The concept of “photocatalysis” was discovered at the beginning of the twentieth century, and research in this area has especially intensified over the past 50 years. Since then, many studies have been conducted and new discoveries made concerning photocatalytic mechanisms, new materials, and new applications such as the valorization of biomass, the inactivation of microorganisms, hydrogen production, and solar fuel. Despite these many years of research, this process remains highly topical, particularly due to its possibility of solving some crucial problems in our society regarding environmental pollution with contaminants of emerging concern (CECs), including pharmaceuticals and personal care products (PPCPs) and microplastics, the sanitary crisis caused by COVID-19, and the energy crisis. Moreover, several fundamental points constantly need to be improved, such as the relation between surface properties, optical properties, and photocatalytic activity, together with the development of new materials or coupling processes, allowing the improvement of charge separation or UV–visible absorption, and studies on upconversion, the use of theoretical chemistry to improve mechanisms, or the development of new structures.
According to the definition of the International Union of Pure and Applied Chemistry (IUPAC) [1], photocatalysis is “Change in the rate of a chemical reaction or its initiation under the action of ultraviolet, visible, or infrared radiation in the presence of a substance—the photocatalyst—that absorbs light and is involved in the chemical transformation of the reaction partners”. Thus, still according to the IUPAC, a photocatalyst is “able to produce, upon absorption of light, chemical transformations of the reaction partners. The excited state of the photocatalyst repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each cycle of such interactions”. To summarize, the basic process of photocatalysis consists of ejecting an electron from the valence band (VB) to the conduction band (CB) of the semiconductor, thereby creating a cationic vacancy, known as a hole (h+), in the valence band. This is due to the UV–vis irradiation of the semiconductor with an energy equal to or greater than its bandgap. These charge carriers (e−/h+) can migrate to the surface of the catalyst, where they are then available to undergo redox reactions with substrates. This is followed by the formation of reactive oxygen species (OH°, O2°−, H2O2, O3, etc.) at the surface of the semiconductor and/or direct oxidation of the polluting species (Figure 1).
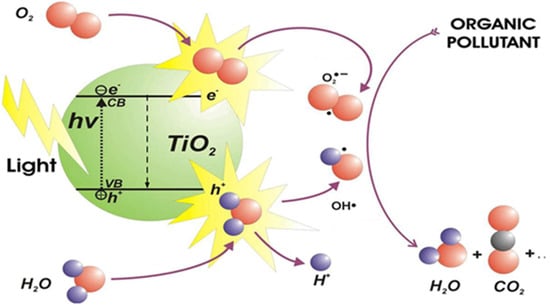
Figure 1.
Schematic diagram of the principles of photocatalysis.
The concept of photocatalysis was first mentioned in 1911 by the German chemist A. Eibner [2]. In his research, Eibner explored the effects of illumination of zinc oxide (ZnO) on the bleaching of Prussian blue pigment. Almost 30 years later, Doodeve et al. discovered that TiO2 could act as a photosensitizer for bleaching dyes in the presence of oxygen [3]. The first review article concerning the degradation of organic molecules by photocatalysis is dated from the mid-1950s. Work was made on the photocatalytic properties of certain metal oxides (ZnO, TiO2, etc.) capable of oxidizing phenol to catechol and then to other organic and mineral products. At that time, the photomineralization of organic pollutants under UV light was already being discussed [4].
In the early 1970s, A. Fujishima and K. Honda made an important discovery by showing that it was possible to produce hydrogen through water electrochemical photolysis using a TiO2 electrode irradiated with ultraviolet light connected to a platinum electrode [5]. In Lyon (France), M. Formenti, S.J.Teichner et al. studied the partial photocatalytic oxidation of alkanes and olefinic hydrocarbons with TiO2 at ambient temperature and atmospheric pressure [6], investigating the impact of the wavelength of UV irradiation, the nature of the catalyst (TiO2 and some other oxides, such as ZnO), the quantum yields, and the selectivities [7]. Their results lead to the suggestions of possible photocatalytic solutions for several environmental problems in the presence of TiO2 [8]. Some years later, P. Pichat and J.M Herrmann contributed to improving the mechanism of photocatalytic degradation by focusing their research in the area of isotopic exchange on a metal oxide, the spillover effect, metal–support interactions, and photoconductivity measurements [9,10].
The development of photocatalysis has garnered significant attention in the last twenty years, with its applications spanning a variety of products and research areas, particularly in environmental and energy-related fields, as illustrated in Figure 2 [11].
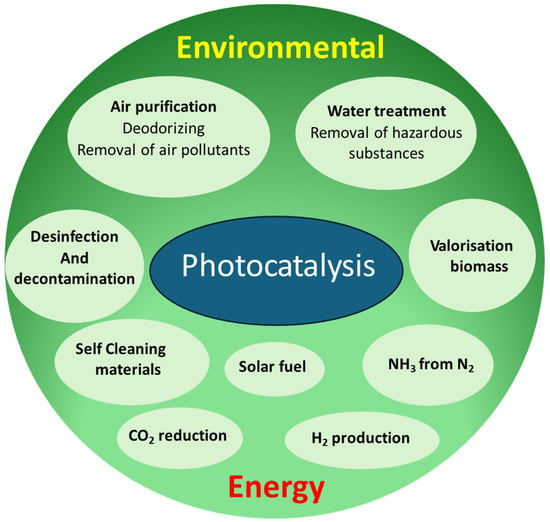
Figure 2.
Photocatalysis applications (adapted from [11]).
In a recent and interesting review published in the journal Catalysts, the authors analyzed the history and past and present trends in photocatalysis research while also outlining potential future scenarios in the medium term [12]. In addition, they observed an evolution of the main themes in the field of photocatalysis and defined four temporal periods in photocatalysis research. To summarize, until 1980, the main research interests were the mechanisms and functionality of semiconductors, primarily TiO2, CdS, and ZnO, and the addition of co-catalysts such as metals and RuO2 for producing H2 and CH4 (via a photo-Kolbe reaction). One of the publications even deals with the reduction of CO2 and N2. In the subsequent period, research shifted predominantly towards water purification, including kinetic analysis, metal doping, and pollutant removal. During this time, TiO2 gained a significantly more prominent role compared to the earlier period. From the end of the 1990s, a lot of work focused on the development of ever more efficient and more complex photocatalysts (Z-schemes with multiple materials, C3N4, ternary semiconductors such as BiVO4, etc.) or on the optimization of existing catalysts (non-metal doping (C, N, F) of TiO2 or ZnO, preparation of crystallized catalysts with dominant faces, etc.). Applications concerned disinfection, self-cleaning surfaces, and the degradation of specific classes of compounds (drugs, endocrine disruptors, phytosanitary products, etc.). Finally, as highlighted by Sordello et al., since this last period, research has expanded to include plasmon resonance and carbon-based materials such as graphene, C3N4, and carbon quantum dots. TiO2 remains the most studied oxide material, often in combination with other materials. Many elements are now considered, including Mo, Cd, In, Ga, Ag, Ce, etc. [12].
In 2023, a search with the keyword “photocatalysis” in the Scopus database yielded nearly 10,000 references (Figure 3). Of these, a third focused on titanium dioxide, and over half were dedicated to the preparation and characterization of photocatalytic materials. A more detailed analysis of photocatalysis applications reveals two major categories: environmental applications (Figure 4a) and energy-related applications (Figure 4b). We observe that research on water treatment remains predominant. However, it is important to highlight the significant increase in the number of articles published in the field of energy, particularly concerning CO2 reduction and hydrogen production.
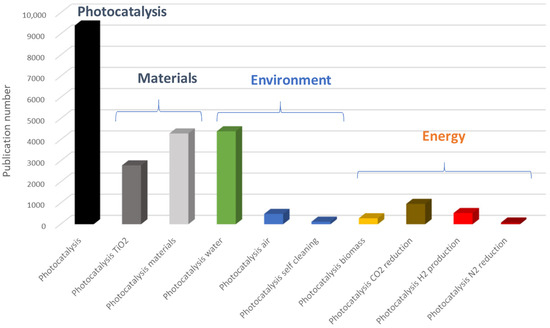
Figure 3.
Number of publications published in 2023 in the field of photocatalysis (Scopus source).
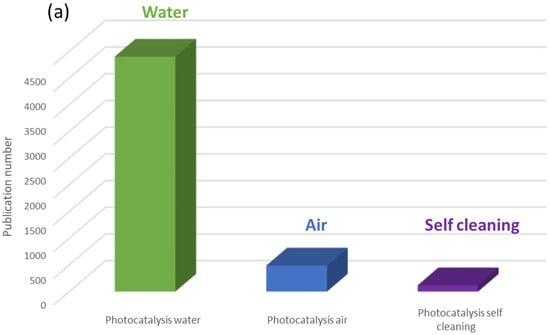
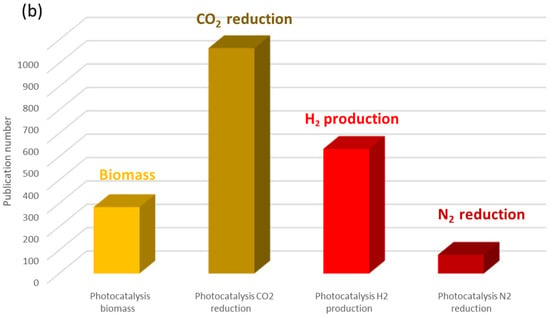
Figure 4.
Number of publications published in 2023 (a) on environmental photocatalysis and (b) on the use of photocatalysis for energy (Scopus source).
Photocatalysis presents major challenges, particularly in terms of catalyst efficiency. Many current photocatalysts are activated only by UV light, limiting their use under natural sunlight and the efficiency of converting photons into chemical reactions. Therefore, efficiency and quantum yield need to be improved. Additionally, materials like titanium dioxide degrade over time, requiring frequent regeneration. The selectivity of reactions is also an issue, as it is essential to minimize unwanted by-products. However, promising prospects are emerging with the development of new materials capable of absorbing visible light and operating under coupled light/temperature conditions. Nanostructure engineering and integration with solar energy systems could improve efficiency and provide sustainable solutions for clean energy production. Furthermore, coupling photocatalysis with other processes could enhance efficiency and selectivity, opening new avenues for environmental applications, energy production, and biomass valorization.
Contributions of This Special Issue
The articles in this Special Issue concern all the fields of application of photocatalysis mentioned above. In the contribution 1, the authors have prepared and characterized TiO2/SiO2 composites via a simple sol–gel method by surface reduction of Ti4+ ions to Ti3+ using titanium isopropoxide as a TiO2 precursor and rice husks as a SiO2 source. The XPS analysis showed the surface reduction of Ti4+ ions to Ti3+ within the TiO2 network via oxygen vacancies after SiO2 introduction, which is beneficial for the photocatalytic reaction. Photocatalytic degradation and mineralization of sodium diclofenac (SDFC) shows that TiO2/SiO2 composites have better activity compared to commercial TiO2-P25.
The contribution 2 is a review on the sustainable production of ultra-low-sulfur fuels through photocatalytic oxidation. Developing highly active photocatalytic systems for the deep purification of fuels from sulfur compounds is a significant task in modern catalysis science. This critical review covers recent progress over the last five years in heterogeneous photocatalytic desulfurization under visible light irradiation. It places specific emphasis on methods to enhance the photocatalytic activity of materials, particularly highlighting the creation of heterojunctions as the most promising approach.
In Contribution 3, Mediouni et al. attempt to establish a correlation between the photocatalytic properties of ZnO and the generation of hydrogen peroxide. The authors have studied the generation of hydrogen peroxide on commercial and synthesized ZnO from different precursors using two model molecules, formic acid (FA) and phenol (Ph). They found that the improved efficiency is accompanied by a high level of H2O2 production and fewer oxygen vacancies, and that the number of moles of H2O2 formed per number of carbon atoms removed is similar to the degradation of FA and Ph with a factor of 1. They compared the formation of H2O2 in the presence of TiO2 rutile and TiO2 anatase with commercial ZnO and demonstrated the impact of the presence of TiO2 on the decomposition of hydrogen peroxide and the formation of phenolic intermediates (which was much lower than with ZnO alone).
Contribution 4 is a review of the history of photocatalytic water oxidation over titanium dioxide (TiO2), accomplished by profiling the research on the molecular mechanism of the oxygen evolution reaction (OER) at the TiO2 surface. Furthermore, to investigate the theoretical approach to the reaction mechanism, some research with density functional theory (DFT) at the anatase (101) surface was illustrated. The results were successfully discussed with the reported mechanism.
In Contribution 5, the authors studied a novel application of photocatalysis to reduce diurnal evaporative fuel vapor emissions from automobiles. They designed and characterized a light-weight annulus photocatalytic device for the oxidation of diurnal evaporative fuel vapor emissions. The study results with TiO2-P25 are promising and demonstrate that the UV LED photocatalytic device is capable of reducing diurnal evaporative fuel vapor emissions from automobiles by 60 wt%, even if the presence of high concentrations of light alkanes and aromatic fuel vapors may limit the longevity of the device due to photocatalyst deactivation.
The title of Contribution 6 is “Adsorption and Photo-Degradation of Organophosphates on Sulfate-Terminated Anatase TiO2 Nanoparticles”. The authors studied the adsorption and photocatalytic degradation of two environmentally relevant model pollutants: trimethyl phosphate (TMP) and triethyl phosphate (TEP). They mainly investigated how these two organophosphates adsorbed anatase TiO2 and sulfate-terminated anatase TiO2 nanoparticles. For this, they used operando diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy and 2D correlation spectroscopy (2D COS). The main conclusions are that both TMP and TEP adsorb dissociatively on anatase TiO2, while on the sulfate-terminated anatase TiO2, TMP and TEP adsorb associatively. They were thus able to precisely determine the photodegradation mechanisms.
The contribution 7 studies one of the booming applications of heterogeneous photocatalysis—the production of hydrogen. Tien et al. have prepared and characterized a novel ZnO/Co3O4 NP photocatalyst for photocatalytic hydrogen evolution with visible light activity. They observed that the hydrogen production rate is much better with ZnO/Co3O4 than their single components.
The contribution 8 is a review on the use of two-dimensional transition metal dichalcogenides (TMDs) for hydrogen production. Due to their adjustable bandgap, near-zero Gibbs free energy, and lower cost compared to noble metal catalysts, these materials are considered as the next generation of H2 evolution electrocatalysts. This review summarizes recent research results on the use of electrocatalysts and photocatalysts for hydrogen evolution reactions based on two-dimensional materials, mainly including MoS2, WS2, and their compounds. The challenges and future development directions of two-dimensional hydrogen evolution reaction electrocatalysts and photocatalysts are also summarized and prospected.
In the contribution 9 of this Special Issue (the ninth), Hussain et al. employed hybrid density functional theory to study the influence of interfacial oxygen (O), sulfur (S), and zinc (Zn) vacancies on the optoelectronic properties of a ZnO/ZnS heterostructure. They showed that the quasi-bandgap of the ZnO/ZnS heterostructure is reduced compared to the ZnO surface; hence, the visible light response is enhanced in the ZnO/ZnS heterostructure.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
List of Contributions
- Dantio Nguela, C.; Manga, N.; Marchal, C.; Abega, A.; Nsami, N.; Robert, D. Effect of Biogenic Silica Behavior in the Incorporation of Mesoporous Anatase TiO2 for Excellent Photocatalytic Mineralization of Sodium Diclofenac. Catalysts. 2022, 12, 1001.
- Belousov, A.; Shafiq, I. Towards the Sustainable Production of Ultra-Low-Sulfur Fuels through Photocatalytic Oxidation. Catalysts 2022, 12, 1036.
- Mediouni, N.; Dappozze, F.; Khrouz, L.; Parola, S.; Amara, A.; Rhaiem, H.; Jaffrezic-Renault, N.; Namour, P.; Guillard, C. Correlation between Photocatalytic Properties of ZnO and Generation of Hydrogen Peroxide—Impact of Composite ZnO/TiO2 Rutile and Anatase. Catalysts 2022, 12, 1445.
- Nosaka, Y. Water Photo-Oxidation over TiO2—History and Reaction Mechanism. Catalysts 2022, 12, 1557.
- Almquist, C.; Kocher, J.; Saxton, K.; Simonson, L.; Danciutiu, A.; Nguyen, P.; Bain, J. A Novel Application of Photocatalysis: A UV-LED Photocatalytic Device for Controlling Diurnal Evaporative Fuel Vapor Emissions from Automobiles. Catalysts 2023, 13, 85.
- Svensson, F.; Österlund, L. Adsorption and Photo-Degradation of Organophosphates on Sulfate-Terminated Anatase TiO2 Nanoparticles. Catalysts 2023, 13, 526.
- Tien, T.; Chen, E. A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2023, 13, 852.
- Yu, H.; Zhang, M.; Cai, Y.; Zhuang, Y.; Wang, L. The Advanced Progress of MoS2 and WS2 for Multi-Catalytic Hydrogen Evolution Reaction Systems. Catalysts 2023, 13, 1148.
- Hussain, S.; Guo, L.; He, T. Influence of Vacancy Defects on the Interfacial Structural and Optoelectronic Properties of ZnO/ZnS Heterostructures for Photocatalysis. Catalysts 2023, 13, 1199.
References
- Braslavsky, S.E. Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Eibner, A. Action of Light on Pigments. Chem. Ztg. 1911, 35, 753–755. [Google Scholar]
- Goodeve, C.F.; Kitchener, J.A. Photosensitisation by titanium dioxide. Trans. Faraday Soc. 1938, 34, 570–578. [Google Scholar] [CrossRef]
- Markham, S.C. Photocatalytic properties of oxides. J. Chem. Educ. 1955, 32, 540–547. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Formenti, M.; Juillet, F.; Teichner, S.J. Photocatalyse hétérogène—Photooxydation ménagée des paraffined et oléfines sur l’anatase à température ambiante. C. R. Acad. Sci. 1970, 270, 138. [Google Scholar]
- Formenti, M.; Juillet, F.; Meriaudeau, P.; Teichner, S.J. Heterogeneous photocatalysis for partial oxidation of paraffins. Chem. Technol. 1971, 1, 680–686. [Google Scholar]
- Formenti, M.; Juillet, F.; Meriaudeau, P.; Teichner, S.J. Preparation in a Hydrogen-Oxygen Flame of Ultrafine Metal Oxide Particles Oxidative Properties Toward Hydrocarbons in the Presence of Ultraviolet Radiation. J. Coll. Interface Sci. 1972, 39, 79–89. [Google Scholar] [CrossRef]
- Courbon, H.; Pichat, P. Occurrence and mechanism of oxygen isotopic exchange catalyzed by ultraviolet-irradiated tin (IV) oxide, zinc oxide and zirconium (IV) oxide at 320 K. CR Seances Acad. Sci. Ser. C 1977, 285, 171. [Google Scholar]
- Herrmann, J.; Disdier, J.; Mozzanega, M.-N.; Pichat, P. Heterogeneous photocatalysis: In situ photoconductivity study of TiO2 during oxidation of isobutane into acetone. J. Catal. 1979, 60, 369–377. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Sordello, F.; Calza, P.; Minero, C.; Malato, S.; Minella, M. More than One Century of History for Photocatalysis from Past, Present and Future Perspectives. Catalysts 2022, 12, 1572–1593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).