Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal
Abstract
1. Introduction
2. Pharmaceutical Pollutants in Water Resources
3. Remediation Method for Pharmaceutical Pollutants
4. Photocatalysis
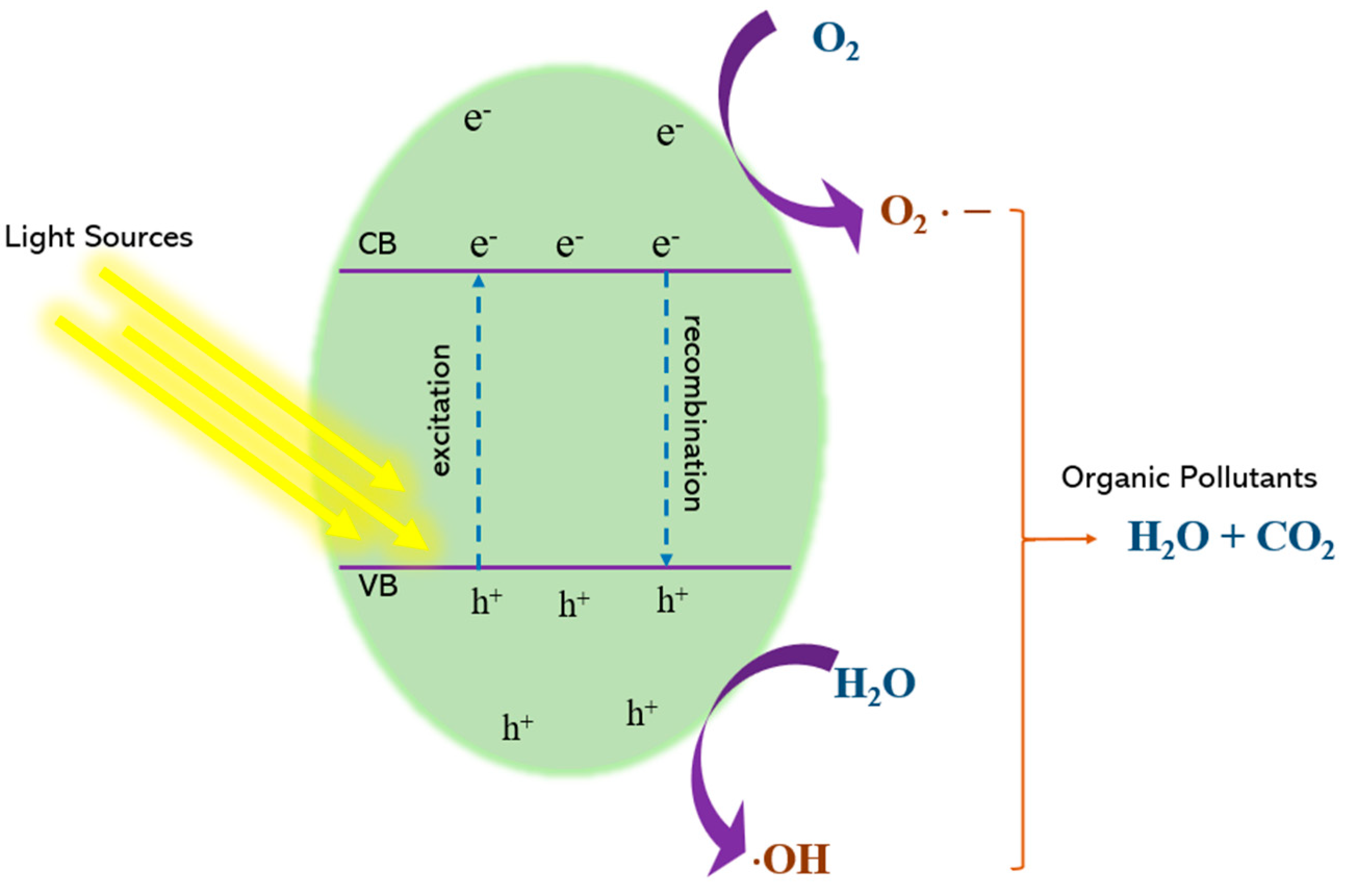
4.1. Principal Mechanism of Photocatalysis
4.2. Affecting Parameters for Photocatalysis Efficiency
4.2.1. Characteristics of the Catalyst
4.2.2. Catalyst Loading and Pollutant Concentration
4.2.3. pH
4.2.4. Light Source, Light Intensity, and Irradiation Time
4.3. Photocatalysis Materials
4.3.1. Metal Oxide
4.3.2. Quantum Dot-Based Photocatalyst
4.3.3. Two-Dimensional Material Photocatalyst
5. Metal Oxide Photocatalysts
5.1. Metal Oxide Morphology
5.1.1. Nanosphere
5.1.2. Nanorods
5.1.3. Nanowires
5.1.4. Nanotube
5.1.5. Nanoflower
5.2. Commonly Used Metal Oxides
5.2.1. Photocatalyst Based on Titanium Oxide
5.2.2. Photocatalyst Based on Zinc Oxide
5.2.3. Photocatalyst Based on Bismuth Oxide
6. Carbon Dots
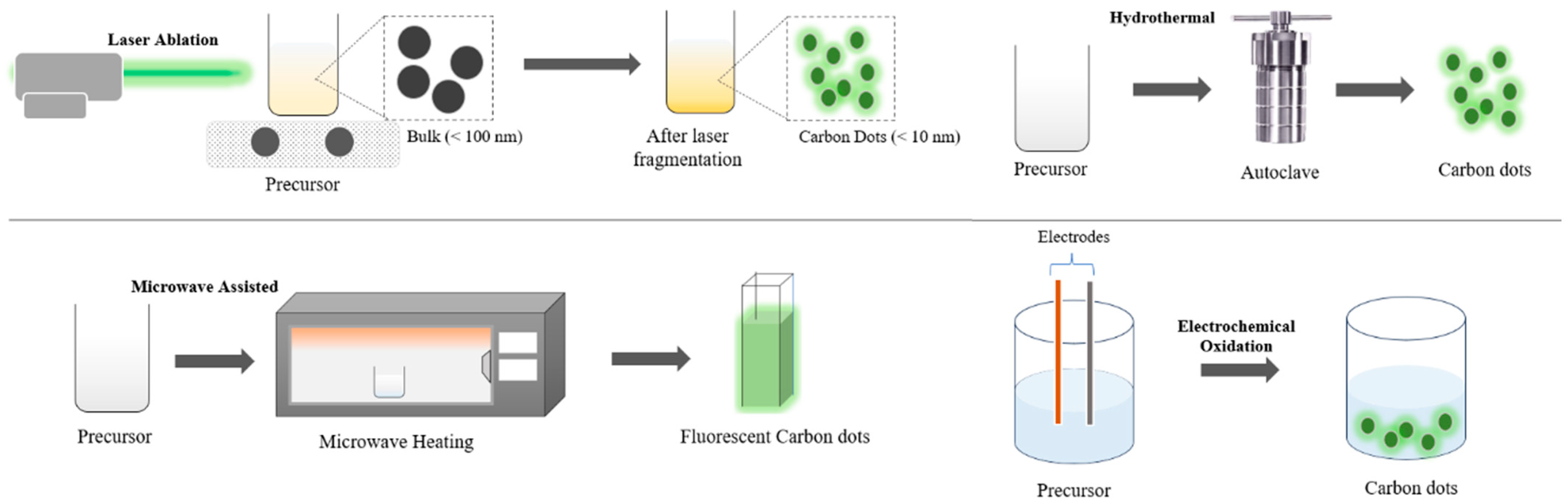
6.1. Synthesis of Carbon Dots
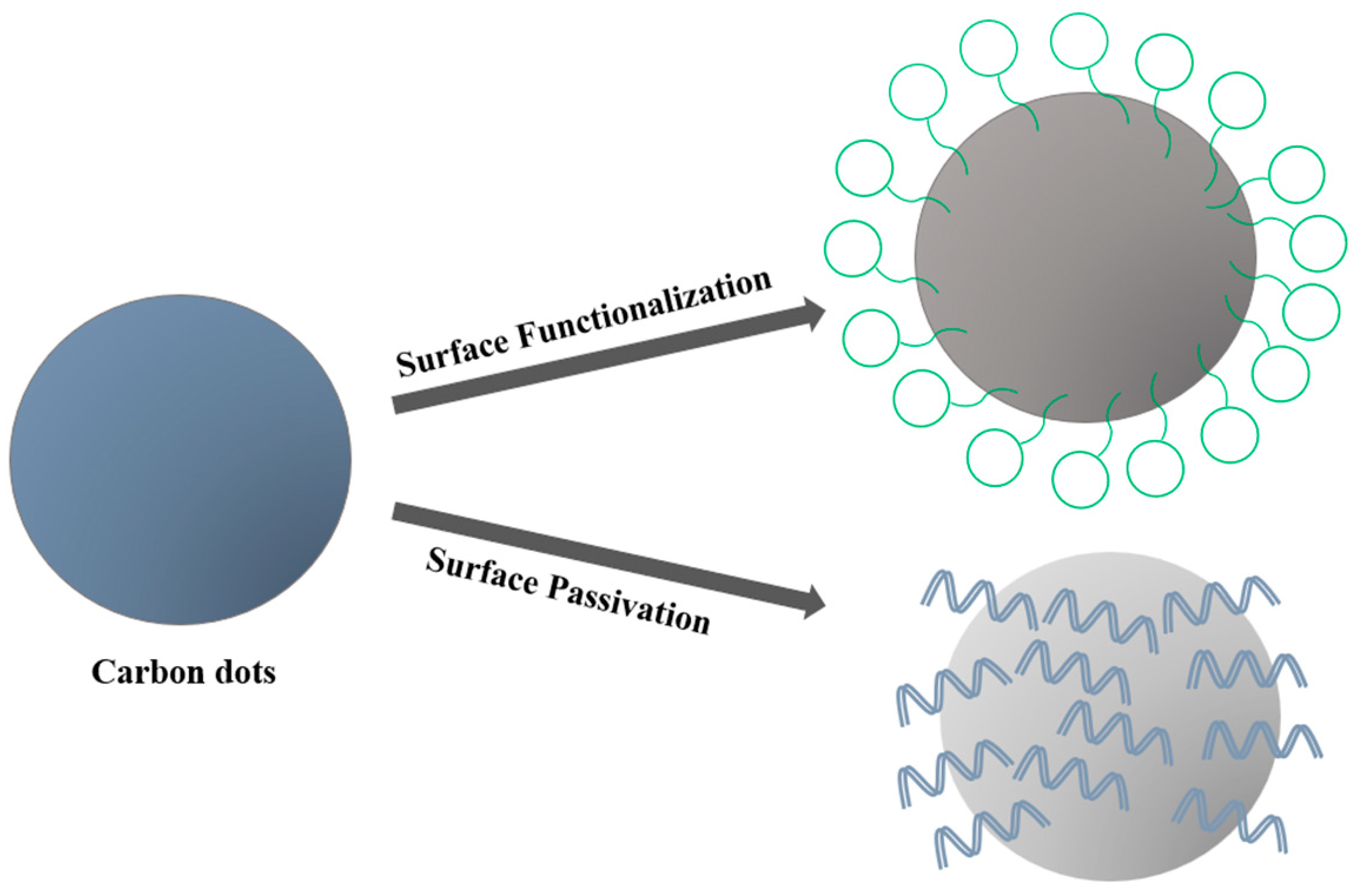
6.2. Properties of Carbon Dots
6.2.1. UV Absorption Property
6.2.2. Fluorescence Property
6.3. Mechanism of Carbon Dots for Photocatalysis
7. Metal Oxide/Carbon Dot Hybrid Materials
7.1. Synthesis Methods of Metal Oxide/Carbon Dots
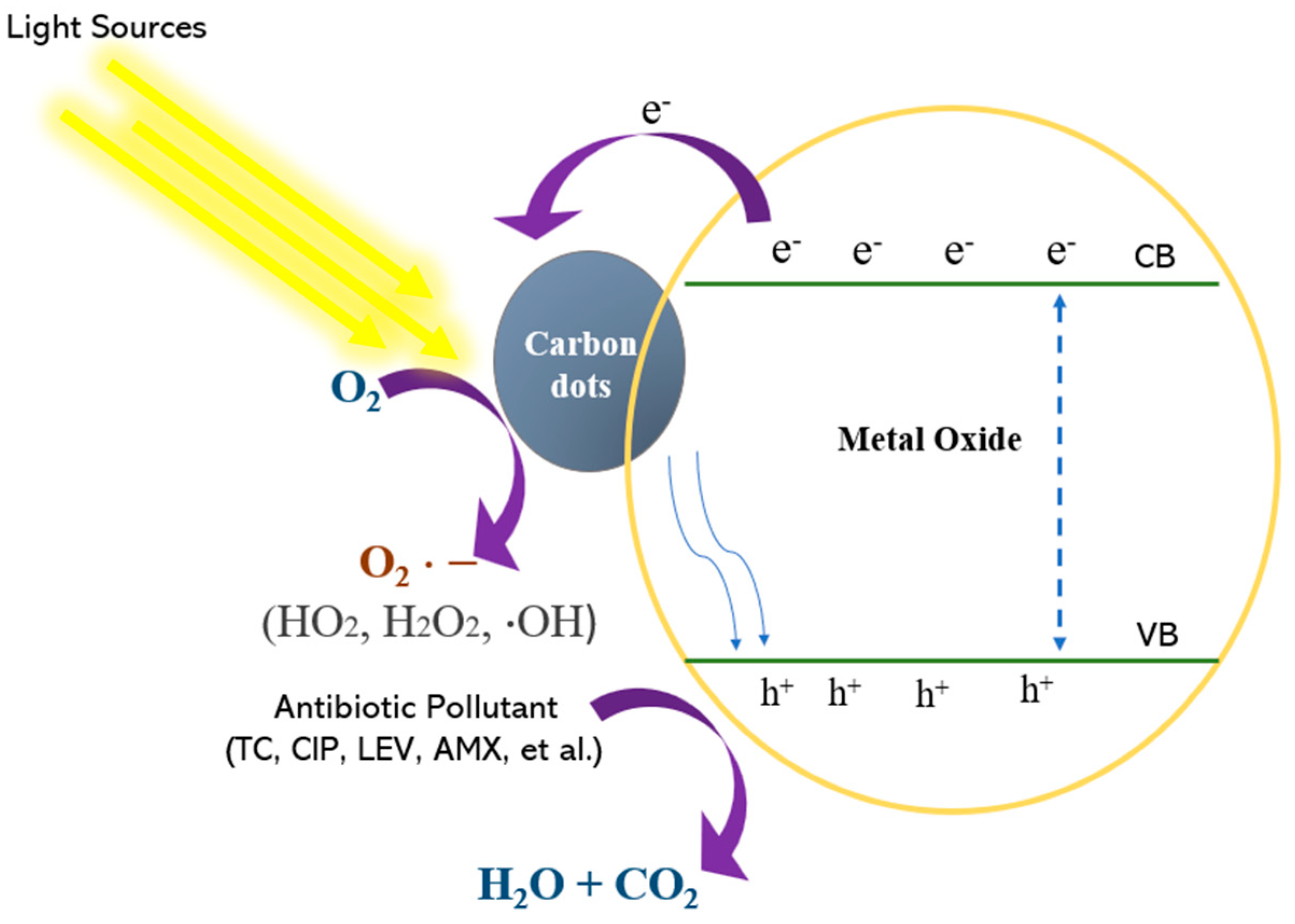
7.2. Mechanism of Metal Oxide/Carbon Dot Photocatalysis
7.3. Application of Metal Oxide/Carbon Dots for Pharmaceutical Pollutant Remediation
7.4. Health Concerns of Metal Oxide/Carbon Dots
| Photocatalyst | Carbon Sources | Antibiotics | Experimental Conditions | Antibiotic Removal | k (min−1) | Ref. |
|---|---|---|---|---|---|---|
| NCDs/ | Citric acid | Tetracycline | [catalyst] = 50 mg [TC] = 5.0 mg/L Light source = 300 W Xe lamp (visible light) | 97% (150 min) | - | [167] |
| -CDs- | Glucose | Tetracycline | [catalyst] = 0.5 g/L (0.01 g) [TC] = 20 mg/L (20 mL) Light source = 800 W Xe lamp (UV–visible light) | 92% (10 min) ~100% (20 min) | 0.09182 | [161] |
| CQD/α-FeOOH | Citric acid | Tetracycline | [catalyst] = 0.25 g/L (50 mg) [TC] = 20 mg/L pH = 3.09–10.31 Light source = 350 W Xe lamp (visible light) | 94.5% (60 min) | - | [162] |
| CQDs/ZnO@HNTs | Citric acid | Tetracycline | [catalyst] = 80 mg [TC] = 20 mg/L (100 mL) Light source = 500 W Xenon lamp (visible light) | 92.48% (90 min) | - | [168] |
| /N-CQDs/ | Ammonium citrate | Tetracycline | [catalyst] = 30 mg [TC] = 10 mg/L (100 mL) Light source = 300 W Xe lamp (visible light) | 88.9% (30 min) | 0.07097 | [163] |
| N-CQDs/ | Ammonium citrate | Tetracycline | [catalyst] = 0.05 g [TC] = 10 mg/L (100 mL) Light source = 300 W Xe lamp (visible light) | 97% (25 min) | 0.10684 | [164] |
| CQDs/ | Ammonium citrate | Tetracycline | [catalyst] = 0.05 g [TC] = 10 mg/L (100 mL) Light source = 300 W Xe lamp (visible light) | 83% (25 min) | 0.0754 | [164] |
| Carbon dots/ | Chroella | Tetracycline | [catalyst] = 15 mg [TC] = 10 mg/L (100 mL) pH = 7 Light source = 500 W Xe lamp | 99.5% (75 min) | 0.075 | [177] |
| CQDs/ | Corn stover | Tetracycline | [catalyst] = 100 mg [TC] = 10 mg/L (90 mL) Light source = Xe lamp (visible light) | 92.49% (100 min) | - | [16] |
| CDs/g- | Citric acid and urea | Tetracycline | [catalyst] = 30 mg [TC] = 20 mg/L (50 mL) Light source = 350 W Xe lamp (visible light) | 88.4% (90 min) | 0.0231 | [169] |
| ZIS/CQDs | Citric acid | Tetracycline | [catalyst] = 20 mg [TC] = 10 mg/L (80 mL) Light source = 250 W Xe lamp (visible light), 350 W mercury lamp (UV) | 67.89% (90 min) | 0.00839 | [140] |
| CDs/g- | Citric acid | Tetracycline | [catalyst] = 20 mg [TC] = 5 mg/L (80 mL) pH = 4 Light source = 500 W Xe lamp (visible light) | 79.3% (210 min) | 0.01315 | [170] |
| // | Citric acid | Tetracycline | [catalyst] = 0.075 g [TC] = 10 mg/L (250 mL) pH = 3 Light source = 150 W Xe Lamp (visible light) | 100% (75 min) | 0.1319 | [171] |
| CQDs/ | Bamboo | Ciprofloxacin | [catalyst] = 50 mg [CIP] = 15 mg/L (100 mL) Light source = 300 W Xe lamp (visible light) | 65% (90 min) | 0.01164 | [172] |
| /CDs/CdTe QDs | PEI | Ciprofloxacin | [catalyst] = 50 g [CIP] = 10 mg/L (100 mL) Light source = 250 W Xe lamp (visible light) | 70% (90 min) | 0.008 | [165] |
| BiOCl/CQDs/rGO | Sucrose | Ciprofloxacin | [catalyst] = 25 mg [CIP] = 20 mg/L (50 mL) Light source = 300 W Xe lamp (visible light) | 87% (100 min) | 0.0146 | [166] |
| ZnO/CD NCs | Trisodium citrate dihydrate | Ciprofloxacin | [catalyst] = 0.6 g/L [CIP] = 12 mg/L (50 mL) pH = 6.3 Light source = natural sunlight | 98% (110 min) | 0.030 | [11] |
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment—unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Srivastava, S.K. Recent advances in removal of pharmaceutical pollutants in wastewater using metal oxides and carbonaceous materials as photocatalysts: A review. RSC Appl. Interfaces 2024, 1, 340–429. [Google Scholar] [CrossRef]
- Shong, B.; Ansari, A.S.; Bent, S.F. Thermally Activated Reactions of Phenol at the Ge(100)-2 × 1 Surface. J. Phys. Chem. C 2020, 124, 23657–23660. [Google Scholar] [CrossRef]
- Ansari, A.A.; Sartale, S.D. Narrow size distributed Ag nanoparticles grown by spin coating and thermal reduction: Effect of processing parameters. Mater. Res. Express 2016, 3, 085023. [Google Scholar] [CrossRef]
- Raya, S.S.; Ansari, A.S.; Kang, S.G.; Lee, H.-B.; Shong, B. Effect of molecular backbone structure on vapor phase coupling reaction between diiso(thio)cyanates with diamines, diols, and dithiols. Prog. Org. Coatings 2020, 140, 105509. [Google Scholar] [CrossRef]
- Khadtare, S.S.; Ansari, A.S.A.; Sartale, S.D.; Jadkar, S.R.; Pathan, H.M. ZnO nanocactus loaded with gold nanoparticles for dye sensitized solar cells. In Proceedings of the 2014 International Renewable and Sustainable Energy Conference (IRSEC), Ouarzazate, Morocco, 17–19 October 2014; pp. 94–96. [Google Scholar]
- Ansari, A.A.; Sartale, S.D. The Calculation of Electronic Parameters of Al/TiO2/p-Si MOS Structure Formed Using TiO2 Thin Films Grown by Thermal Oxidation of Sputtered Ti Films. Adv. Sci. Lett. 2016, 22, 1013–1016. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R. Degradation of antibiotics by advanced oxidation processes: An overview. Sci. Total. Environ. 2020, 701, 135023. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Sharma, S.; Umar, A.; Mehta, S.K.; Ibhadon, A.O.; Kansal, S.K. Solar light driven photocatalytic degradation of levofloxacin using TiO2/carbon-dot nanocomposites. New J. Chem. 2018, 42, 7445–7456. [Google Scholar] [CrossRef]
- Mukherjee, I.; Cilamkoti, V.; Dutta, R.K. Sunlight-Driven Photocatalytic Degradation of Ciprofloxacin by Carbon Dots Embedded in ZnO Nanostructures. ACS Appl. Nano Mater. 2021, 4, 7686–7697. [Google Scholar] [CrossRef]
- Anucha, C.B.; Altin, I.; Bacaksiz, E.; Stathopoulos, V.N. Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies. Chem. Eng. J. Adv. 2022, 10, 100262. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Sendão, R.M.; da Silva, J.C.E.; da Silva, L.P. Photocatalytic removal of pharmaceutical water pollutants by TiO2—Carbon dots nanocomposites: A review. Chemosphere 2022, 301, 134731. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, H.; Yang, Z.; Fang, Y. Visible light degradation of tetracycline using oxygen-rich titanium dioxide nanosheets decorated by carbon quantum dots. Chem. Eng. J. 2021, 408, 127259. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Ma, P.; Guo, Z.; Ma, Q.; Zhao, Q.; Guo, Y.; Zhao, J.; Guan, G. Carbon quantum dots/Cu2O S-scheme heterojunction for enhanced photocatalytic degradation of tetracycline. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 690, 133779. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Bui, H.M.; Nguyen, H.Q. Chapter 7—Heterogeneous Photocatalysis for the Removal of Pharmaceutical Compounds. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Tyagi, R.D., Ngo, H.H., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–183. [Google Scholar]
- Kar, P.; Shukla, K.; Jain, P.; Sathiyan, G.; Gupta, R.K. Semiconductor based photocatalysts for detoxification of emerging pharmaceutical pollutants from aquatic systems: A critical review. Nano Mater. Sci. 2021, 3, 25–46. [Google Scholar] [CrossRef]
- Kutuzova, A.; Dontsova, T.; Kwapinski, W. Application of TiO2-Based Photocatalysts to Antibiotics Degradation: Cases of Sulfamethoxazole, trimethoprim and ciprofloxacin. Catalysts 2021, 11, 728. [Google Scholar] [CrossRef]
- Papaioannou, C.; Geladakis, G.; Kommata, V.; Batargias, C.; Lagoumintzis, G. Insights in Pharmaceutical Pollution: The Prospective Role of eDNA Metabarcoding. Toxics 2023, 11, 903. [Google Scholar] [CrossRef]
- Bayan, E.; Pustovaya, L.; Volkova, M. Recent advances in TiO2-based materials for photocatalytic degradation of antibiotics in aqueous systems. Environ. Technol. Innov. 2021, 24, 101822. [Google Scholar] [CrossRef]
- Gao, J.; Rao, S.; Yu, X.; Wang, L.; Xu, J.; Yang, J.; Liu, Q. Dimensional-matched two dimensional/two dimensional TiO2/Bi2O3 step-scheme heterojunction for boosted photocatalytic performance of sterilization and water splitting. J. Colloid Interface Sci. 2022, 628, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Bhagat, C.; Kumar, M.; Tyagi, V.K.; Mohapatra, P.K. Proclivities for prevalence and treatment of antibiotics in the ambient water: A review. npj Clean Water 2020, 3, 42. [Google Scholar] [CrossRef]
- World Health Organization. Guidance on Wastewater and Solid Waste Management for Manufacturing of Antibiotics; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Mishra, N.S.; Reddy, R.; Kuila, A.; Rani, A.; Nawaz, A.; Pichiah, S.; Mukherjee, P. A Review on Advanced Oxidation Processes for Effective Water Treatment. Curr. World Environ. J. 2017, 12, 469–489. [Google Scholar] [CrossRef]
- Anwer, M.; Faizi, Y. A review on types and applications of advanced oxidation processes. Int. J. Adv. Eng. Manag. 2022, 4, 1059–1070. [Google Scholar]
- Conte, F.; Tommasi, M.; Degerli, S.N.; Forame, E.; Parolini, M.; De Felice, B.; Ramis, G.; Rossetti, I. Comparison of Different Advanced Oxidation Processes (AOPs) and Photocatalysts for the Degradation of Diclofenac. ChemPhotoChem 2024, 8, e202300177. [Google Scholar] [CrossRef]
- Mondal, K.; Sharma, A. Recent advances in the synthesis and application of photocatalytic metal–metal oxide core–shell nanoparticles for environmental remediation and their recycling process. RSC Adv. 2016, 6, 83589–83612. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, S.; Luo, Z. A study on the photocatalytic degradation performance of a [KNbO3]0.9-[BaNi0.5Nb0.5O3−δ]0.1 perovskite. RSC Adv. 2020, 10, 1275–1280. [Google Scholar] [CrossRef]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Chen, F.-Y. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Shanthi, M. Photocatalytic degradation of an organic pollutant by zinc oxide—Solar process. Arab. J. Chem. 2016, 9, S1858–S1868. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Effect of pH on the photocatalytic degradation of remazol brilliant blue dye using zirconia catalyst. Mater. Today Proc. 2020, 31, 260–262. [Google Scholar] [CrossRef]
- Tayyebi, A.; Soltani, T.; Lee, B.-K. Effect of pH on photocatalytic and photoelectrochemical (PEC) properties of monoclinic bismuth vanadate. J. Colloid Interface Sci. 2019, 534, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Josephine, A.J.; Dhas, C.R.; Venkatesh, R.; Arivukarasan, D.; Christy, A.J.; Monica, S.E.S.; Keerthana, S. Effect of pH on visible-light-driven photocatalytic degradation of facile synthesized bismuth vanadate nanoparticles. Mater. Res. Express 2020, 7, 015036. [Google Scholar] [CrossRef]
- Nugroho, F.G.; Agson-Gani, P.A.; Anindita, P.A.; Steky, F.V.; Benu, D.P.; Yuliarto, B.; Dwivany, F.M.; Suendo, V. Prolonging banana shelf life through visible light-induced ethylene scavenging using manganese-decorated TiO2 via KMnO4 reduction. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 691, 133817. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, G. The photocatalytic degradation of methyl green in presence of visible light with photoactive Ni0.10: La0.05: TiO2 nanocomposites. IOSR J. Appl. Chem. 2017, 10, 31–44. [Google Scholar]
- Lu, Y.; Lin, Y.; Wang, D.; Wang, L.; Xie, T.; Jiang, T. A high performance cobalt-doped ZnO visible light photocatalyst and its photogenerated charge transfer properties. Nano Res. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, F.; Zhao, W.; Wang, H.; Liu, Y.; Guan, B. The fabrication and characterization of novel carbon doped TiO2 nanotubes, nanowires and nanorods with high visible light photocatalytic activity. Nanotechnology 2009, 20, 235701. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, X.; Wu, Y.; Wang, X.; Qian, Y. Kinetic study and modeling on photocatalytic degradation of para-chlorobenzoate at different light intensities. Environ. Pollut. 2002, 117, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Z. Study on light intensity in the process of photocatalytic degradation of indoor gaseous formaldehyde for saving energy. Energy Convers. Manag. 2007, 48, 882–889. [Google Scholar] [CrossRef]
- Elaziouti, A.; Ahmed, B. ZnO-assisted photocatalytic degradation of congo Red and benzopurpurine 4B in aqueous solution. J. Chem. Eng. Process Technol. 2011, 2, 1–9. [Google Scholar]
- Wardhani, S.; Purwonugroho, D.; Fitri, C.W.; Prananto, Y.P. Effect of pH and irradiation time on TiO2-chitosan activity for phenol photo-degradation. In Proceedings of the 8th Annual Basic Science International Conference: Coverage of Basic Sciences Toward the World’s Sustainability Challanges, East Java, Indonesia, 6–7 March 2018. [Google Scholar]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R.; Attri, P. Transition Metal Oxides and Their Composites for Photocatalytic Dye Degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Araújo, E.S.; Pereira, M.F.G.; da Silva, G.M.G.; Tavares, G.F.; Oliveira, C.Y.B.; Faia, P.M. A Review on the Use of Metal Oxide-Based Nanocomposites for the Remediation of Organics-Contaminated Water via Photocatalysis: Fundamentals, Bibliometric Study and Recent Advances. Toxics 2023, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Pascariu, P.; Gherasim, C.; Airinei, A. Metal Oxide Nanostructures (MONs) as Photocatalysts for Ciprofloxacin Degradation. Int. J. Mol. Sci. 2023, 24, 9564. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Mishra, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Saleh, T.A.; Aminabhavi, T.M. Band gap tuning and surface modification of carbon dots for sustainable environmental remediation and photocatalytic hydrogen production—A review. J. Environ. Manag. 2019, 250, 109486. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, T.A.; Al-Obaidi, Q.; Abdulla, T.A.; Rasheed, R.T.; Al-Azawi, K.; Meharban, F. A Critical Review of the Photocatalytic Degradation of Pharmaceutical Residues by a TiO2-Based Photocatalyst. Hung. J. Ind. Chem. 2023, 51, 65–75. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Zhao, L.; Meng, Q.; Fan, X.; Ye, C.; Li, X.; Chen, B.; Ramamurthy, V.; Tung, C.; Wu, L. Photocatalysis with Quantum Dots and Visible Light: Selective and Efficient Oxidation of Alcohols to Carbonyl Compounds through a Radical Relay Process in Water. Angew. Chem. Int. Ed. Engl. 2017, 56, 3020–3024. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Zhao, X.; Cheng, Q.; Xing, Y.; Ma, W.; Wang, X.; Zhao, G.; Xu, X. A Mini Review of the Preparation and Photocatalytic Properties of Two-Dimensional Materials. Front. Chem. 2020, 8, 582146. [Google Scholar] [CrossRef]
- Thomas, N.; Mathew, S.; Nair, K.M.; O’Dowd, K.; Forouzandeh, P.; Goswami, A.; McGranaghan, G.; Pillai, S.C. 2D MoS2: Structure, mechanisms, and photocatalytic applications. Mater. Today Sustain. 2021, 13, 100073. [Google Scholar] [CrossRef]
- Quinn, M.D.J.; Ho, N.H.; Notley, S.M. Aqueous Dispersions of Exfoliated Molybdenum Disulfide for Use in Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 12751–12756. [Google Scholar] [CrossRef] [PubMed]
- Naggar, A.H.; Ahmed, A.S.A.; El-Nasr, T.A.S.; Alotaibi, N.F.; Chong, K.F.; Ali, G.A.M. Morphological Dependence of Metal Oxide Photocatalysts for Dye Degradation. Inorganics 2023, 11, 484. [Google Scholar] [CrossRef]
- Kannan, K.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Nanostructured metal oxides and its hybrids for photocatalytic and biomedical applications. Adv. Colloid Interface Sci. 2020, 281, 102178. [Google Scholar] [CrossRef]
- Das, A.; Nikhil, S.K.; Nair, R.G. Influence of surface morphology on photocatalytic performance of zinc oxide: A review. Nano-Struct. Nano-Objects 2019, 19, 100353. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Morais, M.; Nunes, D.; Oliveira, M.J.; Rovisco, A.; Pimentel, A.; Águas, H.; Fortunato, E.; Martins, R. High UV and Sunlight Photocatalytic Performance of Porous ZnO Nanostructures Synthesized by a Facile and Fast Microwave Hydrothermal Method. Materials 2021, 14, 2385. [Google Scholar] [CrossRef]
- Mohtar, S.S.; Aziz, F.; Ismail, A.F.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of Doping and Additive Applications on Photocatalyst Textural Properties in Removing Organic Pollutants: A Review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Q. One-pot synthesis of ZnO/Ag nanospheres with enhanced photocatalytic activity. Mater. Lett. 2010, 64, 389–392. [Google Scholar] [CrossRef]
- Cheng, L.; Zheng, L.; Li, G.; Yin, Q.; Jiang, K. Synthesis of sealed sponge ZnO nanospheres through a novel NH3-evaporation method. Nanotechnology 2008, 19, 075605. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhan, L.; Hong, F.; Song, S.; Lin, Z.; Chen, J.; Jin, M. A visible light-responsive iodine-doped titanium dioxide nanosphere. J. Environ. Sci. 2011, 23, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Hu, H.; Ge, X.; Han, C.; Zhao, D.; Shao, G. One-pot sonochemical fabrication of hierarchical hollow CuO submicrospheres. Ultrason. Sonochemistry 2011, 18, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Ismail, A.A.; Ibrahim, A.A.; Bouzid, H.; Al-Sayari, S.A. Highly efficient photocatalyst based on Ce doped ZnO nanorods: Controllable synthesis and enhanced photocatalytic activity. Chem. Eng. J. 2013, 229, 225–233. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Yan, X.; Yang, Y.; Lei, Y.; Zhou, J.; Huang, Y.; Gu, Y.; Zhang, Y. Structure and photocatalytic activity of Ni-doped ZnO nanorods. Mater. Res. Bull. 2011, 46, 1207–1210. [Google Scholar] [CrossRef]
- Alammar, T.; Mudring, A.-V. Facile ultrasound-assisted synthesis of ZnO nanorods in an ionic liquid. Mater. Lett. 2009, 63, 732–735. [Google Scholar] [CrossRef]
- Sadollahkhani, A.; Ibupoto, Z.H.; Elhag, S.; Nur, O.; Willander, M. Photocatalytic properties of different morphologies of CuO for the degradation of Congo red organic dye. Ceram. Int. 2014, 40, 11311–11317. [Google Scholar] [CrossRef]
- Yin, X.; Liu, L.; Ai, F. Enhanced Photocatalytic Degradation of Methylene Blue by WO3 Nanoparticles Under NIR Light Irradiation. Front. Chem. 2021, 9, 683765. [Google Scholar] [CrossRef]
- Ullah, A.; Rahman, L.; Hussain, S.Z.; Abbas, W.; Tawab, A.; Jilani, A.; Bajwa, S.Z.; Khan, W.S.; Riaz, R.; Hussain, I.; et al. Mechanistic insight of dye degradation using TiO2 anchored α-MnO2 nanorods as promising sunlight driven photocatalyst. Mater. Sci. Eng.: B 2021, 271, 115257. [Google Scholar] [CrossRef]
- Yuan, S.D.; Chen, S.Q.; Zhu, X.; Xiong, P.; Yang, Y.F.; Hu, Z.H.; Xiong, J. Obtaining Ultra-High Surface Area TiO2 Nanorods via Hydrothermally Transformation of Elongated Titanate Nanotubes. J. Nano Res. 2018, 51, 13–23. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, Z.H.; Chen, Z.H.; Shafiq, I.; Zapien, J.A.; Bello, I.; Zhang, W.J.; Lee, S.T. Synthesis, Characterization, and Photocatalytic Application of Different ZnO Nanostructures in Array Configurations. Cryst. Growth Des. 2009, 9, 3222–3227. [Google Scholar] [CrossRef]
- Jia, T.; Wang, W.; Long, F.; Zhengyi, F.; Wang, H.; Zhang, Q. Fabrication, characterization and photocatalytic activity of La-Doped ZnO nanowires. J. Alloy. Compd. 2009, 484, 410–415. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Z.-Y.; Du, G.-H.; Ren, T.-Z.; Bouvy, C.; Halasa, M.; Su, B.-L. Simple approach to highly oriented ZnO nanowire arrays: Large-scale growth, photoluminescence and photocatalytic properties. Nanotechnology 2006, 17, 588–594. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Hu, W.B.; Xia, Y.D.; Smith, E.F.; Zhu, Y.Q.; Dunnill, C.W.; Gregory, D.H. Preparation and characterization of tungsten oxynitride nanowires. J. Mater. Chem. 2007, 17, 4436–4440. [Google Scholar] [CrossRef]
- Sudirman, S.; Atmaja, L.D.P.; Pratama, A.J.; Yuanita, E.; Dharmayani, N.K.T.; Ulfa, M.; Hidayat, R. Highly photocatalytic performance of TiO2 nanowires in the conversion of benzaldehydes to benzoic acid. Acta Chim. Asiana 2023, 6, 315–321. [Google Scholar] [CrossRef]
- Hamisu, A.; Gaya, U.; Gaya, A. Effect of alkali strength on the hydrothermal growth of photoactive TiO2 nanowires. J. Nanostructures 2020, 10, 639–651. [Google Scholar]
- Wan, X.; Liang, X.; Zhang, C.; Li, X.; Liang, W.; Xu, H.; Lan, S.; Tie, S. Morphology controlled syntheses of Cu-doped ZnO, tubular Zn(Cu)O and Ag decorated tubular Zn(Cu)O microcrystals for photocatalysis. Chem. Eng. J. 2015, 272, 58–68. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Jia, L.; Wang, G.; Tang, C. Controllable Preferential-Etching Synthesis and Photocatalytic Activity of Porous ZnO Nanotubes. J. Phys. Chem. C 2008, 112, 11738–11743. [Google Scholar] [CrossRef]
- Rojviroon, T.; Rojviroon, O.; Sirivithayapakorn, S.; Angthong, S.; Thongpool, V. Application of TiO2 nanotubes as photocatalysts for decolorization of synthetic dye wastewater. Water Resour. Ind. 2021, 26, 100163. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Rejani, P.; Beena, B. CuO nano structures as an ecofriendly nano photo catalyst and antimicrobial agent for environmental remediation. Int. J. Nano Dimens. 2018, 9, 145–157. [Google Scholar]
- Choopool, P.; Kooptarnond, K.; Khangkhamano, M.; Rachpech, V. The Effect of Sn(II) Precursor on Morphology and Surface Area of as Synthesis SnO2 Nanotube. Mater. Sci. Forum 2020, 998, 227–232. [Google Scholar] [CrossRef]
- Das, A.; Malakar, P.; Nair, R.G. Engineering of ZnO nanostructures for efficient solar photocatalysis. Mater. Lett. 2018, 219, 76–80. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, H.; Yuan, S.; Jiao, Z.; Zhu, X. Preparation of flower-like ZnO architectures assembled with nanosheets for enhanced photocatalytic activity. J. Colloid Interface Sci. 2016, 462, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lou, F.; Li, M.; Lou, X.; Li, Z.; Zhou, J. Sol−gel-based hydrothermal method for the synthesis of 3D flower-like ZnO microstructures composed of nanosheets for photocatalytic applications. Ceram. Int. 2014, 40, 5507–5514. [Google Scholar] [CrossRef]
- Harris, J.; Silk, R.; Smith, M.; Dong, Y.; Chen, W.-T.; Waterhouse, G.I.N. Hierarchical TiO2 Nanoflower Photocatalysts with Remarkable Activity for Aqueous Methylene Blue Photo-Oxidation. ACS Omega 2020, 5, 18919–18934. [Google Scholar] [CrossRef]
- Liu, M.; Lu, W.-M.; Zhao, L.; Zhou, C.-L.; Li, H.-L.; Wang, W.-J. Fabrication and photocatalytical properties of flower-like TiO2 nanostructures. Trans. Nonferrous Met. Soc. China 2010, 20, 2299–2302. [Google Scholar] [CrossRef]
- He, Z.; Cai, Q.; Fang, H.; Situ, G.; Qiu, J.; Song, S.; Chen, J. Photocatalytic activity of TiO2 containing anatase nanoparticles and rutile nanoflower structure consisting of nanorods. J. Environ. Sci. 2013, 25, 2460–2468. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Martins, P.M.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Lanceros-Mendez, S.; Pereira, L. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Jiang, T.; Cheng, L.; Han, Y.; Feng, J.; Zhang, J. One-pot hydrothermal synthesis of Bi2O3-WO3 p-n heterojunction film for photoelectrocatalytic degradation of norfloxacin. Sep. Purif. Technol. 2020, 238, 116428. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Li, H.; Mu, Y.; Yang, Z. Synthesis and application of Bi2WO6 for the photocatalytic degradation of two typical fluoroquinolones under visible light irradiation. RSC Adv. 2019, 9, 27768–27779. [Google Scholar] [CrossRef] [PubMed]
- Gurung, K.; Ncibi, M.C.; Thangaraj, S.K.; Jänis, J.; Seyedsalehi, M.; Sillanpää, M. Removal of pharmaceutically active compounds (PhACs) from real membrane bioreactor (MBR) effluents by photocatalytic degradation using composite Ag2O/P-25 photocatalyst. Sep. Purif. Technol. 2019, 215, 317–328. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Han, J.; Guo, R. TiO2 nanosheet/NiO nanorod hierarchical nanostructures: P–n heterojunctions towards efficient photocatalysis. J. Colloid Interface Sci. 2020, 562, 313–321. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.-M.; Al-Shirbini, A.-S.; Mohamed, O.; Nasr, O. Photocatalytic degradation of paracetamol over magnetic flower-like TiO2/Fe2O3 core-shell nanostructures. J. Photochem. Photobiol. A Chem. 2017, 347, 186–198. [Google Scholar] [CrossRef]
- Zhao, Q.-E.; Wen, W.; Xia, Y.; Wu, J.-M. Photocatalytic activity of TiO2 nanorods, nanowires and nanoflowers filled with TiO2 nanoparticles. Thin Solid Films 2018, 648, 103–107. [Google Scholar] [CrossRef]
- Nunes, D.; Pimentel, A.; Branquinho, R.; Fortunato, E.; Martins, R. Metal Oxide-Based Photocatalytic Paper: A Green Alternative for Environmental Remediation. Catalysts 2021, 11, 504. [Google Scholar] [CrossRef]
- Abdulkareem, G.A.; Nurul-Asikin, M.; Hin, T.-Y.Y. Nanomaterials: An Overview of Nanorods Synthesis and Optimization. In Nanorods and Nanocomposites; Sasani, G.M., Soumen, D., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 2. [Google Scholar]
- Fu, D.; Han, G.; Meng, C. Size-controlled synthesis and photocatalytic degradation properties of nano-sized ZnO nanorods. Mater. Lett. 2012, 72, 53–56. [Google Scholar] [CrossRef]
- Choudhary, S.; Mohapatra, S. Efficient photocatalytic degradation of antibiotic levofloxacin and organic pollutants in water by Pr doped ZnO nanorods. Chem. Phys. Impact 2024, 9, 100687. [Google Scholar] [CrossRef]
- Mohammed, R.; Ali, M.E.M.; Gomaa, E.; Mohsen, M. Green ZnO nanorod material for dye degradation and detoxification of pharmaceutical wastes in water. J. Environ. Chem. Eng. 2020, 8, 104295. [Google Scholar] [CrossRef]
- Al-Khadhuri, A.; Al-Sabahi, J.; Kyaw, H.H.; Myint, M.T.Z.; Al-Farsi, B.; Al-Abri, M. Photocatalytic degradation toward pharmaceutical pollutants using supported zinc oxide nanorods catalyzed visible light system. Int. J. Environ. Sci. Technol. 2022, 20, 10021–10030. [Google Scholar] [CrossRef]
- Jin, Z.; Meng, F.-L.; Jia, Y.; Luo, T.; Sun, B.; Wang, J.; Liu, J.-H.; Huang, X.-J. Porous TiO2 nanowires derived from nanotubes: Synthesis, characterzation and their enhanced photocatalytic properties. Microporous Mesoporous Mater. 2013, 181, 146–153. [Google Scholar] [CrossRef]
- Rogé, V.; Guignard, C.; Lamblin, G.; Laporte, F.; Fechete, I.; Garin, F.; Dinia, A.; Lenoble, D. Photocatalytic degradation behavior of multiple xenobiotics using MOCVD synthesized ZnO nanowires. Catal. Today 2018, 306, 215–222. [Google Scholar] [CrossRef]
- Nagasundari, S.M.; Muthu, K.; Kaviyarasu, K.; Al Farraj, D.A.; Alkufeidy, R.M. Current trends of Silver doped Zinc oxide nanowires photocatalytic degradation for energy and environmental application. Surfaces Interfaces 2021, 23, 100931. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Guo, Y.; Rhodes, G.; Yeom, J.; Li, H.; Zhang, W. Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: Kinetics, influencing factors, and mechanisms. Environ. Int. 2019, 132, 105105. [Google Scholar] [CrossRef]
- Cha, G.; Schmuki, P.; Altomare, M. Anodic TiO2 nanotube membranes: Site-selective Pt-activation and photocatalytic H2 evolution. Electrochimica Acta 2017, 258, 302–310. [Google Scholar] [CrossRef]
- Bojer, C.; Schöbel, J.; Martin, T.; Ertl, M.; Schmalz, H.; Breu, J. Clinical wastewater treatment: Photochemical removal of an anionic antibiotic (ciprofloxacin) by mesostructured high aspect ratio ZnO nanotubes. Appl. Catal. B Environ. 2017, 204, 561–565. [Google Scholar] [CrossRef]
- Shende, P.; Kasture, P.; Gaud, R. Nanoflowers: The future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Xu, X.; Huang, R.; Qi, W.; Su, R.; He, Z. Enhanced photocatalytic degradation of antibiotics in water over functionalized N,S-doped carbon quantum dots embedded ZnO nanoflowers under sunlight irradiation. Chem. Eng. J. 2020, 382, 123016. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef]
- Velumani, A.; Sengodan, P.; Arumugam, P.; Rajendran, R.; Santhanam, S.; Palanisamy, M. Carbon quantum dots supported ZnO sphere based photocatalyst for dye degradation application. Curr. Appl. Phys. 2020, 20, 1176–1184. [Google Scholar] [CrossRef]
- Wang, K.; Liang, L.; Zheng, Y.; Li, H.; Niu, X.; Zhang, D.; Fan, H. Visible light-driven photocatalytic degradation of organic pollutants via carbon quantum dots/TiO2. New J. Chem. 2021, 45, 16168–16178. [Google Scholar] [CrossRef]
- Parmar, N.; Srivastava, J.K. Process optimization and kinetics study for photocatalytic ciprofloxacin degradation using TiO2 nanoparticle: A comparative study of Artificial Neural Network and Surface Response Methodology. J. Indian Chem. Soc. 2022, 99, 100584. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Wei, S.; Wu, Y.; Zheng, Y.; Yuan, F.; Hou, J. Study on photocatalytic degradation of amoxicillin in wastewater by Bi2WO6/nano-ZnO. Opt. Mater. 2022, 123, 111835. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Rizwan, M.; Bundschuh, J.; Elnahal, A.S.; Li, W. Green synthesized zinc oxide nanoparticles for removal of carbamazepine in water and soil systems. Sep. Purif. Technol. 2024, 334, 125988. [Google Scholar] [CrossRef]
- Qin, K.; Zhao, Q.; Yu, H.; Xia, X.; Li, J.; He, S.; Wei, L.; An, T. A review of bismuth-based photocatalysts for antibiotic degradation: Insight into the photocatalytic degradation performance, pathways and relevant mechanisms. Environ. Res. 2021, 199, 111360. [Google Scholar] [CrossRef]
- Kirubanithy, K.; Jayaraj, S.K.; Beura, R.; Paramasivam, T. Detailed structural and optical studies of the microwave synthesized β-Bi2O3 nanostructured photocatalysts: Photocatalytic applications on anionic and cationic organic dyes. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100629. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Wu, Y.; Shi, W.; Guan, W. Rapid microwave-assisted synthesis of Bi2O3 tubes and photocatalytic properties for antibiotics. Micro Nano Lett. 2013, 8, 177–180. [Google Scholar] [CrossRef]
- Sánchez-Martínez, D.; Juárez-Ramírez, I.; Torres-Martínez, L.M.; de León-Abarte, I. Photocatalytic properties of Bi2O3 powders obtained by an ultrasound-assisted precipitation method. Ceram. Int. 2016, 42, 2013–2020. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef]
- Nugroho, F.G.; Ansari, A.S.; Rochman, N.T.; Khadtare, S.S.; Sree, V.G.; Shrestha, N.K.; Hafiyyan, A.F.; Im, H.; Ahmed, A.T.A. Utilizing Indonesian Empty Palm Fruit Bunches: Biochar Synthesis via Temperatures Dependent Pyrolysis. Nanomaterials 2024, 15, 50. [Google Scholar] [CrossRef]
- Saputra, A.M.A.; Piliang, A.F.R.; Goei, R.; Ramadhan, H.R.; Gea, S. Synthesis, properties, and utilization of carbon quantum dots as photocatalysts on degradation of organic dyes: A mini review. Catal. Commun. 2024, 187, 106914. [Google Scholar] [CrossRef]
- Anpalagan, K.; Yin, H.; Cole, I.; Zhang, T.; Lai, D.T.H. Quantum Yield Enhancement of Carbon Quantum Dots Using Chemical-Free Precursors for Sensing Cr (VI) Ions. Inorganics 2024, 12, 96. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Hoffman, J.; Morgiel, J.; Mościcki, T.; Stobiński, L.; Szymański, Z.; Małolepszy, A. Luminescent Carbon Dots Synthesized by the Laser Ablation of Graphite in Polyethylenimine and Ethylenediamine. Materials 2021, 14, 729. [Google Scholar] [CrossRef]
- Cui, L.; Ren, X.; Wang, J.; Sun, M. Synthesis of homogeneous carbon quantum dots by ultrafast dual-beam pulsed laser ablation for bioimaging. Mater. Today Nano 2020, 12, 100091. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, M.; Kim, H.Y.; Ding, B.; Park, S.-J. A facile ultrasonic-assisted fabrication of nitrogen-doped carbon dots/BiOBr up-conversion nanocomposites for visible light photocatalytic enhancements. Sci. Rep. 2017, 7, 45086. [Google Scholar] [CrossRef]
- Zaib, M.; Akhtar, A.; Maqsood, F.; Shahzadi, T. Green Synthesis of Carbon Dots and Their Application as Photocatalyst in Dye Degradation Studies. Arab. J. Sci. Eng. 2021, 46, 437–446. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.; Guan, W.; Huang, H.; Liu, Y. Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep. Purif. Technol. 2017, 173, 295–303. [Google Scholar] [CrossRef]
- Jusuf, B.N.; Sambudi, N.S.; Isnaeni; Samsuri, S. Microwave-assisted synthesis of carbon dots from eggshell membrane ashes by using sodium hydroxide and their usage for degradation of methylene blue. J. Environ. Chem. Eng. 2018, 6, 7426–7433. [Google Scholar] [CrossRef]
- Jung, H.; Sapner, V.S.; Adhikari, A.; Sathe, B.R.; Patel, R. Recent Progress on Carbon Quantum Dots Based Photocatalysis. Front. Chem. 2022, 10, 881495. [Google Scholar] [CrossRef]
- Alkian, I.; Sutanto, H.; Utami, B.A.; Duri, I.R.; A’yuni, D.Q.; Hadiyanto, H. A Review of Carbon dots (CDs) Application in Sensing and Removing Medical Waste. In Proceedings of the 5th International Conference on Energy, Environmental and Information System (ICENIS 2020), Semarang, Indonesia, 12–13 August 2020; p. 06004. [Google Scholar]
- Aji, M.P.; Wiguna, P.A.; Susanto; Rosita, N.; Suciningtyas, S.A.; Sulhadi, S. Performance of photocatalyst based carbon nanodots from waste frying oil in water purification. In Proceedings of the 3rd International Conference on Advanced Materials Science and Technology (ICAMST 2015), Semarang, Indonesia, 6–7 October 2015; p. 1725. [Google Scholar]
- Prasannan, A.; Imae, T. One-Pot Synthesis of Fluorescent Carbon Dots from Orange Waste Peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Chen, P.; Wang, F.; Chen, Z.-F.; Zhang, Q.; Su, Y.; Shen, L.; Yao, K.; Liu, Y.; Cai, Z.; Lv, W.; et al. Study on the photocatalytic mechanism and detoxicity of gemfibrozil by a sunlight-driven TiO2/carbon dots photocatalyst: The significant roles of reactive oxygen species. Appl. Catal. B Environ. 2017, 204, 250–259. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, Y.; Yang, X.; Li, F.; Li, A.; Liu, Y.; Zhang, J.; Zhou, Z.; Ni, L. Fabricating carbon quantum dots doped ZnIn2S4 nanoflower composites with broad spectrum and enhanced photocatalytic Tetracycline hydrochloride degradation. Mater. Res. Bull. 2018, 97, 158–168. [Google Scholar] [CrossRef]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Lin, Q.; Huang, X.; Zhou, R.; Guo, X.; Xu, W.; Wang, S.; Xu, D.; Chang, H.-T. Electrochemical synthesis of carbon dots with a Stokes shift of 309 nm for sensing of Fe3+ and ascorbic acid. Dye. Pigment. 2021, 185, 108878. [Google Scholar] [CrossRef]
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689. [Google Scholar] [CrossRef]
- Reyes, D.; Camacho, M.; Camacho, M.; Mayorga, M.; Weathers, D.; Salamo, G.; Wang, Z.; Neogi, A. Laser Ablated Carbon Nanodots for Light Emission. Nanoscale Res. Lett. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Chang, K.; Zhu, Q.; Qi, L.; Guo, M.; Gao, W.; Gao, Q. Synthesis and Properties of Nitrogen-Doped Carbon Quantum Dots Using Lactic Acid as Carbon Source. Materials 2022, 15, 466. [Google Scholar] [CrossRef]
- Wan, J.-Y.; Yang, Z.; Liu, Z.-G.; Wang, H.-X. Ionic liquid-assisted thermal decomposition synthesis of carbon dots and graphene-like carbon sheets for optoelectronic application. RSC Adv. 2016, 6, 61292–61300. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Yin, P.; Li, J.; Tang, Y.; Yang, M. Response surface methodology optimization for the synthesis of N, S-codoped carbon dots and its application for tetracyclines detection. Chemosphere 2022, 303, 135145. [Google Scholar] [CrossRef]
- Qi, H.; Huang, D.; Jing, J.; Ran, M.; Jing, T.; Zhao, M.; Zhang, C.; Sun, X.; Sami, R.; Benajiba, N. Transforming waste into value: Pomelo-peel-based nitrogen-doped carbon dots for the highly selective detection of tetracycline. RSC Adv. 2022, 12, 7574–7583. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; He, G.; Li, Z.; He, F.; Gao, F.; Su, Y.; Zhang, L.; Yang, Z.; Zhang, Y. A green heterogeneous synthesis of N-doped carbon dots and their photoluminescence applications in solid and aqueous states. Nanoscale 2014, 6, 10307–10315. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, H.; Alkian, I.; Romanda, N.; Lewa, I.W.L.; Marhaendrajaya, I.; Triadyaksa, P. High green-emission carbon dots and its optical properties: Microwave power effect. AIP Adv. 2020, 10, 055008. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Hafiyyan, A.F.; Lathifah, K.; Rayanisaputri, F.R.H.; Syahidah, S.; Khotimah, R.A.N.; Ahmed, A.T.A.; Ansari, A.S.; Rochman, N.T. Carbon dots based fluorescence sensor for P-nitrophesnol. Mater. Today Proc. 2024, in press. [Google Scholar] [CrossRef]
- Sakdaronnarong, C.; Sangjan, A.; Boonsith, S.; Kim, D.C.; Shin, H.S. Recent Developments in Synthesis and Photocatalytic Applications of Carbon Dots. Catalysts 2020, 10, 320. [Google Scholar] [CrossRef]
- Sahu, Y.; Hashmi, A.; Patel, R.; Singh, A.K.; Susan, A.B.H.; Carabineiro, S.A.C. Potential Development of N-Doped Carbon Dots and Metal-Oxide Carbon Dot Composites for Chemical and Biosensing. Nanomaterials 2022, 12, 3434. [Google Scholar] [CrossRef]
- Bhattacharjee, T.; Konwar, A.; Boruah, J.S.; Chowdhury, D.; Majumdar, G. A sustainable approach for heavy metal remediation from water using carbon dot based composites: A review. J. Hazard. Mater. Adv. 2023, 10, 100295. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Zhang, Z.; Yi, G.; Li, P.; Zhang, X.; Fan, H.; Zhang, Y.; Wang, X.; Zhang, C. A minireview on doped carbon dots for photocatalytic and electrocatalytic applications. Nanoscale 2020, 12, 13899–13906. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, L.; Wu, W.; Wang, S.; Qiang, L. Highly efficient photocatalysis toward tetracycline under simulated solar-light by Ag+-CDs-Bi2WO6: Synergistic effects of silver ions and carbon dots. Appl. Catal. B Environ. 2016, 192, 277–285. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Q.; Liu, P.; Ma, S.; Xie, B.; Yang, K.; Zhao, Y. Novel up-conversion carbon quantum dots/α-FeOOH nanohybrids eliminate tetracycline and its related drug resistance in visible-light responsive Fenton system. Appl. Catal. B Environ. 2020, 263, 118336. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, M.; Yuan, X.; Si, M.; Jiang, L.; Wu, Z.; Wang, H.; Zeng, G. Nitrogen doped carbon quantum dots mediated silver phosphate/bismuth vanadate Z-scheme photocatalyst for enhanced antibiotic degradation. J. Colloid Interface Sci. 2018, 529, 11–22. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, X.; Jiang, L.; Wu, Z.; Chen, X.; Wang, H.; Wang, H.; Zeng, G. Highly efficient photocatalysis toward tetracycline of nitrogen doped carbon quantum dots sensitized bismuth tungstate based on interfacial charge transfer. J. Colloid Interface Sci. 2018, 511, 296–306. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Lu, Y.; Wang, W.; Zhou, Z.; Yan, Y. Constructing carbon dots and CdTe quantum dots multi-functional composites for ultrasensitive sensing and rapid degrading ciprofloxacin. Sens. Actuators B Chem. 2019, 289, 242–251. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Fabrication of a ternary BiOCl/CQDs/rGO photocatalyst: The roles of CQDs and rGO in adsorption-photocatalytic removal of ciprofloxacin. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124758. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Li, W.; Yu, Y.; Liu, M.; Wang, L.; Li, C.; Zhang, X.; Li, X.; Lin, X. Leaf-like BiVO4 nanostructure decorated by nitrogen-doped carbon quantum dots: Binary heterostructure photocatalyst for enhanced photocatalytic performance. Mater. Res. Bull. 2020, 122, 110640. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Xue, J.; Xue, G.; Sheng, X.; Wang, H.; Huo, P.; Yan, Y. CQDS preluded carbon-incorporated 3D burger-like hybrid ZnO enhanced visible-light-driven photocatalytic activity and mechanism implication. J. Catal. 2019, 369, 450–461. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Qian, W.; Hu, W.; Jiang, Z.; Wu, Y.; Li, Z.; Diao, Z.; Li, M. Degradation of Tetracycline Hydrochloride by a Novel CDs/g-C3N4/BiPO4 under Visible-Light Irradiation: Reactivity and Mechanism. Catalysts 2022, 12, 774. [Google Scholar] [CrossRef]
- Amiri, R.; Rezaei, A.; Fattahi, N.; Pirsaheb, M.; Rodríguez-Chueca, J.; Moradi, M. Carbon quantum dots decorated Ag/CuFe2O4 for persulfate-assisted visible light photocatalytic degradation of tetracycline: A comparative study. J. Water Process. Eng. 2022, 47, 102742. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Ma, C.; Zhu, Z.; Liu, Y.; Liu, Z.; Wei, M.; Zhao, X.; Dong, H.; Huo, P.; et al. Bamboo prepared carbon quantum dots (CQDs) for enhancing Bi3Ti4O12 nanosheets photocatalytic activity. J. Alloys Compd. 2018, 752, 106–114. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.; Tung, C.; Zhang, T. Smart Utilization of Carbon Dots in Semiconductor Photocatalysis. Adv. Mater. 2016, 28, 9454–9477. [Google Scholar] [CrossRef] [PubMed]
- Girigoswami, K. Toxicity of Metal Oxide Nanoparticles. Adv. Exp. Med. Biol. 2018, 1048, 99–122. [Google Scholar]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Size-Dependent Effect of Zinc Oxide on Toxicity and Inflammatory Potential of Human Monocytes. J. Toxicol. Environ. Health Part A 2014, 77, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Armaković, S.J.; Armaković, S.; Savanović, M.M. Photocatalytic Application of Polymers in Removing Pharmaceuticals from Water: A Comprehensive Review. Catalysts 2024, 14, 447. [Google Scholar] [CrossRef]
- Zha, Z.; Lai, J.; Li, Y.; Yang, J.; Cui, S.; Li, Y. The degradation of tetracycline by modified BiOCl nanosheets with carbon dots from the chlorella. J. Alloys Compd. 2021, 855, 157454. [Google Scholar] [CrossRef]



| Pollutant (Formula) | Uses | Concentration in WWTPs # | PNECres (µg/L) | PNECeco (µg/L) | |
|---|---|---|---|---|---|
| Influent (µg/L) | Effluent (µg/L) | ||||
| Amoxicillin () | Bacterial infections, and dental abscesses | 0–6.94 | 0–0.0625 | 0.25 | 0.57 |
| Ciprofloxacin () | Treatment of mild-to-moderate infections of the urinary and respiratory tracts induced by sensitive microorganisms | 8.2–4540 | 0–31,000 | 0.023 | 0.45 |
| Norfloxacin () | Curing of urinary tract contagions and prostatitis | 1.11–18.2 | 85–420 | 0.5 | 120 |
| Levofloxacin ( | Therapy for urinary tract infection | 86.7 | 0–300 | 0.25 | 1.52 |
| Tetracycline () | Functioning as an antiprotozoal, antibacterial, and antimicrobial agent; protein synthesis inhibitor; and metabolite of Escherichia coli | 0–48 | NA | 0.1 | 3.2 |
| Water Treatment Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Coagulation | Coagulants function to clump suspended particles together into larger particles. |
|
|
| Ion exchange | Replace unwanted ions with more desirable ions, employing a specialized resin. |
|
|
| Membrane filtration | Physical procedure employing synthetic membranes for separating chemicals. |
|
|
| Electrochemical treatment | Use of electrical current to facilitate chemical reactions that remove contaminants from water. This method typically utilizes electrodes immersed in water, where oxidation and reduction reactions occur at the electrode surfaces. |
|
|
| Electrodialysis | Uses an electric field to selectively remove ions from water. It involves passing water through a series of alternating ion-exchange membranes and applying an electric current. |
|
|
| Reverse osmosis | Uses a semi-permeable membrane to remove contaminants from water. |
|
|
| Adsorption | A mass transfer process of accumulation of chemicals from the liquid phase into the solid phase of adsorbent. |
|
|
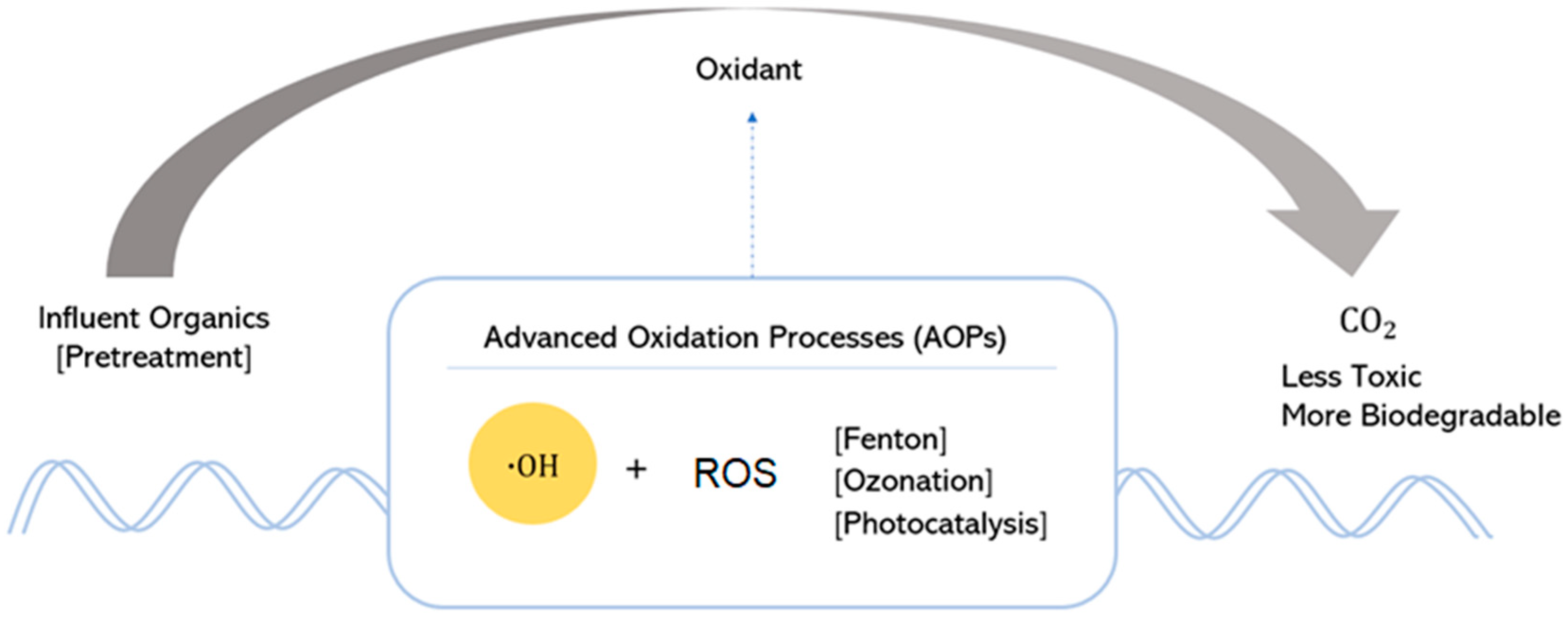
| Advanced oxidation processes | Processes based on the utilization of highly reactive chemical species that are efficient in oxidizing and degrading organic compounds. |
|
|
| AOPs | ROS | Description | Advantages | Disadvantages |
|---|---|---|---|---|
| Fenton-based | Fe(OOH)+ FeO− •OH •OOH | ) with iron salts (typically ferrous iron, Fe2+). |
|
|
| Ozonation | O2•− O3•− | ), which are highly reactive and capable of oxidizing contaminants. |
|
|
| Electrochemical | Utilizes electrodes immersed in water to induce oxidation and reduction reactions, facilitating the breakdown of organic contaminants and pathogens. |
|
| |
| Photocatalysis | •OOH | ) with significant oxidizing potential. |
|
|
| Morphology | Metal Oxide | Methods | Precursors | Experimental Conditions | Size | Surface Area (m2/g) | Ref. |
|---|---|---|---|---|---|---|---|
| Nanosphere | ZnO | Solvothermal | Zn(CH3COO)2∙2H2O, AgNO3 | T = 160 °C pH = 9.3 | Diameter: 400 nm to 500 nm | [62] | |
| NH3 evaporation | Zn(NO3)2, NH4OH | Evaporation = 50 °C Precipitation = 125 °C pH = 5.4–8.0 | Diameter: 80 nm to 130 nm | 66.12 | [63] | ||
| TiO2 | Hydrothermal | Ti(SO4)2, glucose | T = 180 °C | Diameter: 300 nm to 500 nm | 76 | [64] | |
| CuO | Ultrasound-assisted | Cu(CH3COO).2H2O, CH4ON2 | T = 80 °C pH = 5.94 to 5.08 | Diameter: 400 nm to 500 nm | 59.60 | [65] | |
| Nanorods | ZnO | Hydrothermal | (Zn(Ac)2), Ce(NO3)2.6H2O | T = 130 °C pH = 10 | Length: 900 nm to 3 μm Diameter: 70 nm to 85 nm | 2.8 | [66] |
| Hydrothermal | Zn(Ac)2.2H2O, Ni(Ac)2.4H2O | T = 140 °C | Diameter: 15 nm to 20 nm Length: 150 nm to 400 nm | [67] | |||
| Hydrothermal | (CH3CO2)2Zn·2H2O, (CH3CO2)2Co·4H2O | T = 220 °C | Length: 0.2 μm to 1.5 μm Diameter: 90 nm | [41] | |||
| Ultrasound-assisted | Zn(CH3COO)2.2H2O, NaOH, ionic liquid [C4mim][Tf2N] | T = 25 °C | Diameter: 20 nm Length: 50 nm to 100 nm | 49.93 | [68] | ||
| CuO | Hydrothermal | Cu(NO3)2·2.5H2O, C6H12N4 | Low temperature | Length: 10 nm to 20 nm Diameter: - | [69] | ||
| WO3 | Wet chemicals | Na2WO4.2H2O | T = 180 °C pH = 1.5 | Length: 2 μm Diameter: 2 μm | [70] | ||
| TiO2 | Hydrothermal | Ti(OBu)4, KMnO4, HCl, isopropanol | T = 80 °C pH = 1.3 | [71] | |||
| Hydrothermal | TiO2, NaOH | T = 150 °C Calcination = 600 °C pH = 6.5 | Diameter: 7 nm | 49.6 to 64.2 | [42] | ||
| Hydrothermal | Na2O7Ti3 | T = 180 °C pH = 0, 0.5, 1, 2, 7 rpm = 0, 500, 900 | Diameter: 5 nm Length: 100 nm to 1200 nm | 26.67 to 109.81 | [72] | ||
| Nanowires | ZnO | Hydrothermal | Zinc foil, C2H8N2 | T = 170 °C | Length: 3 um Diameter: 120 nm | [73] | |
| Solvothermal | (Zn(Ac)2·2H2O), (La(NO3)3·6H2O) | T = 150 °C Alkaline condition | Diameter: 15 nm to 25 nm | [74] | |||
| Hydrothermal | ZnSO4, NH4Cl | T = 60 °C pH = 11.7 | Diameter: 50 nm to 200 nm Length: 5 μm to 6 μm | [75] | |||
| WO3 | Solvothermal Thermal reaction | WCl6, cyclohexanol, NH3 pure gas | T = 200 °C (solvothermal) 923 K (thermal reaction), 400 sccm min−1 | Diameter: 5 nm | 151 | [76] | |
| TiO2 | Hydrothermal | TiO2, NaOH, KOH, NH4OH | T = 180 °C Neutral pH | Diameter: 25 nm to 30 nm Length: 54.35 nm | [77] | ||
| Hydrothermal | TiO2, NaOH | Hydrothermal = 150 °C Calcination = 500 °C pH = 6.5 | Diameter: 6 nm | 90.5 to 111.1 | [42] | ||
| Hydrothermal | TiO2 P25, NaOH/KOH/NH3 | Hydrothermal = 180 °C Calcination = 450 °C pH = 7 | Diameter: 5 nm to 20 nm Length: 74 nm to 367 nm | 143.42 to 228.34 | [78] | ||
| Nanotube | ZnO | Co-precipitation | (Zn(CH3COO)2·2H2O, Cu(CH3COO)2·H2O, (HOCH2CH2)3N | T = 92 °C pH = 6.5 | Length: 2.1 μm Diameter: 0.21 μm | [79] | |
| Hydrothermal | ZnCl2, NH3 | T = 95 °C | Diameter: 250 nm Length: 500 nm | [80] | |||
| TiO2 | Electrochemical | Ti | Voltage = 20–50 V | Length: - Diameter: 40 nm to 75 nm | [81] | ||
| Hydrothermal | TiO2, NaOH | Hydrothermal = 150 °C Calcination = 400 °C pH = 6.5 | Outer diameter: 9 nm to 15 nm Inner diameter: 4 nm to 7 nm | 146.3 to 189.6 | [42] | ||
| CuO | Microwave-assisted | Cu(CH3COO)2.H2O | Calcination = 100 °C pH = 7 | Mean crystallite size: 14 nm | 55.90 | [82] | |
| SnO2 | Hydrothermal | SnCl2⋅2H2O, HCl | T = 200 °C pH = 7 | Diameter: 6.6 nm | 61.99 | [83] | |
| Nanoflower | ZnO | Microwave-assisted | Zn(CH3COO)2.2H2O, KOH | T = 180 °C | Length: 600 nm to 800 nm Diameter: 150 nm to 200 nm | 31.75 | [84] |
| Hydrothermal | Zn(Ac)2·2H2O, SDS | T = 100 °C | Diameter: 10 μm to 12 μm | 22.7 | [85] | ||
| Sol–gel | Zn(NO3)2·6H2O, citric acid | T = 100 °C pH = 14 | Diameter: 2 μm to 3 μm | 11.05 | [86] | ||
| TiO2 | Hydrothermal | Ti(OBu)4, acetic acid | T = 140 °C | Length: 10 nm Diameter: 4.5 μm | 79.2 | [87] | |
| Hydrothermal | Ti, NaOH, H2O2, HNO3 | T = 150 °C pH = 7 | - | 134.7 | [88] | ||
| Hydrothermal | Ti(OBu)4, HCl | T = 180 °C | Diameter: 800 nm | 68 to 185 | [89] | ||
| CuO | Microwave-assisted | Cu(CH3COO)2. H2O, NaOH, HMT | T = 90 °C | Mean crystallite size: 12 nm | 65.34 | [82] |
| Method | Carbon Sources | Application | Ref |
|---|---|---|---|
| Laser ablation | Graphite | [128] | |
| Polyvinylpyrrolidone (PVP) | |||
| Dimethylsulfoxide (DMSO) | [129] | ||
| Ultrasonication | Citric acid | Removal of methylene blue | [130] |
| Elettaria cardamomum | Removal of methylene blue and Congo red | [131] | |
| Electrochemical | Graphite | Removal of tetracycline | [132] |
| Microwave-assisted | Egg shell | Removal of methylene blue | [133] |
| D-glucose | Removal of methyl orange | [134] | |
| Sugar cane juice | Removal of methylene blue | [134] | |
| Citric acid | Removal of ciprofloxacin | [135] | |
| Hydrothermal | Waste frying oil | Removal of methylene blue | [136] |
| Orange peels | Removal of naphthol black | [137] | |
| Lemon peel waste | Removal of methylene blue | [138] | |
| Citric acid | Removal of gemfibrozil | [139] | |
| L-ascorbic acid | Removal of levofloxacin | [10] | |
| Citric acid | Removal of tetracycline | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, A.S.; Azzahra, G.; Nugroho, F.G.; Mujtaba, M.M.; Ahmed, A.T.A. Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal. Catalysts 2025, 15, 134. https://doi.org/10.3390/catal15020134
Ansari AS, Azzahra G, Nugroho FG, Mujtaba MM, Ahmed ATA. Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal. Catalysts. 2025; 15(2):134. https://doi.org/10.3390/catal15020134
Chicago/Turabian StyleAnsari, Abu Saad, Griszha Azzahra, Fairuz Gianirfan Nugroho, Momin M. Mujtaba, and Abu Talha Aqueel Ahmed. 2025. "Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal" Catalysts 15, no. 2: 134. https://doi.org/10.3390/catal15020134
APA StyleAnsari, A. S., Azzahra, G., Nugroho, F. G., Mujtaba, M. M., & Ahmed, A. T. A. (2025). Oxides and Metal Oxide/Carbon Hybrid Materials for Efficient Photocatalytic Organic Pollutant Removal. Catalysts, 15(2), 134. https://doi.org/10.3390/catal15020134







