One-Step Fe/N Co-Doping for Efficient Catalytic Oxidation and Selective Non-Radical Pathway Degradation in Sludge-Based Biochar
Abstract
1. Introduction
2. Results and Discussion
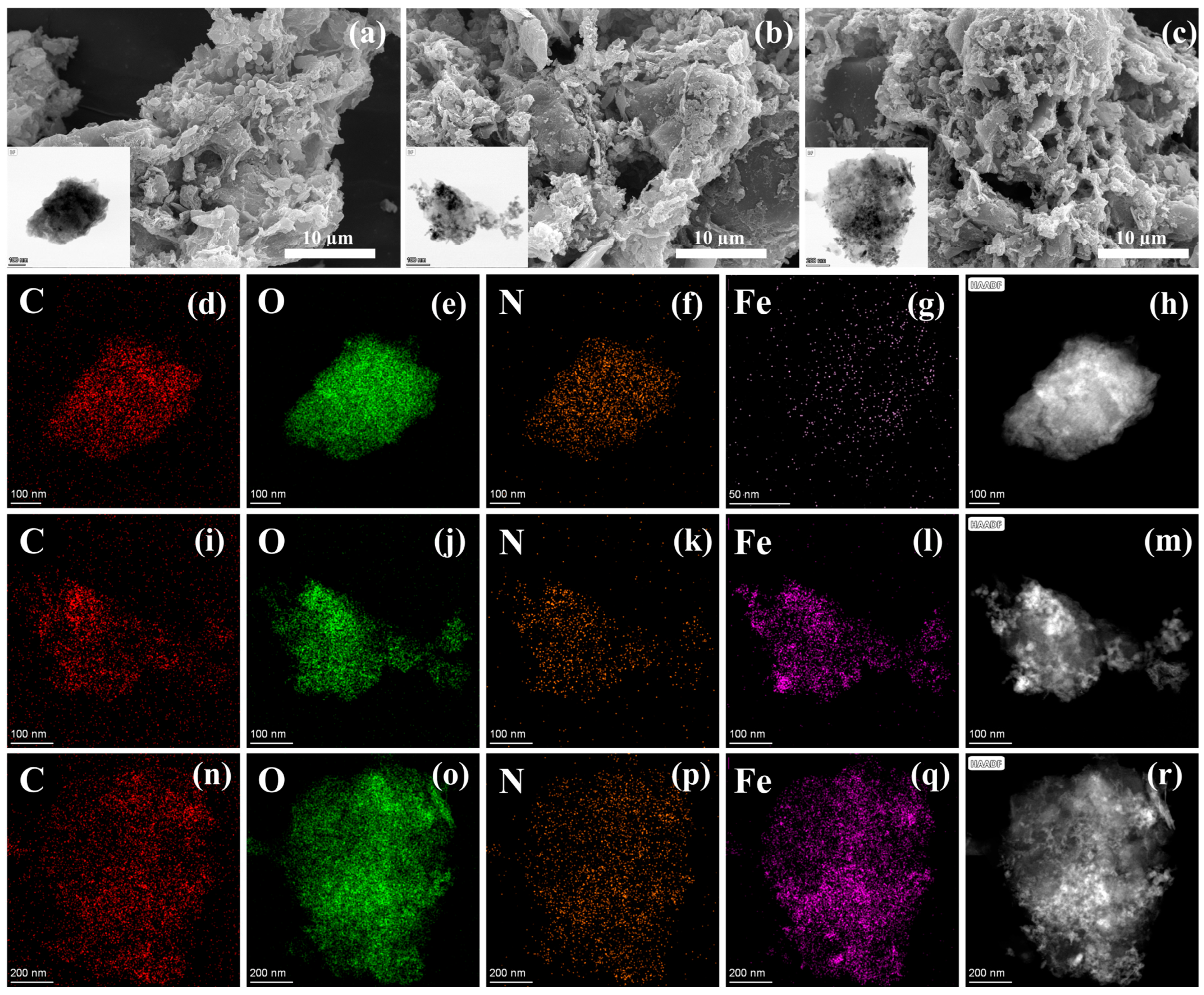
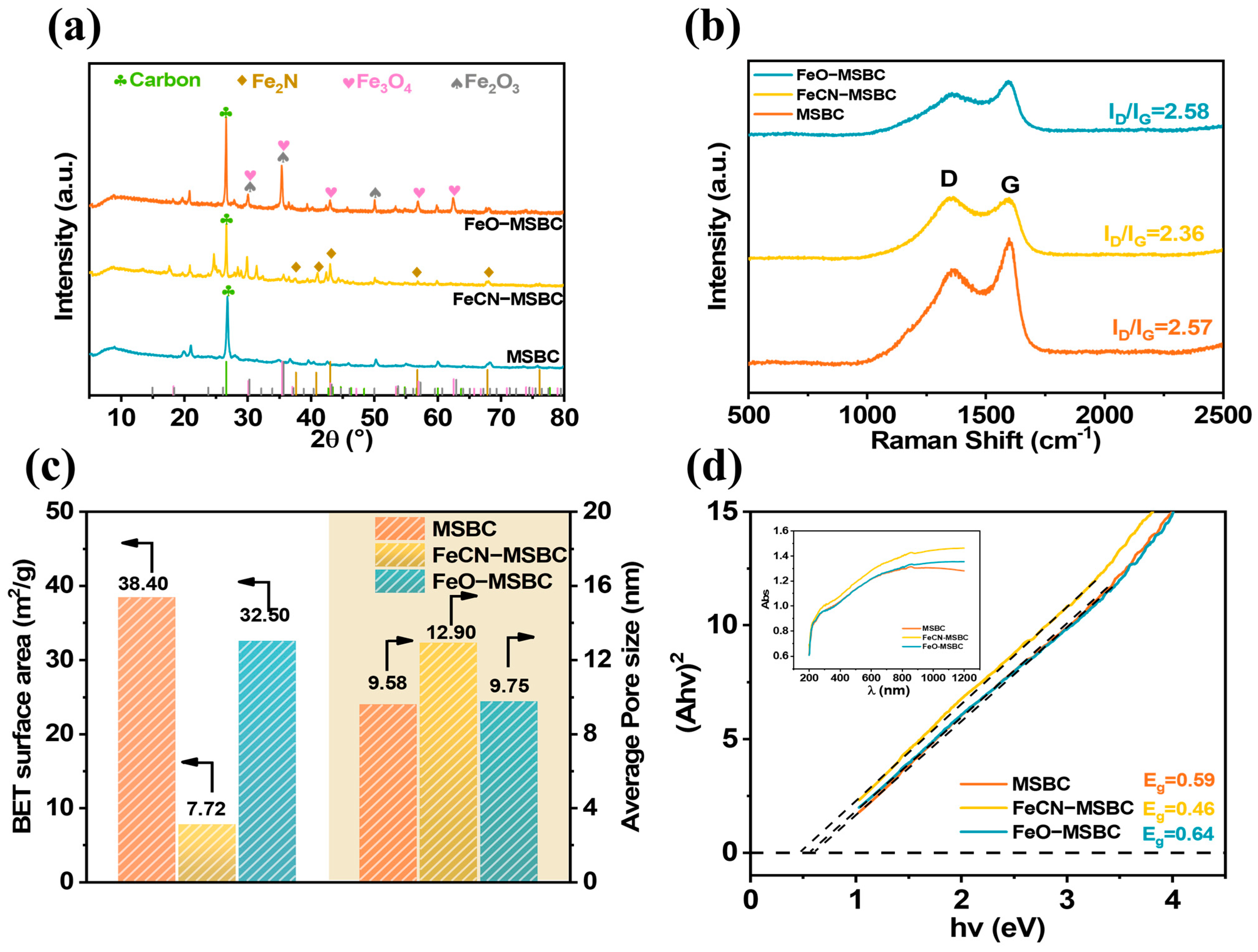
2.1. Characterizations of MSBC with Different Fe Ball Milling Treatment
2.2. Distinguishing Key Factors Affecting the Phenol Degradation in the MSBC/PMS Systems
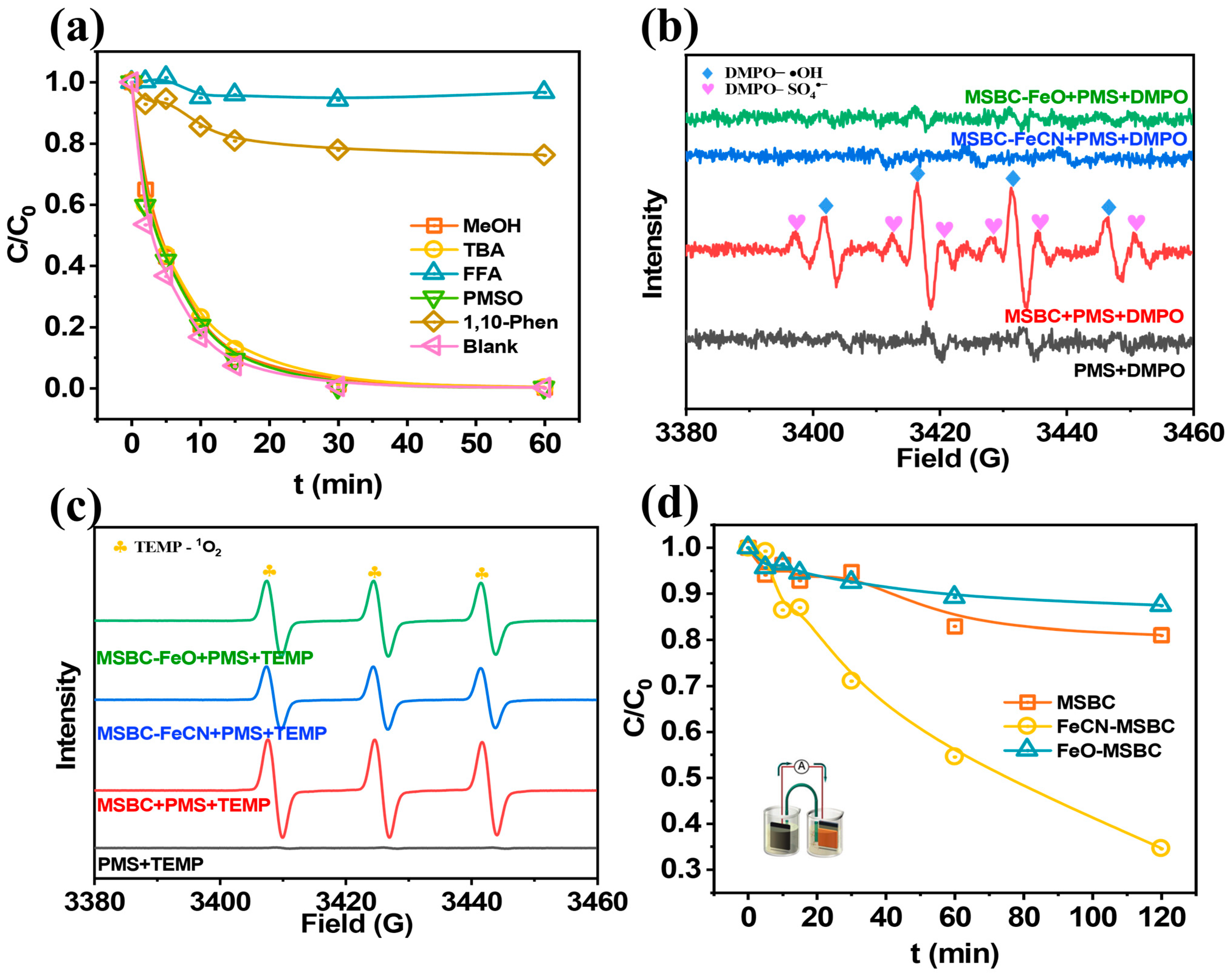
2.3. Examinations of the Dominant Reactive Species in FeCN-MSBC/PMS System
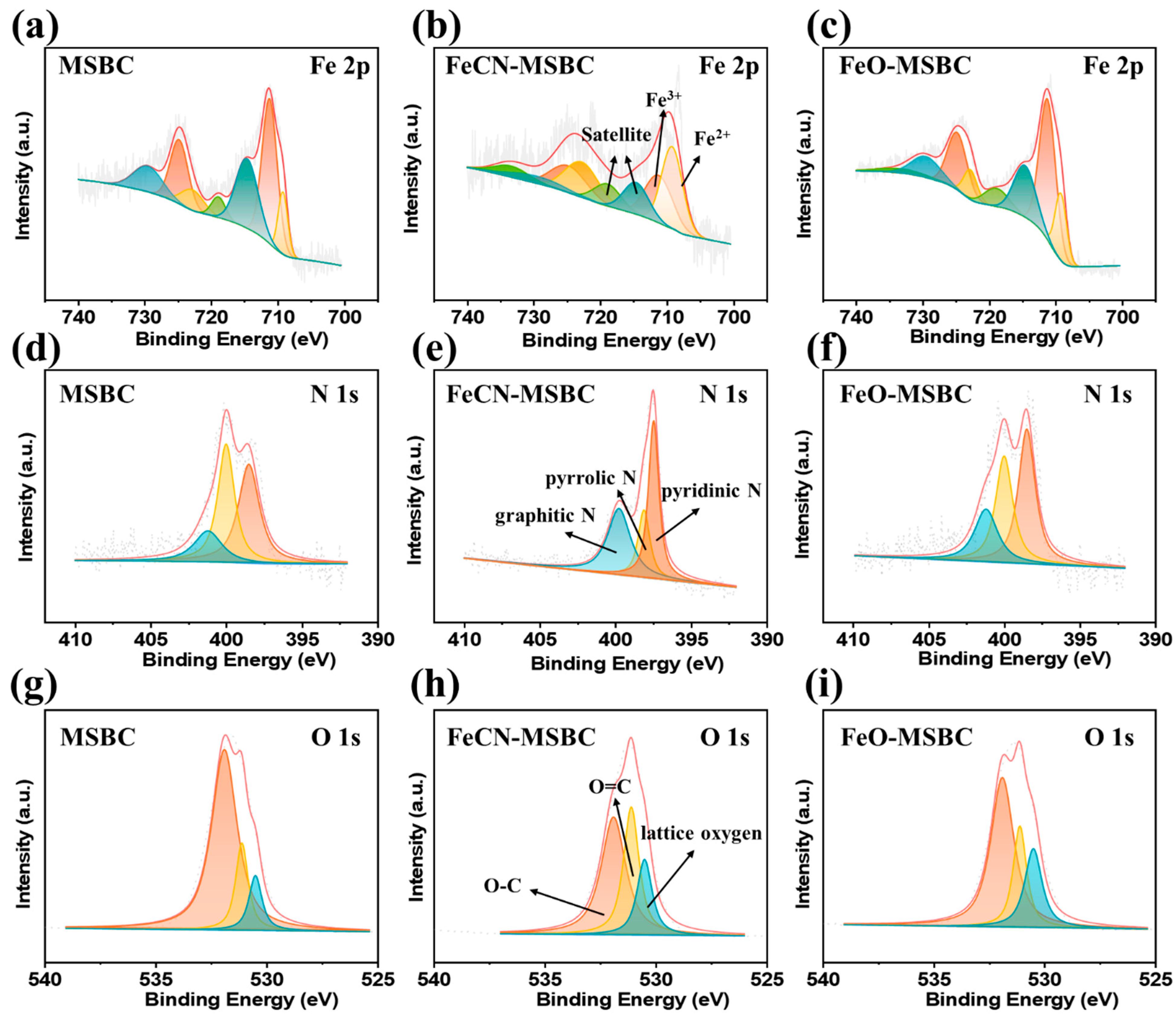
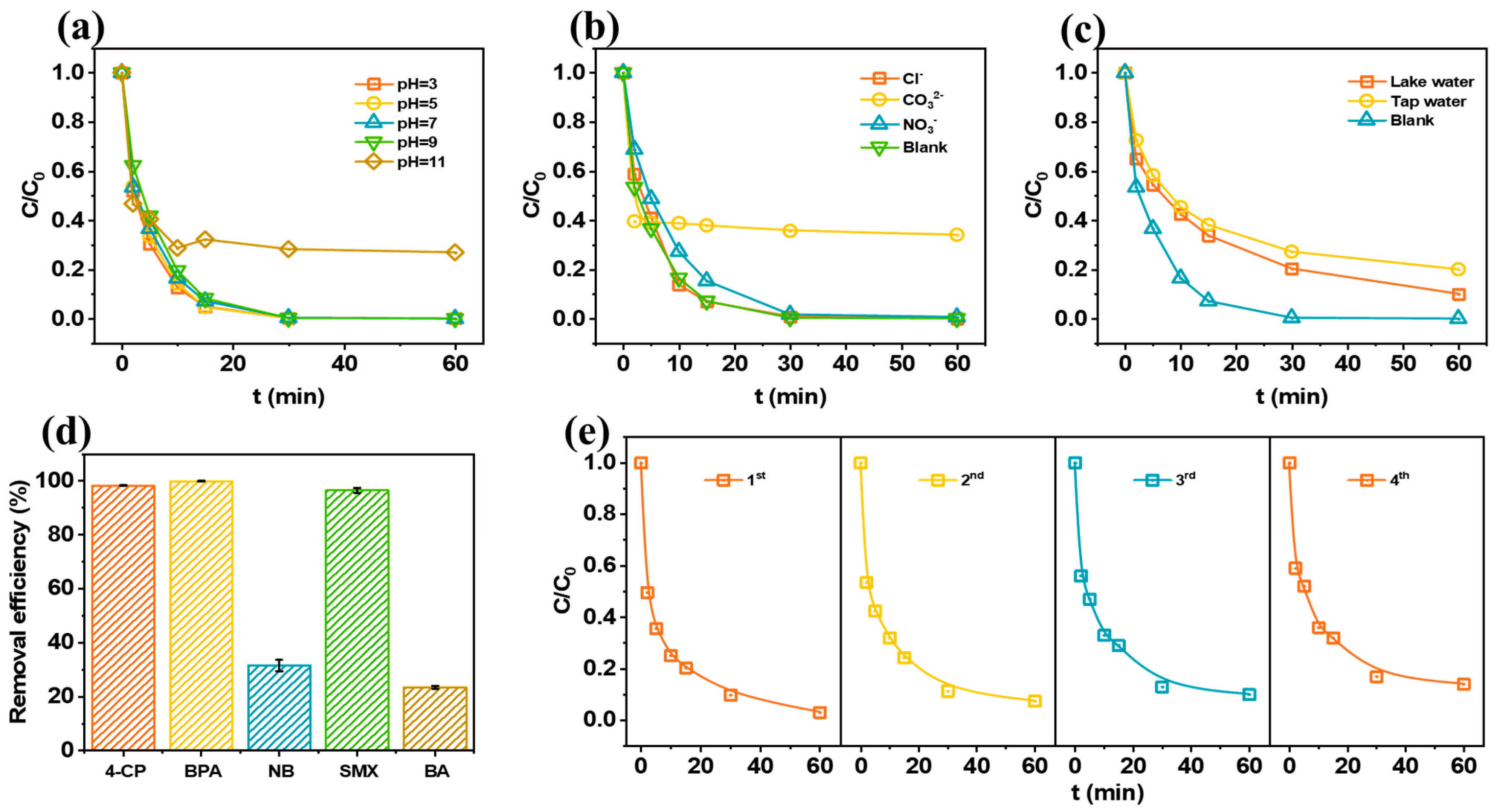
2.4. Key Factors Affecting the Catalytic Mechanism of the FeCN-MSBC/PMS System
2.5. Application of FeCN-MSBC/PMS in Complex Water Matrices
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Biochars
3.3. Degradation Experiments
3.4. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Samer, M. Biological and Chemical Wastewater Treatment. In Wastewater Treatment Engineering; InTech: Rijeka, Croatia, 2015; pp. 1–50. [Google Scholar]
- Tang, J.; Zhang, C.; Shi, X.; Sun, J.; Cunningham, J.A. Municipal wastewater treatment plants coupled with electrochemical, biological and bio-electrochemical technologies: Opportunities and challenge toward energy self-sufficiency. J. Environ. Manag. 2019, 234, 396–403. [Google Scholar] [CrossRef]
- Mantis, I.; Voutsa, D.; Samara, C. Assessment of the environmental hazard from municipal and industrial wastewater treatment sludge by employing chemical and biological methods. Ecotoxicol. Environ. Saf. 2005, 62, 397–407. [Google Scholar] [CrossRef]
- Deleris, S.; Geaugey, V.; Camacho, P.; Debellefontaine, H.; Paul, E. Minimization of sludge production in biological processes: An alternative solution for the problem of sludge disposal. Water Sci. Technol. 2002, 46, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ødegaard, H.; Paulsrud, B.; Karlsson, I. Wastewater sludge as a resource: Sludge disposal strategies and corresponding treatment technologies aimed at sustainable handling of wastewater sludge. Water Sci. Technol. 2002, 46, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A sustainable paradigm of sewage sludge biochar: Valorization, opportunities, challenges and future prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Goldan, E.; Nedeff, V.; Barsan, N.; Culea, M.; Tomozei, C.; Panainte-Lehadus, M.; Mosnegutu, E. Evaluation of the use of sewage sludge biochar as a soil amendment—A review. Sustainability 2022, 14, 5309. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage sludge derived biochar for environmental improvement: Advances, challenges, and solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical review of the science and sustainability of persulphate advanced oxidation processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, Z.; Duan, X.; Long, M.; Spinney, R.; Dionysiou, D.D.; Xiao, R.; Alvarez, P.J. Merits and limitations of radical vs. nonradical pathways in persulfate-based advanced oxidation processes. Environ. Sci. Technol. 2023, 57, 12153–12179. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Liang, C.; Guo, Y.-Y.; Chien, Y.-C.; Wu, Y.-J. Oxidative degradation of MTBE by pyrite-activated persulfate: Proposed reaction pathways. Ind. Eng. Chem. Res. 2010, 49, 8858–8864. [Google Scholar] [CrossRef]
- Fang, G.-D.; Dionysiou, D.D.; Wang, Y.; Al-Abed, S.R.; Zhou, D.-M. Sulfate radical-based degradation of polychlorinated biphenyls: Effects of chloride ion and reaction kinetics. J. Hazard. Mater. 2012, 227, 394–401. [Google Scholar] [CrossRef]
- Saien, J.; Soleymani, A.; Sun, J. Parametric optimization of individual and hybridized AOPs of Fe2+/H2O2 and UV/S2O82− for rapid dye destruction in aqueous media. Desalination 2011, 279, 298–305. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zhou, Y.; Feng, H.; Ren, X.; Tang, J.; Wang, J.; Deng, L.; Shao, B. Non-radical oxidation by N, S, P co-doped biochar for persulfate activation: Different roles of exogenous P/S doping, and electron transfer path. J. Clean. Prod. 2022, 374, 133995. [Google Scholar] [CrossRef]
- Yu, J.; Gong, Z.; Wang, S.; Zhong, H.; Tao, Y.; Hou, Y.; Fu, Q.; Yang, H.; Li, J.; Wang, J. Two major deactivation mechanisms in carbon-based advanced oxidation processes (AOPs) dominated by electron-transfer pathway (ETP). Appl. Catal. B Environ. Energy 2025, 364, 124850. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, M.; Shi, Q.; Song, Y.; Yang, L.; Zhang, J.; Li, Z.; Zhu, W. Sludge-derived biochar applied in peroxymonosulfate (PMS) activation: Reactive oxygen species (ROS) dominated process and characteristics. J. Environ. Chem. Eng. 2023, 11, 111365. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater. Chem. Eng. J. 2019, 356, 350–358. [Google Scholar] [CrossRef]
- Udayanga, W.C.; Veksha, A.; Giannis, A.; Lisak, G.; Lim, T.-T. Effects of sewage sludge organic and inorganic constituents on the properties of pyrolysis products. Energy Convers. Manag. 2019, 196, 1410–1419. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Kang, J.; Wang, S. N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. Acs Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Liu, P.; Bai, X.; Du, X.; Jin, P.; Yang, L.; Jin, X.; Shi, X.; Wang, Y. Peroxymonosulfate activation by nitrogen-doped biochar from sawdust for the efficient degradation of organic pollutants. Chem. Eng. J. 2020, 387, 124065. [Google Scholar] [CrossRef]
- Byambaa, B.; Kim, E.-J.; Seid, M.G.; An, B.-M.; Cho, J.; Aung, S.L.; Song, K.G. Synthesis of N-doped sludge biochar using the hydrothermal route-enabled carbonization method for the efficient degradation of organic pollutants by peroxymonosulfate activation. Chem. Eng. J. 2023, 456, 141037. [Google Scholar] [CrossRef]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Li, Y.; Wei, Y.; Xu, G.; Hu, F. High efficiency degradation of tetracycline by peroxymonosulfate activated with Fe/NC catalysts: Performance, intermediates, stability and mechanism. Environ. Res. 2022, 205, 112538. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, B.; Liu, J.; Sillanpää, M.; Kim, Y. The oxidation treatment of pharmaceutical wastewater in H2O2 and PMS system by Iron-containing biochar originated from excess sludge. J. Water Process Eng. 2024, 58, 104833. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Liu, H.; Liu, H.; Tao, J.; Cui, M.H.; Zheng, Z.; Wen, D.; Zhan, X. Fe(x)N produced in pharmaceutical sludge biochar by endogenous Fe and exogenous N doping to enhance peroxymonosulfate activation for levofloxacin degradation. Water Res. 2022, 224, 119022. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Kan, E. FeCl3-activated biochar catalyst for heterogeneous Fenton oxidation of antibiotic sulfamethoxazole in water. Chemosphere 2022, 306, 135554. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Zhang, Y. Modification of biochar by Fe2O3 for the removal of pyridine and quinoline. Environ. Technol. 2018, 39, 1470–1480. [Google Scholar] [CrossRef]
- Luo, J.; Yi, Y.; Fang, Z. Nitrogen-rich magnetic biochar prepared by urea was used as an efficient catalyst to activate persulfate to degrade organic pollutants. Chemosphere 2023, 339, 139614. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Zhou, H. Preparation of N-doped biochar from sewage sludge and melamine for peroxymonosulfate activation: N-functionality and catalytic mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhong, X.; Gao, L.; Hu, J.; Peng, W.; Wang, X.; Zhou, G.; Xu, B. Asymmetric coordination of bimetallic Fe–Co single-atom pairs toward enhanced bifunctional activity for rechargeable zinc–air batteries. ACS Nano 2024, 18, 13006–13018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Liu, J.; Zhang, L.; Abazari, R.; Li, T.-T.; Qian, J. Iron phthalocyanine coupled with Co-Nx sites in carbon nanostraws for Zn-Air batteries. Chem. Eng. J. 2025, 503, 158343. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Yang, P.; Wan, Y.; Wang, D.; Xu, H.; Liu, L.; Xiao, L.; Li, R.; Wang, G. Atomically dispersed Fe-NC catalyst with densely exposed Fe-N4 active sites for enhanced oxygen reduction reaction. Chem. Eng. J. 2024, 485, 149529. [Google Scholar] [CrossRef]
- Ahmed, S.; Yoon, W.; Jo, H.; Irshad, M.; Khan, M.K.; Kim, J. Structure-activity relationship and deactivation behavior of iron oxide during CO2 hydrogenation. Chem. Eng. J. 2024, 499, 156104. [Google Scholar] [CrossRef]
- Liang, C.; Sun, H.; Ling, C.; Liu, X.; Li, M.; Zhang, X.; Guo, F.; Zhang, X.; Shi, Y.; Cao, S.; et al. Pyrolysis temperature-switchable Fe-N sites in pharmaceutical sludge biochar toward peroxymonosulfate activation for efficient pollutants degradation. Water Res. 2023, 228 Pt A, 119328. [Google Scholar] [CrossRef]
- Bayoka, H.; Snoussi, Y.; Bhakta, A.K.; El Garah, M.; Khalil, A.M.; Jouini, M.; Ammar, S.; Chehimi, M.M. Evidencing the synergistic effects of carbonization temperature, surface composition and structural properties on the catalytic activity of biochar/bimetallic composite. J. Anal. Appl. Pyrolysis 2023, 173, 106069. [Google Scholar] [CrossRef]
- Urbonaite, S.; Hälldahl, L.; Svensson, G. Raman spectroscopy studies of carbide derived carbons. Carbon 2008, 46, 1942–1947. [Google Scholar] [CrossRef]
- Ni, Z.; Fan, H.; Fan, X.; Wang, H.; Zheng, Z.; Feng, Y.; Wu, Y.; Shen, Z. High temperature Raman spectroscopy studies of carbon nanowalls. J. Raman Spectrosc. 2007, 38, 1449–1453. [Google Scholar] [CrossRef]
- Chen, C.; Sun, K.; Huang, C.; Yang, M.; Fan, M.; Wang, A.; Zhang, G.; Li, B.; Jiang, J.; Xu, W. Investigation on the mechanism of structural reconstruction of biochars derived from lignin and cellulose during graphitization under high temperature. Biochar 2023, 5, 51. [Google Scholar] [CrossRef]
- Liu, H.; Ye, C.; Xu, Y.; Wang, Q. Effect of activation conditions and iron loading content on the catalytic cracking of toluene by biochar. Energy 2022, 247, 123409. [Google Scholar] [CrossRef]
- Yang, Y.; Banerjee, G.; Brudvig, G.W.; Kim, J.-H.; Pignatello, J.J. Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ. Sci. Technol. 2018, 52, 5911–5919. [Google Scholar] [CrossRef]
- Zhu, R.; Shi, Y.; Zhou, Y.; Xia, Y.; Qi, Q.; Liang, X.; Kuang, M.; Jia, Z.; Wang, M.; Yu, C. Highly reactive iron/nickel bimetallic biochar composites for highly efficient remediation of Cr (vi). New J. Chem. 2025, 49, 4427–4437. [Google Scholar] [CrossRef]
- Sun, Y.; Jing, Y.; Wang, Z.; Huang, M.; Oh, W.-D.; Wu, X.; Zhou, T. Regulating sulfur vacancies formation on mechanochemically modified pyrite to steer non-radical molecular oxygen activation for water purification. Sep. Purif. Technol. 2025, 353, 128572. [Google Scholar] [CrossRef]
- Zhang, L.S.; Jiang, X.H.; Zhong, Z.A.; Tian, L.; Sun, Q.; Cui, Y.T.; Lu, X.; Zou, J.P.; Luo, S.L. Carbon Nitride Supported High-Loading Fe Single-Atom Catalyst for Activation of Peroxymonosulfate to Generate 1O2 with 100 % Selectivity. Angew. Chem. Int. Ed. 2021, 60, 21751–21755. [Google Scholar] [CrossRef]
- Wang, B.-B.; Shi, X.; Liu, X.-T.; Zou, J.-T.; Li, H.-J.; Peng, D.-C.; He, F. Insight into the fenton-induced degradation process of extracellular polymeric substances (EPS) extracted from activated sludge. Chemosphere 2019, 234, 318–327. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Li, J.; Wei, Z.; Duan, W.; Chen, F. Enhancing effects of sludge biochar on aerobic granular sludge for wastewater treatment. Processes 2022, 10, 2385. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Q.; Chen, Y.; Guo, T.; Zhang, Y.; Huang, Z.; Hu, H.; Gan, T. Single-atom Fe catalysts anchored on graphitized chitin-derived carbon for improved anti-interference ability of Fenton-like reaction via non-radical pathway. Appl. Surf. Sci. 2024, 660, 160020. [Google Scholar] [CrossRef]

| Date of Drawing | TSS g/L | VSS g/L | Moisture Content | pH | CST s L/g |
|---|---|---|---|---|---|
| 20 February 2024 | 31.56 | 24.12 | 93.5% | 6.53 | 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Ding, S.; Huang, M.; Oh, W.-d.; Wu, X.; Zhou, T. One-Step Fe/N Co-Doping for Efficient Catalytic Oxidation and Selective Non-Radical Pathway Degradation in Sludge-Based Biochar. Catalysts 2025, 15, 934. https://doi.org/10.3390/catal15100934
Gong Z, Ding S, Huang M, Oh W-d, Wu X, Zhou T. One-Step Fe/N Co-Doping for Efficient Catalytic Oxidation and Selective Non-Radical Pathway Degradation in Sludge-Based Biochar. Catalysts. 2025; 15(10):934. https://doi.org/10.3390/catal15100934
Chicago/Turabian StyleGong, Zupeng, Shixuan Ding, Mingjie Huang, Wen-da Oh, Xiaohui Wu, and Tao Zhou. 2025. "One-Step Fe/N Co-Doping for Efficient Catalytic Oxidation and Selective Non-Radical Pathway Degradation in Sludge-Based Biochar" Catalysts 15, no. 10: 934. https://doi.org/10.3390/catal15100934
APA StyleGong, Z., Ding, S., Huang, M., Oh, W.-d., Wu, X., & Zhou, T. (2025). One-Step Fe/N Co-Doping for Efficient Catalytic Oxidation and Selective Non-Radical Pathway Degradation in Sludge-Based Biochar. Catalysts, 15(10), 934. https://doi.org/10.3390/catal15100934







