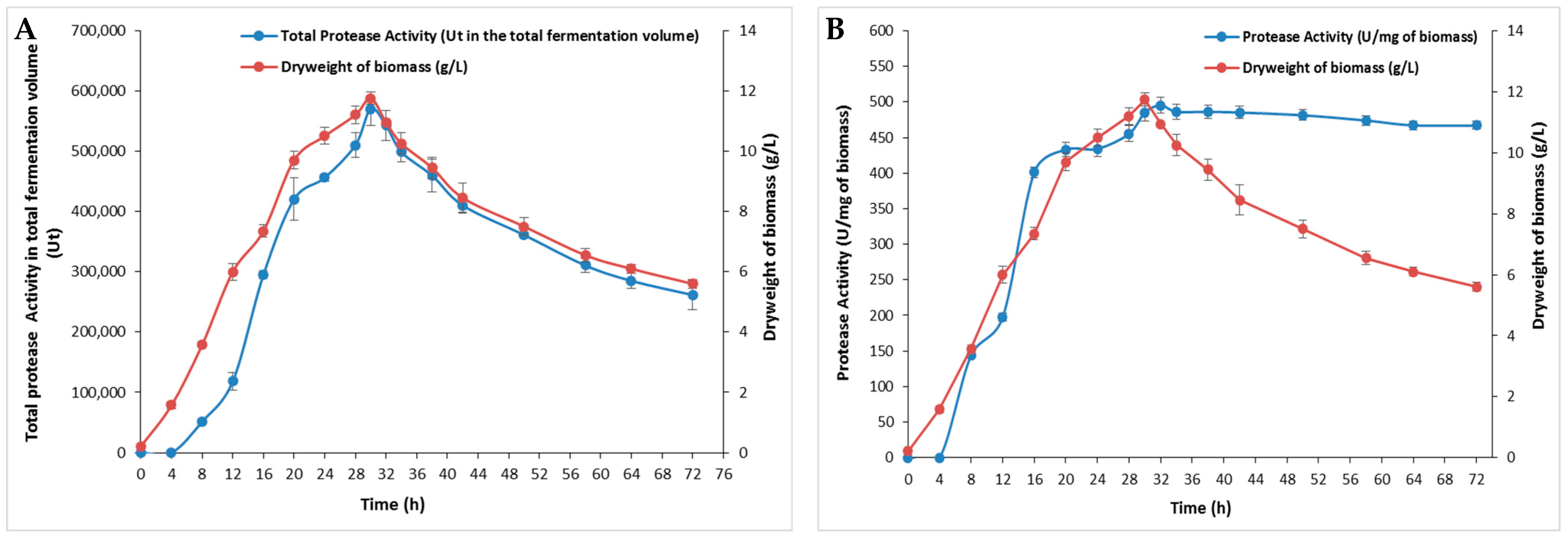
The effect of incubation time on bacterial growth and protease production was performed for 72 h and relative results are depicted in Figure 1. The protease production was proportional to bacterial growth with an optimum of 495 U/mg, which corresponded to the highest biomass of 11.75 ± 0.2 g/L at 30 h (Figure 1B). This occurrence may have been due to the presence of sufficient nutrients essential for bacterial growth in the initial stages of incubation [19]. After 30 h, a gradual decrease in both enzymes and biomass was observed (Figure 1). The incubation period distinctly highlights the enzyme’s role as a primary metabolite being synthesized during the logarithmic phase of bacterial growth [20]. As bacteria in the exponential phase consume nutrient sources, they enter the stationary phase, where the cell number does not increase, leading to reduced secretion of extracellular enzymes. During this phase, nutrient-depleted cultures accumulate metabolic waste products, eventually progressing to the death phase and further decreasing extracellular enzyme levels [21]. Additionally, factors such as nutrient availability, quorum sensing (a cell density-dependent signaling mechanism), the growth phase, osmolarity, pH, and temperature (37–43 °C) have been reported to influence bacterial protease production [22].
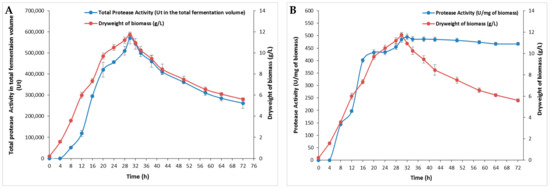
Figure 1.
The effect of incubation time on S. aureus Pr production at different times. (A) The total protease activity in the total fermentation volume (100 mL) and (B) protease activity (U/mg of biomass) at different times during 72 h. The means of three replicates are represented with their corresponding SD (±).
The impact of incubation time on microbial enzyme production is widely reported, with durations ranging from 24 h to a week, depending on the microorganism type and other cultural conditions. The maximum protease activity of S. sciuri (TKMFT 8) isolated from soil and water samples collected from manufacturing units in India was recorded after 48 h of incubation, reaching 215.66 ± 1.98 U/mL [20]. The highest level of production of an extracellular protease (67.57 U/mL) from S. simulans QB7 was reached after 36 h of incubation followed by a progressive decrease. The highest protease activity from S. aureus S-2 isolated from chicken waste was observed after 24 h [23]. S. epidermidis BP9 isolated from Harbin dry sausages exhibited a maximum level of protease activity after 48 h [24] while the maximum biosynthesis of proteases for S. auriculari P18 was observed at 72 h [25].
2.1.2. Effect of pH and Temperature on Protease Production
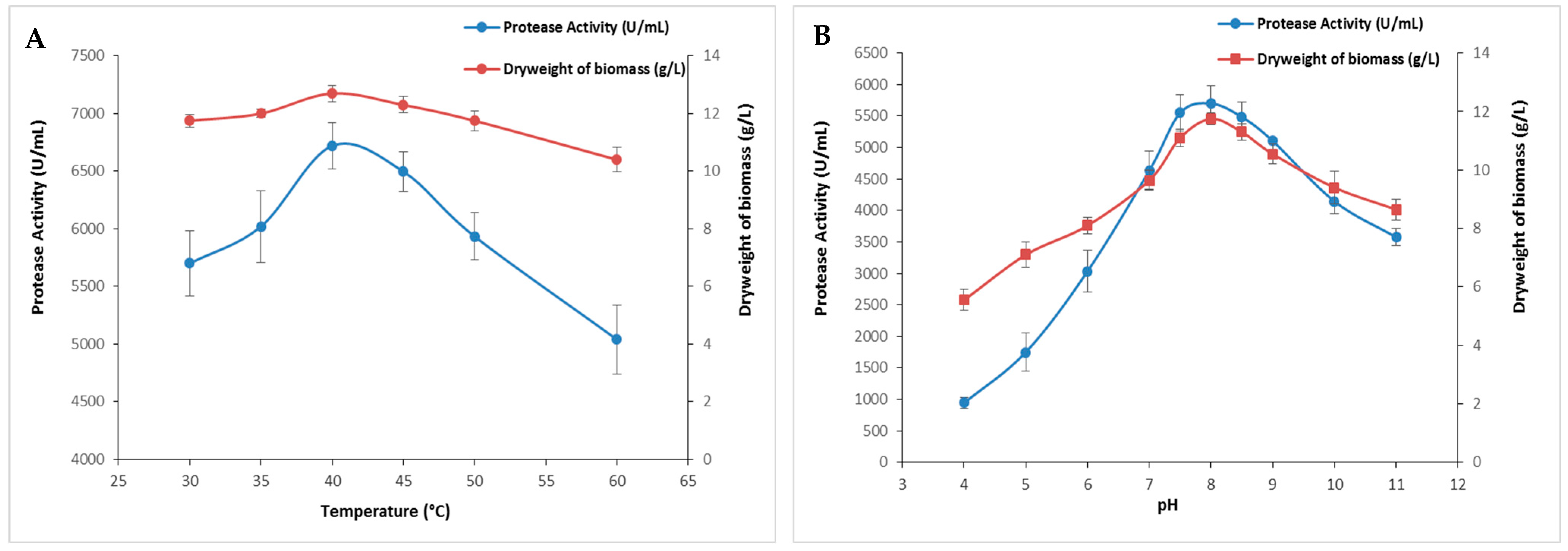
Temperature stands as a pivotal factor in governing microbial growth and metabolism, consequently influencing the expression and activity of microbial proteases. Growth temperature strongly affects protease synthesis either by influencing the rate of biochemical reactions or inducing or repressing their production [26]. In this regard, the impact of temperature on protease production was evaluated across a broad temperature spectrum, ranging from 30 to 60 °C. The current findings presented in Figure 2A showed a rise in enzymatic activity alongside an increase in biomass as temperature rises with maximum protease activity (6720 ± 197 U/mL) at 40 °C. It is noteworthy that enzymatic activity was relatively affected above 45 °C (Figure 2A). Indeed, at 50 and 60 °C, protease activity was reduced to 5935 ± 205 U/mL and 5040 ± 296 U/mL, respectively.
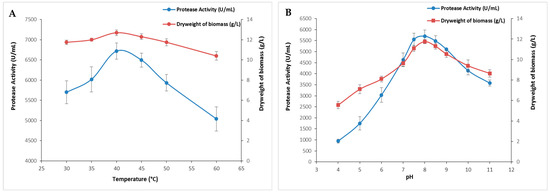
Figure 2.
The effect of temperature and pH on S. aureus protease production. The cultures were incubated at several temperatures ranging from 30 to 60 °C (A) or different initial pH values of the medium (B). The means of three replicates are represented with their corresponding SD (±).
The initial pH value influenced the transport of different growth factors that regulate microbial metabolism, resulting in a detrimental effect on protease production. This is a critical physical parameter because it regulates numerous enzymatic processes and the transport of molecules across the cell membrane [27]. Figure 2B illustrates the influence of the medium’s initial pH on the protease production activity. The enzymatic activity and the biomass increased within the pH range of 4.0 to 6.5, and then decreased steadily until a pH of 9.0. Protease activity increased between a pH of 4.0 and 8.0 and peaked at a pH of 8.5 (5700 ± 282 U/mL), followed by a gradual decline until a pH of 11 (Figure 2B). The decrease could be attributed to changes in the pH of the medium, which disturb the system’s equilibrium by altering the concentrations of H+ and OH− ions and impacting the availability of nutrients [27]. These results were in line with previous research indicating that both initial pH and temperature have a notable impact on microbial protease production. Maximum protease activity was reached at temperatures of 32 °C, 37 °C, and 45 °C and pH values of 6.5, 7.0, and 9.0 for S. simulans QB7 [25], S. aureus S-2 [23], and S. auricularis [19], respectively.
2.1.3. Effect of Carbon and Nitrogen Sources on Protease Production
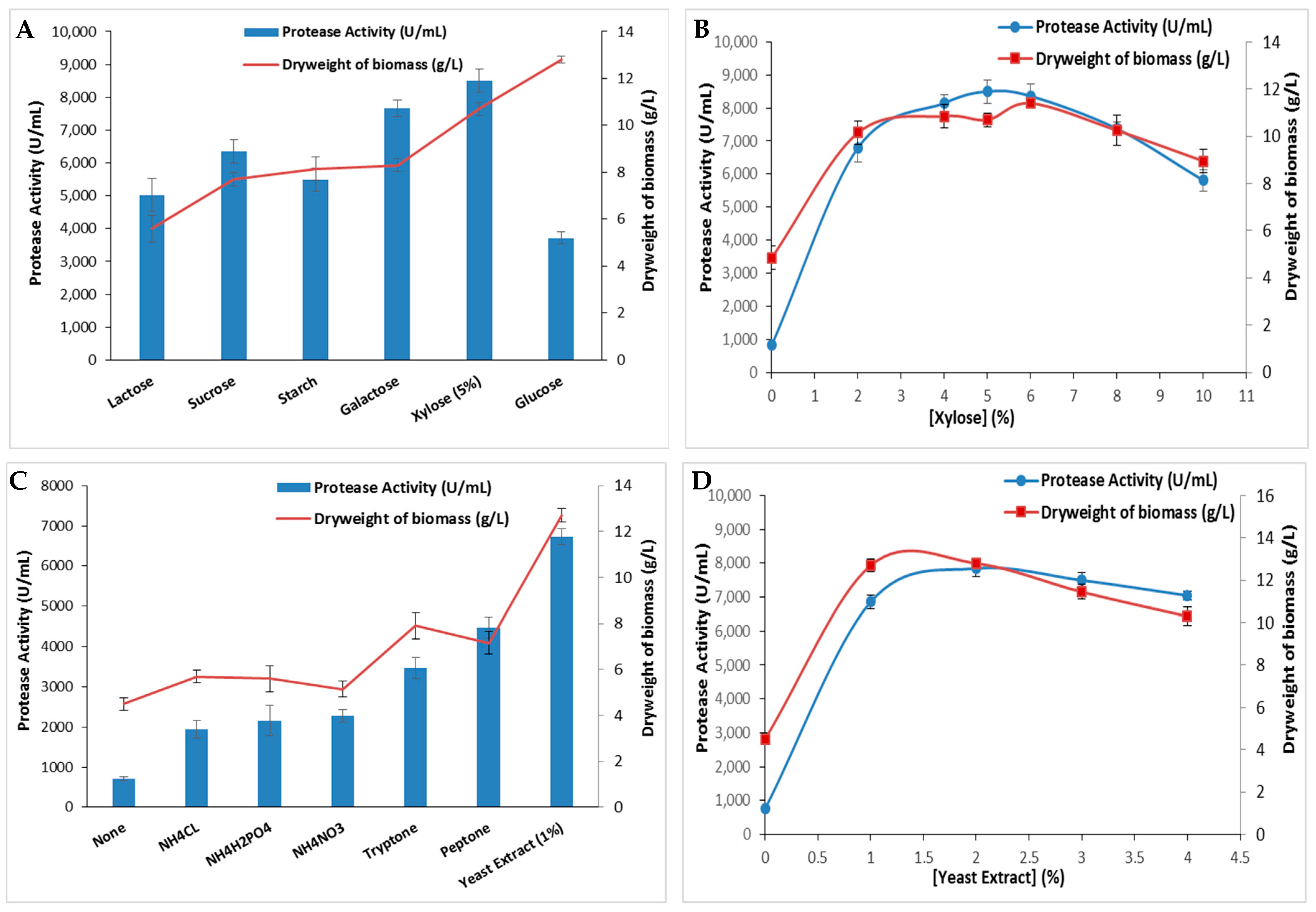
The effect of several carbon sources on protease activity was investigated using glucose, lactose, starch, sucrose, galactose, and xylose at a 5% concentration. Figure 3A indicated that the protease activity was strongly affected by the tested carbon sources while xylose provided the highest protease activity (8500 ± 335 U/mL) and the highest dry weight of biomass (10.7 ± 0.28 g/L). This activity was 2.3 times higher than that obtained with glucose (3710 ± 183 U/mL), which is the preferred carbon source for the majority of microorganisms. As the xylose concentrations were varied up to 10%, the protease activity as well as the dry weight of biomass increased proportionally with the rising concentrations, peaked at 5%, and subsequently declined gradually (Figure 3B). In contrast, S. sciuri (TKMFT 8) exhibited a preference for glucose at a 1% concentration over sucrose, lactose, and maltose, resulting in an enzymatic activity level of 195.21 ± 1.3 U/mL [20]. In comparison to pure carbon sources such as glucose, galactose, sucrose, lactose, maltose, citric acid, and trisodium citrate, S. epidermidis achieved its highest protease production level when fermented with 1% of industrial waste molasses [28].
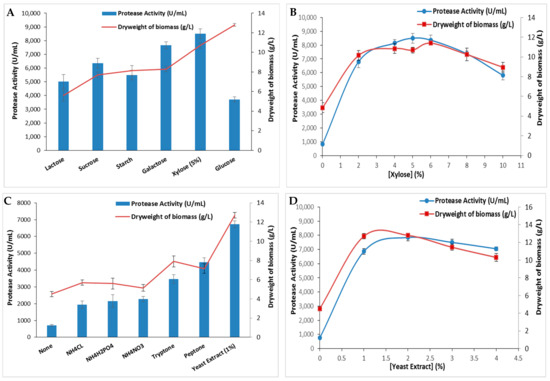
Figure 3.
The extracellular protease production by S. aureus strain and bacterial dry weight of biomass using different carbon sources (A) (at a 5% concentration) and (B) a nitrogen source (at a 1% concentration). The variation in protease activity and bacterial dry weight of biomass is shown with varying concentrations of the most effective carbon (C) and nitrogen (D) sources. The means of three replicates are represented with their corresponding SD (±).
Both the nature and qualities of nitrogen sources play a significant role in bacterial metabolism. Nitrogen is involved in synthesizing biomass, including enzymes, nucleotides, and secondary metabolites essential for microorganism growth and metabolism [29]. The effect of several organic and inorganic nitrogen sources on protease production by S. aureus ALA1 strain was also analyzed. Current data presented in Figure 3C reveal that protease production was influenced by nitrogen sources in the following order at a 1% concentration: yeast extract > peptone > tryptone > NH4NO3 > NH4H2PO4 > NH4CL. The highest enzymatic activity (6720 ± 197 U/mL) and dry weight of biomass (12.8 ± 0.28 g/L) was achieved with the yeast extract-supplemented medium (Figure 3C). The variation in yeast extract concentrations in the S. aureus culture medium demonstrated that 2% was the optimal concentration, allowing a maximal protease activity of 7670 ± 254 U/mL and a maximal dry weight of biomass (12.8 ± 0.14 g/L) (Figure 3D). Beyond this concentration, enzymatic activity decreased slightly. Similar results were found with S. auricularis p18, which preferred yeast extract at a 0.7% concentration rather than peptone as the nitrogen source to exhibit the maximal protease activity [25]. Among the nitrogen sources examined, beef extract was identified as the optimal nitrogen source for eliciting the highest protease activity for S. sciuri (TKMFT 8) [20]. However, S. epidermidis displayed the highest protease biosynthesis when 1% of peptone was used as the nitrogen source in the mineral medium supplemented with 1.0% of molasses. When various organic and inorganic nitrogen sources were substituted for peptone, S. epidermidis achieved maximum protease production when cultivated in a medium containing 1.0% of ammonium nitrate [28].
2.2. Purification of S. aureus Protease S. aureus Pr
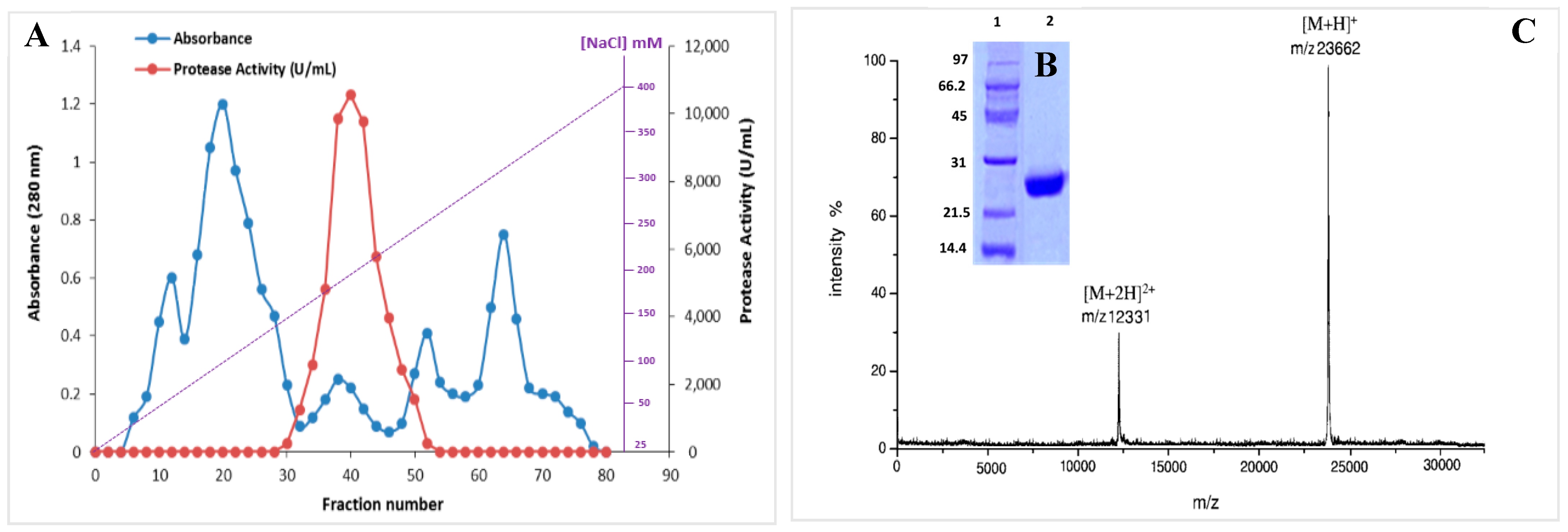
In order to produce the S. aureus Pr, the specified bacterial strain S. aureus ALA1 was cultured in the optimized medium (5% xylose, 2% yeast extract, 5 g/L KH2PO4, 0.2/L CaCl2, 0.1 g/L FeSO4•7H2O, and 3 g/L NaCl (pH 8.5)). The purification of S. aureus Pr was performed following the protocol outlined in the Material and Methods section. Briefly, a heat and acidic treatment (80 °C for 10 min) was applied for the supernatant culture, followed by ammonium sulfate (NH4)2SO4 precipitation fractionation from 25% to 65% (w/v) and overnight dialysis. Then, the supernatant was injected into a Mono Q-Sepharose column. After washing, the proteins adsorbed onto the column were eluted by a linear gradient of NaCl from 25 to 400 mM (Figure 4A). Eluted fractions with protease activity were subjected to analysis via SDS-PAGE, revealing a single band with an apparent molecular mass of approximately 25 kDa (Figure 4B). The molecular mass was accurately determined by MALDI-TOF analysis, indicating a singular peak with a molecular mass of 23,662 Da (Figure 4C). Table 1 outlines the specific activity and recovery rates of S. aureus Pr following each purification step. Purified S. aureus Pr was purified with a recovery rate of 38.2% and a purification factor of 78.6 (Table 1). Under the optimized culture conditions, 100 mL of S. aureus supernatant culture facilitated the generation of 5 mg of pure enzyme. The purified protease exhibited a specific activity of 64,270 U/mg using casein as the substrate under standard conditions.
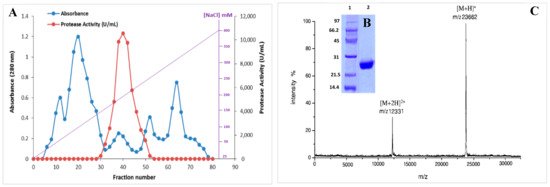
Figure 4.
(A) Chromatography profile of S. aureus Pr on a Mono Q-Sepharose column. (B) SDS-PAGE analysis and (C) MALDI-TOF spectrum of S. aureus Pr, 1: size marquer, 2: purified S. aureus Pr.
The S. epidermidis BP9 protease underwent a purification factor of 32.88, with a recovery yield of 21.57% and a specific activity of 75.96 U/mg. SDS-PAGE analysis of the purified S. epidermidis BP9 protease revealed a distinct single band, approximately 24.0 kDa in size [24] (Table 2). The protease isolated from S. simulans QB7, with a recovery rate of 14.75%, displayed a specific activity of 66.33 U/mg after purification and an apparent molecular weight of 47 kDa [19]. Another extracellular protease with a molecular weight of 21.5 kDa was isolated from S. xylosus A2 in a two-step purification process with a purification fold of 14.23, a yield of 14.7%, and displaying a specific activity of 33.3 U/mg [30]. The protease from a new strain of S. saprophyticus (28 kDa) was successfully purified, achieving 42.66-fold purification through a combination of 70% to 80% ammonium sulfate precipitation and gel-permeable column chromatography [31]. (Table 2) Similarly, after 80% saturation of ammonium sulfate of the crude enzyme of S. sciuri, dialysis, and gel filtration on a Sephacryl S-200 high-resolution column, the specific activity of the purified metalloprotease increased up to 154.34 U/mg at a 15.8% yield [1] (Table 2).
Through Edman degradation, the first 35 amino acid residues from the purified S. aureus Pr N-terminal sequence were determined. The obtained sequence “NH2- VILPNDNRHQIFNTTQGHYDLLSFIYIPINGGYMSGSG-COOH” showed high identity (89%) with those reported for serine proteases produced by several strains of S. epidermidis (accession number MBF9302351.1 and MDU3980336.1) and Moraxella sp. (accession number TWV79723.1).

Table 1.
Purification table of S. aureus Pr from 100 mL of culture of S. aureus ALA-1.
Table 1.
Purification table of S. aureus Pr from 100 mL of culture of S. aureus ALA-1.
| Purification Step | Total (a) Activity (Units) | Protein (b) (mg) | Specific Activity (U/mg) | Activity Recovery (%) | Purification Factor |
|---|---|---|---|---|---|
| Culture supernatant | 840,000 | 1028 | 817.1 | 100 | 1 |
| Heat and pH treatment (10 min at 80 °C and pH 3) | 714,000 | 215 | 3320.9 | 85 | 4 |
| (NH4)2SO4 Precipitation (25–65%) | 535,500 | 49 | 11,900 | 63.4 | 14.6 |
| Mono Q-Sephadex | 321,300 | 5 | 64,270 | 38.2 | 78.6 |
(a): One unit is defined as the amount of enzyme that catalyzes the hydrolysis of the substrate to yield one µg of amino acid equivalent to tyrosine per min under the specified experimental conditions. (b): Protein concentration was measured using the Bradford method [32].

Table 2.
Biochemical characterization of purified proteases from Staphylococcus strains (ND: not determined).
Table 2.
Biochemical characterization of purified proteases from Staphylococcus strains (ND: not determined).
| Protease Origin | pH Optimal | Optimal Temperature (°C) | Molecular Weight (kDa) | References |
|---|---|---|---|---|
| S. epidermidis BP9 | 6.0 | 50 | 24.0 | [24] |
| S. simulans QB7 | 7.0 | 50 | 47 | [19] |
| S. xylosus | 6.0 | 50 | 21.5 | [30] |
| S. saprophyticus | 11.0 | 30–60 | 28 | [31] |
| S. sciuri | 5.6 | 70 | ND | [20] |
| S. aureus S2 | 8.0 | 50 | 30 | [23] |
2.3. Biochemical Characterization of S. aureus Pr
2.3.1. Effect of pH and Temperature on S. aureus Pr Activity and Stability
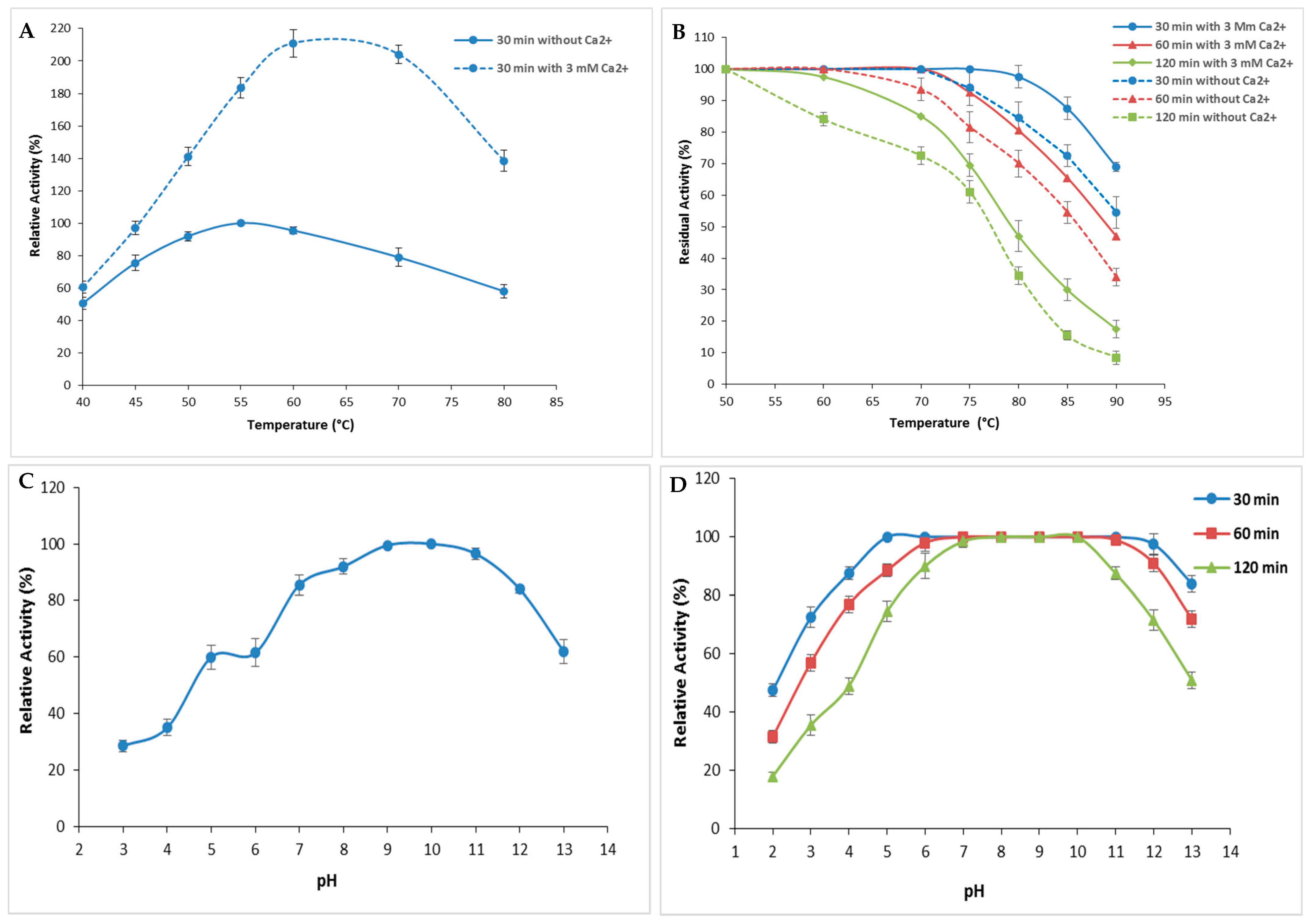
The performance and endurance of S. aureus Pr in response to variations of the physicochemical factors pH and temperature were investigated. Current findings presented in Figure 5A showed that S. aureus Pr exhibited its maximal activity corresponding to an SA of 64,270 U/mg at 50 °C when no Ca2+ was added (Figure 5A). However, in the presence of 3 mM Ca2+, the peak shifted to 60 °C (Figure 5A). It is clear that enzymatic activity increased significantly in the presence of calcium (Figure 5A) since, even at the highest temperature, S. aureus Pr remained active. This improved thermostability could be due to the hydrophobic interactions within the protease amino acids mainly charged and aromatic residues [3].
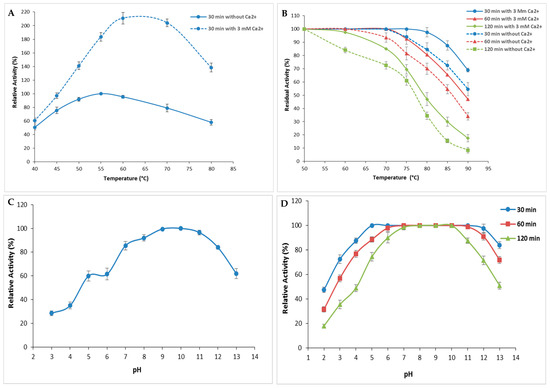
Figure 5.
Evaluation of pH and temperature effects on the activity and the stability of S. aureus Pr. (A) The variation of protease activity with a variation of temperature from 40 to 80 °C. The enzymatic activity was measured during 30 min without Ca2+ and with 3 mM of Ca2+. (B) The stability of S. aureus Pr at different temperatures during 30, 60, and 120 min in the absence and in the presence of Ca2+. (C) The effect of acidic and alkali pH on S. aureus Pr activity. (D) The stability of S. aureus Pr with a pH variation from 2.0 to 13.0 during 30, 60, and 120 min. The means of three replicates are represented with their corresponding SD (±).
In order to enhance the characterization of enzyme stability under high temperatures, residual activity was also assessed following incubation at different temperatures (50–90 °C) either with or without 3 mM of Ca2+, for different time periods of 30, 60, and 120 min (Figure 5B). As depicted in Figure 5B, S. aureus Pr exhibited stability, retaining 100% of its initial activity (corresponding to an SA of 64,270 U/mg) after 30 min and 60 min of incubation at 70 °C, regardless of the presence of Ca2+. However, after 120 min of incubation, the enzyme’s stability decreased, retaining 85% activity (corresponding to an SA of 54,629.5 U/mg) at 70 °C, and gradually declined to 20% (SA: 12,845 U/mg) at 90 °C (Figure 5B). Notably, the presence of calcium ions significantly enhanced the thermal stability as described with most microbial proteases [3]. Indeed, metal ions serve to safeguard the protease from thermal denaturation and are crucial in preserving its active conformation, even at elevated temperatures. The presence of Ca2+ is recognized for its ability to enhance protease activation by improving thermostability [10].
S. aureus Pr exhibited high relative activity at a large range of pH values ranging from 6.0 to 12.0 with an optimum at a pH of 10.0. This relative activity decreased slightly to 96.5% and 84% at a pH of 11.0 and 12.0, respectively (Figure 5C) [31]. These relative activities correspond to an SA of 62,020.5 and 53,986.5 U/mg, respectively. This result made S. aureus Pr protease well suited for utilization in environments of weak acid fermentation, as well as for the hydrolysis of meat proteins into bioactive peptides and amino acids. Moreover, purified S. aureus Pr was interestingly highly stable and retained its total activity in the pH range of 6.0 to 10.0 after time periods of 30, 60, and 120 min (Figure 5D). At higher pH values, the activity decreased progressively to reach 84% (53,986.5 U/mg), 72% (46,274.4 U/mg), and 51% (34,307.7 U/mg) after incubation for 30, 60, and 120 min, respectively. This remarkable stability at extreme pH and temperature values was observed with different proteases purified from various strains of Staphylococcus (Table 2). The protease from S. saprophyticus BUU1 maintained over 70% of its relative activity across a broad pH range of 3.0 to 12.0, with an optimum observed at a pH of 11.0. This protease exhibited significant stability across the same pH range, retaining nearly 90% of its initial activity even after 6 h of incubation. The optimal temperature was found to be between 30 °C and 60 °C while 70% of its initial activity was maintained after incubation at 70–80 °C for 48 h [31]. Likewise, the partially purified protease from S. sciuri maintained its catalytic activity across a temperature spectrum spanning from 20 to 90 °C, with optimal performance at 70 °C and a pH of 5.6 when utilizing azocasein as a substrate. The enzyme, which was thermostable up to 50 °C, gradually lost up to 51% of its initial activity at 90 °C [1]. The protease produced by S. simulans QB7 was stable within temperatures ranging from 20 to 60 °C, reached the highest activity at a pH of 7.0, and maintained more than 50% of its catalytic efficiency at a pH of 4.0–9.0 [19]. The optimal pH and temperature for the protease activity from S. aureus S-2 were 8.0 and 50 °C, respectively. This enzyme was active at a broad range of pH from 5.0 to 9.0 and temperature values from 30 to 90 °C [23]. Additionally, the extracellular protease from S. epidermidis reached maximal activity at a pH of 6.0 and 50 °C and was stable at a pH of 4.0–9.0 and 20–40 °C [24].
2.3.2. Effect of Stabilizers Addition on S. aureus Pr Thermal Stability
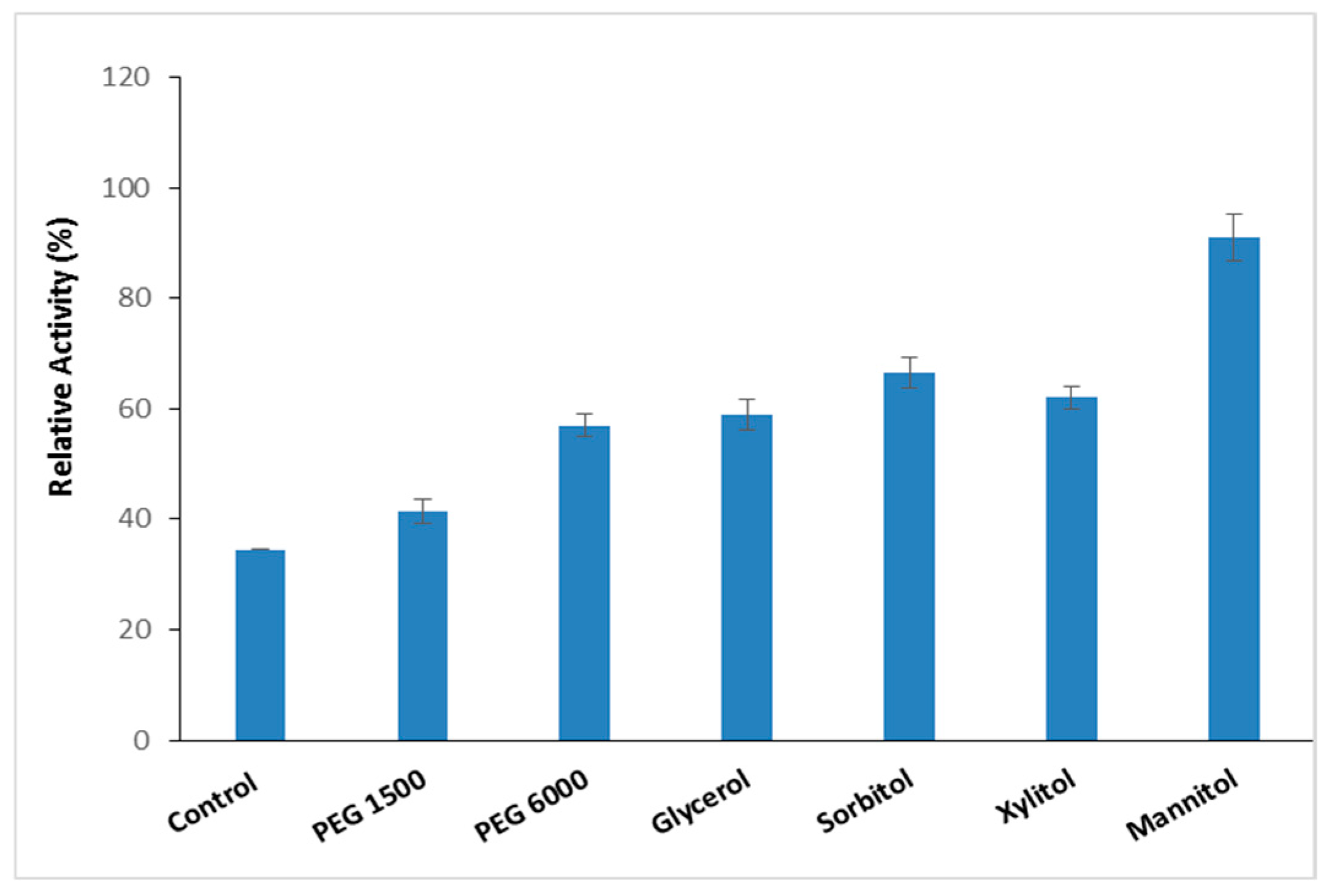
Thermostable proteases are pivotal in biotechnological and industrial applications because of their ability to withstand denaturing agents and chemicals [3]. Numerous molecules are recognized as additives that protect proteins from heat-induced inactivation by stabilizing thermally unfolded proteins [33]. To improve the thermal resilience of the purified S. aureus Pr at high temperatures, the impact of different thermal stabilizer additives (Polyethylene Glycol PEG 1500, PEG 6000, glycerol, sorbitol, xylitol, and mannitol) at a concentration of 10% was evaluated at 90 °C over a period of 1 h (Figure 6).
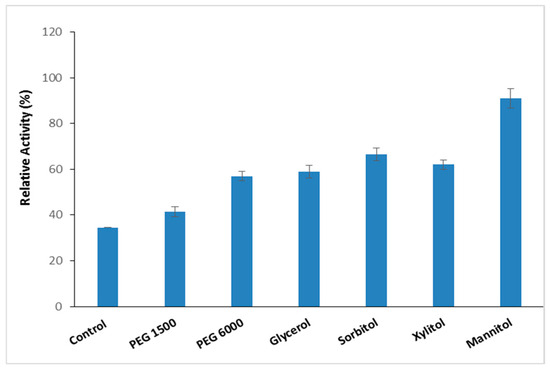
Figure 6.
The effect of stabilizer addition on S. aureus Pr thermal stability enhancement. The residual activity was measured after incubation of S. aureus Pr with 10% of different stabilizer additives (PEG 1500, PEG 6000, glycerol, sorbitol, xylitol, and mannitol) at 90° C during a period of 1 h. The means of three replicates are represented with their corresponding SD (±).
The data presented in Figure 6 indicate that PEG1500 and PEG6000 enhanced the stability of S. aureus Pr at 90 °C to 41% (26,350.7 U/mg) and 57% (36,633.9 U/mg), respectively. According to Padma and Ananthanarayan [34], polyethylene glycols have the capability to reinforce the hydrophobic bonds among amino acids, thereby enabling the protein structure to maintain organization for an extended duration. Glycerol, sorbitol, and xylitol increased thermal stability by up to 60% (Figure 6). It seemed that the protective effect of polyols can be due to the hydrophobic interactions within protein molecules [35]. At a concentration of 10%, mannitol emerged as the most effective additive among those tested, enhancing the stability of S. aureus Pr to 91 ± 4.2% (SA 58,485.7 U/mg) compared to 34.5 ± 2.1% (22,173.15 U/mg) without any additive after 1 h at 90 °C (Figure 6). Table 3 indicates that the presence of 3 mM of Ca2+ with the mannitol improved thermal stability to 100% ± 0.5 (SA 64,270 U/mg) and 70% ± 4.23 (SA 44,998 U/mg) after 1 h and 2 h incubation. The present findings are in line with previous studies indicating mannitol’s role as a stabilizing agent. Mannitol functions by reinforcing hydrophobic interactions among non-polar amino acid residues of enzymes, thereby promoting protein rigidity and resistance to thermal deactivation [36]. Moreover, the presence of metal ions is among the factors contributing to the stability of thermophilic proteases, which serve to enhance molecular stability [3].

Table 3.
The effect of a combination of mannitol and Ca2+ on the thermal stability of S. aureus Pr. The means of three replicates are represented with their corresponding SD (±).
2.3.3. Stability Study
Impacts of Inhibitors, Reducing Agents, and Metallic Ions on S. aureus Pr Stability
The effect of natural and synthetic inhibitors, metallic ions, as well as chelating agents on S. aureus Pr stability was evaluated. Table 4 illustrates the comparative inhibitory impacts of different compounds. S. aureus Pr was strongly inhibited by serine proteases inhibitors Phenylmethylsulfonyl Fluoride (PMSF) and 3,4-Dichloroisocoumarin (DFB) at 5 mM, with residual activities of 8% ± 1.41 (SA 5141 U/mg) and 6% ± 1.41 (SA 3856 U/mg), respectively (Table 4). This implied that a serine residue participates in the S. aureus Pr catalytic activity. Indeed, the serine residue within the proteinases’ active site undergoes irreversible acylation by PMSF or Diisopropyl Fluorophosphate (DIFP), leading to enzyme inactivation. Consequently, reagents like PMSF or DIFB function as inhibitors of serine proteases [37]. Furthermore, 5,5′-Dithiobis(2-nitrobenzoic acid) or thiol BTNB and Benzamidine (trypsin competitive reagent) reagents at 10 mM had almost no effect on S. aureus Pr activity (Table 4) while the metalloprotease inhibitor EDTA at 10 mM or Ethylene Glycol Tetraacetic Acid (EGTA) at 2 mM did not affect the purified S. aureus Pr (Table 4). This finding suggested that S. aureus Pr does not require metallic cofactors. Interestingly, the enzyme’s stability in the presence of EDTA is beneficial for its utilization as a detergent additive since detergents feature high levels of chelating agents. These agents serve as water softeners and aid in stain removal by binding specifically to metal ions, rendering them inaccessible in the detergent solution [38].

Table 4.
The impacts of various inhibitors and reducing agents on S. aureus Pr stability. The protease activity measured without any inhibitor or reducing agent served as the control (100%). The residual activity was measured under standard conditions. The means of three replicates are represented with their corresponding SD (±).
Interestingly, S. aureus Pr was fully active in the presence of reducing agents β-Mercaptoethanol (β-ME at 5 mM) and Dithiothreitol (DTT at 10 mM) with residual activities of 98.5 ± 2.1% (SA 63,305.95 U/mg) and 100 ± 4.2% (SA 64,270 U/mg), respectively (Table 4). This could indicate that S. aureus Pr belonged to a thiol-dependent protease like proteases produced by Exiguobacterium sp. SKPB5 and Bacillus mojavensis, with an increased activity of two-fold in the presence of β-ME and DTT [12,39]. Moreover, a lower concentration of β-ME may facilitate the unveiling of concealed active sites and facilitate the restoration of the protease’s disulfide bond. This subsequently leads to a notable increase in protease activity [24].
S. aureus Pr retained 89 ± 2.8% (SA 57,521.65 U/mg) and 63.5 ± 3.5% (SA 40, 811.45 U/mg) of its initial activity after incubation with 60 mM of sodium dodecyl sulfate (SDS) and 6 M of urea, respectively (Table 4). Its stability in the presence of SDS indicated S. aureus Pr’s suitability as a detergent constituent. However, the decrease in enzymatic activity could be due to the change in the enzyme conformation by SDS, leading to improper 3D folding and subsequent enzyme inactivation [11]. After exposure to 0.5% of SDS, the protease derived from S. sciuri maintained 52.7% of its initial catalytic activity [1].
The decrease in activity observed in the presence of the chaotropic agent urea at high concentrations might be linked to potential interactions between urea and the enzyme surface. These interactions could lead to the weakening of intermolecular bonds within enzyme molecules or the displacement of water molecules from the protein’s hydration shell in aqueous solutions [40].
Moreover, the evaluation of metal ions’ impact on S. aureus Pr activity was conducted as they are constituents of proteases’ active sites. A significant enhancement of S. aureus Pr activity was observed, increasing by 220.5 ± 6.36%; 202.5 ± 6.36%; and 162 ± 4.24% in the presence of Ca2+, Mg2+, and Zn2+, respectively at a concentration of 3 mM (Table 5), suggesting the promotion of the substrate binding at the active site by the presence of these cations [41]. A slight improvement in enzymatic activity was observed when Cu2+, Co2+, Fe2+, and Mn2+ were added (Table 5). However, Cd2+, Hg2+, and especially Ni2+ strongly deactivated S. aureus Pr up to 34.21 ± 2.8%, 21 ± 2.8%, and 12 ± 1.4%, respectively, which might indicate that these cations decreased the affinity between the protease and substrates [42] (Table 5). This result could be explained by the fact that Ca2+ is widely recognized for its ability to enhance both proteolytic activity and thermal stability, potentially by safeguarding enzymes from thermal denaturation and crucially contributing to the maintenance of their active conformation [31,41]. Mg2+ typically functions as a salt or ion bridge, preserving histidine within active sites and upholding the rigid conformation of the enzyme molecule [31]. Similar findings were observed with the S. epidermidis BP9 protease whose relative activity was strongly enhanced to 289.0% and 137.4% by adding 10 mM of Ca2+ and Cu2+, respectively, while it was significantly reduced in the presence of 5 mM and 10 mM of Fe2+ [40]. The S. simulans QB7 protease, which was activated by adding 10 mM of Ca2+ up to 195.89%, was strongly inhibited in the presence of Cu2+, Co2+, Fe2+, and Fe3+ [19]. Likewise, the activity of the thermotolerant alkaline metalloprotease from S. sciuri was enhanced by Ca2+ and Mg2+ while being reduced by Cu2+ and Hg2+ at 10 mM [1]. Similarly, the protease activity of S. saprophyticus which was stimulated by about 44% with Ca2+ was strongly inhibited by Hg2+ after 48 h ofincubation [31].

Table 5.
The impacts of various bivalent cations on S. aureus Pr stability. The protease activity measured without any bivalent cations was taken as the control (100%). The relative activity was measured under standard conditions. The means of three replicates are represented with their corresponding SD (±).
Impacts of Surfactants, Oxidizing Agents, and Organic Solvents on S. aureus Pr Stability
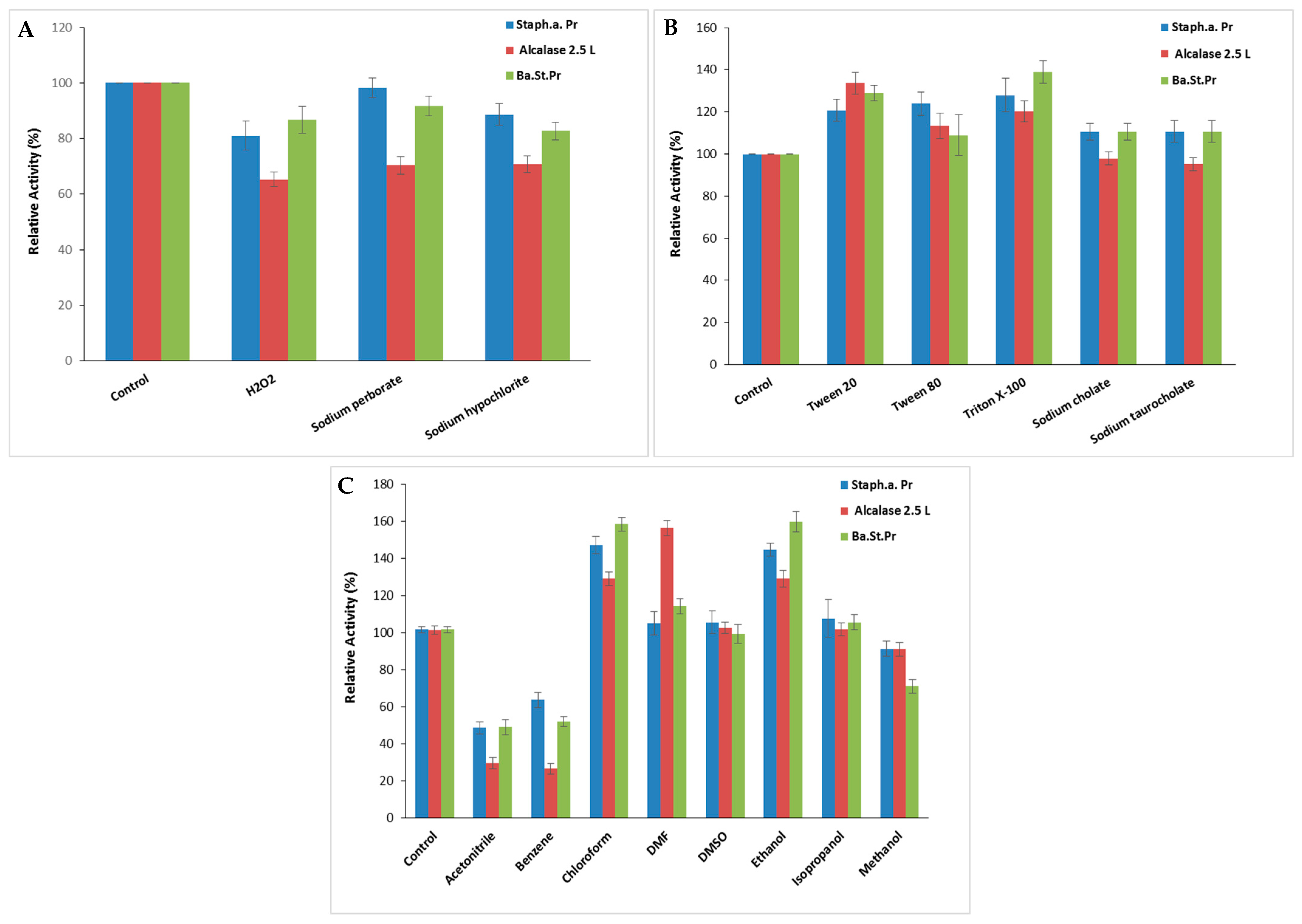
The stability of S. aureus Pr in the presence of surfactants and oxidizing agents is a crucial characteristic for its potential utilization in detergent formulations. S. aureus Pr maintained about 81 ± 5.29% (SA: 52,058.7 U/mg), 98 ± 3.51% (SA: 63,177.4 U/mg), and 88.6 ± 4.04% (SA: 56,943.2 U/mg) of its maximal activity following exposure to H2O2, sodium perborate, and sodium hypochlorite at 5%, respectively (Figure 7A), against approximately 70 ± 3.05% of Alcalase 2.5 L activity when incubated under comparable treatment conditions. Increased protease activity due to H2O2 might arise from the additional oxidation of cysteine, tyrosine, or tryptophan residues following methionine, which are prone to oxidative inactivation [31].
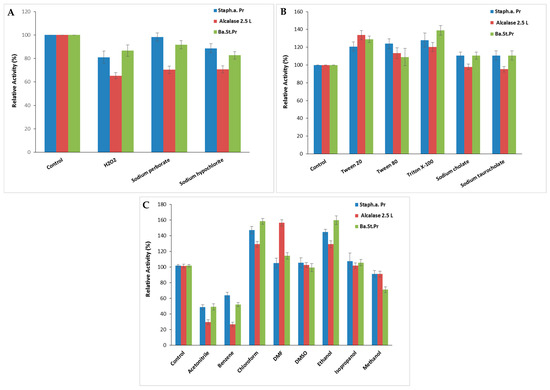
Figure 7.
The impact of oxidizing agents (A), surfactants (B), and organic solvents (C) on S. aureus Pr stability. The residual activity was measured at the optimal condition after incubation of the purified protease with each appropriate agent for 1 h. The commercial Alcalase 2.5 L and Ba.St.Pr were taken as positive controls. The means of three replicates are represented with their corresponding SD (±).
As seen in the case of the positive control, Alcalase 2.5 L and the Bacillus stearothermophilus protease (Ba.St.Pr), enhanced stability was noted in the presence of Tween 20, Tween 80, Triton X-100, sodium cholate, and sodium taurocholate at 15%, with residual activities ranging between 110 and 124%, respectively (Figure 7B). The specific activities of Alcalase 2.5 L and Ba.St.Pr were 24,915 103 U/mg and 59,022 U/mg, respectively. According to Sasithorn [31], Tween 80 had little effect on the protease activity by changing the protease conformation or its interfacial properties.
Furthermore, when contemplating the utilization of proteases in organic synthesis, it is essential to examine their stability in organic solvents. Therefore, various solvents were tested at a concentration of 25% for 24 h to study the stability of S. aureus Pr. As illustrated in Figure 7C, S. aureus Pr was remarkably stable in the presence of chloroform (147 ± 4.58%) and ethanol (144 ± 3.51%) followed by isopropanol, Dimethylformamide (DMF) (105 ± 6.24%) and Dimethyl Sulfoxide (DMSO) (106 ± 6.02%). Thus, S. aureus Pr’s remarkable stability in organic solvents supported its potential application in organic synthesis. However, S. aureus Pr was less stable in the presence of acetonitrile and benzene, retaining only 48 ± 3.2% and 63 ± 4% of its initial activity, respectively (Figure 7C). The overall trend of the stability profile was comparable for both proteases Alcalase 2.5 L and Ba.St.Pr, with slight differences in stability observed with some solvents like isopropanol, DMSO, and DMF (Figure 7C). Possibly, the operational mechanism of these solvents was to impede the efficient interaction between enzymes and substrates. Hydrophilic solvents might have the ability to dissolve proteases, potentially leading to increased inactivation. The solubility of proteases in short-chain alcohols likely diminishes the formation of a new liquid phase at moderate concentrations, thereby contributing to enzyme inactivation [31].
Similar observations were described with various proteases derived from Staphylococcus strains. Indeed, the protease from S. sciuri retained 93.5 and 100.4% of the initial catalytic activity after incubation with 0.5% of Triton X100 and Tween 20, respectively [1]. The purified protease from S. saprophyticus was highly stable in H2O2, sodium perborate, triton X100, and Tween 80 when tested at 1% for 1 h. In the presence of methanol, ethanol, and isopropanol, this protease maintained nearly 60% of its initial activity, whereas benzene, hexanes, heptane, and hexadecane had a minimal impact on its catalytic activity [31].
Stability and Compatibility of S. aureus Pr with Solid and Liquid Commercial Laundry Detergents
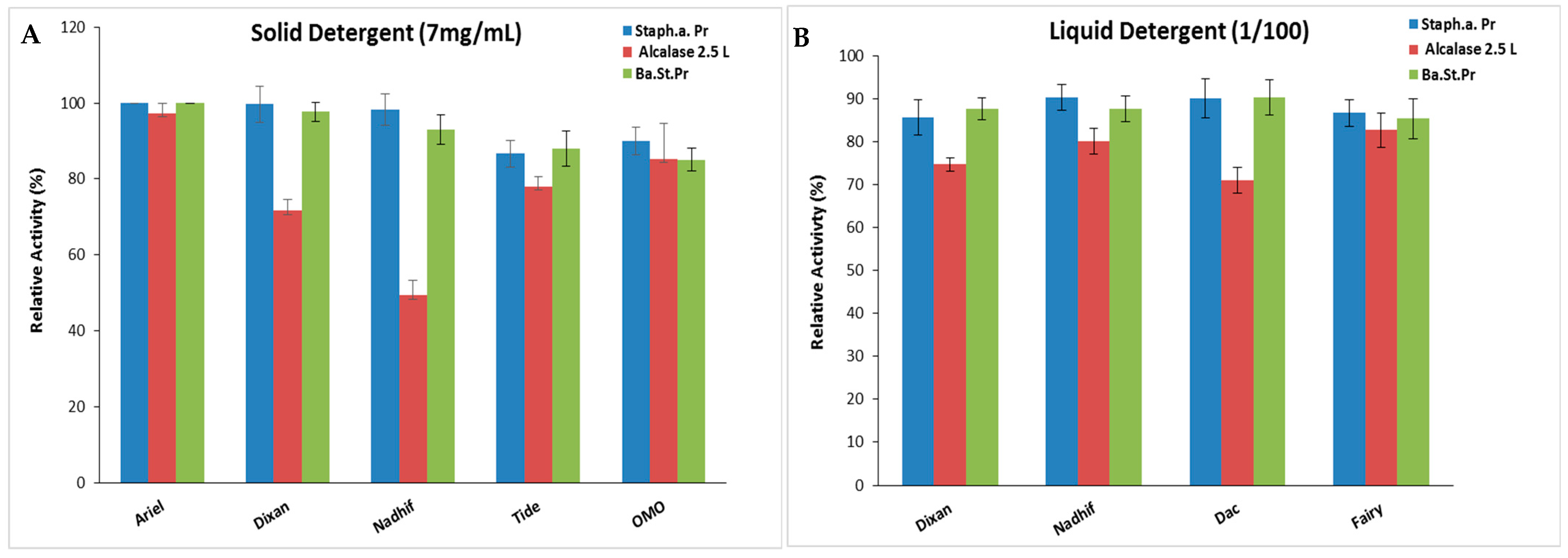
Incorporating proteases into detergents significantly enhances their cleaning effectiveness while reducing the usage of surface-active substances, consequently lowering the pollution burden. An effective detergent protease should demonstrate stability and compatibility with the surfactants and oxidizing agents typically present in detergent formulations [43]. As S. aureus Pr exhibited notable stability in surfactants, oxidizing agents, and organic solvents commonly found in detergent compositions, its suitability and compatibility with various forms of commercial detergents, including liquids and solids, were assessed. Following the denaturation of endogenous proteolytic enzymes, both liquid and solid commercial detergents were diluted in tap water to achieve final concentrations of 7 mg/mL and 1/100, respectively, mimicking typical washing conditions. Subsequently, S. aureus Pr was incubated with the respective detergent under agitation, followed by the measurement of its residual activity. Alcalase 2.5 L and Ba.St.Pr were used as positive controls for comparative analysis.
The present findings suggest that S. aureus Pr demonstrated exceptional stability and compatibility with the liquid and solid laundry detergents tested in this study (Figure 8). This stability was comparable to that of Ba.St.Pr, but much better than that of the commercial protease. In fact, S. aureus Pr maintained its full initial activity when incubated with several solid detergents, while Alcalase 2.5 L exhibited residual activities ranging from 49% to 97% (Figure 8A). When incubated with liquid detergents, S. aureus Pr retained ~ 90% of its maximal activity, against 70–82% in the case of Alcalase 2.5 L (Figure 8B). This characteristic has been extensively documented with numerous microbial proteases, particularly those from Bacillus strains, with some commercialized under the brand name Alcalase® (from Bacillus licheniformis), Durazym® (from Bacillus spp. GMO), Esperase® (Bacillus halodurans), Neutrase® (from Bacillus amyloliquefaciens), etc. [44].
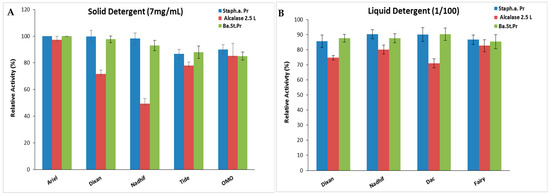
Figure 8.
The stability and compatibility of S. aureus Pr with solid (A) and liquid (B) commercial laundry detergents assessed in comparison to commercial Alcalase 2.5 L and Ba.St.Pr. Residual activities were measured under the same optimal conditions. The means of three replicates are represented with their corresponding.
3. Materials and Methods
3.1. Protease Activity Assay
Protease functionality was evaluated using the approach outlined by McDonald and Chen [45]. A mixture of 2 mL of a 1% (w/v) casein solution purchased from Sigma Aldrich (St. Quentin-Fallavier, France) used as the substrate dissolved in 100 mM of glycine-NaOH buffer (pH 10.0) (Sigma Aldrich, St. Quentin-Fallavier, France) was incubated with 1 mL of enzymes at 55 °C for 15 min. Following this, 3 mL of 10% TCA solution (Sigma Aldrich, St. Quentin-Fallavier, France) was added in order to terminate the reaction. The precipitated proteins (non-hydrolyzed) were removed by centrifugation (Bio-Rad, Hercules, CA, USA) at 5000× g for 20 min. The control sample comprised 2 mL of a 1% (w/v) casein solution, 1 mL of enzyme, and 3 mL of 10% TCA (w/v). Next, 1 mL of the clear supernatant containing the incubated enzyme/substrate was blended with 5 mL of alkaline copper reagent (Sigma Aldrich, St. Quentin-Fallavier, France). After a 15 min incubation at room temperature, 0.5 mL of the Folin–Ciocalteu reagent (Sigma Aldrich, St. Quentin-Fallavier, France) was diluted in a 1:1 ratio and allowed to stand for 30 min. The absorbance of the resulting supernatant was taken at 700 nm against a blank control. The enzyme activity was determined by the amount of enzymes releasing the equivalent of one µg of tyrosine per min under the defined assay conditions.
3.2. Optimization of S. aureus Culture Conditions
The impact of various factors on bacterial growth and protease production by S. aureus was examined including carbon and nitrogen sources, temperature, initial pH, and duration of incubation. These parameters were assessed following the protocol outlined by Sepahy and Jabalameli [46] with some minor adjustments.
3.2.1. Effects of Incubation Time
S. aureus growth and protease production were assessed at varying time intervals during incubation up to 72 h at 40 °C in a medium including 5 g of yeast extract, 5 g of bactopeptone, 5 g of KH2PO4, 0.2 g of CaCl2, 0.1 g of FeSO4·7H2O, and 3 g of NaCl (Sigma Aldrich, St. Quentin-Fallavier, France) dissolved in 1 L of distilled water (pH of 8.0). For the optimization study, samples were collected regularly at intervals of either 4 or 8 h. At each time point, growth, measured by optical density (OD) at 600 nm, and protease activity were determined utilizing a spectrophotometer (Bio-Rad, Hercules, CA, USA). Samples were diluted appropriately to ensure the readings were within the linear range of the instrument.
3.2.2. Effects of pH and Temperature
An inoculum of 8 × 108 CFU/mL was introduced into the sterilized medium previously used. Subsequently, it was subjected to individual incubations at different pH levels spanning from 4.0 to 11.0 and temperatures ranging from 30 °C to 60 °C for a duration of 30 h, with continuous agitation at 200 rpm. The assessment included the evaluation of both protease activity determined utilizing a spectrophotometer (Bio-Rad, Hercules, CA, USA) and bacterial biomass monitored by measuring the OD at 600 nm.
3.2.3. Effects of Carbon and Nitrogen Sources
Media were prepared in 50 mL flasks, each adjusted to a pH of 8.0 and containing 5% (w/v) of chosen carbon sources. Briefly, glucose, lactose, starch, sucrose, galactose, and xylose (Sigma Aldrich, St. Quentin-Fallavier, France) were individually examined for protease activity and dry weight measurement. These carbon source media underwent sterilization at 121 °C for 15 min before being inoculated with 8 × 108 CFU/mL of S. aureus. Incubation was followed at 40 °C for 30 h with agitation set at 200 rpm.
Growth media were formulated with 1% (w/v) concentrations of the following specified nitrogen sources: yeast extract, peptone, tryptone, NH4NO3, NH4H2PO4, and NH4Cl (Sigma Aldrich, St. Quentin-Fallavier, France). These media were adjusted to a pH of 8.0 and sterilized through autoclaving. Subsequently, they were inoculated with 8 × 108 CFU/mL of bacteria and subjected to a 30 h incubation period at 40 °C.
3.3. Protease Purification
A 30 h culture of S. aureus, totaling 100 mL (5% xylose, 2% yeast extract, 5 g/L of KH2PO4, 0.2/L of CaCl2, 0.1 g/L of FeSO4•7H2O, and 3 g/L of NaCl (pH 8.5) (Sigma Aldrich, St. Quentin-Fallavier, France)), underwent centrifugation (Bio-Rad, Hercules, CA, USA) at 9000× g for 30 min. The resulting supernatant was subjected to heat and acidic treatment (80 °C and pH 3.0 for 10 min), followed by fractionation via (NH4)2SO4 (Sigma Aldrich, St. Quentin-Fallavier, France) precipitation ranging from 25% to 65% (w/v). The precipitated pellet was then recovered in 50 mM of Tris-HCl buffer containing 2 mM of CaCl2 (Sigma Aldrich, St. Quentin-Fallavier, France) at a pH of 8.0. Following overnight dialysis, the supernatant was introduced into a Mono Q Sephadex column (30 cm × 2.5 cm) (Applied Biosystems, Foster City, CA, USA). After washing, proteins adsorbed onto the column were eluted using a linear gradient of NaCl (Sigma Aldrich, St. Quentin-Fallavier, France) ranging from 25 to 400 mM. Fractions exhibiting the highest protease activity were collected, lyophilized, and stored at 4 °C until further utilization.
3.4. Protein Analysis
The protein concentration was determined at 595 nm using Bio-Rad DC Protein Assays (Hercules, CA, USA), following the Bradford method [32], and utilizing crystalline bovine serum albumin (Sigma Aldrich, St. Quentin-Fallavier, France) as a reference standard. The purity and molecular weight of the isolated protease were estimated using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with 15% polyacrylamide gels, and β-mercaptoethanol (Sigma Aldrich, St. Quentin-Fallavier, France) was included as a reducing agent. Additionally, the N-terminal sequence of the protease was identified using Edman’s degradation technique, as outlined by Hewick et al. [47] using a protein sequencer (Applied Biosystems (Carlsbad, CA, USA) Protein sequencer ABI Procise 492/610A) fitted with a 140C HPLC system (Applied Biosystems), following established operating procedures. Amino acid residues were identified as distinct signals during the analysis.
3.5. Biochemical Characterization of Purified S. aureus Pr
3.5.1. Effects of pH and Temperature on Activity and Stability
The impact of pH and temperature on both the activity and stability of the purified S. aureus Pr was investigated in the presence and in the absence of Ca2+ ions at 3 mM. Initially, different buffers at a concentration of 200 mM, covering pH values from 3.0 to 13.0, were employed to evaluate protease activity at 55 °C.
The pH stability of S. aureus Pr was investigated by incubating the enzyme for 30, 60, and 120 min in different buffers (200 mM) at pH values ranging from 2.0 to 13.0. The residual protease activity was then determined in triplicate using the standard assay method after eliminating denatured proteins.
The impact of temperature on protease activity was assessed by examining enzyme activity across a temperature range of 40 °C to 80 °C at a pH of 10.0.
To assess thermal stability, S. aureus Pr was exposed to high temperatures ranging from 50 °C to 90 °C, at a pH of 10.0 for durations of 30, 60, and 120 min. Residual protease activity was then determined in triplicate using the standard assay method after eliminating denatured proteins.
3.5.2. Stability Study
Impact of Organic Solvents
The organic solvent stabilities of S. aureus Pr were examined by incubating the enzyme preparation to 25% (v/v) of various organic solvents for 24 h at 40 °C. The tested organic solvents included acetonitrile, benzene, chloroform, DMF, DMSO, ethanol, isopropanol, and methanol (Sigma Aldrich, St. Quentin-Fallavier, France). The residual proteolytic activities were then assessed at 55 °C and a pH of 10.0. The control (100%) for this study consisted of the reaction mixture without any organic solvent. A commercial protease (Alcalase 2.5 L, type DX, Novozymes A/S, Copenhagen, Denmark) and a protease previously purified from Bacillus Stearothermophilus strain named Ba.St.Pr served as positive controls.
Impact of Surfactants and Oxidizing Agents
The impact of various neutral and charged surfactants (15%), including Tween 20, Tween 80, Triton X100, NaDC, and NaTDC (Sigma Aldrich, St. Quentin-Fallavier, France), on the stability of S. aureus Pr was investigated. The enzyme preparation was subjected to different surfactants for 24 h at 40 °C, with continuous shaking at 160 rpm. Subsequently, the residual enzyme activity was assessed at 55 °C and a pH of 10.0.
Furthermore, the protease stability in the presence of oxidizing agents such as H2O2, sodium perborate, and sodium hypochlorite (5%) (Sigma Aldrich, St. Quentin-Fallavier, France) was also evaluated. The residual enzyme activity was measured under identical conditions and compared with the positive control (Alcalase 2.5 L, type DX, Novozymes A/S, Copenhagen, Denmark) and Ba.St.Pr as well as with the negative control (the reaction mixture without any additives).
Impact of Liquid and Solid Detergent
To assess the stability and compatibility of S. aureus Pr with detergents, liquid and solid commercial detergents were diluted in tap water to achieve a final concentration of 7 mg/mL, mimicking typical washing conditions. The inherent proteolytic enzymes present in these laundry detergents were deactivated through heat treatment of the diluted detergents at 90 °C for 1 h. Subsequently, 15 U/mL corresponding to 0.23 µg of the protease was subjected to shaking incubation with each laundry detergent for 1 h at 40 °C. The residual enzyme activity was then determined at a pH of 10.0 and 55 °C, and compared with that of commercial Alcalase 2.5 L type Dx (Novozymes A/S, Copenhagen, Denmark) and Ba.St.Pr.
4. Conclusions
In this study, an extracellular serine protease isolated from the S. aureus strain ALA1 exhibited intriguing characteristics suitable for industrial use. It demonstrated notable stability across various temperatures and pH levels, along with exceptional compatibility with a wide array of commercial laundry detergents. Additionally, the enzyme displayed heightened tolerance towards organic solvents, surfactants, and oxidizing agents better than that of the industrial protease commercial Alcalase 2.5 L. With all these promising attributes in mind, this protease held potential for diverse industrial applications, including its use in leather industries, silk degumming, and the photographic and chemical industries, as well as in the medical field. Despite the significant role already played by microbial proteases in industry, researchers continue to seek more efficient wild-type biocatalysts that are compatible with process parameters and industrial requirements. Considering the commercial success of these enzymes, their potential is vast, and their application in future processes is expected to grow.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The author extends their appreciation to the researchers’ Supporting Project number (RSP2024R237), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Conflicts of Interest
The author declares no conflict of interest.
References
- Abu-Khudir, R.; Salem, M.M.; Allam, N.G.; Ali, E.M. Production, Partial Purification, and Biochemical Characterization of a Thermotolerant Alkaline Metallo-Protease from Staphylococcus sciuri. Appl. Biochem. Biotechnol. 2019, 189, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, X.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial Proteases and Their Applications. Front. Microbiol. 2023, 14, 1236368. [Google Scholar] [CrossRef] [PubMed]
- Barzkar, N.; Homaei, A.; Hemmati, R.; Patel, S. Thermostable Marine Microbial Proteases for Industrial Applications: Scopes and Risks. Extremophiles 2018, 22, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Sharma, A.K.; Kumar, R.; Chanalia, P.; Gupta, B. Serine Proteases: Classification, Catalytic Mechanism, Types, and Industrial Applications. In Serine Proteases: Role in Human Health and Disease; De Gruyter: Berlin, Germany, 2023; Volume 1. [Google Scholar]
- Patel, S. A Critical Review on Serine Protease: Key Immune Manipulator and Pathology Mediator. Allergol. Immunopathol. 2017, 45, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Dadshahi, Z.; Homaei, A.; Zeinali, F.; Sajedi, R.H.; Khajeh, K. Extraction and Purification of a Highly Thermostable Alkaline Caseinolytic Protease from Wastes Penaeus vannamei Suitable for Food and Detergent Industries. Food Chem. 2016, 202, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Bizuye, A.; Sago, A.; Admasu, G.; Getachew, H.; Kassa, P.; Amsaya, M. Isolation, Optimization and Characterization of Protease Producing Bacteria from Soil and Water in Gondar Town, North West Ethiopia. Int. J. Bacteriol. Virol. Immunol. 2014, 1, 020–024. [Google Scholar]
- Razzaq, A.; Shamsi, S.; Ali, A.; Ali, Q.; Sajjad, M.; Malik, A.; Ashraf, M. Microbial Proteases Applications. Front. Bioeng. Biotechnol. 2019, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Dubey, C.K.; Mishra, J.; Nagar, A.; Gupta, M.; Sharma, A.; Kumar, S.; Mishra, V.; Pandey, H.P. Microbial Protease: An Update on Sources, Production Methods, and Applications. In Bioactive Microbial Metabolites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 233–260. [Google Scholar]
- Veloorvalappil, N.J.; Robinson, B.S.; Selvanesan, P.; Sasidharan, S.; Kizhakkepawothail, N.U.; Sreedharan, S.; Prakasan, P.; Moolakkariyil, S.J.; Sailas, B. Versatility of Microbial Proteases. Adv. Enzym. Res. 2013, 2013, 36957. [Google Scholar]
- Niyonzima, F.N.; More, S. Detergent-Compatible Proteases: Microbial Production, Properties, and Stain Removal Analysis. Prep. Biochem. Biotechnol. 2015, 45, 233–258. [Google Scholar] [CrossRef]
- Kamran, A.; Rehman, H.U.; Qader, S.A.U.; Baloch, A.H.; Kamal, M. Purification and Characterization of Thiol Dependent, Oxidation-Stable Serine Alkaline Protease from Thermophilic Bacillus sp. J. Genet. Eng. Biotechnol. 2015, 13, 59–64. [Google Scholar] [CrossRef]
- Banik, R.M.; Prakash, M. Laundry Detergent Compatibility of the Alkaline Protease from Bacillus cereus. Microbiol. Res. 2004, 159, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Ray, A.K. Impact of Microbial Proteases on Biotechnological Industries. Biotechnol. Genet. Eng. Rev. 2017, 33, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.; Putatunda, C.; Kumar, A.; Bhatia, R.; Walia, A. Microbial Proteases: Ubiquitous Enzymes with Innumerable Uses. 3 Biotech 2021, 11, 428. [Google Scholar] [CrossRef] [PubMed]
- Dubin, G. Extracellular Proteases of Staphylococcus spp. Biol. Chem. 2002, 383, 1075–1086. [Google Scholar] [CrossRef]
- Sharma, M.; Gat, Y.; Arya, S.; Kumar, V.; Panghal, A.; Kumar, A. A Review on Microbial Alkaline Protease: An Essential Tool for Various Industrial Approaches. Ind. Biotechnol. 2019, 15, 69–78. [Google Scholar] [CrossRef]
- Ben Bacha, A.; Moubayed, N.M.; Al-Assaf, A. An Organic Solvent-Stable Lipase from a Newly Isolated Staphylococcus aureus ALA1 Strain with Potential for Use as an Industrial Biocatalyst. Biotechnol. Appl. Biochem. 2016, 63, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, H.; Lu, K.; Zhu, Q.; Wu, J. Purification of Extracellular Protease from Staphylococcus simulans QB7and Its Ability in Generating Antioxidant and Anti-inflammatory Peptides from Meat Proteins. Nutrients 2022, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Sony, I. Optimization of Culture Conditions for Protease Production from Staphylococcus sciuri (TKMFT 8). Int. J. Adv. Sci. Res. Manag. 2019, 4, 1–7. [Google Scholar]
- Navarro Llorens, J.M.; Tormo, A.; Martínez-García, E. Stationary Phase in Gram-Negative Bacteria. FEMS Microbiol. Rev. 2010, 34, 476–495. [Google Scholar] [CrossRef]
- Lindsay, S.; Oates, A.; Bourdillon, K. The Detrimental Impact of Extracellular Bacterial Proteases on Wound Healing. Int. Wound J. 2017, 14, 1237–1247. [Google Scholar] [CrossRef]
- Akram, M.; Shafaat, S.; Bukhari, D.A.; Rehman, A. Characterization of a Thermostable Alkaline Protease from Staphylococcus aureus S-2 Isolated from Chicken Waste. Pak. J. Zool. 2014, 46, 1125–1132. [Google Scholar]
- Wang, H.; Liu, J.; Chen, Q.; Kong, B.; Sun, F. Biochemical Properties of Extracellular Protease from Staphylococcus epidermidis Isolated from Harbin Dry Sausages and Its Hydrolysis of Meat Protein. Food Biosci. 2021, 42, 101130. [Google Scholar] [CrossRef]
- Bholay, A.; More, S.; Patil, V.; Niranjan, P. Bacterial Extracellular Alkaline Proteases and Its Industrial Applications. Int. Res. J. Biol. Sci 2012, 1, 1–5. [Google Scholar]
- Gupta, A.; Khare, S. Enhanced Production and Characterization of a Solvent Stable Protease from Solvent Tolerant Pseudomonas aeruginosa PseA. Enzym. Microb. Technol. 2007, 42, 11–16. [Google Scholar] [CrossRef]
- Jadhav, H.; Sayyed, R.; Shaikh, S.; Bhamre, H.; Sunita, K.; El Enshasy, H. Statistically Designed Bioprocess for Enhanced Production of Alkaline Protease in Bacillus cereus HP_RZ17. J. Sci. Ind. Res. 2020, 79, 491–498. [Google Scholar]
- Qureshi, A.S.; Dahot, M.U. Production of Proteases by Staphylococcus epidermidis EFRL 12 Using Cost Effective Substrate (Molasses) as a Carbon. Pak. J. Biotechnol. 2009, 6, 55–60. [Google Scholar]
- Alonazi, M.; Krayem, N.; Alzahrani, A.A.; Horchani, H.; Ben Bacha, A. Novel Phospholipase C with High Catalytic Activity from a Bacillus stearothermophilus Strain: An Ideal Choice for the Oil Degumming Process. Processes 2023, 11, 3310. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Kong, B.; Liu, Q.; Xia, X.; Sun, F. Purification and Characterization of the Protease from Staphylococcus xylosus A2 Isolated from Harbin Dry Sausages. Foods 2022, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Uttatree, S.; Charoenpanich, J. Purification and Characterization of a Harsh Conditions-Resistant Protease from a New Strain of Staphylococcus saprophyticus. Agric. Nat. Resour. 2018, 52, 16–23. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pandhare, J.; Zog, K.; Deshpande, V.V. Differential Stabilities of Alkaline Protease Inhibitors from Actinomycetes: Effect of Various Additives on Thermostability. Bioresour. Technol. 2002, 84, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.V.; Ananthanarayan, L. Enzyme Stability and Stabilization—Aqueous and Non-Aqueous Environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Steuer, C.; Heinonen, K.H.; Kattner, L.; Klein, C.D. Optimization of Assay Conditions for Dengue Virus Protease: Effect of Various Polyols and Nonionic Detergents. J. Biomol. Screen. 2009, 14, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Spier, M.R.; Siepmann, F.B.; Staack, L.; Souza, P.Z.; Kumar, V.; Medeiros, A.B.; Soccol, C.R. Impact of Microbial Growth Inhibition and Proteolytic Activity on the Stability of a New Formulation Containing a Phytate-Degrading Enzyme Obtained from Mushroom. Prep. Biochem. Biotechnol. 2016, 46, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Si, J.-B.; Jang, E.-J.; Charalampopoulos, D.; Wee, Y.-J. Purification and Characterization of Microbial Protease Produced Extracellularly from Bacillus subtilis FBL-1. Biotechnol. Bioprocess Eng. 2018, 23, 176–182. [Google Scholar] [CrossRef]
- Salem, R.B.; Abbassi, M.S.; Cayol, J.-l.; Bourouis, A.; Mahrouki, S.; Fardeau, M.-L.; Belhadj, O. Thermophilic Bacillus Licheniformis Rbs 5 Isolated from Hot Tunisian Spring Co-Producing Alkaline and Thermostable [α]-Amylase and Protease Enzymes. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 557. [Google Scholar]
- Esakkiraj, P.; Meleppat, B.; Lakra, A.K.; Ayyanna, R.; Arul, V. Cloning, Expression, Characterization and Application of Protease Produced by Bacillus cereus PMW8. RSC Adv. 2016, 6, 38611–38616. [Google Scholar] [CrossRef]
- Sharma, A.K.; Kikani, B.A.; Singh, S.P. Biochemical, Thermodynamic and Structural Characteristics of a Biotechnologically Compatible Alkaline Protease from a Haloalkaliphilic, Nocardiopsis dassonvillei OK-18. Int. J. Biol. Macromol. 2020, 153, 680–696. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Wu, J.; Zhang, Y.; Zhang, G.; Wen, T. Purification and Characterization of a Surfactant-Stable High-Alkaline Protease from Bacillus sp. B001. Bioresour. Technol. 2010, 101, 7100–7106. [Google Scholar] [CrossRef]
- Sun, F.; Li, Q.; Liu, H.; Kong, B.; Liu, Q. Purification and Biochemical Characteristics of the Protease from Lactobacillus brevis R4 Isolated from Harbin Dry Sausages. LWT 2019, 113, 108287. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Arafat, H.H.; Isaac, G.S. Laundry Detergent Compatibility and Dehairing Efficiency of Alkaline Thermostable Protease Produced from Aspergillus terreus under Solid-State Fermentation. J. Oleo Sci. 2020, 69, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Gürkök, S. Microbial Enzymes in Detergents: A Review. Int. J. Sci. Eng. Res. 2019, 10, 75–81. [Google Scholar]
- McDonald, C.; Chen, L.L. The Lowry Modification of the Folin Reagent for Determination of Proteinase Activity. Anal. Biochem. 1965, 10, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Akhavan Sepahy, A.; Jabalameli, L. Effect of Culture Conditions on the Production of an Extracellular Protease by Bacillus sp. Isolated from Soil Sample of Lavizan Jungle Park. Enzym. Res. 2011, 2011, 219628. [Google Scholar] [CrossRef]
- Hewick, R.M.; Hunkapiller, M.W.; Hood, L.E.; Dreyer, W.J. A Gas-Liquid Solid Phase Peptide and Protein Sequenator. J. Biol. Chem. 1981, 256, 7990–7997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).