Ligand-Engineered Mn-MOFs Derived Mn2O3 for Enhanced Carbon Dioxide Conversion to Ethylene Urea
Abstract
1. Introduction
2. Results
2.1. Catalysts Characterization
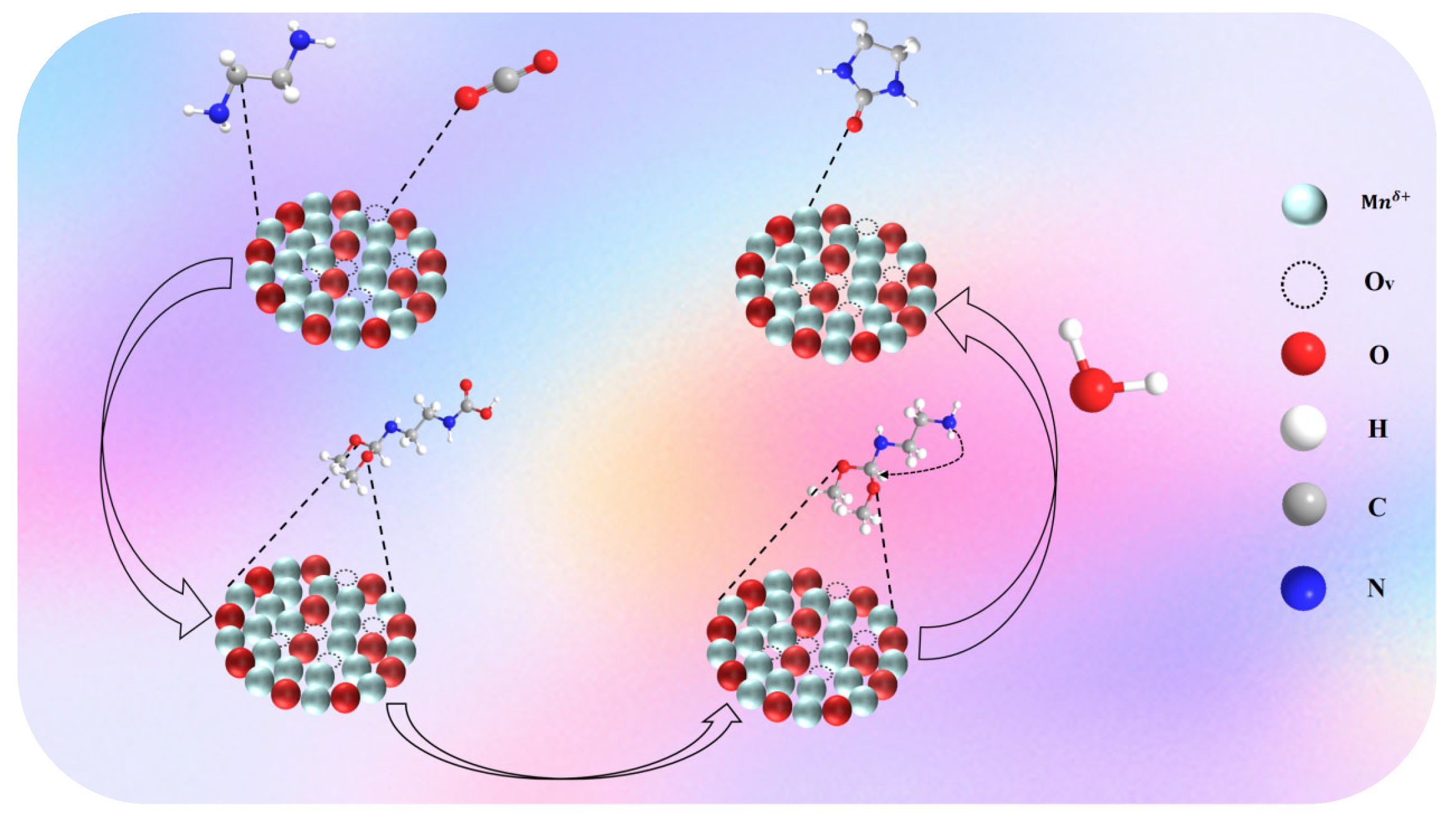
2.2. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of MOF-Derived Mn2O3 Catalysts
3.3. Catalytic Performance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nwabueze, Q.A.; Leggett, S. Advancements in the Application of CO2 Capture and Utilization Technologies—A Comprehensive Review. Fuels 2024, 5, 508–532. [Google Scholar] [CrossRef]
- Sun, S.; Sun, H.; Williams, P.T.; Wu, C. Recent advances in integrated CO2capture and utilization: A review. Sustain. Energy Fuels 2021, 5, 4546–4559. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, P.; Zhang, H.; Zhang, S.; Tan, X.; Ye, F.; Gu, J.; Zou, J.-J.; Wang, D. A comprehensive review on photo-thermal co-catalytic reduction of CO2 to value-added chemicals. Fuels 2024, 362, 130906. [Google Scholar] [CrossRef]
- Latsiou, A.I.; Charisiou, N.D.; Frontistis, Z.; Goula, M.A. From CO2 to value added chemicals: The promise of single atom catalysts. Int. J. Hydrogen Energy 2024, 92, 465–481. [Google Scholar] [CrossRef]
- Tedeeva, M.A.; Kustov, A.L.; Batkin, A.M.; Garifullina, C.; Zalyatdinov, A.A.; Yang, D.; Dai, Y.; Yang, Y.; Kustov, L.M. Catalytic systems for hydrogenation of CO2 to methanol. Mol. Catal. 2024, 566, 114403. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Wang, W.; Xin, J.; Mi, X.; Yang, G.; Su, M.; Zhang, S.; Li, H. Research progress on CO2 catalytic conversion to value-added oxygenates. J. Fuel Chem. Technol. 2024, 52, 496–511. [Google Scholar] [CrossRef]
- Obasanjo, C.A.; Gao, G.; Crane, J.; Golovanova, V.; de Arquer, F.P.G.; Dinh, C.-T. High-rate and selective conversion of CO2 from aqueous solutions to hydrocarbons. Nat. Commun. 2023, 14, 3176. [Google Scholar] [CrossRef]
- Wang, F.; Da, B.; Wan, T.; Zhang, Y.; Liu, N.; Ma, Q.; Xu, J.; Xue, B.; Wei, X. CeO2/Beta composite catalysts in dimethyl carbonate synthesis from CO2: Effect of preparation method on surface oxygen species. React. Kinet. Catal. Lett. 2024, 138, 845–858. [Google Scholar] [CrossRef]
- Wang, L.; Qi, C.; Xiong, W.; Jiang, H. Recent advances in fixation of CO2 into organic carbamates through multicomponent reaction strategies. Chin. J. Catal. 2022, 43, 1598–1617. [Google Scholar] [CrossRef]
- Yabushita, M.; Fujii, R.; Nakagawa, Y.; Tomishige, K. Thermodynamic and Catalytic Insights into Non-Reductive Transformation of CO2 with Amines into Organic Urea Derivatives. ChemCatChem 2024, 16, e202301342. [Google Scholar] [CrossRef]
- Ou, J.; Wang, Y.; Liu, F.; Zhao, T. Catalytic conversion of CO2 to cyclic carbonates: A metal-free approach using urea-functionalized ionic salts grafted in ordered mesoporous SiO2. Sep. Purif. Technol. 2024, 360, 131254. [Google Scholar] [CrossRef]
- Reedijk, J.; Mulder, T.M.; Smit, J.A. The coordination chemistry of 2-imidazolidinone. Inorg. Chim. Acta 1975, 13, 219–227. [Google Scholar] [CrossRef]
- Rahman, F.; Yang, X.; Motswaiso, F.; Takanashi, I.; Kameda, T.; Rahman, M.T.; Saito, Y.; Kumagai, S.; Yoshioka, T. Effective synthesis of ethylene urea from CO2 adsorbed cerium doped Mg–Al layered double hydroxide. J. Clean. Prod. 2023, 434, 140191. [Google Scholar] [CrossRef]
- Casnati, A.; Motti, E.; Mancuso, R.; Gabriele, B.; Della Ca’, N. Recent Advances in the Catalytic Synthesis of Imidazolidin-2-ones and Benzimidazolidin-2-ones. Catalysts 2019, 9, 28. [Google Scholar] [CrossRef]
- Rahman, F.; Kunii, Y.; Kameda, T.; Rahman, M.T.; Saito, Y.; Kumagai, S.; Yoshioka, T. Efficacy of zirconium hydroxide and cerium hydroxide for carbon dioxide adsorption and subsequent ethylene urea synthesis. J. Clean. Prod. 2025, 493, 144956. [Google Scholar] [CrossRef]
- Motswaiso, F.; Suzuki, U.; Sawaguchi, K.; Rahman, F.; Kameda, T.; Kumagai, S.; Saito, Y.; Yoshioka, T. Synthesis of ethylene urea using carbon-dioxide-adsorbed titanium–zirconium mixed oxides. Environ. Chall. 2024, 16, 100970. [Google Scholar] [CrossRef]
- Wang, F.; Jin, Y.; Xue, Y.; Cui, L.; Yu, S.; Liu, N.; Ma, Q.; Xu, J.; Xue, B. Ceria-based nanorods catalysts for efficient catalytic production of ethylene urea from CO2 and ethylenediamine: The influence of doped zirconium ions. Res. Chem. Intermed. 2024, 50, 2175–2186. [Google Scholar] [CrossRef]
- Wang, F.; Da, B.; Jin, Y.; Sanwal, P.; Cui, L.; Chen, S.; Xu, J.; Xue, B.; Li, G. Insight into the effect of manganese oxidation state on the synthesis of ethylene urea from CO2 and ethylenediamine. J. Environ. Sci. 2024, 155, 37–47. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Chen, J.; Niu, H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts 2022, 12, 775. [Google Scholar] [CrossRef]
- Cheng, L.; He, Y.; Gong, M.; He, X.; Ning, Z.; Yu, H.; Jiao, Z. MOF-derived synthesis of Co3O4 nanospheres with rich oxygen vacancies for long-term stable and highly selective n-butanol sensing performance. J. Alloys Compd. 2021, 857, 158205. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem. Eng. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.-C. Tuning the Topology and Functionality of Metal−Organic Frameworks by Ligand Design. Accounts Chem. Res. 2010, 44, 123–133. [Google Scholar] [CrossRef]
- Guillerm, V.; Maspoch, D. Geometry Mismatch and Reticular Chemistry: Strategies To Assemble Metal–Organic Frameworks with Non-default Topologies. J. Am. Chem. Soc. 2019, 141, 16517–16538. [Google Scholar] [CrossRef]
- Ramírez, A.; Hillebrand, P.; Stellmach, D.; May, M.M.; Bogdanoff, P.; Fiechter, S. Evaluation of MnOx, Mn2O3, and Mn3O4 electro-deposited films for the oxygen evolution reaction of water. J. Phys. Chem. C. 2014, 118, 14073–14081. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Li, S.; Zhang, J.; Yang, X.; Shen, P.; Guo, L.; Wei, R.; Zhang, J.; Xiao, G. 3D-monoclinic M–BTC MOF (M = Mn, Co, Ni) as highly efficient cat-alysts for chemical fixation of CO2 into cyclic carbonates. J. Ind. Eng. Chem. 2018, 58, 296–303. [Google Scholar] [CrossRef]
- Zhang, P.; Zhan, Y.; Cai, B.; Hao, C.; Wang, J.; Liu, C.; Meng, Z.; Yin, Z.; Chen, Q. Shape-controlled synthesis of Mn3O4 nanocrystals and their catalysis of the degradation of methylene blue. Nano Res. 2010, 3, 235–243. [Google Scholar] [CrossRef]
- Mei, J.; Shen, Y.; Wang, Q.; Shen, Y.; Li, W.; Zhao, J.; Chen, J.; Zhang, S. Roles of Oxygen Species in Low-Temperature Catalytic o-Xylene Oxidation on MOF-Derived Bouquetlike CeO. ACS Appl. Mater. Interfaces 2022, 14, 35694–35703. [Google Scholar] [CrossRef]
- Jithul, K.; Tamilarasi, B.; Pandey, J. In-situ growth of γ-Mn2O3 on activated carbon cloth for enhanced bifunctional electrocatalysis of ORR and OER. Mater. Chem. Phys. 2025, 341, 130955. [Google Scholar] [CrossRef]
- Noei, H.; Qiu, H.; Wang, Y.; Löffler, E.; Wöll, C.; Muhler, M. The identification of hydroxyl groups on ZnO nanoparticles by infrared spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, G.E.; Achache, M.; El Haddaoui, H.; El-Haddar, S.; Draoui, K.; Bouchta, D.; Choukairi, M. Carbon paste electrode modified with Mn2O3 nanoparticles for simultaneous detection of heavy metals Cd(II) and Pb(II) in wastewater sample. J. Water Process Eng. 2025, 75, 107928. [Google Scholar] [CrossRef]
- Qu, G.; Yang, Y.; Xu, Y.; Zhao, C.; Ning, P. In situ pyrolysis of Mn-doped MOF-74 metal–organic framework derived MCNOx catalysts for enhanced low-temperature catalytic performance of toluene. New J. Chem. 2024, 48, 16917–16930. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, Y.J.; Kim, H.; Hong, M.J.; Moon, J.; Kim, Y.J.; Mun, J.; Kimi, K.J. Stable immobilization of lithium polysulfides using three-dimensional ordered mesoporous Mn2O3 as the host material in lithium–sulfur batteries. Carbon Energy 2024, 6, e487. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Zheng, T.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Promoted catalytic transformation of polycyclic aromatic hydrocarbons by MnO2 polymorphs: Synergistic effects of Mn3+ and oxygen vacancies. Appl. Catal. B Environ. 2020, 272, 119030. [Google Scholar] [CrossRef]
- Yu, M.; Li, M.; Zhang, X.; Ge, Z.; Xu, E.; Wang, L.; Yin, B.; Dou, Y.; Yang, Y.; Zhang, X.; et al. Coupling photo-catalytic reduction and biosynthesis towards sustainable CO2 upcycling. Angew. Chem. Int. Ed. 2025, 64, e202423995. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.; Badu, S.A.; Saravanamurugan, S. Surface Acidic Species-Driven Reductive Amination of Furfural with Ru/T-ZrO. ChemSusChem 2025, 18, e202401277. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Hasegawa, Y.; Ishimoto, M.; Toyosaki, T.; Matsuda, H. Carbonylation of amines by carbon dioxide in the presence of an organoantimony catalyst. J. Org. Chem. 1992, 57, 7339–7342. [Google Scholar] [CrossRef]
- Koizumi, H.; Takeuchi, K.; Matsumoto, K.; Fukaya, N.; Sato, K.; Uchida, M.; Matsumoto, S.; Hamura, S.; Choi, J.-C. One-pot catalytic synthesis of urea derivatives from alkyl ammonium carbamates using low concentrations of CO. Commun. Chem. 2021, 4, 66. [Google Scholar] [CrossRef]
- Primo, A.; Aguado, E.; Garcia, H. CO2-Fixation on Aliphatic α,ω-Diamines to Form Cyclic Ureas, Catalyzed by Ceria Nanoparticles that were Obtained by Templating with Alginate. ChemCatChem 2012, 5, 1020–1023. [Google Scholar] [CrossRef]
- Tamura, M.; Noro, K.; Honda, M.; Nakagawa, Y.; Tomishige, K. Highly efficient synthesis of cyclic ureas from CO2 and diamines by a pure CeO2 catalyst using a 2-propanol solvent. Green Chem. 2013, 15, 1567–1577. [Google Scholar] [CrossRef]
- Kulal, N.; John, C.; Shanbhag, G.V. Rational design of bifunctional catalyst from KF and ZnO combination on alumina for cyclic urea synthesis from CO2 and diamine. Appl. Catal. A Gen. 2020, 598, 117550. [Google Scholar] [CrossRef]
- Kulal, N.; Vetrivel, R.; Gopinath, C.S.; Ravindran, R.K.; Rao, V.N.; Shetty, M.; Shrikanth, R.; Rangappa, D.; Shanbhag, G.V. Green route for carbonylation of amines by CO2 using Sn-Ni-O bifunctional catalyst and theoretical study for finding best suited active sites. Chem. Eng. J. 2021, 419, 129439. [Google Scholar] [CrossRef]
- More, G.S.; Srivastava, R. Efficient Activation of CO2 over Ce-MOF-derived CeO2 for the Synthesis of Cyclic Urea, Urethane, and Carbamate. Ind. Eng. Chem. Res. 2021, 60, 12492–12504. [Google Scholar] [CrossRef]
- Wang, F.; Jin, Y.; Zhang, Y.; Liang, X.; Liu, N.; Wei, X.; Ke, Y.; Xu, J.; Xue, B. Shape-dependency activity of Nanostructured α-Mn2O3 in direct synthesis of ethylene urea from C2. J. Ind. Eng. Chem. 2025, 151, 524–533. [Google Scholar] [CrossRef]
- Mao, X.; Dai, J.; Chen, Y.; Xiong, C.; Ji, H. Hydrogen annealing-regulated oxygen vacancies in Co-doped ceria for efficient synthesis of dimethyl carbonate from CO. Chem. Eng. J. 2025, 521, 166761. [Google Scholar] [CrossRef]
- Tamura, M.; Honda, M.; Nakagawa, Y.; Tomishige, K. Direct conversion of CO2 with diols, aminoalcohols and diamines to cyclic carbonates, cyclic carbamates and cyclic ureas using heterogeneous catalysts. J. Chem. Technol. Biotechnol. 2013, 89, 19–33. [Google Scholar] [CrossRef]

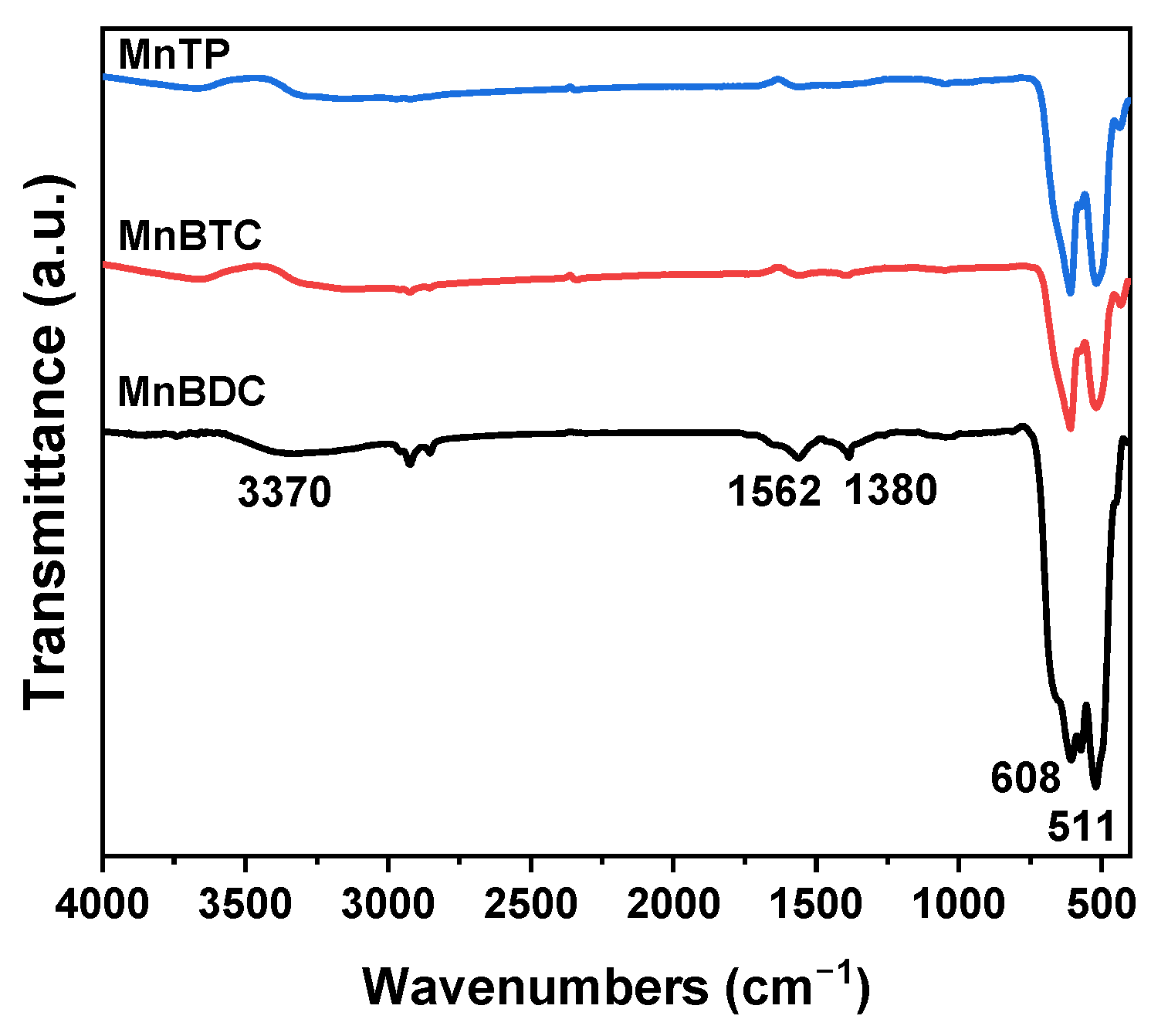
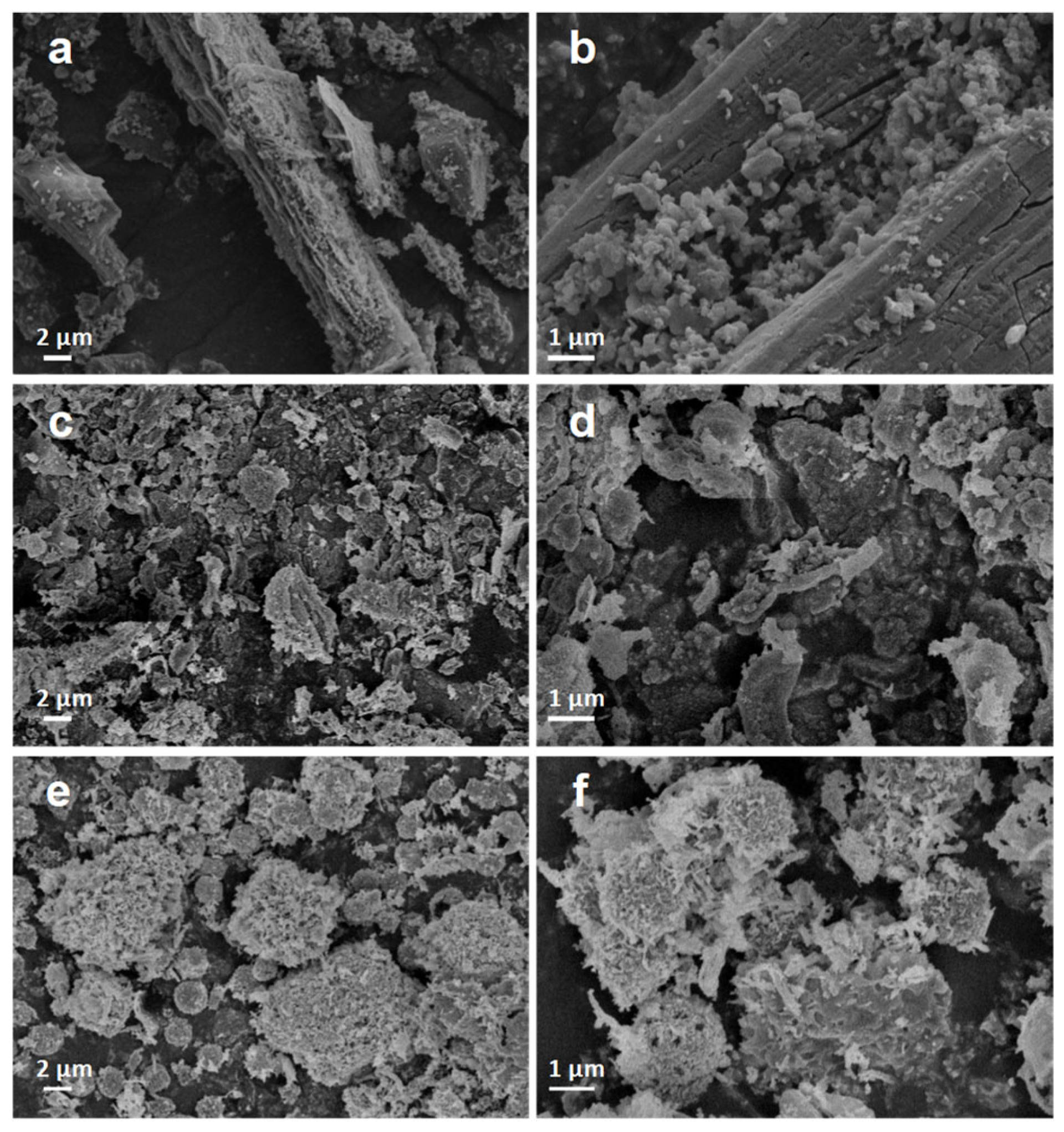

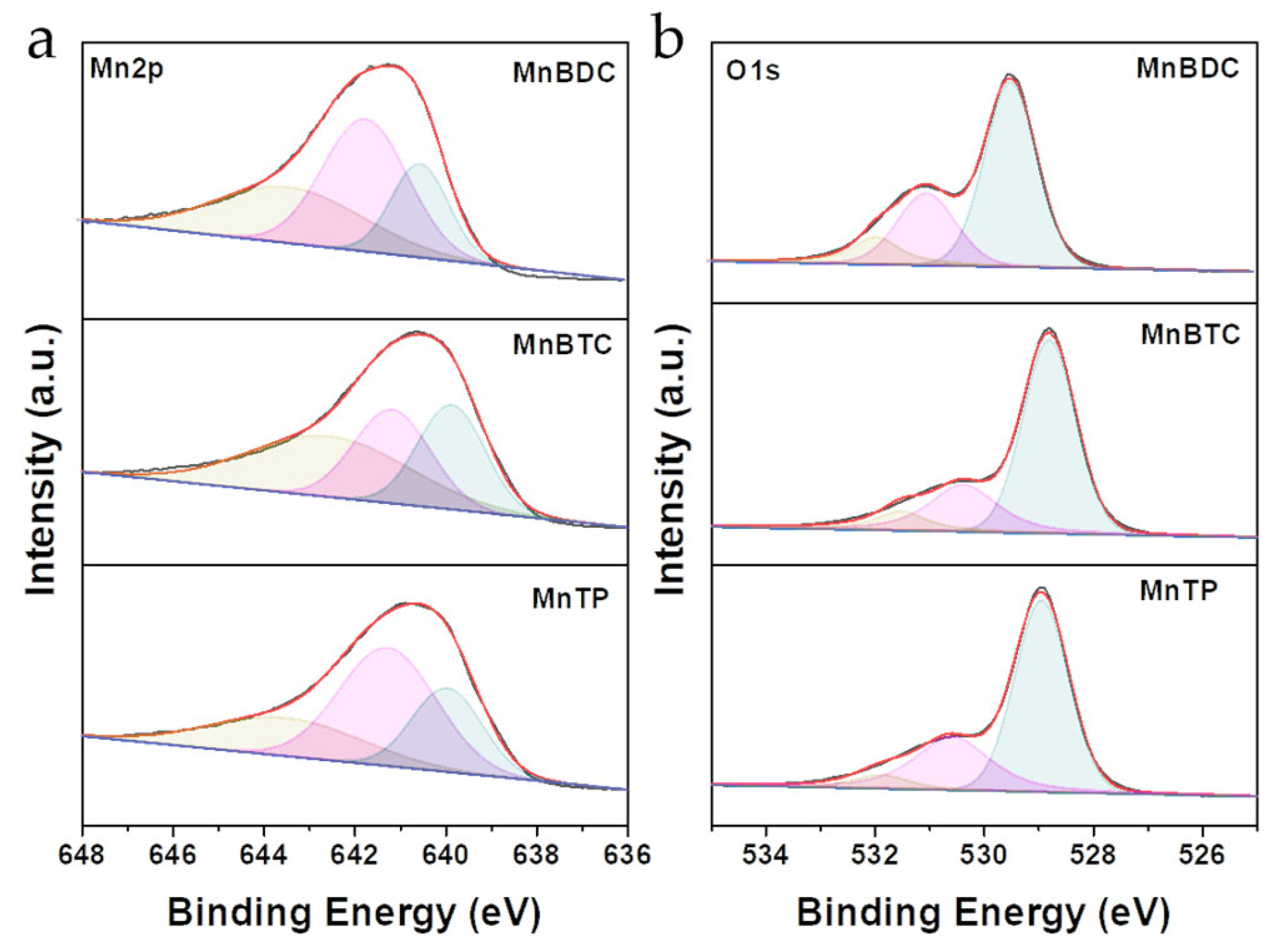

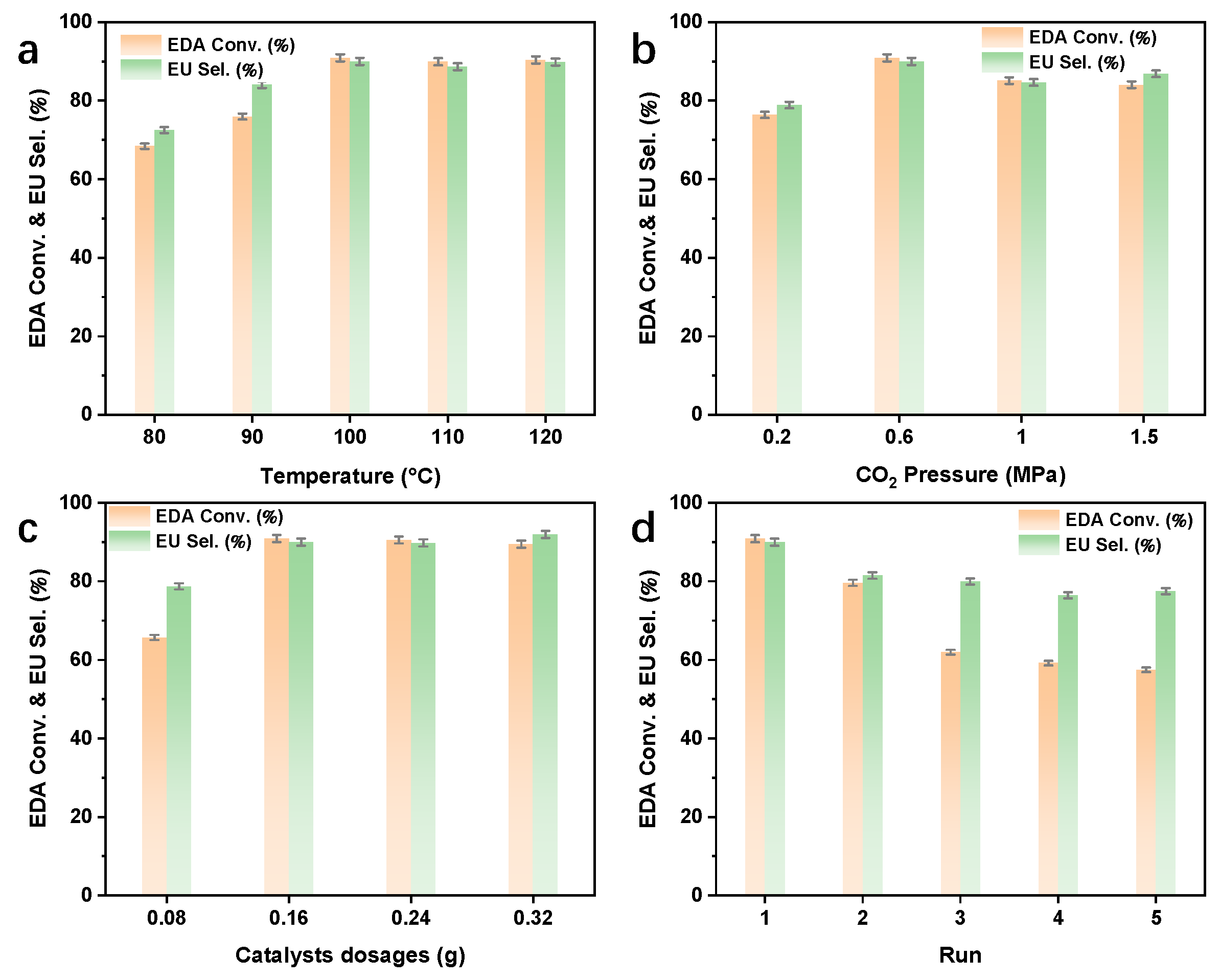

| Catalyst | Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) | Mn3+ (%) | OV (%) |
|---|---|---|---|---|---|
| MnBDC | 45.5 | 0.15 | 11.2 | 38.2 | 26.1 |
| MnBTC | 17.8 | 0.07 | 16.2 | 45 | 27.5 |
| MnTP | 19.7 | 0.08 | 16.2 | 49.4 | 31.2 |
| MnTP-R5 | - | - | - | 41.5 | 18.8 |
| Entry | Catalyst | T (°C) | t (min) | PCO2 (MPa) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ph3SbO/P4S10 | 150 | 720 | 4.9 | 85 | [36] |
| 2 | Cp2Ti(OTf)2 | 170 | 900 | - | 99 | [37] |
| 3 | CeO2 | 160 | 480 | 0.7 | 37 | [38] |
| 4 | CeO2 | 160 | 720 | 0.5 | 96 | [39] |
| 5 | 2.4ZnO/0.49KF/Al2O3 | 180 | 240 | 1 | 86 | [40] |
| 6 | Sn1.1-Ni-O-600 | 160 | 240 | 0.25 | 84 | [41] |
| 7 | MOF-derived CeO2 | 160 | 720 | 0.5 | 94 | [42] |
| 8 | MnO2 | 160 | 120 | 0.6 | 22 | [18] |
| 9 | Mn3O4 | 160 | 120 | 0.6 | 4.4 | |
| 10 | Mn2O3 | 160 | 120 | 0.6 | 81 | |
| 11 | Mn2O3-NC | 140 | 20 | 0.6 | 70 | [43] |
| 12 | Mn2O3-NO | 140 | 20 | 0.6 | 55 | |
| 13 | Mn2O3-NS | 140 | 20 | 0.6 | 90 | |
| 14 | MnBDC | 100 | 1 | 0.6 | 48 | this work |
| 15 | MnBTC | 100 | 1 | 0.6 | 71 | |
| 16 | MnTP | 100 | 1 | 0.6 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Zhang, Y.; Yin, J.; Chen, Y.; Deng, G.; Jin, Y.; Xu, J.; Xue, B.; Wang, F. Ligand-Engineered Mn-MOFs Derived Mn2O3 for Enhanced Carbon Dioxide Conversion to Ethylene Urea. Catalysts 2025, 15, 933. https://doi.org/10.3390/catal15100933
Tang J, Zhang Y, Yin J, Chen Y, Deng G, Jin Y, Xu J, Xue B, Wang F. Ligand-Engineered Mn-MOFs Derived Mn2O3 for Enhanced Carbon Dioxide Conversion to Ethylene Urea. Catalysts. 2025; 15(10):933. https://doi.org/10.3390/catal15100933
Chicago/Turabian StyleTang, Junxi, Yue Zhang, Jun Yin, Yiwen Chen, Guocheng Deng, Yulong Jin, Jie Xu, Bing Xue, and Fei Wang. 2025. "Ligand-Engineered Mn-MOFs Derived Mn2O3 for Enhanced Carbon Dioxide Conversion to Ethylene Urea" Catalysts 15, no. 10: 933. https://doi.org/10.3390/catal15100933
APA StyleTang, J., Zhang, Y., Yin, J., Chen, Y., Deng, G., Jin, Y., Xu, J., Xue, B., & Wang, F. (2025). Ligand-Engineered Mn-MOFs Derived Mn2O3 for Enhanced Carbon Dioxide Conversion to Ethylene Urea. Catalysts, 15(10), 933. https://doi.org/10.3390/catal15100933








