The Influence of the ZrO2 Crystal Phase on Cu/ZrO2-Al2O3 Catalysts in Methanol Steam Reforming
Abstract
:1. Introduction
2. Results
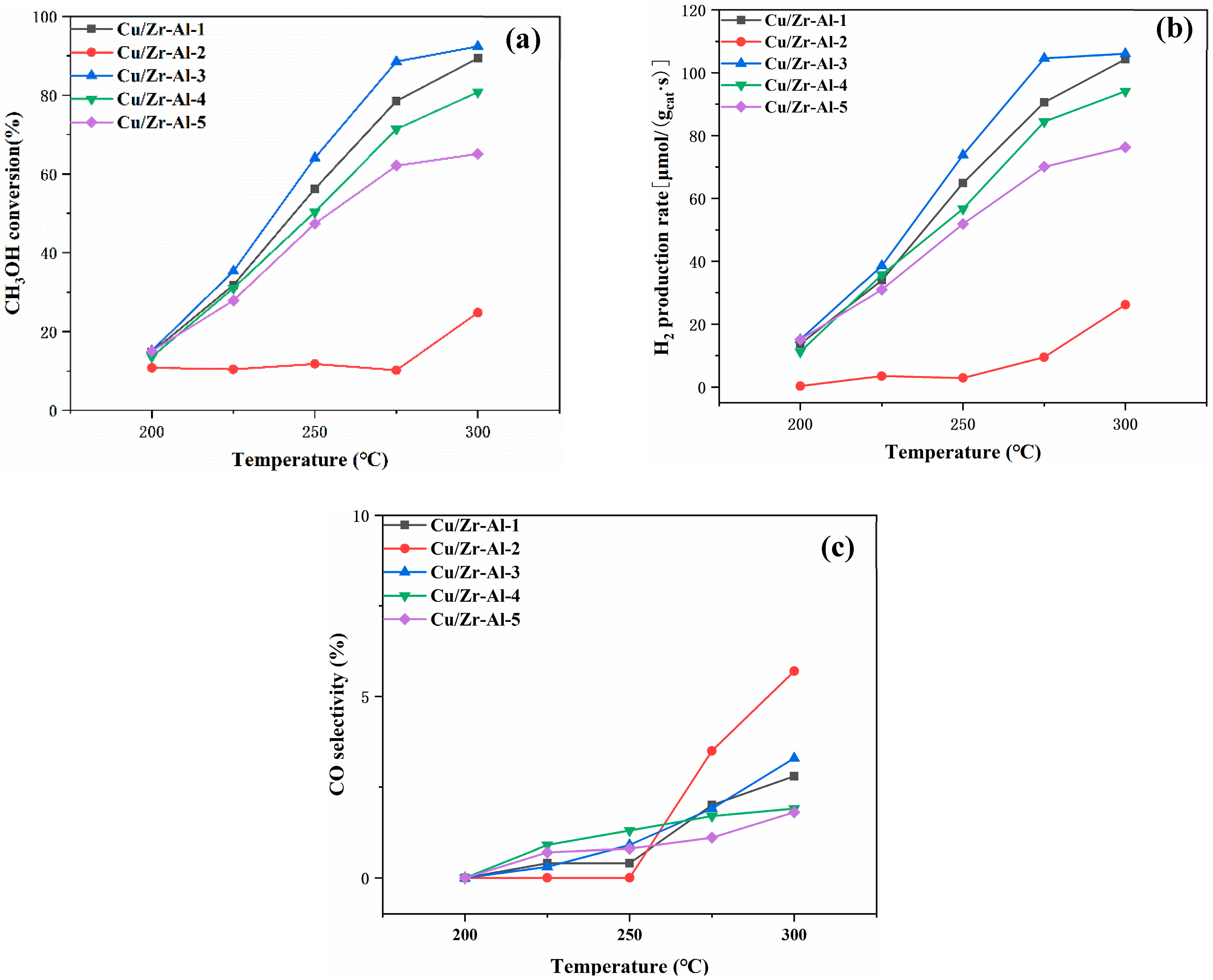
2.1. Catalyst Activity for MSR
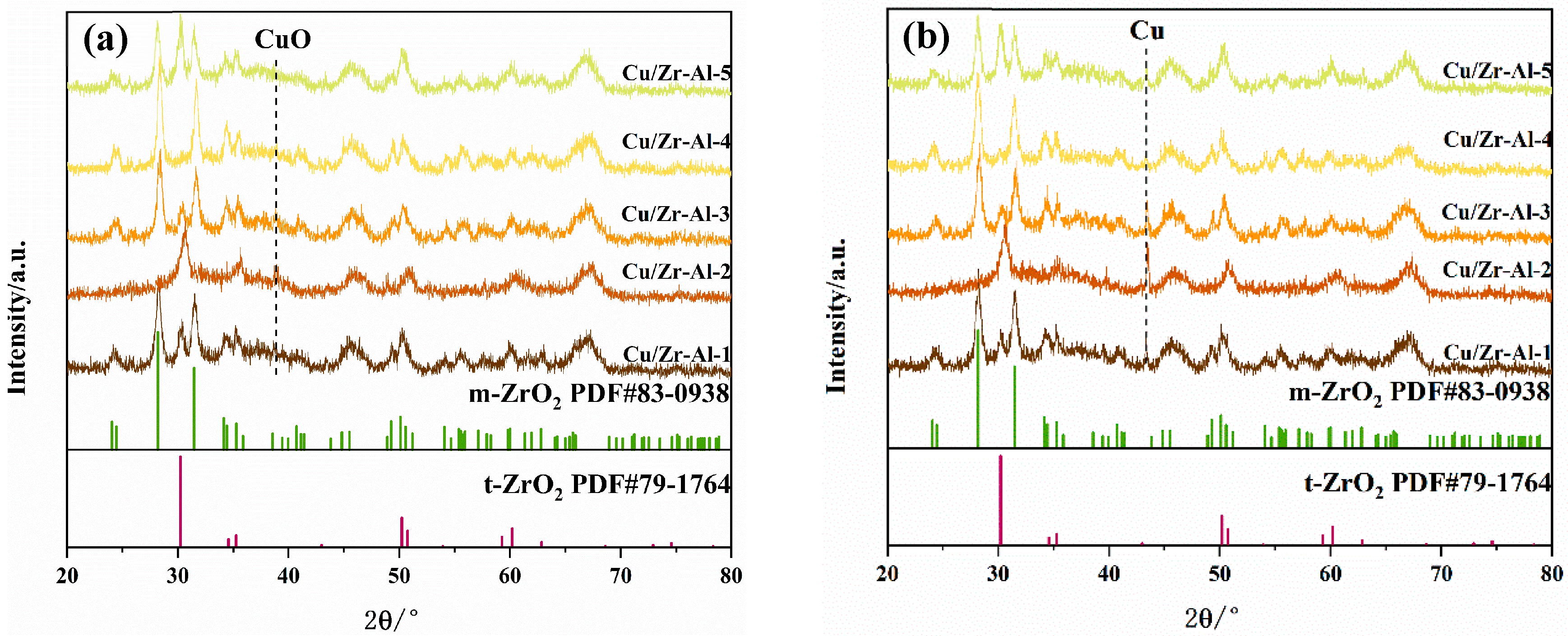
2.2. XRD Analysis
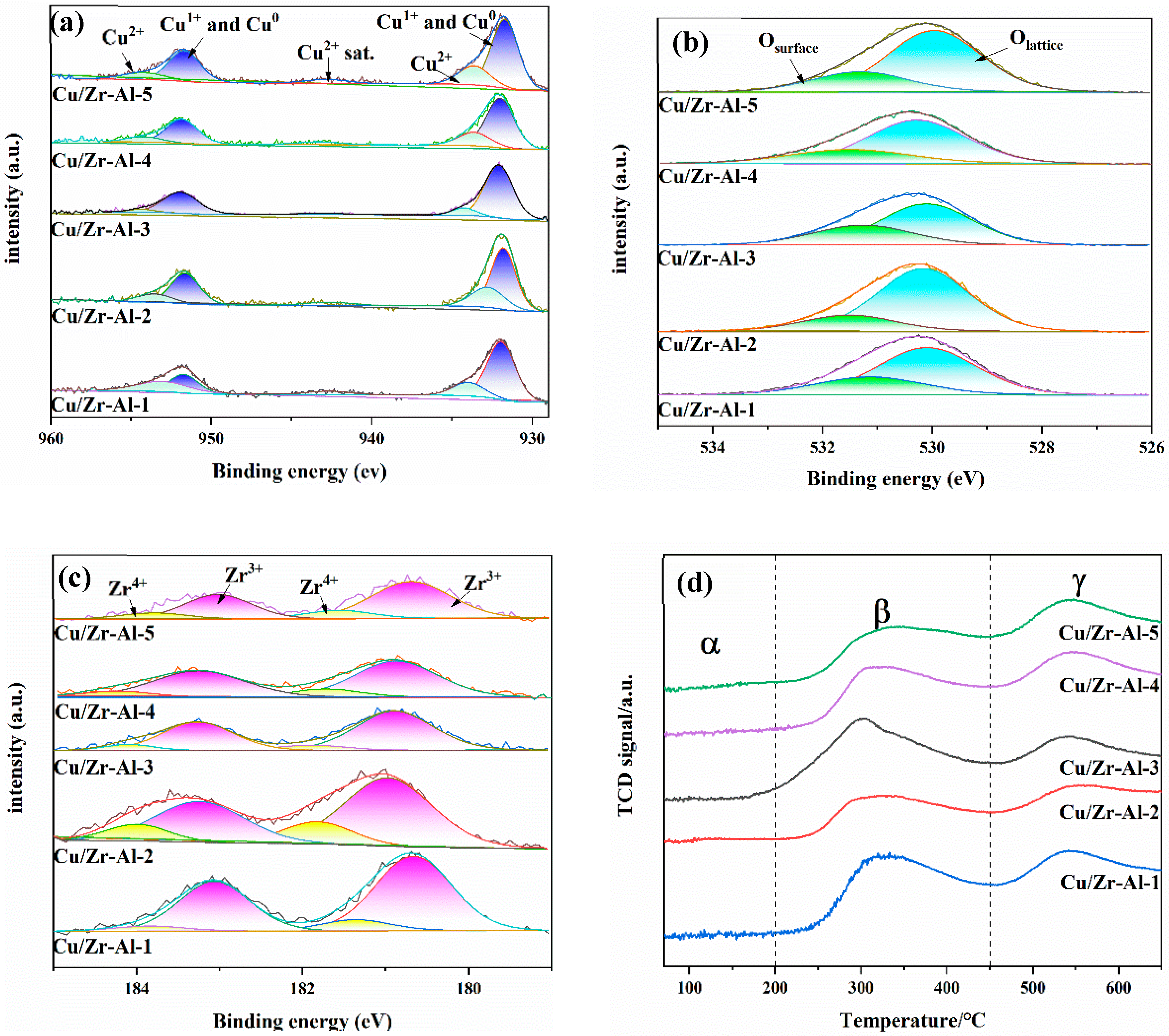
2.3. H2-TPR Analysis
2.4. BET Surface Area and ICP-AES Analysis
2.5. XPS and AES Analysis
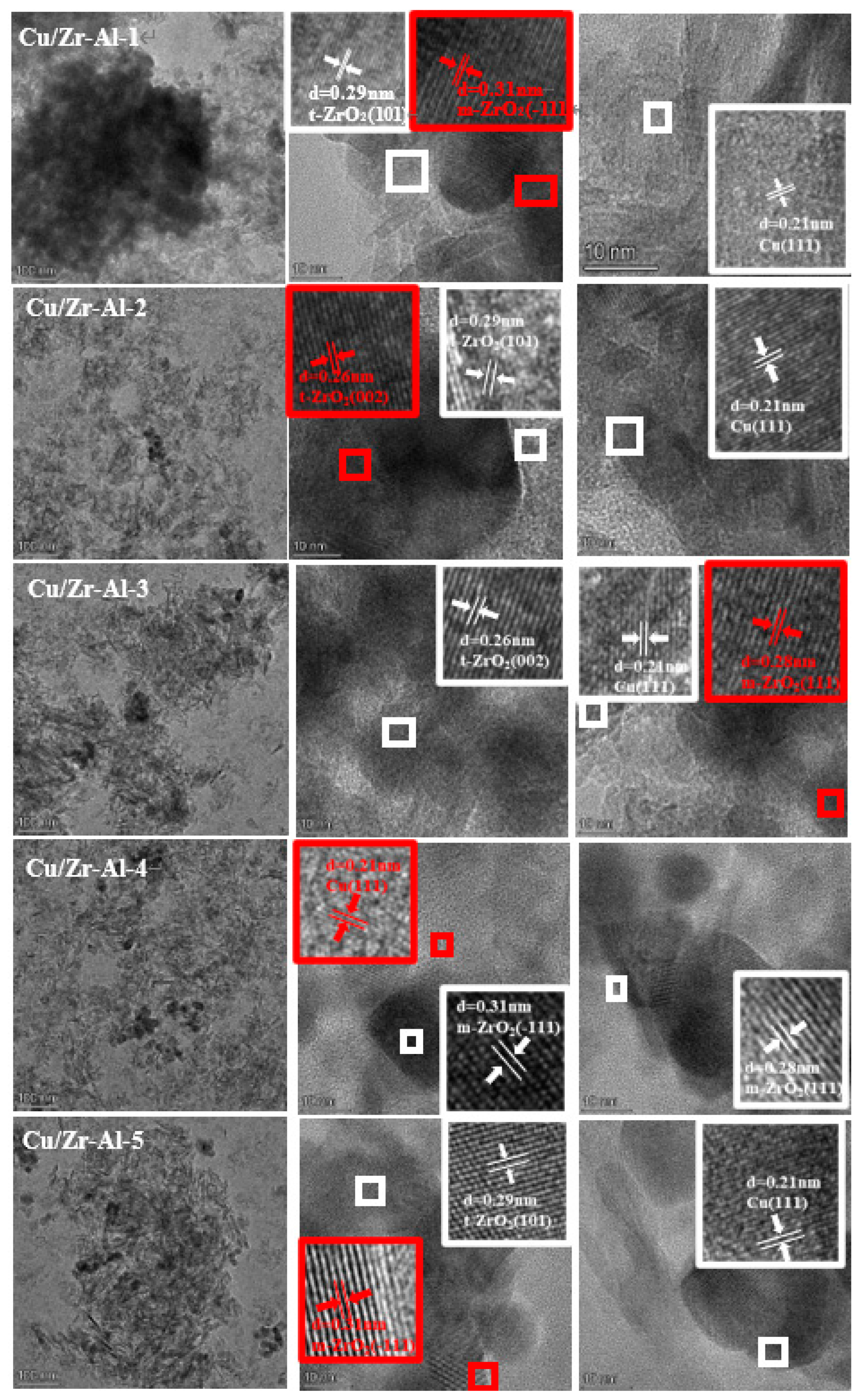
2.6. TEM and HRTEM Analysis
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Synthesis of ZrO2-Al2O3
4.3. Synthesis of Cu/ZrO2-Al2O3
4.4. Catalyst Activity Study
4.5. Catalyst Characterizations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwon, B.W.; Kim, G.S.; Oh, J.H.; Jang, S.C.; Yoon, S.P.; Han, J.; Nam, S.W.; Ham, H.C. H2 Production from Reforming Biogas (CH4 + CO2) in the Ni-doped Sr0.92 Y0.08 TiO3 Perovskite Catalyst. Energy Procedia 2017, 105, 1942–1947. [Google Scholar] [CrossRef]
- Liu, H.; Hadjltaief, H.B.; Benzina, M.; Gálvez, M.E.; Da Costa, P. Natural clay based nickel catalysts for dry reforming of methane: On the effect of support promotion (La, Al, Mn). Int. J. Hydrogen Energy 2019, 44, 246–255. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C–H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Nunes, P.; Oliveira, F.; Hamacher, S.; Almansoori, A. Design of a hydrogen supply chain with uncertainty. Int. J. Hydrogen Energy 2015, 40, 16408–16418. [Google Scholar] [CrossRef]
- Balcombe, P.; Speirs, J.; Johnson, E.; Martin, J.; Brandon, N.; Hawkes, A. The carbon credentials of hydrogen gas networks and supply chains. Renew. Sustain. Energy Rev. 2018, 91, 1077–1088. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J. Effect of microwave double absorption on hydrogen generation from methanol steam reforming. Int. J. Hydrogen Energy 2010, 35, 1987–1997. [Google Scholar] [CrossRef]
- Silva, H.; Mateos-Pedrero, C.; Ribeirinha, P.; Boaventura, M.; Mendes, A. Low-temperature methanol steam reforming kinetics over a novel CuZrDyAl catalyst. React. Kinet. Mech. Catal. 2015, 115, 321–339. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Mateos-Pedrero, C.; Boaventura, M.; Sousa, J.; Mendes, A. CuO/ZnO/Ga2O3 catalyst for low temperature MSR reaction: Synthesis, characterization and kinetic model. Appl. Catal. B Environ. 2018, 221, 371–379. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Basile, A.; Parmaliana, A.; Tosti, S.; Iulianelli, A.; Gallucci, F.; Espro, C.; Spooren, J. Hydrogen production by methanol steam reforming carried out in membrane reactor on Cu/Zn/Mg-based catalyst. Catal. Today 2008, 137, 17–22. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Cacciola, G.; Antonucci, V.; Freni, S. Technology up date and new strategies on fuel cells. J. Power Sources 2001, 100, 67–79. [Google Scholar] [CrossRef]
- King, J.M.; O’Day, M.J. Applying fuel cell experience to sustainable power products. J. Power Sources 2000, 86, 16–22. [Google Scholar] [CrossRef]
- Ramirez, D.; Beites, L.; Blazquez, F.; Ballesteros, J. Distributed generation system with PEM fuel cell for electrical power quality improvement. Int. J. Hydrogen Energy 2008, 33, 4433–4443. [Google Scholar] [CrossRef]
- Stone, C.; Morrison, A.E. From curiosity to “power to change the world”. Solid State Ion. 2002, 152-153, 1–13. [Google Scholar] [CrossRef]
- Dhar, H.P.; Christner, L.G.; Kush, A.K. Nature of CO Adsorption during H2 Oxidation in Relation to Modeling for CO Poisoning of a Fuel Cell Anode. J. Electrochem. Soc. 1987, 134, 3021–3026. [Google Scholar] [CrossRef]
- Ranganathan, E.S.; Bej, S.K.; Thompson, L.T. Methanol steam reforming over Pd/ZnO and Pd/CeO2 catalysts. Appl. Catal. A Gen. 2005, 289, 153–162. [Google Scholar] [CrossRef]
- Suwa, Y.; Ito, S.-i.; Kameoka, S.; Tomishige, K.; Kunimori, K. Comparative study between Zn–Pd/C and Pd/ZnO catalysts for steam reforming of methanol. Appl. Catal. A Gen. 2004, 267, 9–16. [Google Scholar] [CrossRef]
- Conant, T.; Karim, A.; Lebarbier, V.; Wang, Y.; Girgsdies, F.; Schlogl, R.; Datye, A. Stability of bimetallic Pd–Zn catalysts for the steam reforming of methanol. J. Catal. 2008, 257, 64–70. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Avgouropoulos, G.; Ioannides, T. Steady-state isotopic transient kinetic analysis of steam reforming of methanol over Cu-based catalysts. Appl. Catal. B Environ. 2009, 88, 490–496. [Google Scholar] [CrossRef]
- Wu, G.-S.; Mao, D.-S.; Lu, G.-Z.; Cao, Y.; Fan, K.-N. The Role of the Promoters in Cu Based Catalysts for Methanol Steam Reforming. Catal. Lett. 2009, 130, 177–184. [Google Scholar] [CrossRef]
- Clancy, P.; Breen, J.P.; Ross, J.R.H. The preparation and properties of coprecipitated Cu–Zr–Y and Cu–Zr–La catalysts used for the steam reforming of methanol. Catal. Today 2007, 127, 291–294. [Google Scholar] [CrossRef]
- Rameshan, C.; Stadlmayr, W.; Penner, S.; Lorenz, H.; Memmel, N.; Havecker, M.; Blume, R.; Teschner, D.; Rocha, T.; Zemlyanov, D.; et al. Hydrogen production by methanol steam reforming on copper boosted by zinc-assisted water activation. Angew. Chem. Int. Ed. 2012, 51, 3002–3006. [Google Scholar] [CrossRef] [PubMed]
- Alejo, L.; Lago, R.; Pena, M.A.; Fierro, J.L.G. Partial oxidation of methanol to produce hydrogen over Cu-Zn-based catalysts. Appl. Catal. A Gen. 1997, 162, 281–297. [Google Scholar] [CrossRef]
- Paglia, G.; Buckley, C.E.; Udovic, T.J.; Rohl, A.L.; Jones, F.; Maitland, C.F.; Connolly, J. Boehmite-Derived γ-Alumina System. 2. Consideration of Hydrogen and Surface. Effects. Chem. Mater. 2004, 16, 1914–1923. [Google Scholar] [CrossRef]
- Byun, M.Y.; Kim, J.S.; Park, D.W.; Lee, M.S. Influence of calcination temperature on the structure and properties of Al2O3 as support for Pd catalyst. Korean, J. Chem. Eng. 2018, 35, 1083–1088. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.I.; Kim, T.H.; Ko, C.H.; Park, H.C.; Song, I.K. Hydrogen production by steam reforming of methanol in a micro-channel reactor coated with Cu/ZnO/ZrO2/Al2O3 catalyst. J. Power Sources 2006, 159, 1296–1299. [Google Scholar] [CrossRef]
- Witoon, T.; Chalorngtham, J.; Dumrongbunditkul, P.; Chareonpanich, M.; Limtrakul, J. CO2 hydrogenation to methanol over Cu/ZrO2 catalysts: Effects of zirconia phases. Chem. Eng. J. 2016, 293, 327–336. [Google Scholar] [CrossRef]
- Tada, S.; Katagiri, A.; Kiyota, K.; Honma, T.; Kamei, H.; Nariyuki, A.; Uchida, S.; Satokawa, S. Cu Species Incorporated into Amorphous ZrO2 with High Activity and Selectivity in CO2-to-Methanol Hydrogenation. J. Phys. Chem. C 2018, 122, 5430–5442. [Google Scholar] [CrossRef]
- Samson, K.; Śliwa, M.; Socha, R.P.; Góra-Marek, K.; Mucha, D.; Rutkowska-Zbik, D.; Paul, J.F.; Ruggiero-Mikołajczyk, M.; Grabowski, R.; Słoczyński, J. Influence of ZrO2 Structure and Copper Electronic State on Activity of Cu/ZrO2 Catalysts in Methanol Synthesis from CO2. ACS Catal. 2014, 4, 3730–3741. [Google Scholar] [CrossRef]
- Song, M.; Zeng, W.; Li, L.; Wu, X.; Li, G.; Hu, C. Effect of the Zr/Al molar ratio on the performance of Cu/ZrO2–Al2O3 catalysts for methanol steam reforming. Ind. Eng. Chem. Res. 2023, 62, 3898–3908. [Google Scholar] [CrossRef]
- Lv, X.; Cheng, D.H.; Shou, X.K.; Liu, J.C.; Xu, H.T. Bifunctional catalyst Ga2O3-mZrO2/SAPO-34 for CO2 hydrogenation: Ga2O3-mZrO2 improving light olefins. Catal. Sci. Technol. 2024, 14, 3652–3659. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Li, G.; Hu, C. Insights into the Influence of ZrO2 Crystal Structures on Methyl Laurate Hydrogenation over Co/ZrO2 Catalysts. ACS Catal. 2021, 11, 7099–7113. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Azenha, C.; Pacheco Tanaka, D.A.; Sousa, J.M.; Mendes, A. The influence of the support composition on the physicochemical and catalytic properties of Cu catalysts supported on Zirconia-Alumina for methanol steam reforming. Appl. Catal. B Environ. 2020, 277, 119243. [Google Scholar] [CrossRef]
- Chen, M.; Sun, G.; Wang, Y.; Liang, D.; Li, C.; Wang, J.; Liu, Q. Steam reforming of methanol for hydrogen production over attapulgite-based zeolite-supported Cu-Zr catalyst. Fuel 2022, 314, 122733. [Google Scholar] [CrossRef]
- Snyder, R.L. The Use of Reference Intensity Ratios in X-Ray Quantitative Analysis. Powder Diffr. 1992, 7, 186–193. [Google Scholar] [CrossRef]
- Yu, Q.; Yao, X.; Zhang, H.; Gao, F.; Dong, L. Effect of ZrO2 addition method on the activity of Al2O3-supported CuO for NO reduction with CO: Impregnation vs. coprecipitation. Appl. Catal. A Gen. 2012, 423, 42–51. [Google Scholar] [CrossRef]
- Mohtashami, Y.; Taghizadeh, M. Performance of the ZrO2 promoted Cu-ZnO catalyst supported on acetic acid-treated MCM-41 in methanol steam reforming. Int. J. Hydrogen Energy 2019, 44, 5725–5738. [Google Scholar] [CrossRef]
- Luo, M.-F.; Fang, P.; He, M.; Xie, Y.-L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Liang, C.; Li, X.; Qu, Z.; Tade, M.; Liu, S. The role of copper species on Cu/γ-Al2O3 catalysts for NH3–SCO reaction. Appl. Surf. Sci. 2012, 258, 3738–3743. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Yang, B.; Guo, L. A highly active and selective mesostructured Cu/AlCeO catalyst for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 571, 51–60. [Google Scholar] [CrossRef]
- Liu, R.; Wang, C.A. Synthesis of aluminum-doped mesoporous zirconia with improved thermal stability. Microporous Mesoporous Mater. 2014, 186, 1–6. [Google Scholar] [CrossRef]
- Bin, F.; Wei, X.; Li, B.; Hui, K.S. Self-sustained combustion of carbon monoxide promoted by the Cu-Ce/ZSM-5 catalyst in CO/O2/N2 atmosphere. Appl. Catal. B Environ. 2015, 162, 282–288. [Google Scholar] [CrossRef]
- Liu, P.; Hensen, E.J. Highly efficient and robust Au/MgCuCr2O4 catalyst for gas-phase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.M.d.; Silva, C.M.; Moreno-Tost, R.; Farias, T.L.; Jiménez-López, A.; Rodríguez-Castellón, E. A study of copper-exchanged mordenite natural and ZSM-5 zeolites as SCR–NOx catalysts for diesel road vehicles: Simulation by neural networks approach. Appl. Catal. B Environ. 2009, 88, 420–429. [Google Scholar] [CrossRef]
- Azenha, C.; Lagarteira, T.; Mateos-Pedrero, C.; Mendes, A. Production of hydrogen from methanol steam reforming using CuPd/ZrO2 catalysts–Influence of the catalytic surface on methanol conversion and CO selectivity. Int. J. Hydrogen Energy 2021, 46, 17490–17499. [Google Scholar] [CrossRef]
- Li, H.; Tian, H.; Chen, S.; Sun, Z.; Liu, T.; Liu, R.; Assabumrungrat, S.; Saupsor, J.; Mu, R.; Pei, C.; et al. Sorption enhanced steam reforming of methanol for high-purity hydrogen production over Cu-MgO/Al2O3 bifunctional catalysts. Appl. Catal. B Environ. 2020, 276, 119052. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Moroz, E.M.; Kriventsov, V.V.; Larina, T.V.; Boronin, A.I.; Dolgikh, L.Y.; Strizhak, P.E. Structure and state of copper oxide species supported on Yttria-stabilized zirconia. J. Phys. Chem. C 2009, 113, 21368–21375. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Mu, M.; Ding, F.; Liu, Z.; Guo, X.; Song, C. Organic acid-assisted preparation of highly dispersed Co/ZrO2 catalysts with superior activity for CO2 methanation. Appl. Catal. B Environ. 2019, 254, 531–540. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Liu, B.; Liu, J.; Wang, L.; Xiao, Y.; Xu, Y.; Liu, X. Insights into the Influence of CeO2 Crystal Facet on CO2 Hydrogenation to Methanol over Pd/CeO2 Catalysts. ACS Catal. 2020, 10, 11493–11509. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Yu, P.; Hu, C. Temperature-tuned selectivity to alkanes or alcohol from ethyl palmitate deoxygenation over zirconia-supported cobalt catalyst. Fuel 2020, 278, 118295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Li, L.; Hu, C.; Da Costa, P. Dry reforming of methane over Ni–ZrOx catalysts doped by manganese: On the effect of the stability of the structure during time on stream. Appl. Catal. A Gen. 2021, 617, 118120. [Google Scholar] [CrossRef]
- Cheng, Z.; Jiang, C.; Sun, X.; Lan, G.; Wang, X.; He, L.; Li, Y.; Tang, H.; Li, Y. Insights into the inducing effect of aluminum on Cu–ZnO synergy for methanol steam reforming. Ind. Eng. Chem. Res. 2022, 61, 11699–11707. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.; Chen, C.; Yue, L.; Qiao, N.; Shen, Q.; Chen, J.; Hao, Z. Facile preparation of 3D ordered mesoporous CuOx–CeO2 with notably enhanced efficiency for the low temperature oxidation of heteroatom-containing volatile organic compounds. RSC Adv. 2013, 3, 19639–19656. [Google Scholar] [CrossRef]
- Yang, H.; Zeng, Y.; Zhou, Y.; Du, X.; Li, D.; Hu, C. One-step synthesis of highly active and stable Ni-ZrO2 catalysts for the conversion of methyl laurate to alkanes. J. Catal. 2022, 413, 297–310. [Google Scholar] [CrossRef]
- Fu, M.; Yue, X.; Ye, D.; Ouyang, J.; Huang, B.; Wu, J.; Liang, H. Soot oxidation via CuO doped CeO2 catalysts prepared using coprecipitation and citrate acid complex-combustion synthesis. Catal. Today 2010, 153, 125–132. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Abbandanak, M.H. Production of hydrogen via methanol steam reforming over mesoporous CeO2–Cu/KIT-6 nanocatalyst: Effects of polar aprotic tetrahydrofuran solvent and ZrO2 promoter on catalytic performance. Int. J. Hydrogen Energy 2022, 47, 16362–16374. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Khanchi, M.; Mousavian, S.M.A.; Soltanali, S. Methanol steam reforming over Cu supported on SiO2, amorphous SiO2-Al2O3, and Al2O3 catalysts: Influence of support nature. Int. J. Energy Res. 2021, 46, 3057–3071. [Google Scholar] [CrossRef]
- Shahmohammadi, N.; Rezaei, M.; Alavi, S.M.; Akbari, E. Methanol steam reforming over highly efficient CuO–Al2O3 nanocatalysts synthesized by the solid-state mechanochemical method. Int. J. Hydrogen Energy 2023, 48, 13139–13150. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, W.; Lan, G.; Sun, X.; Wang, X.; Jiang, C.; Li, Y. High-performance Cu/ZnO/Al2O3 catalysts for methanol steam reforming with enhanced Cu-ZnO synergy effect via magnesium assisted strategy. J. Energy Chem. 2021, 63, 550–557. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Zeng, C.; Liu, Q.; Hu, H.; Huang, Q.; Chen, X. Preparation of CuZnZrAl catalysts by coprecipitation-ammonia method for methanol steam reforming and the effect of promoters Y and Ce. Mol. Catal. 2023, 537, 112887. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y. Influence of ceria existence form on deactivation behavior of Cu-Ce/SBA-15 catalysts for methanol steam reforming. Int. J. Hydrogen Energy 2023, 48, 1323–1336. [Google Scholar] [CrossRef]
- Abate, S.; Mebrahtu, C.; Giglio, E.; Deorsola, F.; Bensaid, S.; Perathoner, S.; Pirone, R.; Centi, G. Catalytic Performance of γ-Al2O3–ZrO2–TiO2–CeO2 Composite Oxide Supported Ni-Based Catalysts for CO2 Methanation. Int. J. Hydrogen Energy 2016, 55, 4451–4460. [Google Scholar] [CrossRef]
- Zeng, W.; Li, L.; Song, M.; Wu, X.; Li, G.; Hu, C. The effect of different atmosphere treatments on the performance of Ni/Nb–Al2O3 catalysts for methane steam reforming. Int. J. Hydrogen Energy 2023, 48, 6358–6369. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Y.; Du, X.; Li, L.; Song, M.; Zeng, W.; Li, G.; Hu, C. Carbon resistance of xNi/HTASAO5 catalyst for the production of H2 via CO2 reforming of methane. Int. J. Hydrogen Energy 2021, 46, 20835–20847. [Google Scholar] [CrossRef]




| Catalyst | Cu Content a (wt%) | SBET b (m2·g−1) | VBJH c (cm3·g−1) | Dp d (nm) | Cu Dispersion e (%) | Cu Surface e Area (m2/gcat) | Cu Particle Size f (nm) | ZrO2 Particle Size f (nm) |
|---|---|---|---|---|---|---|---|---|
| Cu/Zr-Al-1 | 5.1 | 116.4 | 0.38 | 12.7 | 4.63 | 5.40 | 35.8 | 16.0 |
| Cu/Zr-Al-2 | 4.5 | 86.4 | 0.24 | 9.7 | 1.98 | 2.24 | 35.5 | 13.8 |
| Cu/Zr-Al-3 | 5.1 | 106.7 | 0.39 | 13.6 | 7.42 | 8.48 | 31.0 | 16.6 |
| Cu/Zr-Al-4 | 5.1 | 113.9 | 0.39 | 13.3 | 2.19 | 2.55 | 32.3 | 16.3 |
| Cu/Zr-Al-5 | 4.7 | 117.6 | 0.40 | 13.0 | 2.01 | 2.35 | 26.9 | 17.2 |
| Catalysts | Cu2+ (%) | Cu1+ and Cu0 (%) | Zr3+ (%) | Zr4+ (%) | OL a (%) | OS b (%) | Ov c | Ov d (mmol/g) |
|---|---|---|---|---|---|---|---|---|
| Cu/Zr-Al-1 | 21.0 | 79.0 | 87.7 | 12.3 | 70.4 | 29.6 | 0.296 | 0.102 |
| Cu/Zr-Al-2 | 31.1 | 68.9 | 79.8 | 20.2 | 79.5 | 20.5 | 0.205 | 0.056 |
| Cu/Zr-Al-3 | 10.7 | 89.3 | 89.1 | 10.9 | 65.8 | 34.2 | 0.342 | 0.136 |
| Cu/Zr-Al-4 | 23.9 | 76.1 | 85.6 | 14.4 | 71.7 | 28.3 | 0.283 | 0.075 |
| Cu/Zr-Al-5 | 25.5 | 74.5 | 83.2 | 16.8 | 74.6 | 25.4 | 0.254 | 0.063 |
| Catalyst | Temperature (°C) | S/C a | Space-Time Ratio (h−1) | Conversion (%) | H2 Production Rate (μmol/gcat·s) | CO Selectivity (%) | References |
|---|---|---|---|---|---|---|---|
| ZrO2-CeO2-Cu/KIT-6 | 300 | 2 | 2 (WHSV) | 96 | SH2 = 99.8% | 0.7 | [59] |
| Cu/ZnO-3Al | 250 | 1 | 15.3 (WHSV) | 57.3 | - | 1.3 | [55] |
| Cu/ZnAl-R10 | 225 | 1.3 | 6 (WHSV) | 67 | - | - | [60] |
| 1Cu/1Zr/AZ | 400 | 1.2 | 10.8 (WHSV) | 90.6 | 182.0 | 3.2 | [36] |
| Cu/ASA1 | 300 | 0.5 | 1.8 (WHSV) | 91.0 | SH2 = 96.8% | 9.6 | [61] |
| CuO-Al2O3 | 350 | 5 | 36,000(GHSV) | 72.2 | SH2 = 87.5% | 0.5 | [62] |
| CuZnAl-5Mg | 200 | 1 | 3.84 (WHSV) | 70 | 47.2 | 1.0 | [63] |
| Cu/ZnCeZrAl | 250 | 1.2 | 2.6 (WHSV) | 96.1 | - | 0.37 | [64] |
| Cu-Ce/SBA-15 | 270 | 1.2 | 3 (WHSV) | 90.1 | - | 2.1 | [65] |
| Cu/ZrO2-8Al203 | 300 | 1 | 9.9 (WHSV) | 84.5 | 98.7 | 1.5 | [32] |
| Cu/Zr-Al-3 | 275 | 1 | 9.9 (WHSV) | 88.5 | 104.6 | 1.9 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Li, L.; Wu, X.; Cai, H.; Li, G.; Hu, C. The Influence of the ZrO2 Crystal Phase on Cu/ZrO2-Al2O3 Catalysts in Methanol Steam Reforming. Catalysts 2024, 14, 480. https://doi.org/10.3390/catal14080480
Song M, Li L, Wu X, Cai H, Li G, Hu C. The Influence of the ZrO2 Crystal Phase on Cu/ZrO2-Al2O3 Catalysts in Methanol Steam Reforming. Catalysts. 2024; 14(8):480. https://doi.org/10.3390/catal14080480
Chicago/Turabian StyleSong, Mouxiao, Li Li, Xueshuang Wu, Haiqing Cai, Guiying Li, and Changwei Hu. 2024. "The Influence of the ZrO2 Crystal Phase on Cu/ZrO2-Al2O3 Catalysts in Methanol Steam Reforming" Catalysts 14, no. 8: 480. https://doi.org/10.3390/catal14080480




