Green Catalysts and/or Green Solvents for Sustainable Multi-Component Reactions
Abstract
1. Introduction
2. Multi-Component Reactions (MCRs)
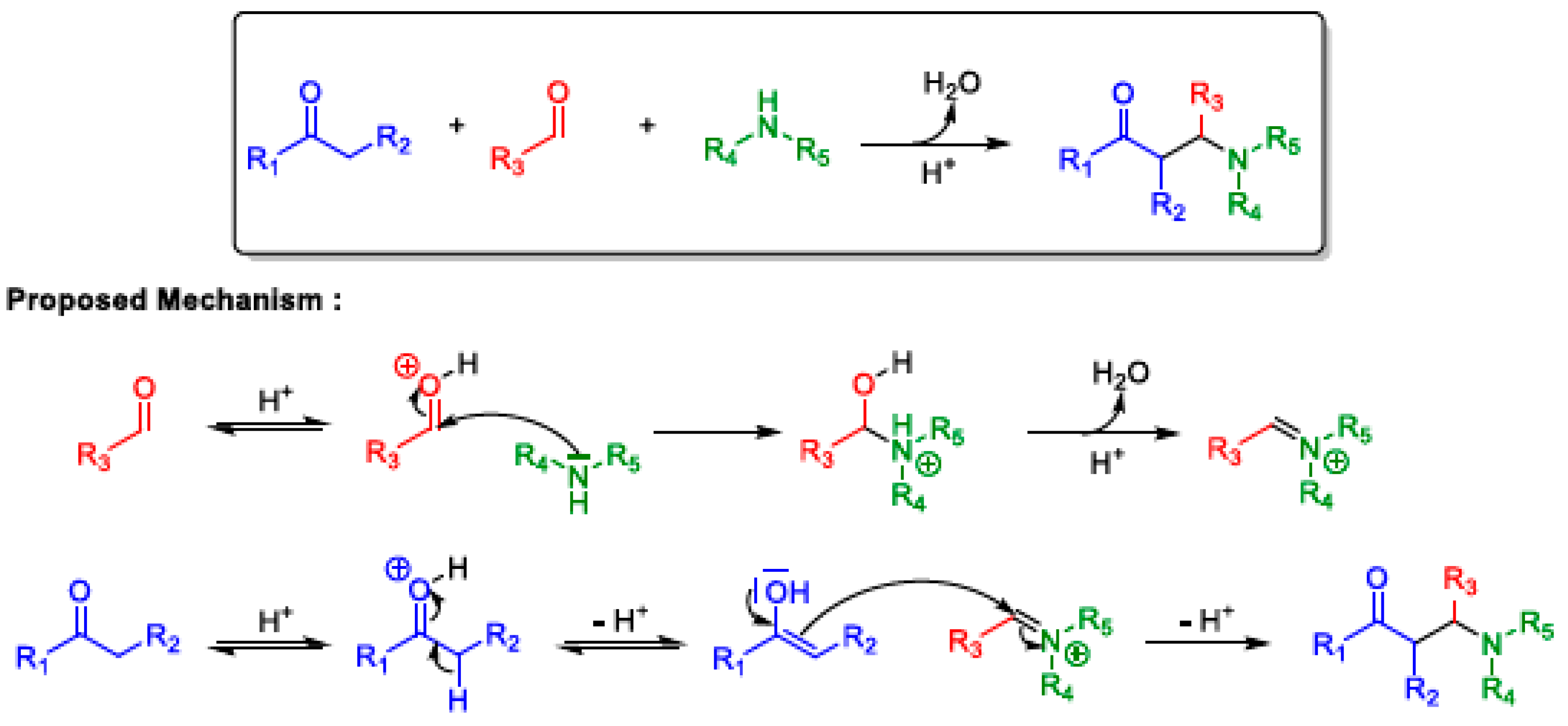
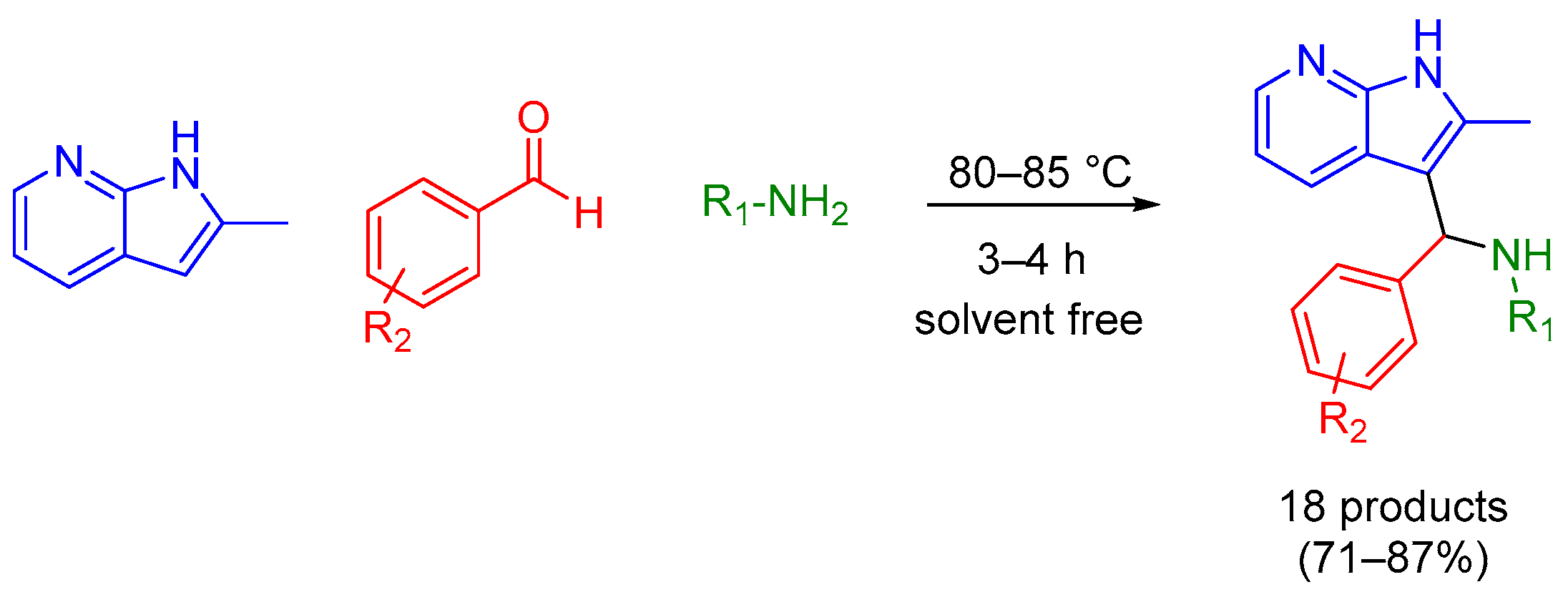
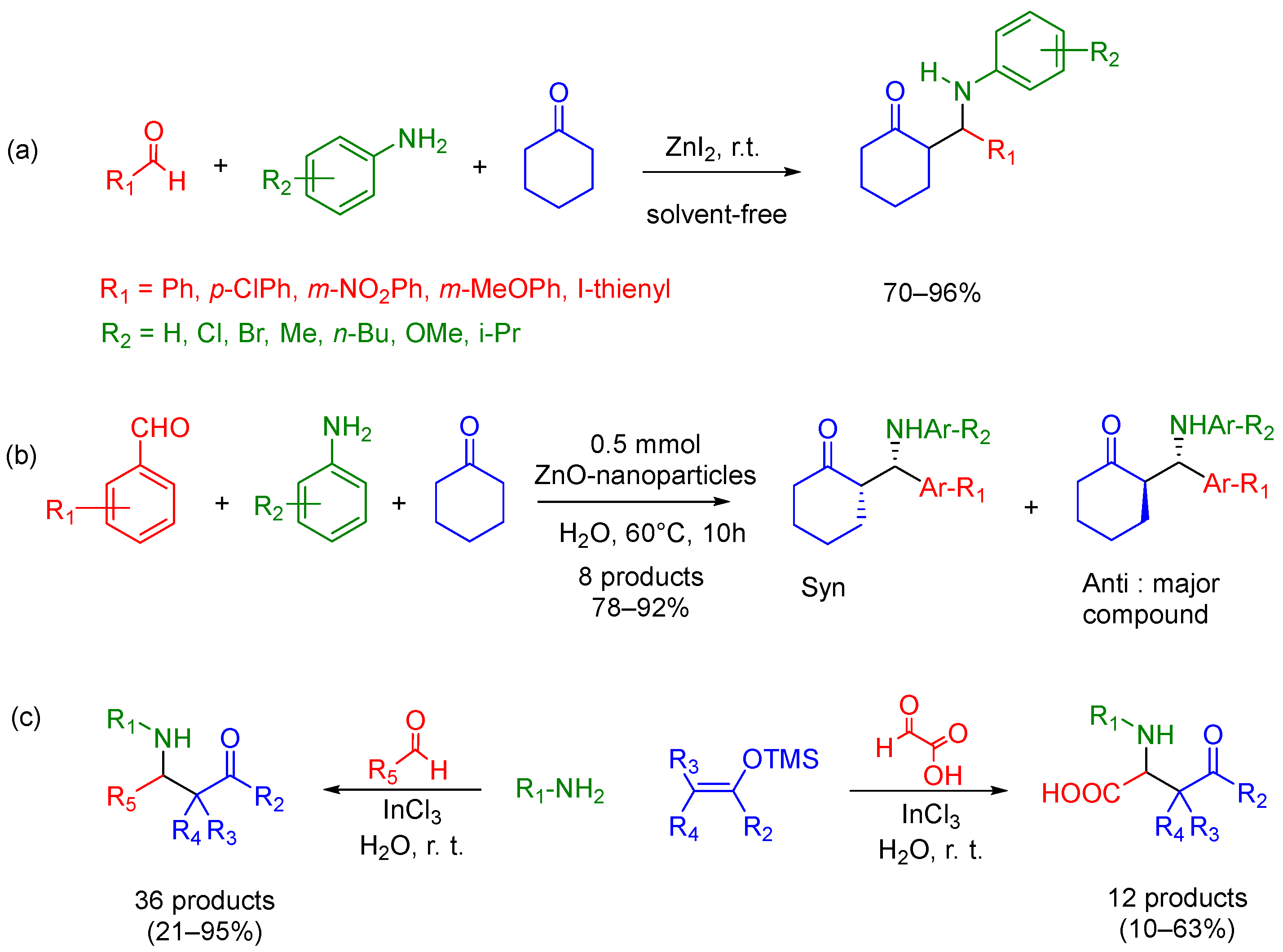
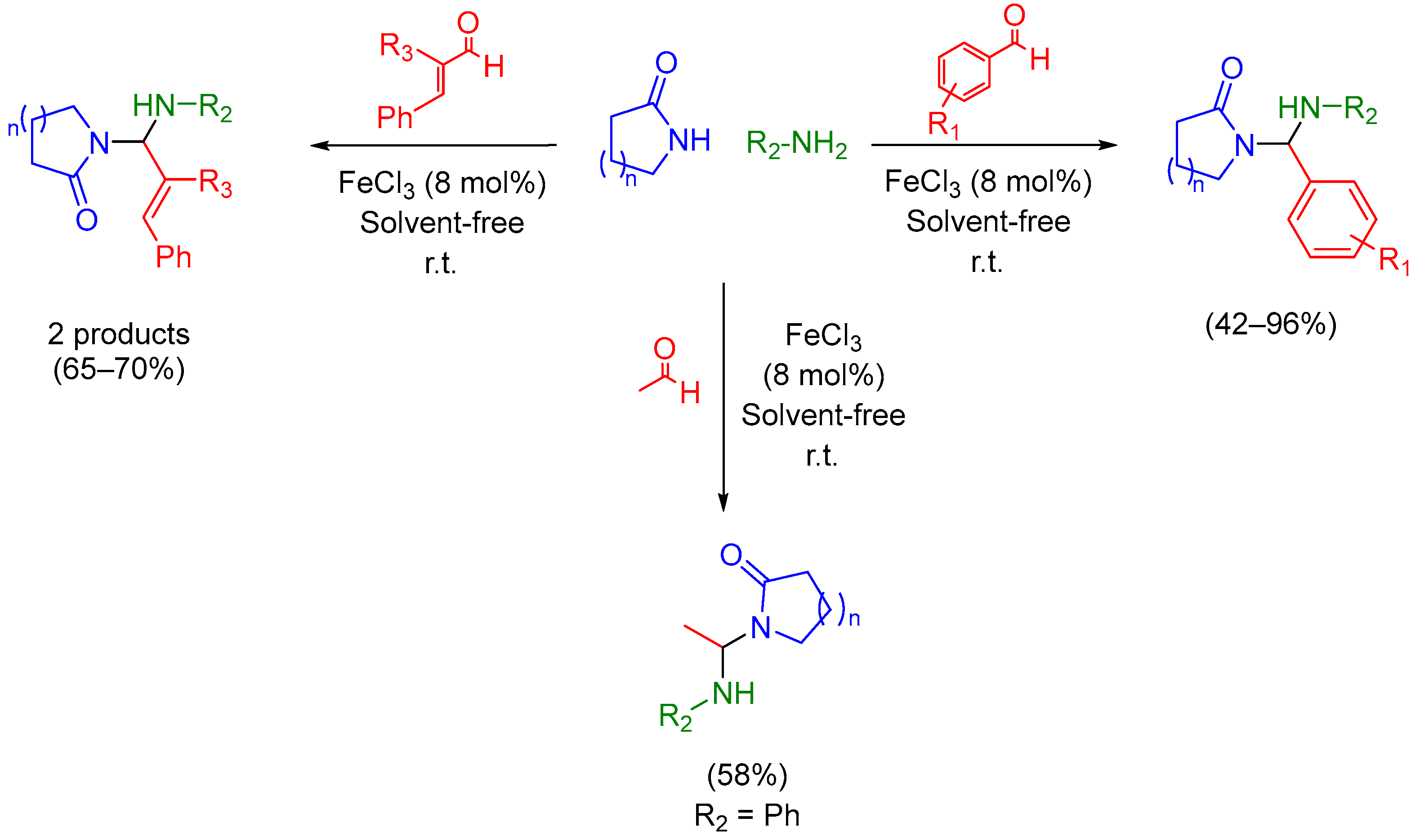
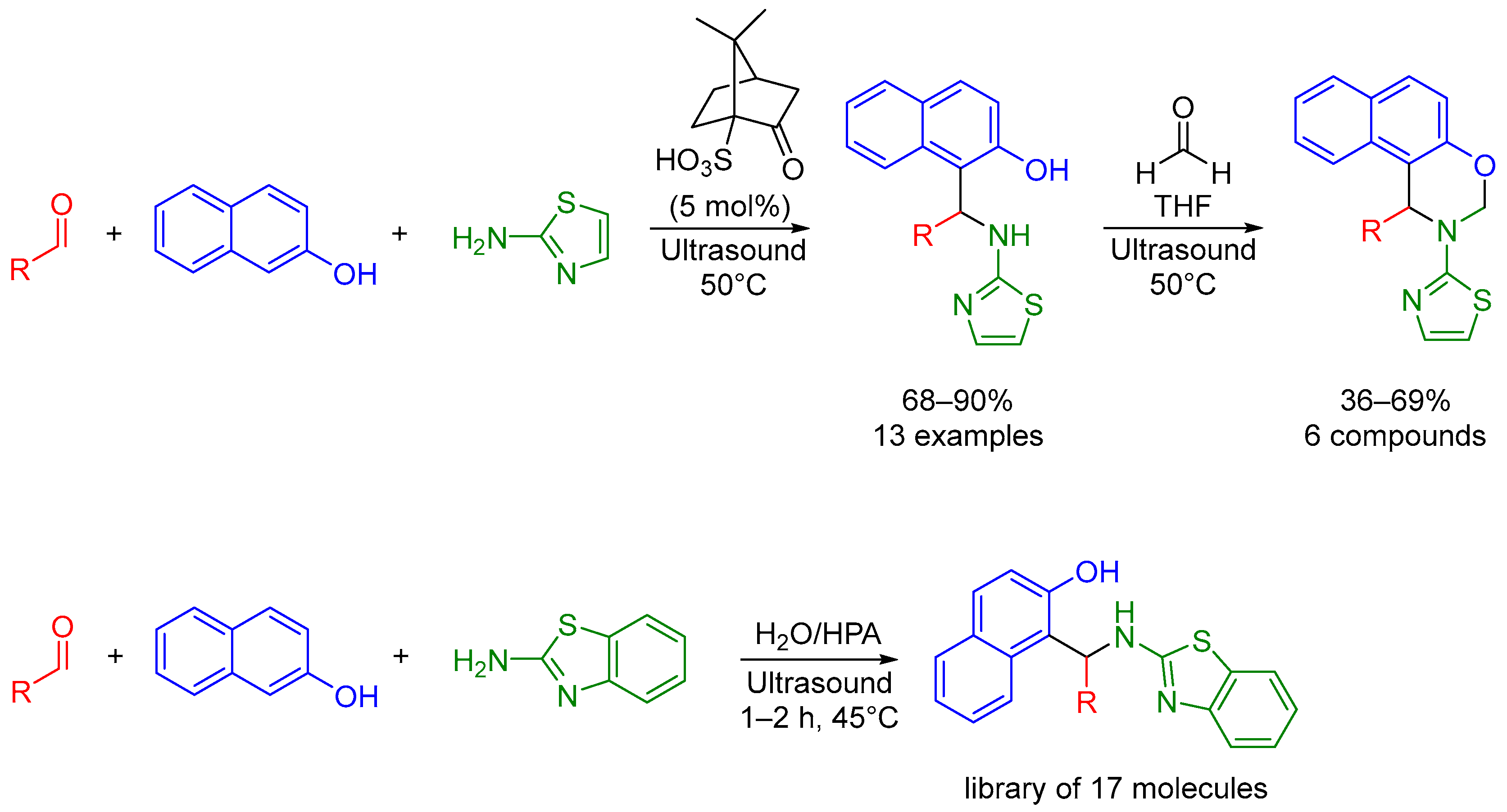
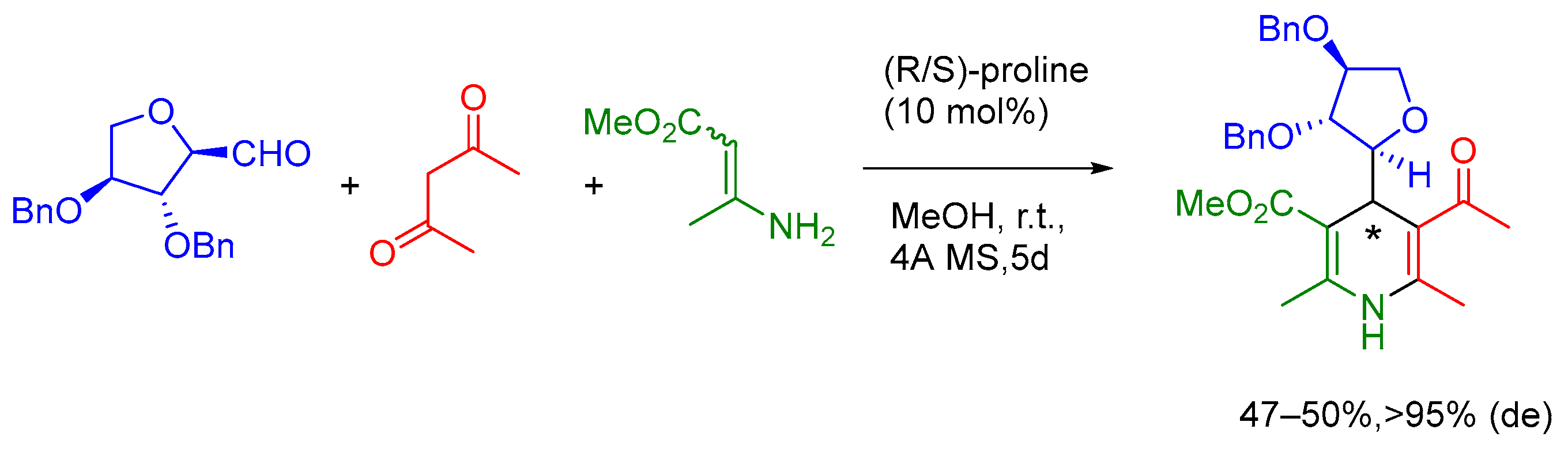
2.1. Mannich 3-MCR
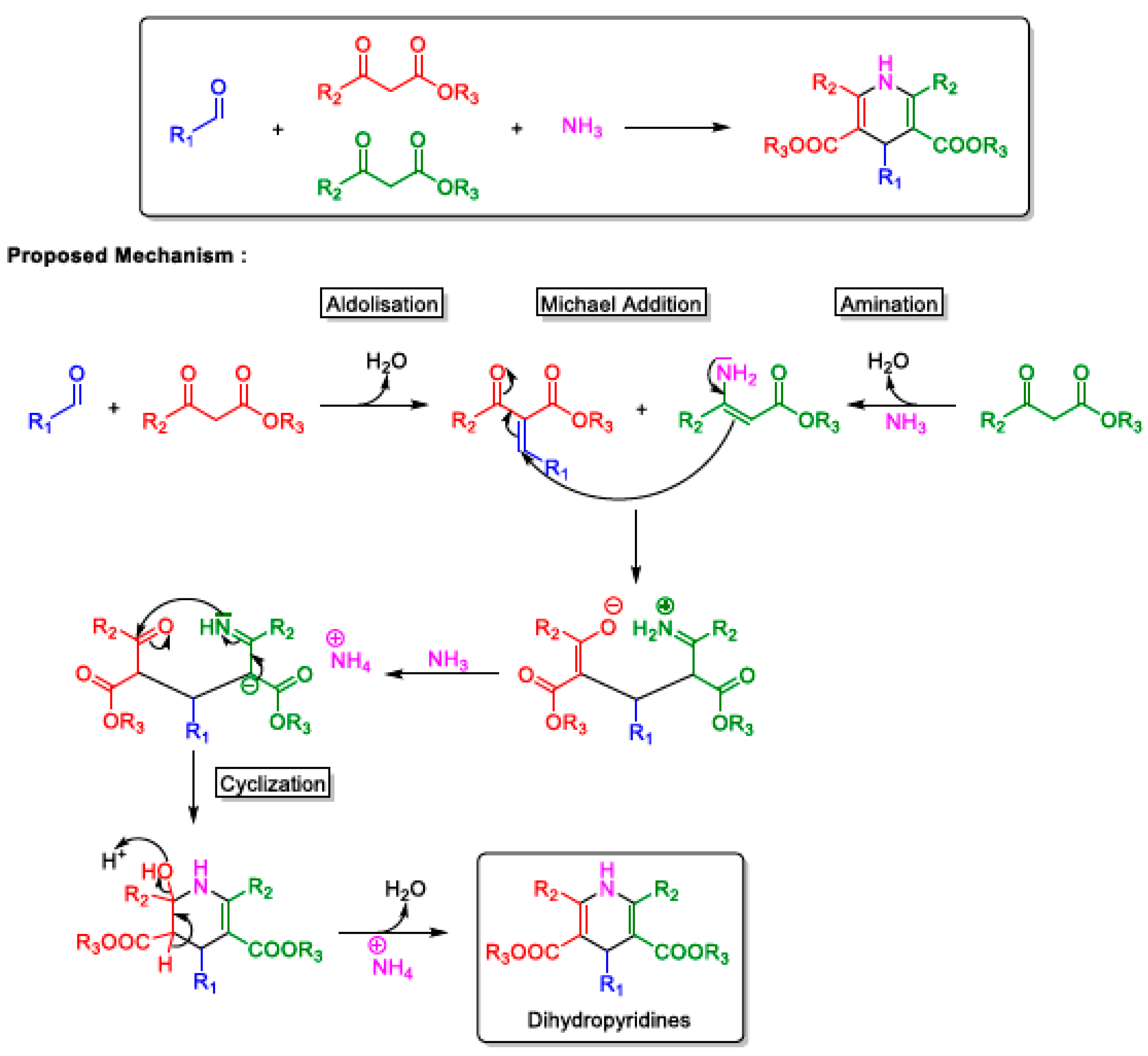
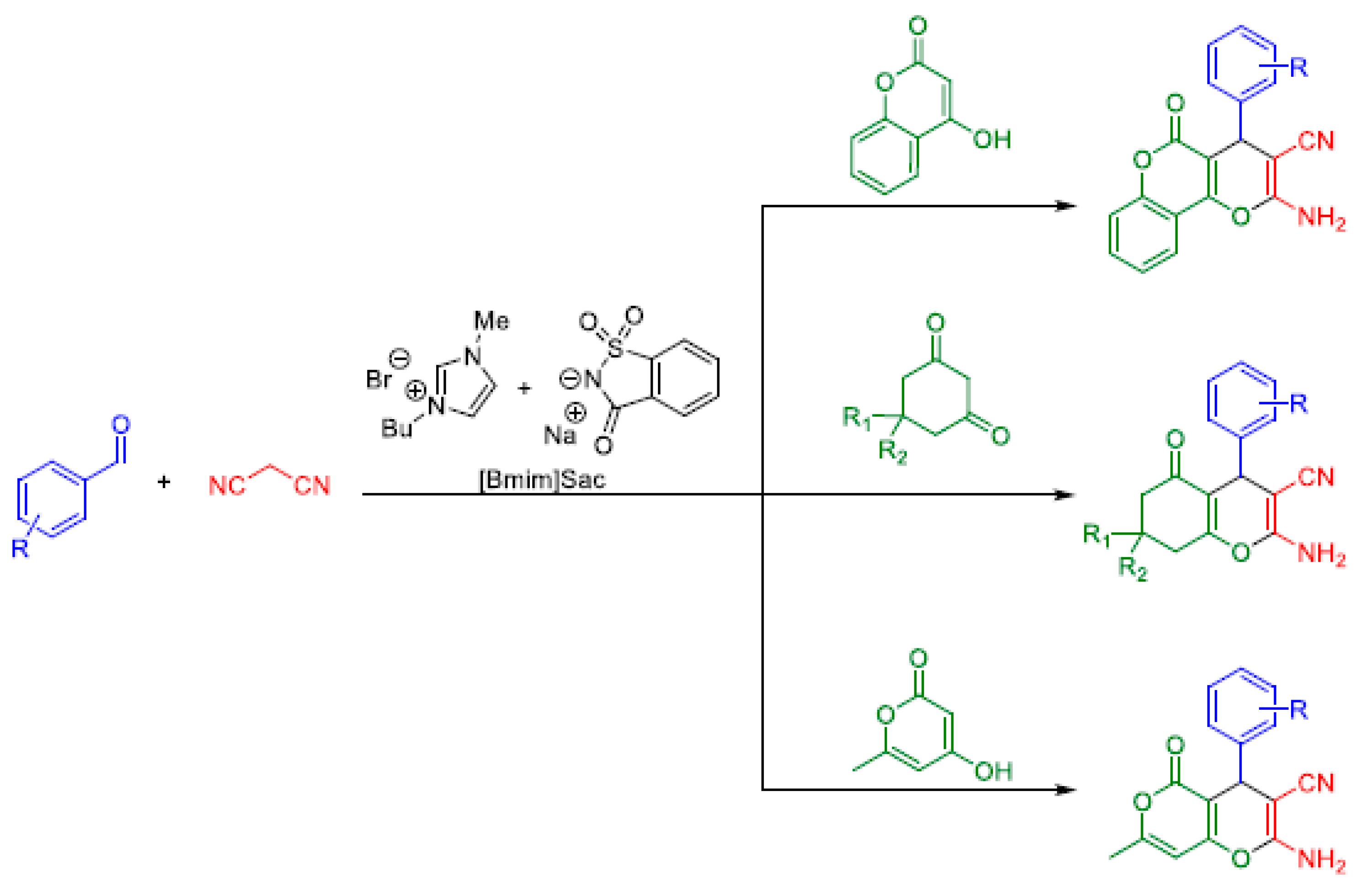
2.2. Hantzsch MCRs
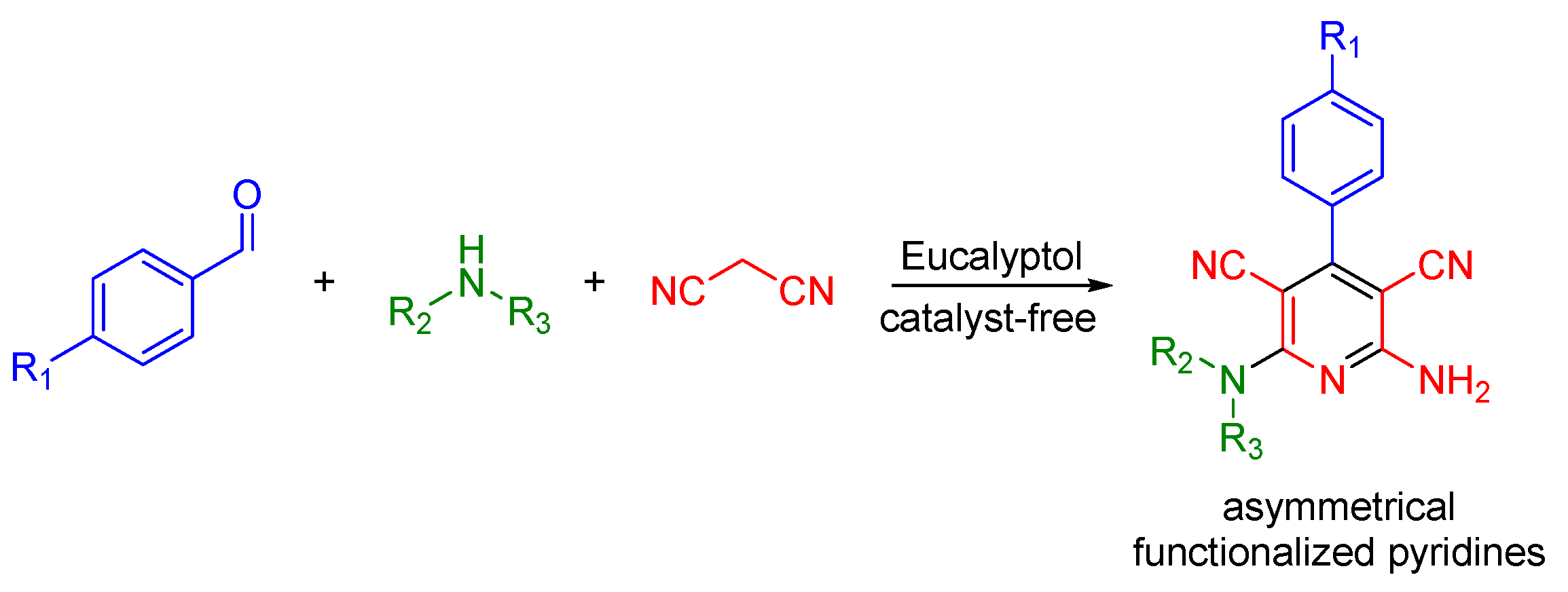
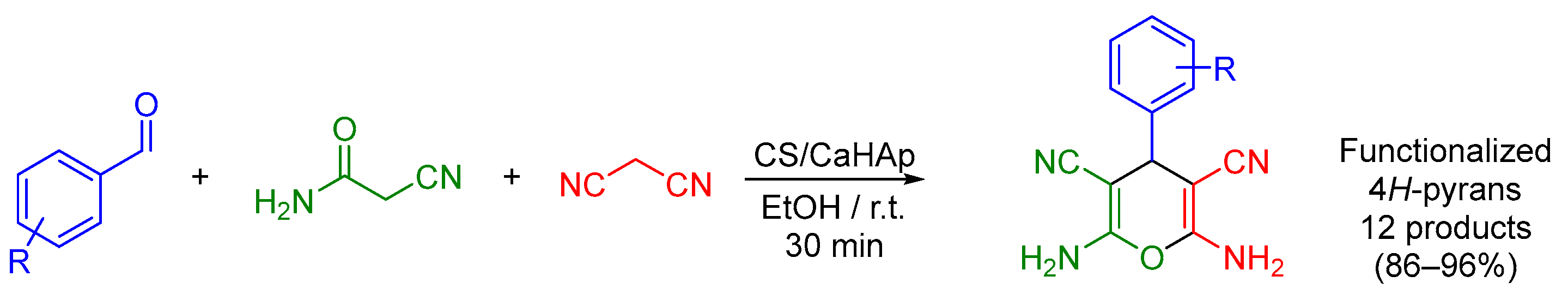
Variants of the Hantzsch MCR
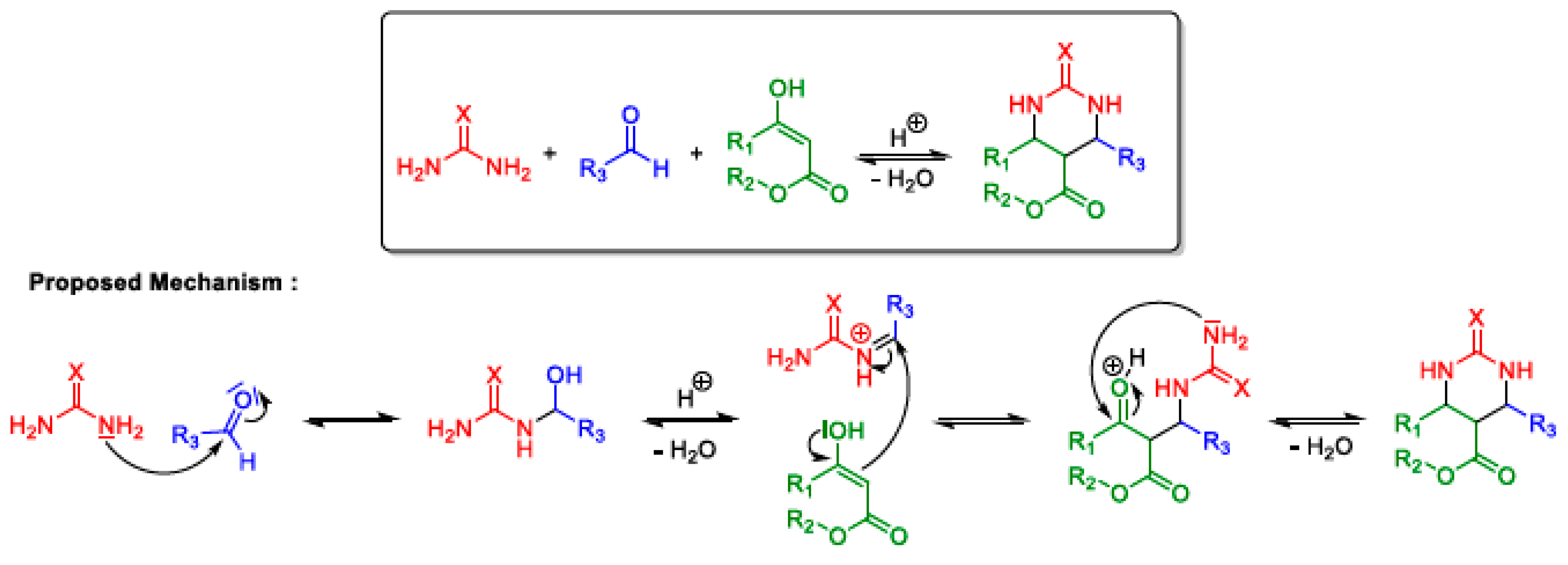
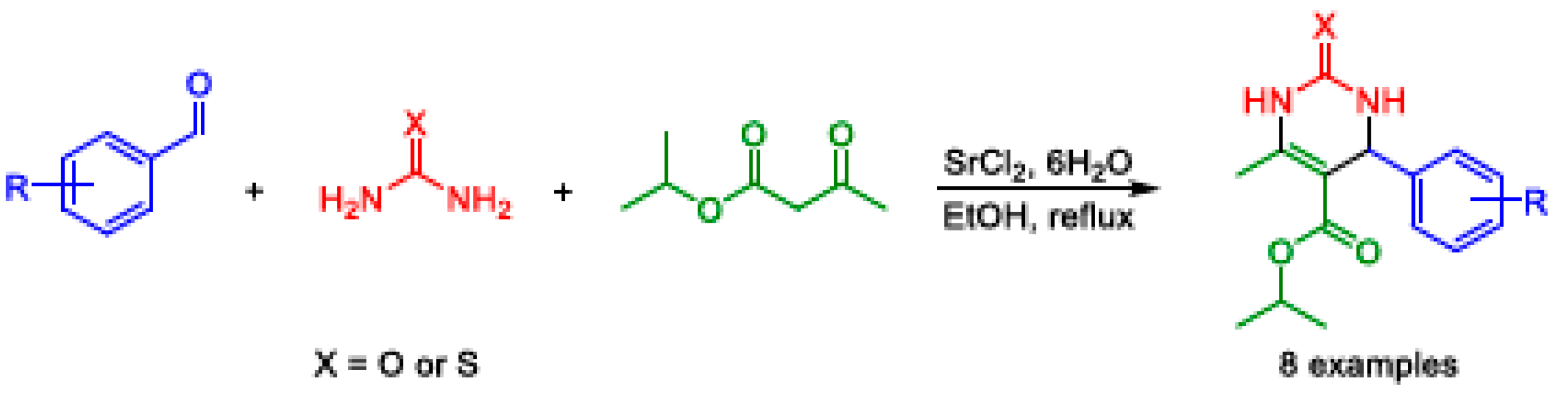
2.3. Biginelli MCR
2.4. Ugi MCR
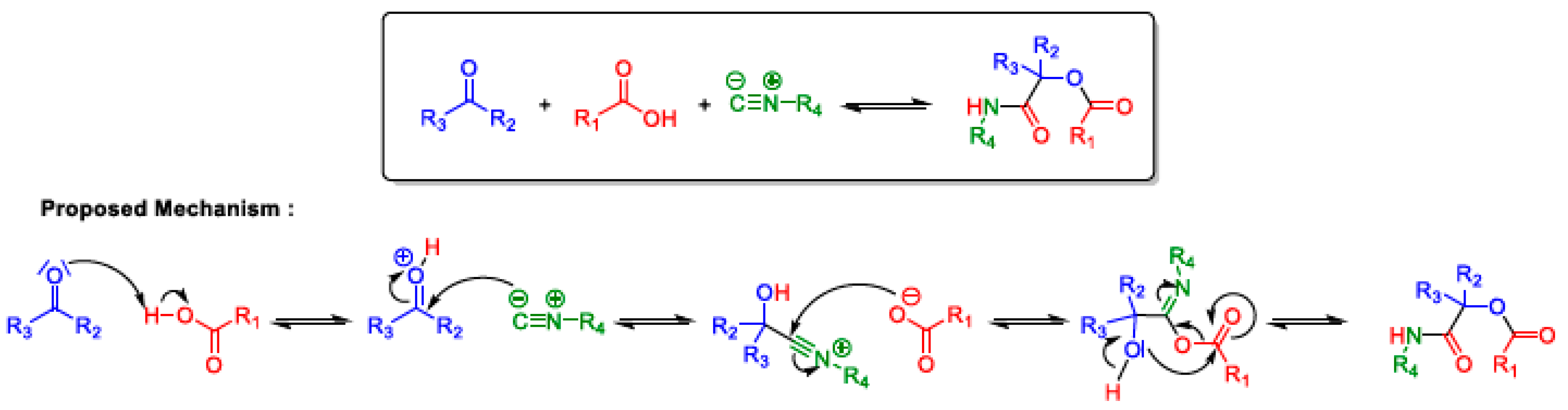
2.5. Passerini MCR
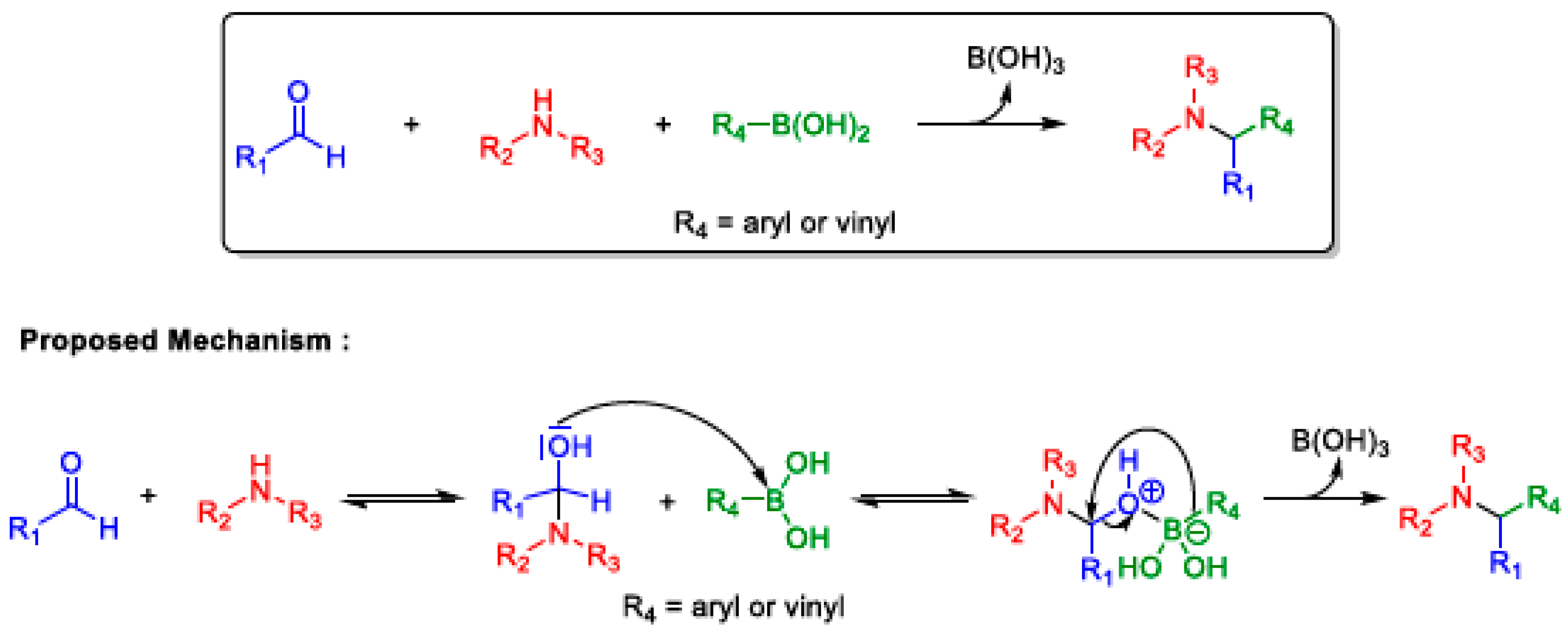
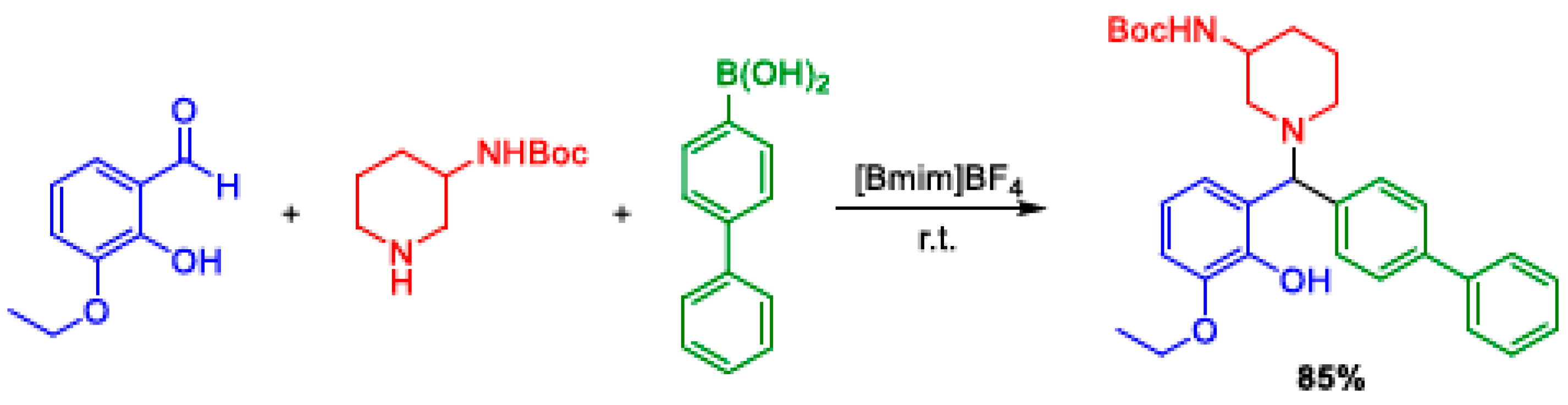
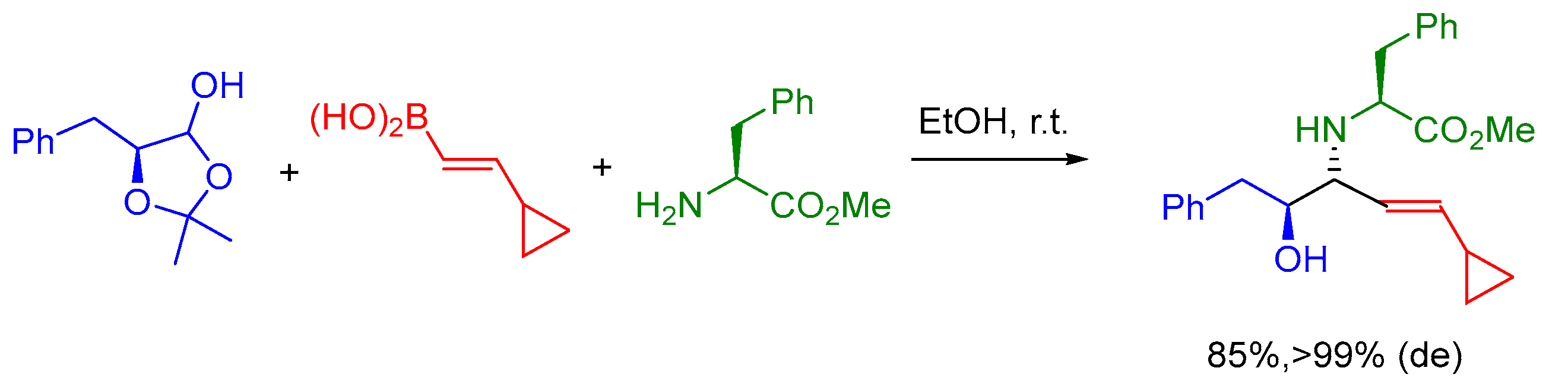
2.6. Petasis MCR
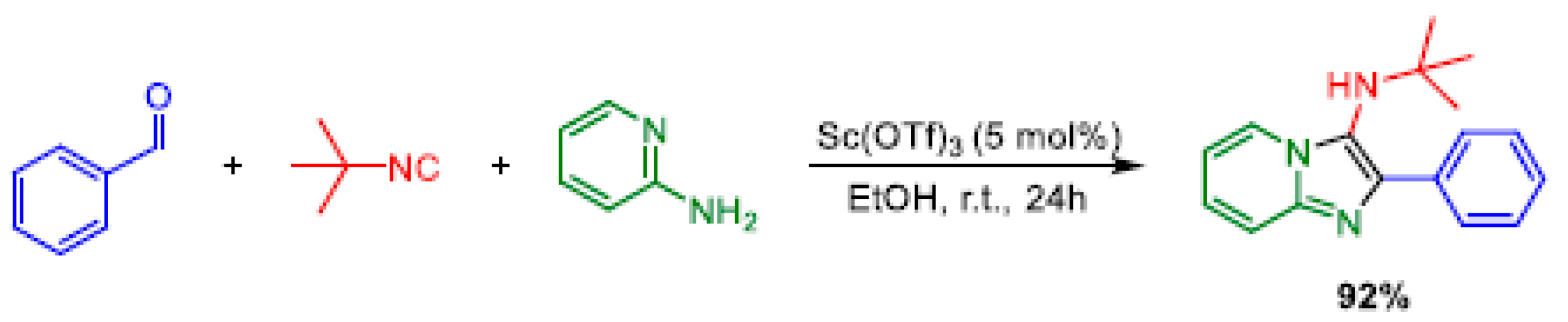
2.7. Groebke-Blackburn-Bienaymé: GBB-3-CR
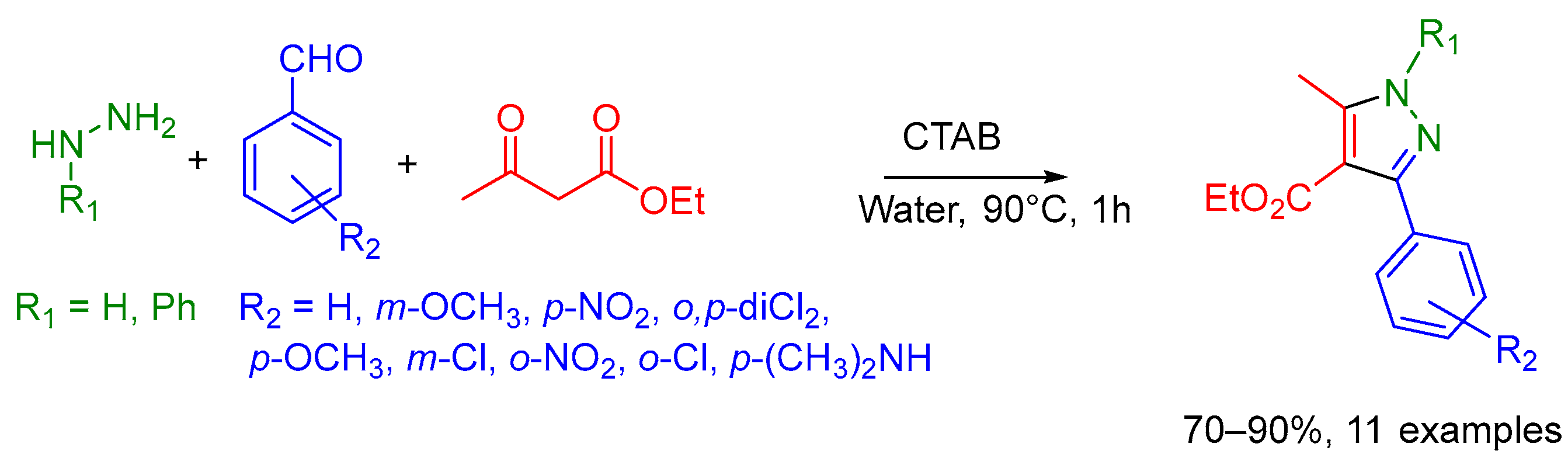
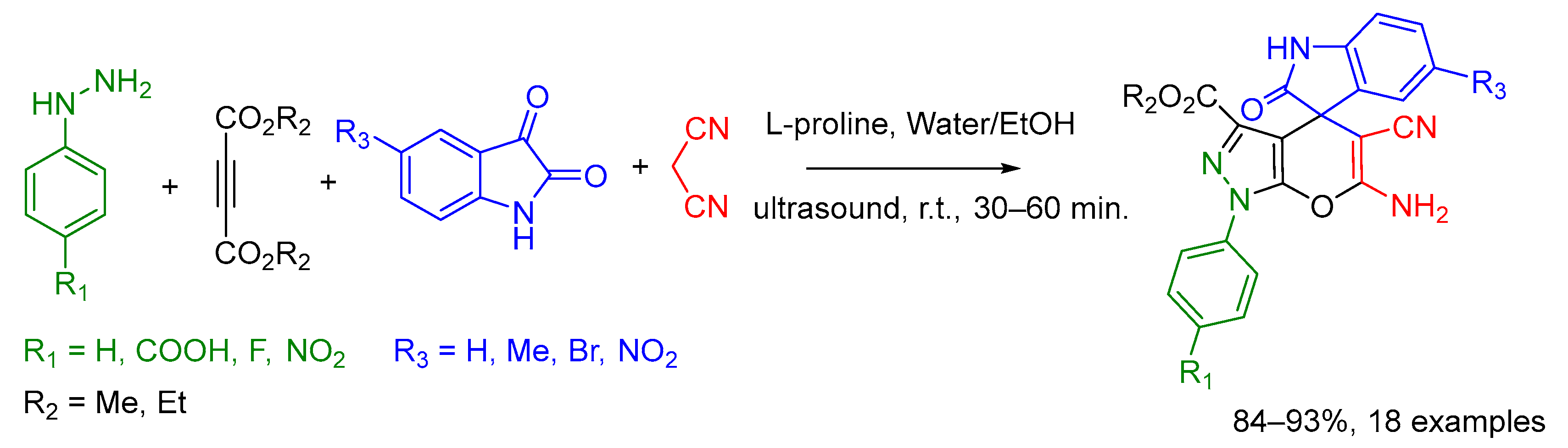
2.8. Others Examples of Undefined MCR
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dömling, A.; Ugi, I. Multicomponent Reactions with Isocyanides. Angew. Chem. Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid-state peptide synthesis. I. Synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Pellissier, H. Stereocontrolled Domino Reactions. Chem. Rev. 2013, 113, 442–524. [Google Scholar] [CrossRef]
- Zhu, J.; Bienayme, H. Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Orru, R.V.A.; Ruijter, E. Synthesis of Heterocycles via Multicomponent Reactions. In Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2010; Volumes I and II. [Google Scholar]
- Mannich, C.; Krösche, W. Ueber ein Kondensationsprodukt aus Formaldehyd, Ammoniak und Antipyrin. Arch. Pharm. 1912, 250, 647–667. [Google Scholar] [CrossRef]
- Filho, J.F.A.; Lemos, B.C.; de Souza, A.S.; Pinheiro, S.; Greco, S.J. Multicomponent Mannich reactions: General aspects, methodologies and applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar] [CrossRef]
- Hantzsch, A. Ueber die Synthese pyridinartiger Verbindungen aus Acetessigaẗher und Aldehydammoniak. Justus Liebigs. Ann. Chem. 1882, 215, 1–82. [Google Scholar] [CrossRef]
- Debache, A.; Boulcina, R.; Belfaitah, A.; Rhouati, S.; Carboni, B. One-Pot Synthesis of 1,4-Dihydropyridines via a Phenylboronic Acid Catalyzed Hantzsch Three-Component Reaction. Synlett 2008, 2008, 509–512. [Google Scholar] [CrossRef]
- Biginelli, P. Ueber Aldehyduramide des Acetessigaẗhers. Eur. J. Inorg. Chem. 2006, 24, 1317–1319. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Pinheiro, D.L.J.; Carvalho-Silva, V.H.; Fioramonte, M.; Gozzo, F.C.; da Silva, W.A.; Amarante, G.W.; Neto, B.A.D. Combined Role of the Asymmetric Counteranion-Directed Catalysis (ACDC) and Ionic Liquid Effect for the Enantioselective Biginelli Multicomponent Reaction. J. Org. Chem. 2018, 83, 12143–12153. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versammlungs- berichte. Angew. Chem. 1959, 71, 373–388. [Google Scholar] [CrossRef]
- Van der Heijden, G.; Jong, J.A.W.S.; Ruijter, E.; Orru, R.V.A. 2-Bromo-6-isocyanopyridine as a Universal Convertible Isocyanide for Multicomponent Chemistry. Org. Lett. 2016, 18, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Passerini, M. Sopra gli isonitrili (I). Composto del p-isonitril- azobenzolo con acetone ed acido acetico. Gazz. Chim. Ital. 1921, 51, 126–129. [Google Scholar]
- Yang, K.; Zhang, F.; Fang, T.; Li, C.; Li, W.; Song, Q. Passerini- type Reaction of Boronic Acids Enables α-hydroxyketones Synthesis. Nat. Commun. 2021, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Givskov, M.; Nielsen, T.E. Reactivity and Synthetic Applications of Multicomponent Petasis Reactions. Chem. Rev. 2019, 119, 11245–11290. [Google Scholar] [CrossRef]
- Boltjes, A.; Dömling, A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019, 7007–7049. [Google Scholar] [CrossRef]
- Dongare, S.B.; Chavan, H.V.; Bhale, P.S.; Mule, Y.B.; Kotmale, A.S.; Bandgar, B.P. A catalyst- and solvent-free multicomponent synthesis of 7-azagramine analogues via a Mannich type reaction. Chin. Chem. Lett. 2016, 27, 99–103. [Google Scholar] [CrossRef]
- Abonia, R.; Castillo, J.C.; Garay, A.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; D’Vries, R. A facile synthesis of stable β-amino-N-/O-hemiacetals through a catalyst-free three-component Mannich-type reaction. Tetrahedron Lett. 2017, 58, 1490–1494. [Google Scholar] [CrossRef]
- Yang, Z.-K.; Chen, Q.-H.; Wang, F.-P. Construction of AE ring system for the C19-diterpenoid alkaloids with a 5β-hydroxyl group. Tetrahedron 2011, 67, 4192–4195. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, C.; Liu, M.; Chu, Y.; Zhou, L.; Hu, C.; Ye, D. First total synthesis of (+)-Carainterol A. Tetrahedron Lett. 2010, 51, 1870–1872. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Gurung, L.; Jog, P.V.; Tanaka, S.; Thomas, T.E.; Ganesh, N.; Haiges, R.; Mathew, T.; Olah, G.A. Direct Synthesis of Diverse β-Fluoroethylamines by a Multicomponent Protocol. Chem. Eur. J. 2013, 19, 3579–3583. [Google Scholar] [CrossRef]
- Salter, M.M.; Kobayashi, J.; Shimizu, Y.; Kobayashi, S. Direct-Type Catalytic Three-Component Mannich Reactions Leading to an Efficient Synthesis of α,β-Diamino Acid Derivatives. Org. Lett. 2006, 8, 3533–3536. [Google Scholar] [CrossRef]
- Garib, A.; Pesyan, N.N.; Vojdanifard, I.; Jahangir, M.; Roshani, M.; Moghadasi, S. Synthesis of β-amino carbonyl compounds using ZnO nanoparticles as a green, effective and reusable catalyst. Bulg. Chem. Commun. 2014, 46, 486–496. [Google Scholar]
- Loh, T.P.; Chen, S.L. InCl3-Catalyzed Three-Component Asymmetric Mannich-Type Reaction in Methanol. Org. Lett. 2002, 4, 3647–3650. [Google Scholar] [CrossRef]
- Loh, T.P.; Liung, S.B.K.W.; Tan, K.L.; Wei, L.L. Three Component Synthesis of β-Amino Carbonyl Compounds Using Indium Trichloride-Catalyzed One-pot Mannich-type Reaction in Water. Tetrahedron 2000, 56, 3227–3237. [Google Scholar] [CrossRef]
- Behbahani, F.K.; Ziarani, L.M. One pot three-component Mannich reaction catalyzed by FePO4. Eur. Chem. Bull. 2013, 2, 782–784. [Google Scholar] [CrossRef]
- Sathesh, V.; Sathishkumar, M.; Ramachandran, G.; Rathore, R.S.; Sathiyanarayanan, K.I. A green approach for the one-pot multi-component synthesis of N-substituted γ, δ and ε-lactams involving C–N bond formation catalyzed by FeCl3. RSC Adv. 2013, 3, 23035–23045. [Google Scholar] [CrossRef]
- Behbahani, F.K.; Ziaei, P. A Green Route for the One-Pot Synthesis of 1,2-Disubstituted Benzimidazoles Using Iron(III) Phosphate under Solventless Conditions. Chin. J. Chem. 2012, 30, 65–70. [Google Scholar] [CrossRef]
- Halli, J.; Manolikakes, G. Iron-Catalyzed Three-Component Synthesis of α-Amino Acid Derivatives. Eur. J. Org. Chem. 2013, 33, 7471–7475. [Google Scholar] [CrossRef]
- Xu, H.; Liao, W.M.; Li, H.H. A mild and efficient ultrasound-assisted synthesis of diaryl ethers without any catalyst. Ultrason. Sonochem. 2007, 14, 779–782. [Google Scholar] [CrossRef]
- Pelit, E.; Turgut, Z. (+)-CSA Catalyzed Multicomponent Synthesis of 1-[(1,3-Thiazol-2-ylamino)methyl]-2-naphthols and Their Ring-Closure Reaction under Ultrasonic Irradiation. J. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, X.; Lu, Y. Direct asymmetric three-component organocatalytic anti-selective Mannich reactions in a purely aqueous system. Org. Biomol. Chem. 2007, 5, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-W.; Luo, J.; Lu, Y. Asymmetric catalysis with chiral primary amine-based organocatalysts. Chem. Commun. 2009, 14, 1807–1821. [Google Scholar] [CrossRef]
- Dziedzic, P.; Ibrahem, I.; Cordova, A. Direct catalytic asymmetric three-component Mannich reactions with dihydroxyacetone: Enantioselective synthesis of amino sugar derivatives. Tetrahedron Lett. 2007, 49, 803–807. [Google Scholar] [CrossRef]
- Campos, J.F.; Ferreira, V.; Berteina-Raboin, S. Eucalyptol: A bio-based solvent for the synthesis of O,S,N-Heterocycles. Application to Hiyama Coupling, Cyanation, and Multicomponent Reactions Catalysts 2021, 11, 222. [Google Scholar] [CrossRef]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green. Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.K.; Khan, A.T. Synthesis of fully substituted pyridines and dihydropyridines in a highly chemoselective manner utilizing a multicomponent reaction (MCR) strategy. RSC Adv. 2014, 4, 53752–53760. [Google Scholar] [CrossRef]
- Maddila, S.; Gangu, K.K.; Maddila, S.N.; Jonnalagadda, S.B. A facile, efficient, and sustainable chitosan/CaHAp catalyst and one-pot synthesis of novel 2,6-diamino-pyran-3,5-dicarbonitriles. Mol. Divers. 2017, 21, 247–255. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, X.; Yang, Z.; Qi, C. Novel macroporous palladium cation crosslinked chitosan membrane for heterogeneous catalysis application. Inter. J. Biolog. Macromol. 2014, 68, 189–197. [Google Scholar] [CrossRef]
- Sharma, H.; Srivastava, S. Anion–cation co-operative catalysis by artificial sweetener saccharine-based ionic liquid forsustainable synthesis of 3,4-dihydropyrano[c]chromenes, 4,5-dihydropyrano [4,3-b]pyran and tetrahydrobenzo[b]pyrans in aqueous medium. RSC Adv. 2018, 8, 38974–38979. [Google Scholar] [CrossRef]
- Ducatti, D.R.B.; Massi, A.; Noseda, M.D.; Duarte, M.E.R.; Dondoni, A. DihydropyridineC-glycoconjugates by organocatalytic Hantzsch cyclocondensation. Stereoselective synthesis of α-threofuranoseC-nucleoside enantiomers. Org. Biomol. Chem. 2009, 7, 1980–1986. [Google Scholar] [CrossRef]
- Wang, Z.; Huynh, H.K.; Han, B.; Krishnamurthy, R.; Eschenmoser, A. 2,6-Diamino-5,8-diaza-7,9-dicarba-purine. Org. Lett. 2003, 5, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. Aldehyde-urea derivatives of aceto-and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Kappe, C.O. 100 years of the Biginelli dihydropyrimidine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Panda, S.S.; Khanna, P.; Khanna, L. Biginelli Reaction: A Green Perspective. Curr. Org. Chem. 2012, 16, 507–520. [Google Scholar] [CrossRef]
- Chopda, L.V.; Dave, P.N. Recent Advances in Homogeneous and Heterogeneous Catalyst in Biginelli Reaction from 2015-19: A Concise Review. Chem. Sel. 2020, 5, 5552–5572. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Parallel synthesis of dihydropyrimidinones using Yb(III)-resin and polymer-supported scavengers under solvent-free conditions. A green chemistry approach to the Biginelli reaction. Tetrahedron Lett. 2001, 42, 7975–7978. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, C.; Wang, J. An efficient synthesis of 4-substituted pyrazolyl-3,4-dihydropyrimidin-2(1H)-(thio)ones catalyzed by Mg(ClO4)2 under ultrasound irradiation. J. Mol. Catal. A Chem. 2006, 253, 207–211. [Google Scholar] [CrossRef]
- Ahn, B.J.; Gang, M.S.; Chae, K.; Oh, Y.; Shin, J.; Chang, W. A microwave-assisted synthesis of 3,4-dihydropyrimidin-2-(1H)-ones catalyzed by FeCl3-supported Nanopore Silica under solvent-free conditions. J. Ind. Eng. Chem. 2008, 14, 401–405. [Google Scholar] [CrossRef]
- Chitra, S.; Devanathan, D.; Pandiarajan, K. Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur. J. Med. Chem. 2010, 45, 367–371. [Google Scholar] [CrossRef]
- Marinescu, M. Biginelli Reaction Mediated Synthesis of Antimicrobial Pyrimidine Derivatives and Their Therapeutic Properties. Molecules 2021, 26, 6022. [Google Scholar] [CrossRef]
- Yadav, L.D.S.; Awasthi, C.; Rai, V.K.; Rai, A. Biorenewable and mercaptoacetylating building blocks in the Biginelli reaction: Synthesis of thiosugar-annulated dihydropyrimidines. Tetrahedron Lett. 2007, 48, 4899–4902. [Google Scholar] [CrossRef]
- Ugi, I. Versuche Mit Isonitrilen. Angew. Chem., Int. Ed. 1959, 71, 386. [Google Scholar]
- Alvim, H.G.O.; da Silva Junior, E.N.; Neto, B.A.D. What do we know about multicomponent reactions? Mechanisms and trends for the Biginelli, Hantzsch, Mannich, Passerini and Ugi MCRs. RSC Adv. 2014, 4, 54282–54299. [Google Scholar] [CrossRef]
- Madej, A.; Paprocki, D.; Koszelewski, D.; Zadlo-Dobrowolska, A.; Brzozowska, A.; Walde, P.; Ostaszewski, R. Efficient Ugi reactions in an aqueous vesicle system. RSC Adv. 2017, 7, 33344–33354. [Google Scholar] [CrossRef]
- Rocha, R.O.; Rodrigues, M.-O.; Neto, B.A.D. Review on the Ugi Multicomponent Reaction Mechanism and the Use of Fluorescent Derivatives as Functional Chromophores. ACS Omega 2020, 5, 972–979. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Zhang, W. Ugi Four-Component Reactions Using Alternative Reactants. Molecules 2023, 28, 1642. [Google Scholar] [CrossRef]
- Linderman, R.J.; Binet, S.; Petrich, S.R. Enhanced Diastereoselectivity in the Asymmetric Ugi Reaction Using a New “Convertible” Isonitrile. J. Org. Chem. 1999, 64, 336–337. [Google Scholar] [CrossRef]
- Ugi, I.; Demharter, A.; Hörl, W.; Smid, T. Ugi reactions with trifunctional α-amino acids, aldehydes, isocyanides and alcohols. Tetrahedron 1996, 52, 11657–11664. [Google Scholar] [CrossRef]
- Ugi, I.; Hörl, W.; Hanusch-Kompa, C.; Smid, T.; Herdtweck, E. MCR 6: Chiral 2,6-Piperazinedione via UGI reactions with α-amino acids, carbonyl compounds, isocyanides and alcohols. Heterocycles 1998, 47, 965–975. [Google Scholar] [CrossRef]
- Kadzimirsz, D.; Hildebrandt, D.; Merz, K.; Dyker, G. Isoindoles and dihydroisoquinolines by gold-catalyzed intramolecular hydroamination of alkynes. Chem. Commun. 2006, 6, 661–662. [Google Scholar] [CrossRef]
- Andreana, P.R.; Liu, C.C.; Schreiber, S.L. Stereochemical Control of the Passerini Reaction. Org. Lett. 2004, 6, 4231–4233. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Wang, M.-X.; Wang, D.-X.; Zhu, J. Asymmetric synthesis of 5-(1-hydroxyalkyl)tetrazoles by catalytic enantioselective Passerini-type reactions. Angew. Chem., Int. Ed. 2008, 47, 9454–9457. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Fan, Y. Catalytic, Enantioselective α-Additions of Isocyanides: Lewis Base Catalyzed Passerini-Type Reactions. J. Org. Chem. 2005, 70, 9667–9676. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.; Hell, Z.; Pirault-Roy, L. Catalytic activity of metal-doped porous materials in the salicylaldehyde Petasis-BoronoMannich reaction. Monatsh. Chem. 2016, 147, 749–753. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Rasool, N.; Ahmad, M.; Ali, K.G. Recent trends in the chemistry of Sandmeyer reaction: A review. Mol. Divers. 2022, 26, 1837–1873. [Google Scholar] [CrossRef]
- Bering, L.; Antonchick, A.P. Regioselective metal-free cross-coupling of quinoline N-oxides with boronic acids. Org. Lett. 2015, 17, 3134–3137. [Google Scholar] [CrossRef]
- Abonia, R.; Garay, A.; Castillo, J.C.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; Butassi, E.; Zacchino, S. Design of two alternative routes for the synthesis of naftifine and analogues as potential antifungal agents. Molecules 2018, 23, 520. [Google Scholar] [CrossRef]
- Reddy, B.N.; Rani, C.R.; Reddy, S.M.; Pathak, M. An efficient and green La(OTf)3 catalyzed Petasis Borono–Mannich reaction for the synthesis of tertiary amines. Res. Chem. Intermed. 2016, 42, 7533–7549. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.H. Lewis acid promoted highly diastereoselective Petasis borono-Mannich reaction: Efficient synthesis of optically active β,γ-unsaturated α-amino acids. Org. Lett. 2012, 14, 2062–2065. [Google Scholar] [CrossRef]
- Saeed, S.; Munawar, S.; Ahmad, S.; Mansha, A.; Zahoor, A.F.; Irfan, A.; Irfan, A.; Kotwica-Mojzych, K.; Soroka, M.; Głowacka, M.; et al. Recent Trends in the Petasis Reaction: A Review of Novel Catalytic Synthetic Approaches with Applications of the Petasis Reaction. Molecules 2023, 28, 8032. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.S.; Lakshmi, P.N. Ionic liquid accelerated Petasis reaction: A green protocol for the synthesis of alky-laminophenols. J. Mol. Catal. A Chem. 2007, 274, 101–104. [Google Scholar] [CrossRef]
- Azizi, N.; Farhadi, E. Straightforward and rapid Petasis multicomponent reactions in deep eutectic solvent. Curr. Res. Green Sustain. Chem. 2020, 4, 100220. [Google Scholar] [CrossRef]
- Nun, P.; Martinez, J.; Lamaty, F. Microwave-assisted neat procedure for the Petasis reaction. Synthesis 2010, 12, 2063–2068. [Google Scholar] [CrossRef]
- Ayaz, M.; Dietrich, J.; Hulme, C. A novel route to synthesize libraries of quinoxalines via Petasis methodology in two synthetic operations. Tetrahedron Lett. 2011, 52, 4821–4823. [Google Scholar] [CrossRef]
- Kumagai, N.; Muncipinto, G.; Schreiber, S.L. Short Synthesis of Skeletally and Stereochemically Diverse Small Molecules by Coupling Petasis Condensation Reactions to Cyclization Reactions. Angew. Chem. Int. Ed. 2006, 45, 3635–3638. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.F.D.; Anjos, N.S.; Gibeli, M.M.; Silva, G.A.; Fernandes, P.C.S.; Fiorentino, E.S.C.; Longo, L.S., Jr. A Comparative Study on the Groebke-Blackburn-Bienaymé Three-Component Reaction Catalyzed by Rare Earth Triflates under Microwave Heating. J. Braz. Chem. Soc. 2020, 31, 1434–1444. [Google Scholar] [CrossRef]
- Ganta, R.K.; Kerru, N.; Maddila, S.; Jonnalagadda, S.B. Advances in pyranopyrazole scaffolds’s syntheses using sustainable catalysts—A review. Molecules 2021, 26, 3270. [Google Scholar] [CrossRef]
- Singh, S.; Yadav, S.; Minakshi; Pundeer, R. Green Synthesis of Pyrazoles: Recent Developments in Aqueous Methods. Synopen 2023, 7, 297–312. [Google Scholar] [CrossRef]
- Bansal, R.; Soni, P.K.; Gupta, N.; Bhagyawant, S.S.; Halve, A.K. Synthesis and Antibacterial Screening of Some Pyrazole Derivatives Catalyzed by Cetyltrimethylammoniumbromide (CTAB). Curr. Org. Synth. 2021, 18, 225–231. [Google Scholar] [CrossRef]
- Liju, W.; Ablajan, K.; Jun, F. Rapid and efficient one-pot synthesis of spiro[indoline-3,4′-pyrano [2, 3-c]pyrazole] derivatives catalyzed by l-proline under ultrasound irradiation. Ultrason. Sonochem. 2015, 22, 113–118. [Google Scholar] [CrossRef]
- Saha, A.; Payra, S.; Banerjee, S. One-pot multicomponent synthesis of highly functionalized bio-active pyrano [2,3-c]pyrazole and benzylpyrazolyl coumarin derivatives using ZrO2 nanoparticles as a reusable catalyst. Green Chem. 2015, 17, 2859–2866. [Google Scholar] [CrossRef]
- Sikandar, S.; Zahoor, A.F.; Ahmad, S.; Anjum, M.N.; Ahmad, M.N.; Shah, M.S.U. L-Cysteine Catalyzed Environmentally BenignOne-Pot Multicomponent Approach Towards the Synthesis of Dihydropyrano [2,3-c]Pyrazole Derivatives. Curr. Org. Synth. 2020, 17, 457–463. [Google Scholar] [CrossRef]
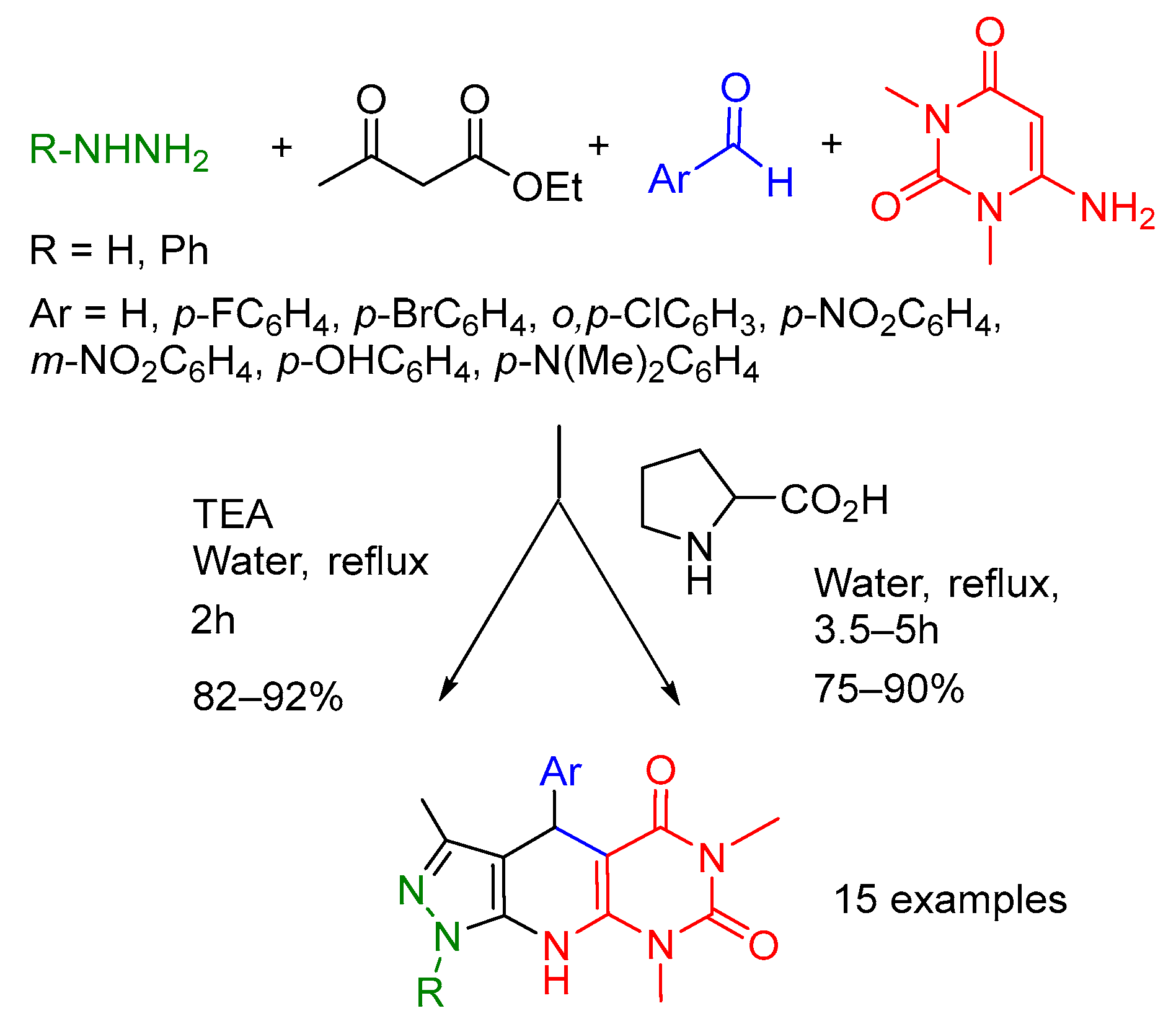
- Daraie, M.; Heravi, M.M. Molecular diversity of four-component synthesis of pyrazole-basedpyrido [2,3-d]pyrimidine-diones in water: A green synthesis. ARKIVOC 2016, (iv), 328–338. [Google Scholar] [CrossRef]
- Govindaraju, S.; Tabassum, S.; Pasha, M.A. Citric-Acid-Catalyzed Green and Sustainable Synthesis of Novel Functionalized Pyrano [2, 3-e]pyrimidin- and Pyrano [2, 3-d]pyrazol-amines in Water via One-Pot Multicomponent Approaches. ChemistrySelect 2018, 3, 3832–3838. [Google Scholar] [CrossRef]
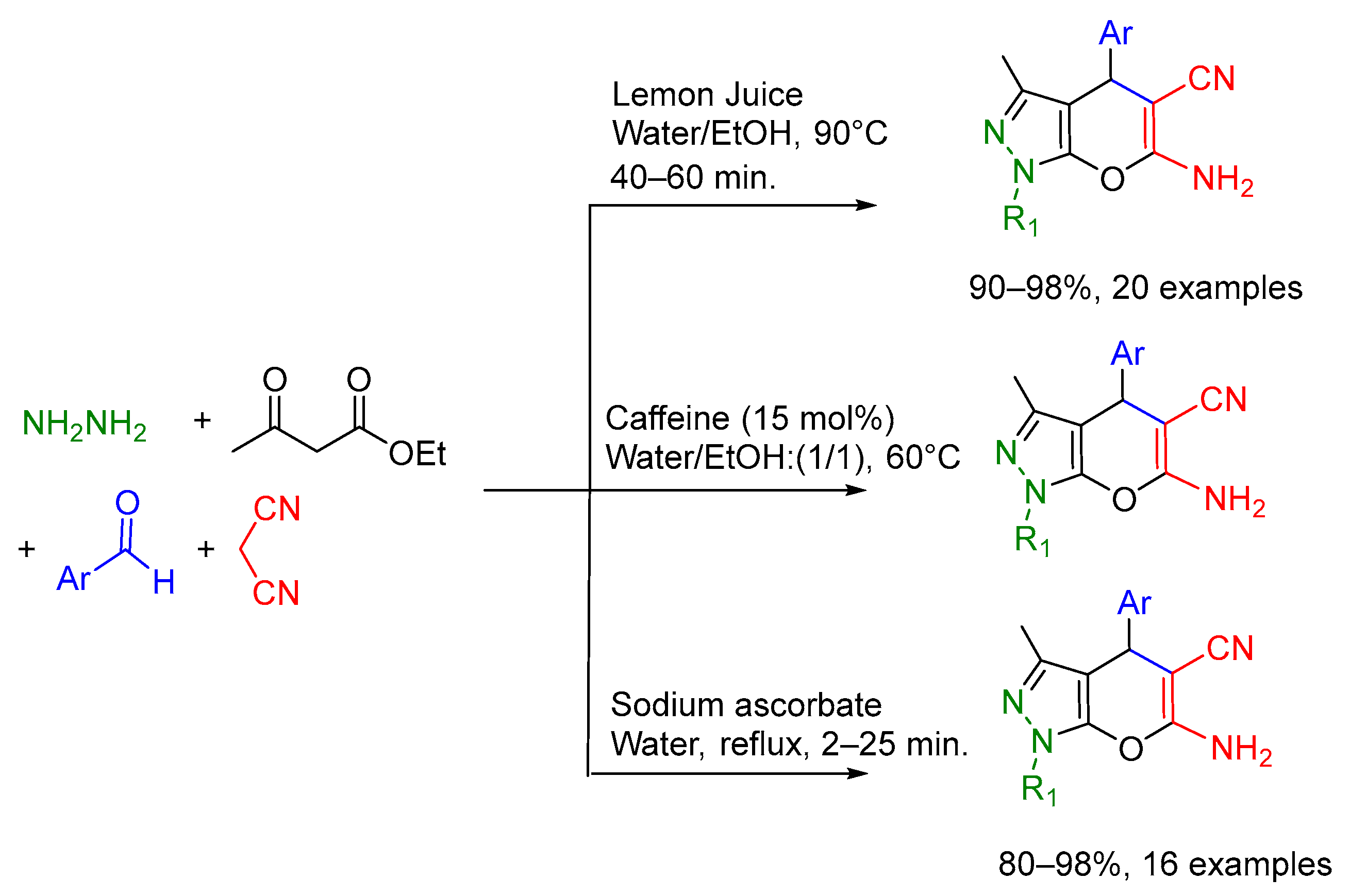
- Mohamadpour, F. Caffeine as a Naturally Green and Biodegradable Catalyst for Preparation of Dihydropyrano [2,3-c]pyrazoles. Org. Prep. Proced. Int. 2020, 52, 453–457. [Google Scholar] [CrossRef]
- Kiyani, H.; Bamdad, M. Sodium ascorbate as an expedient catalyst for green synthesis of polysubstituted 5-aminopyrazole-4-carbonitriles and 6-amino-1,4-dihydropyrano [2,3-c]pyrazole-5-carbonitriles. Res. Chem. Intermed. 2018, 44, 2761–2778. [Google Scholar] [CrossRef]
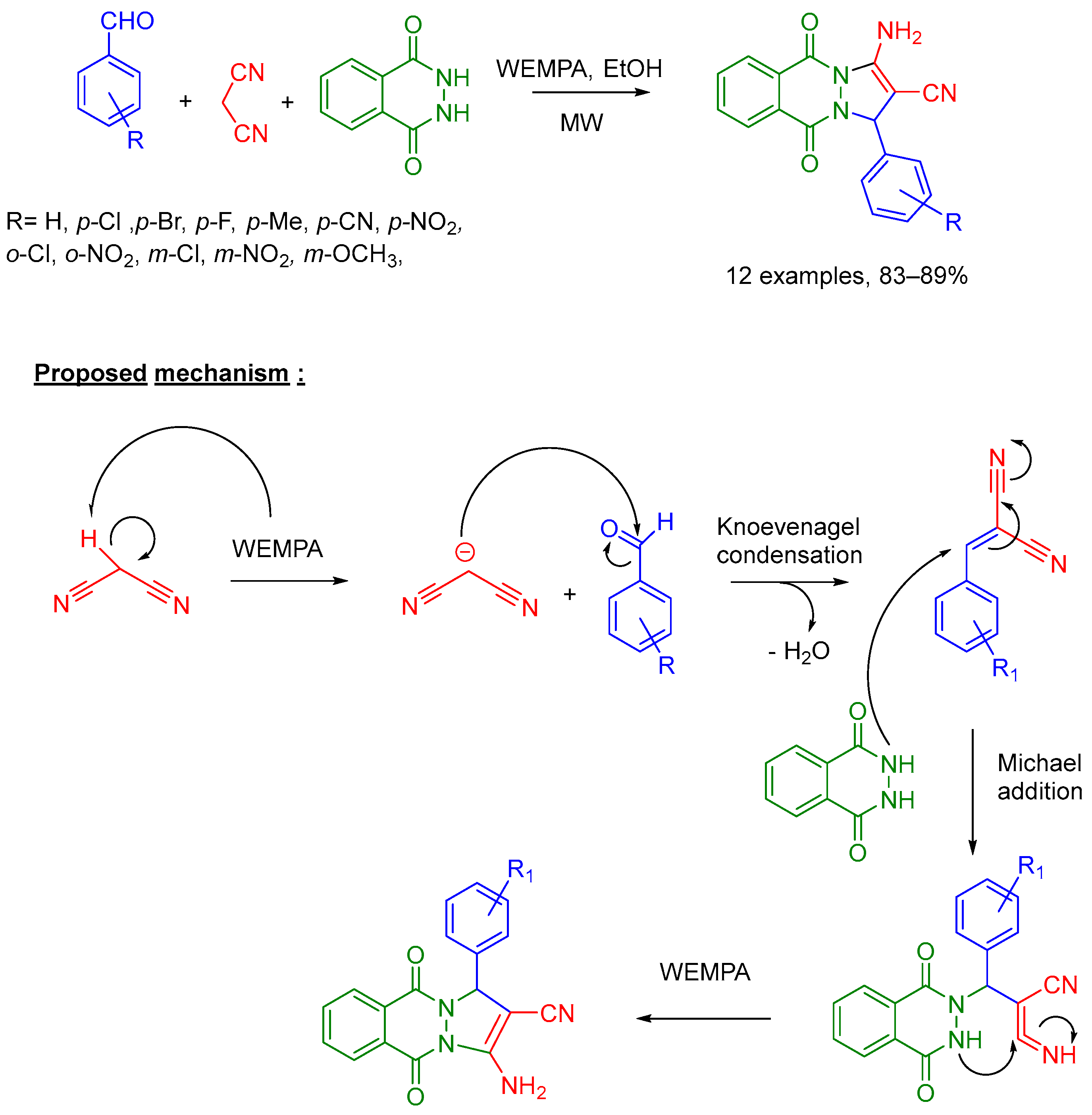
- Hiremath, P.B.; Kamanna, K. Microwave-Accelerated Facile Synthesis of 1H-Pyrazolo [1,2-b]Phthalazine-5,10-Dione Derivatives Catalyzed by WEMPA. Polycycl. Aromat. Compd. 2022, 42, 2162–2178. [Google Scholar] [CrossRef]



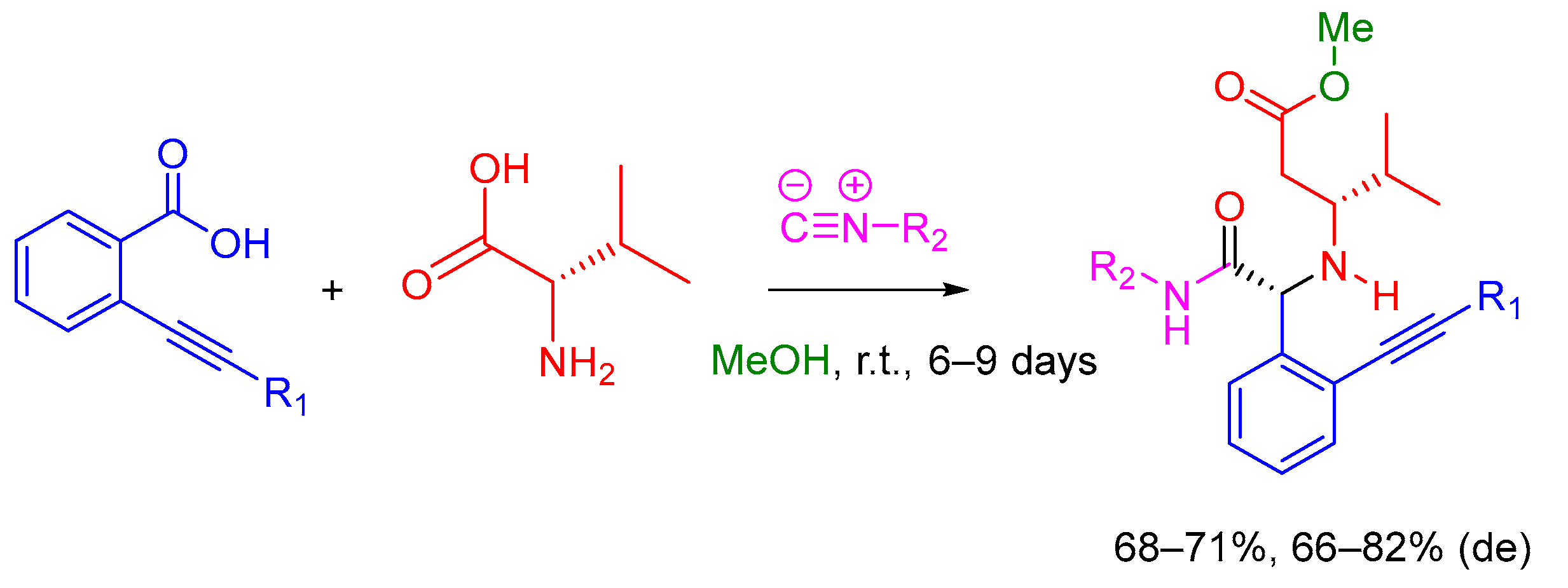

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messire, G.; Caillet, E.; Berteina-Raboin, S. Green Catalysts and/or Green Solvents for Sustainable Multi-Component Reactions. Catalysts 2024, 14, 593. https://doi.org/10.3390/catal14090593
Messire G, Caillet E, Berteina-Raboin S. Green Catalysts and/or Green Solvents for Sustainable Multi-Component Reactions. Catalysts. 2024; 14(9):593. https://doi.org/10.3390/catal14090593
Chicago/Turabian StyleMessire, Gatien, Emma Caillet, and Sabine Berteina-Raboin. 2024. "Green Catalysts and/or Green Solvents for Sustainable Multi-Component Reactions" Catalysts 14, no. 9: 593. https://doi.org/10.3390/catal14090593
APA StyleMessire, G., Caillet, E., & Berteina-Raboin, S. (2024). Green Catalysts and/or Green Solvents for Sustainable Multi-Component Reactions. Catalysts, 14(9), 593. https://doi.org/10.3390/catal14090593







