Engineering Photocatalytic Membrane Reactors for Sustainable Energy and Environmental Applications
Abstract
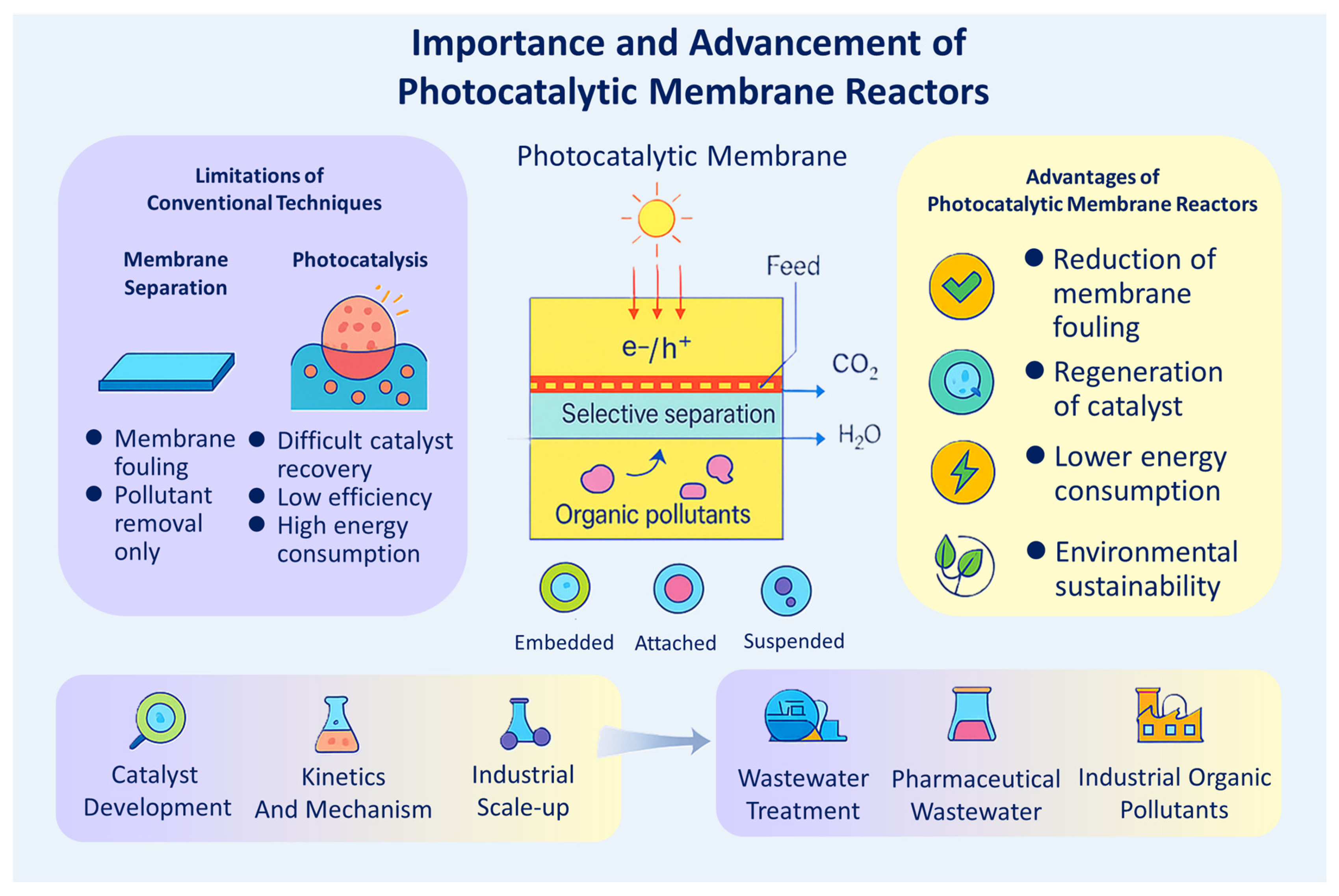
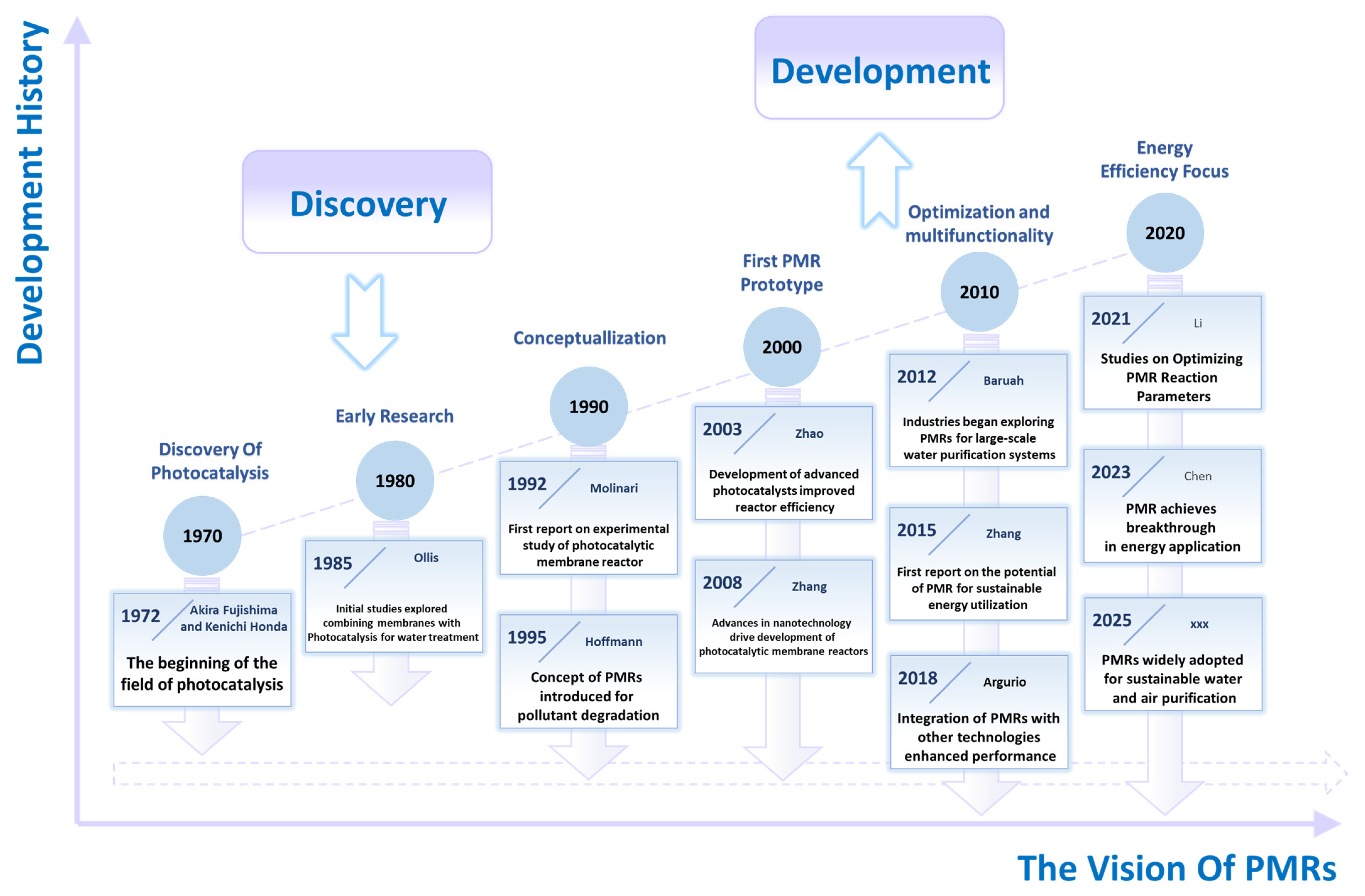
1. Introduction
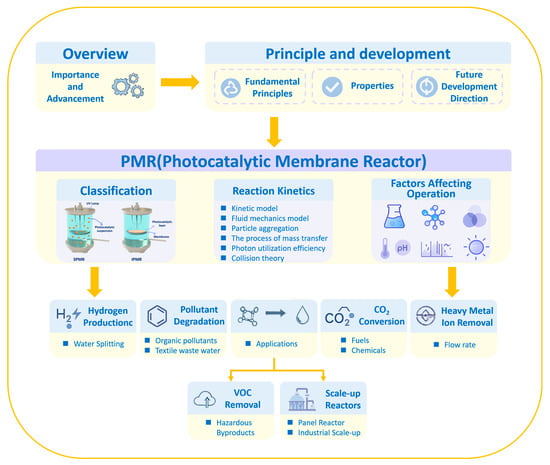
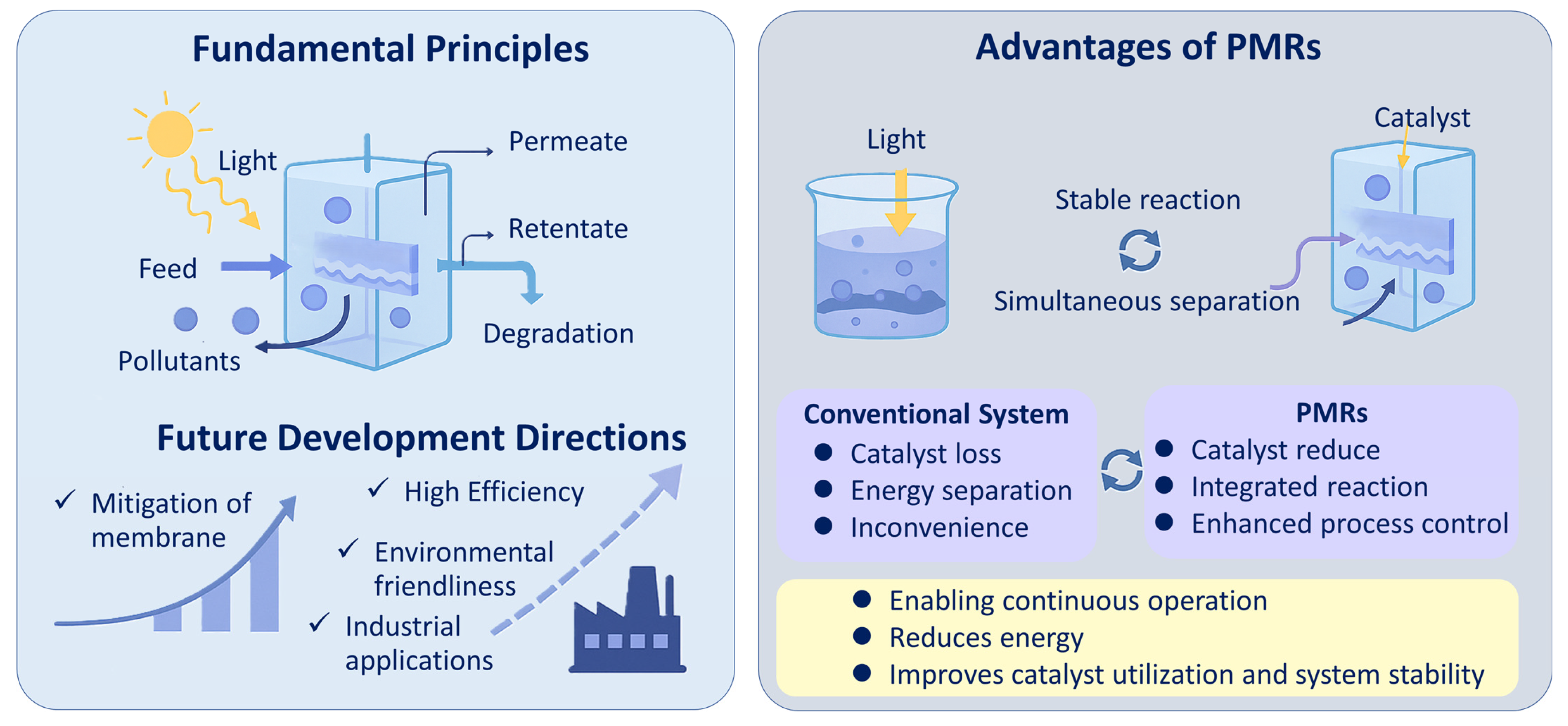
2. Principles and Developments of PMRs
3. Photocatalytic Membrane Reactors
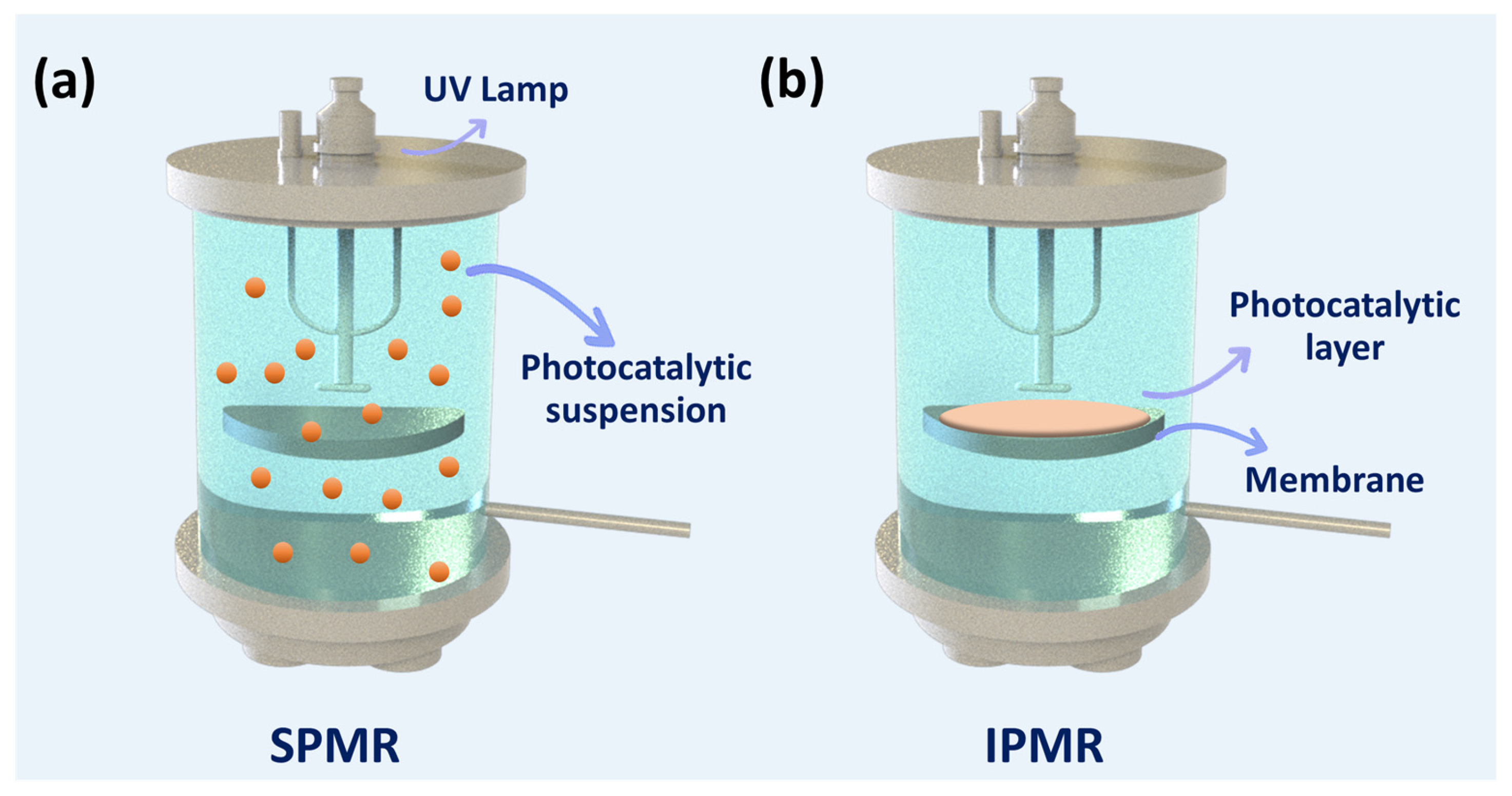
3.1. Classifications
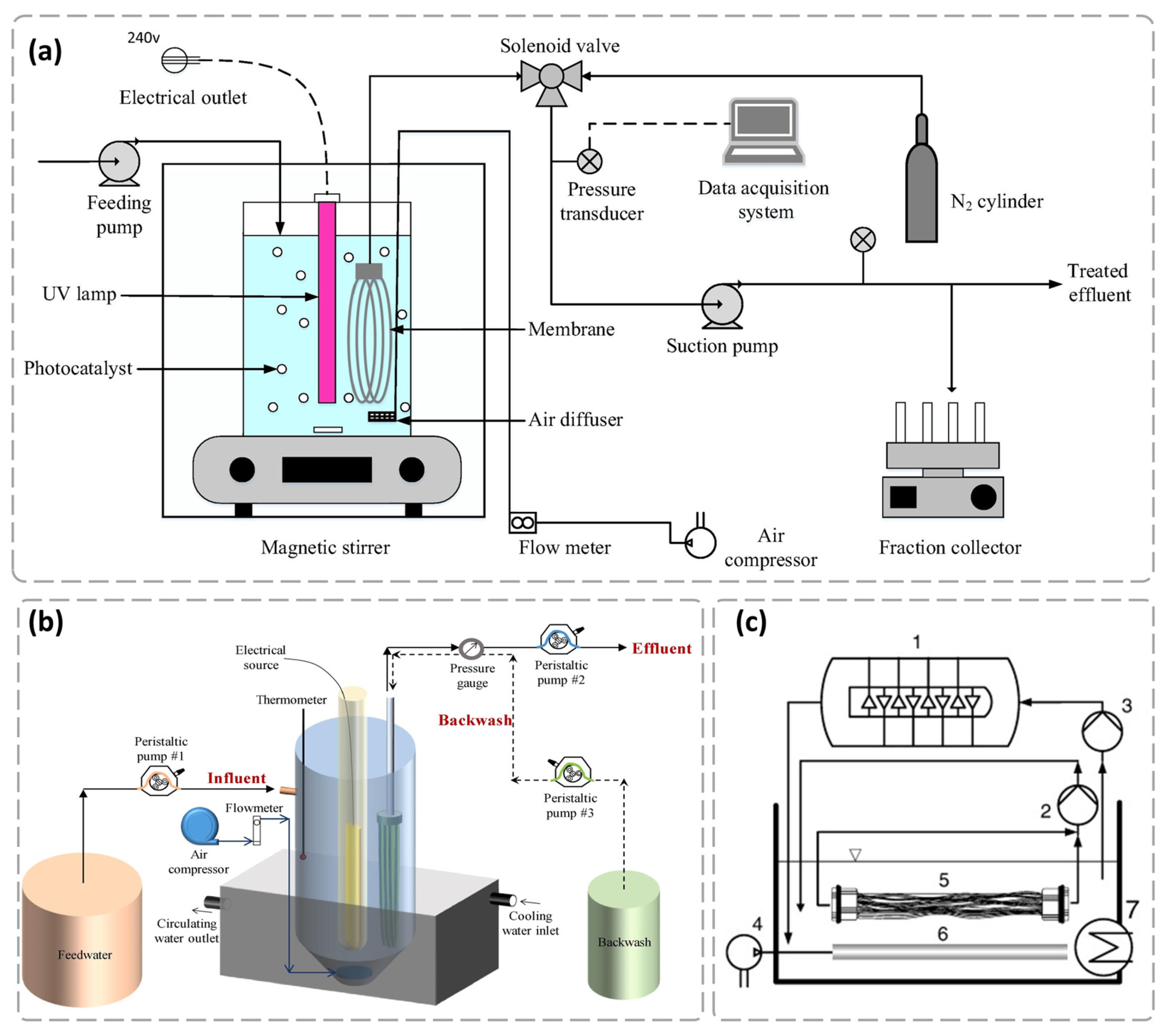
3.1.1. Slurry Photocatalytic Membrane Reactor (SPMR)
3.1.2. Immobilized Photocatalytic Membrane Reactor (IPMR)
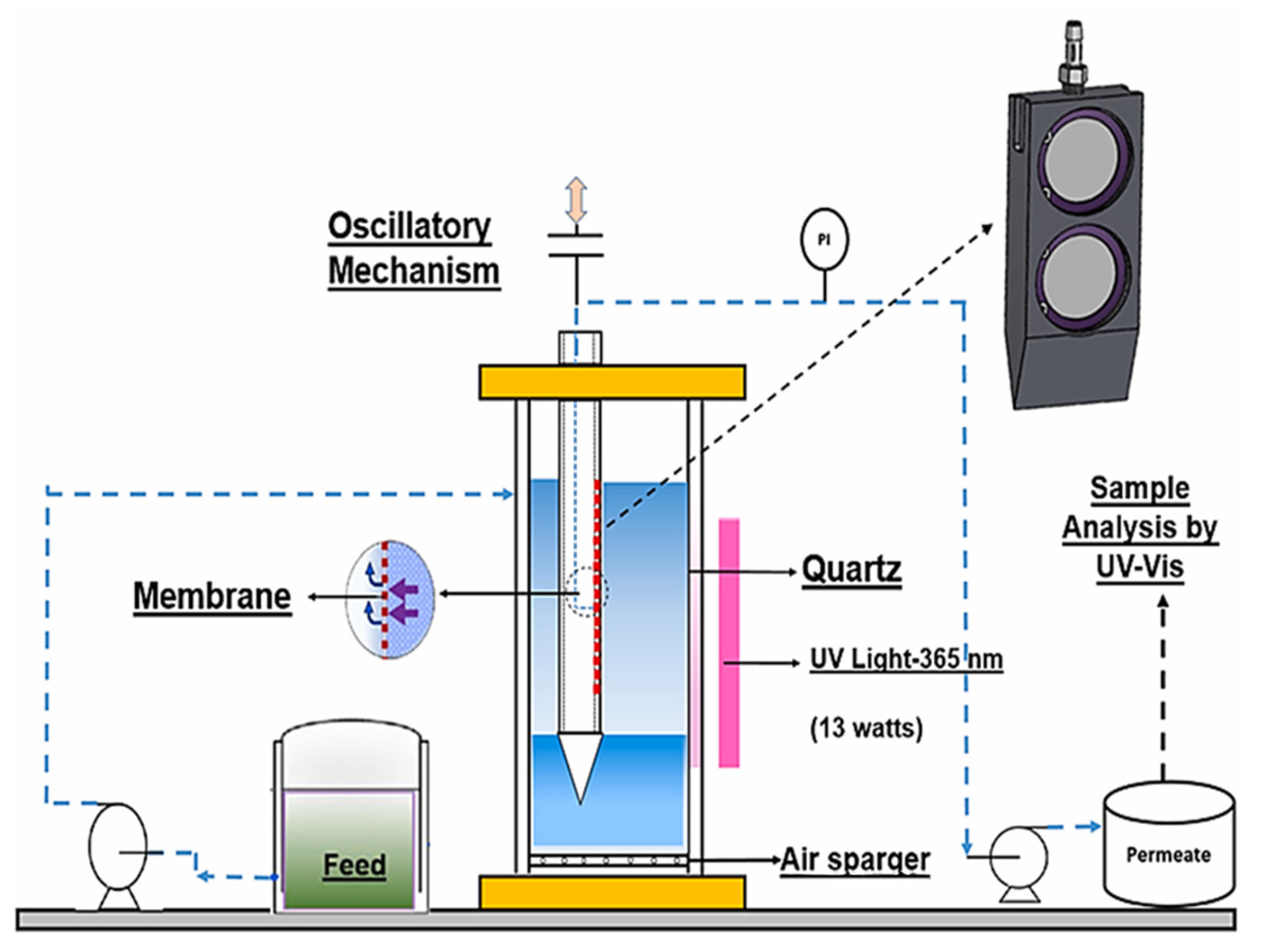
3.1.3. Other Types
Submerged Photocatalytic Membrane
Oscillating Photocatalytic Membrane
3.2. Kinetics in PMRs
3.2.1. Kinetic Model
3.2.2. Fluid Mechanics Model
3.2.3. Particle Aggregation
3.2.4. Mass Transmission
3.2.5. Photon Utilization
3.2.6. Collision Theory
3.3. Factors Affecting PMR Performances

4. Advances of PMRs in Different Applications
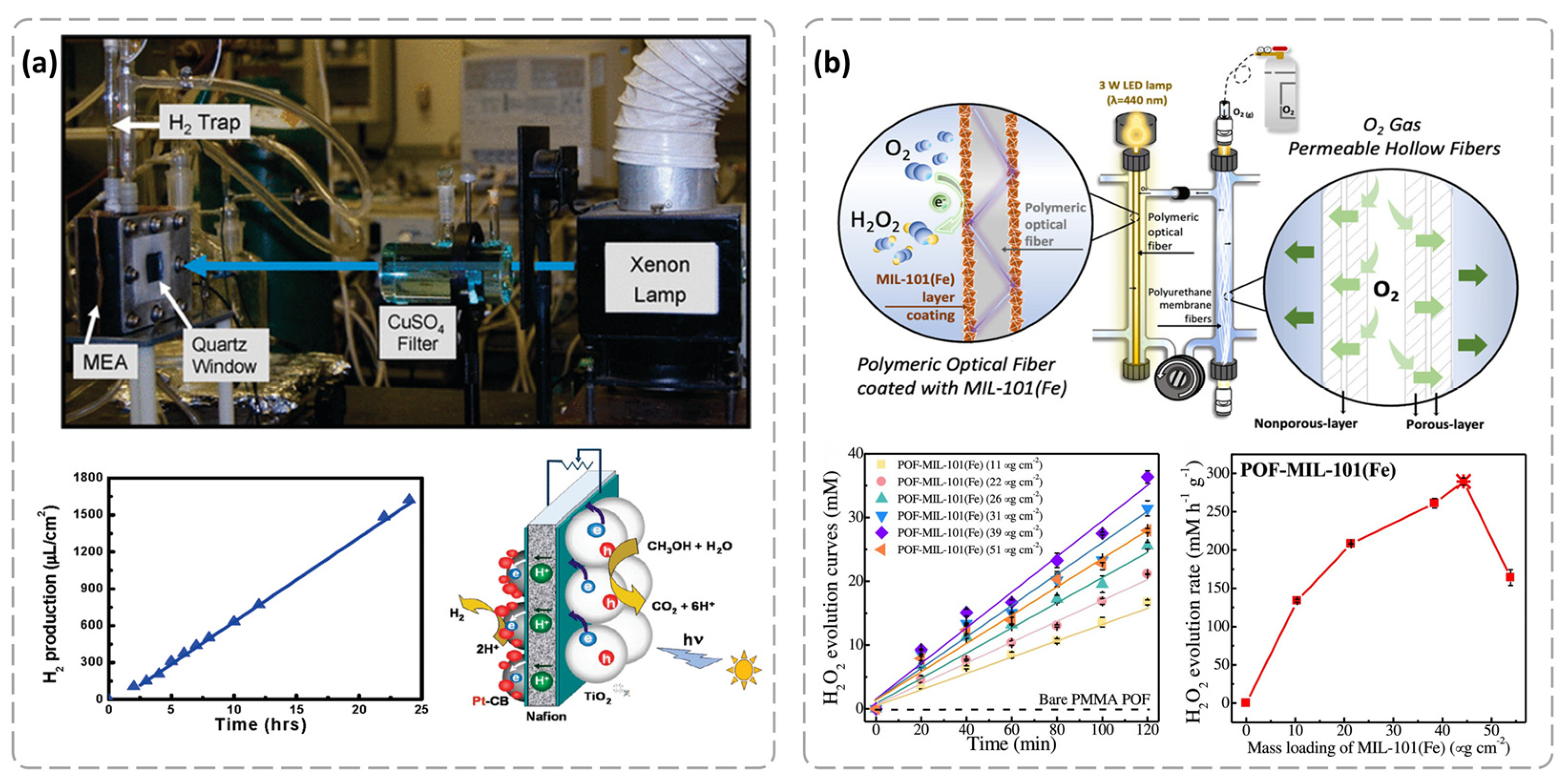
4.1. Photocatalytic Water Splitting to Produce Hydrogen
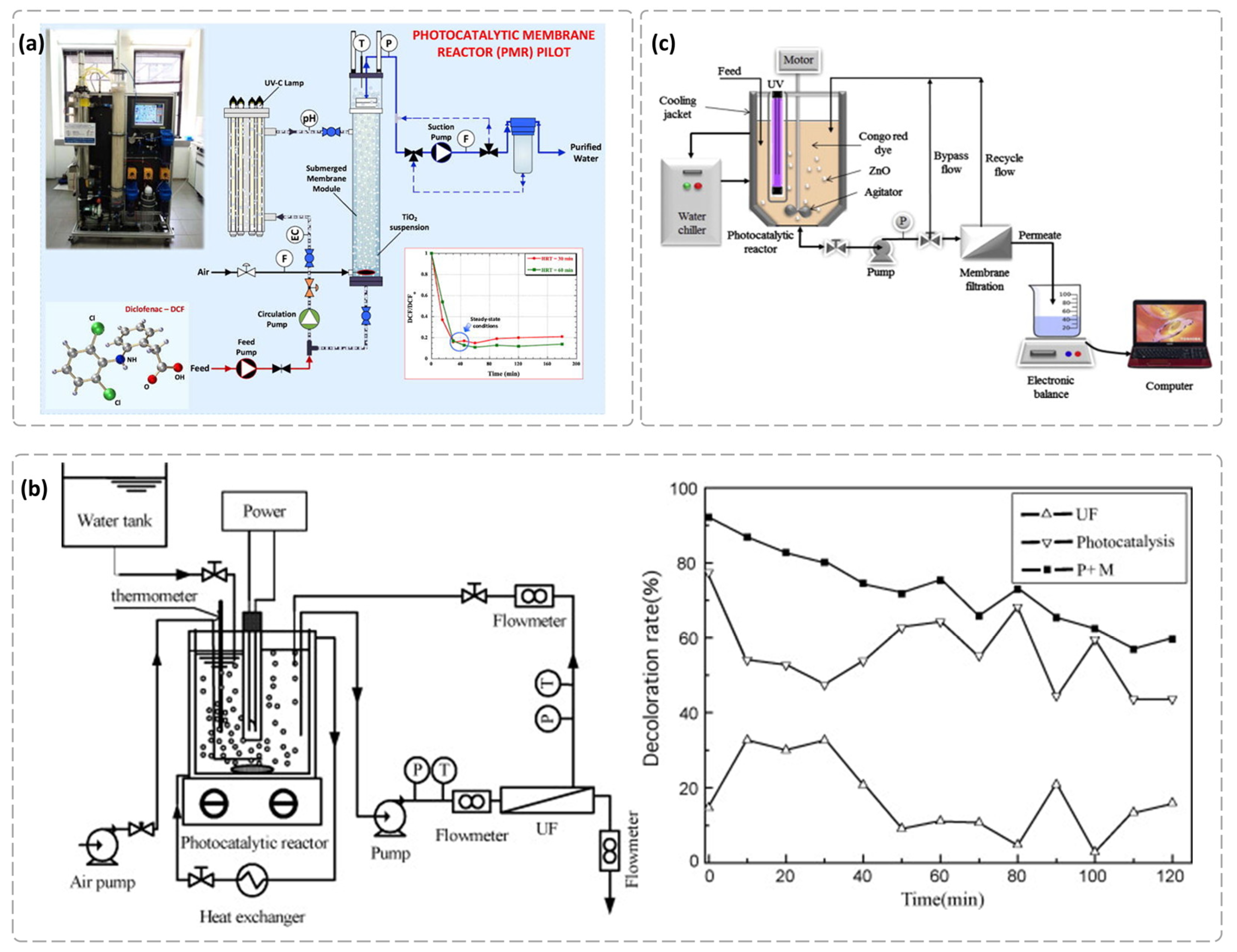
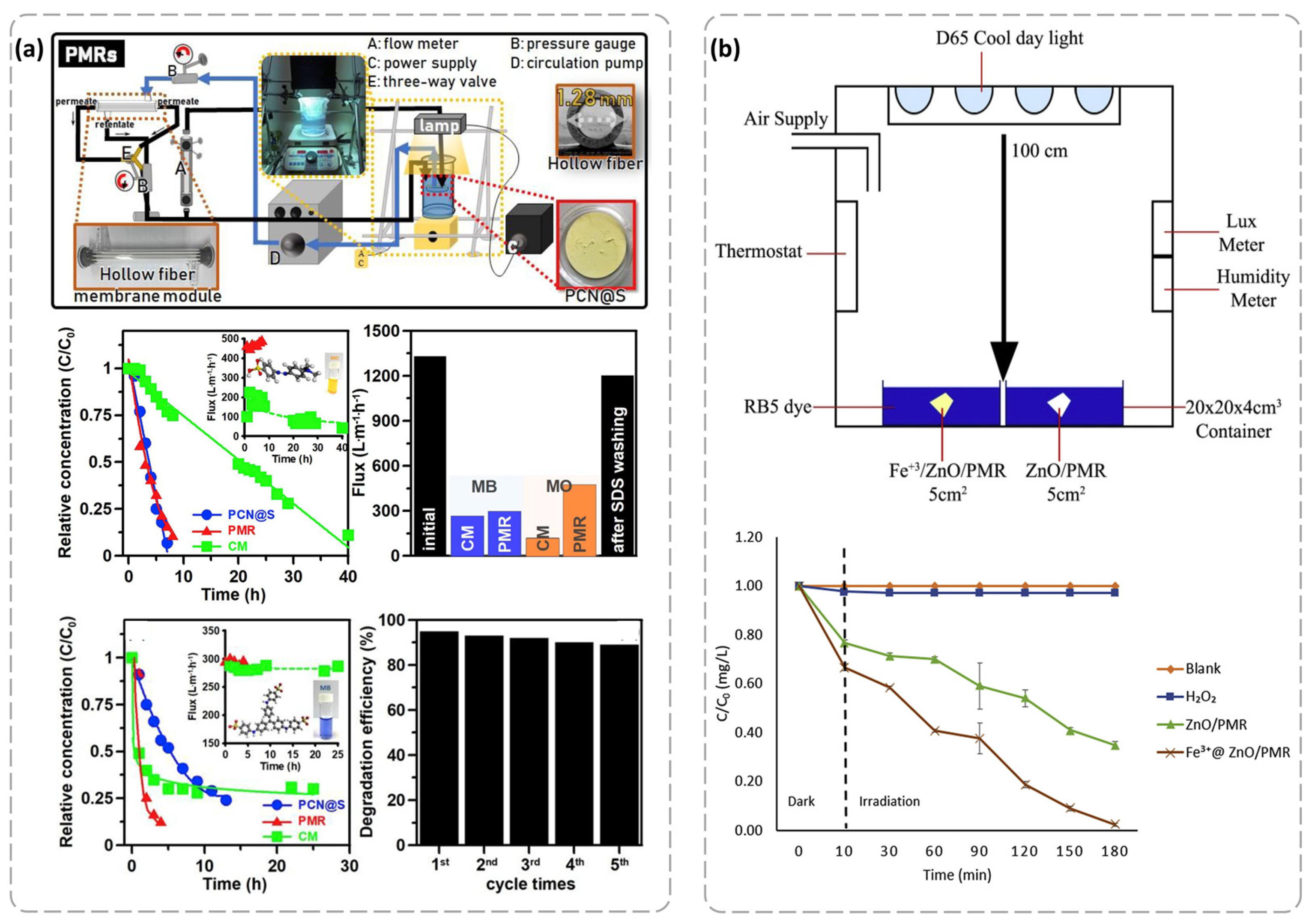
4.2. Removal of Organic Pollutants from Water
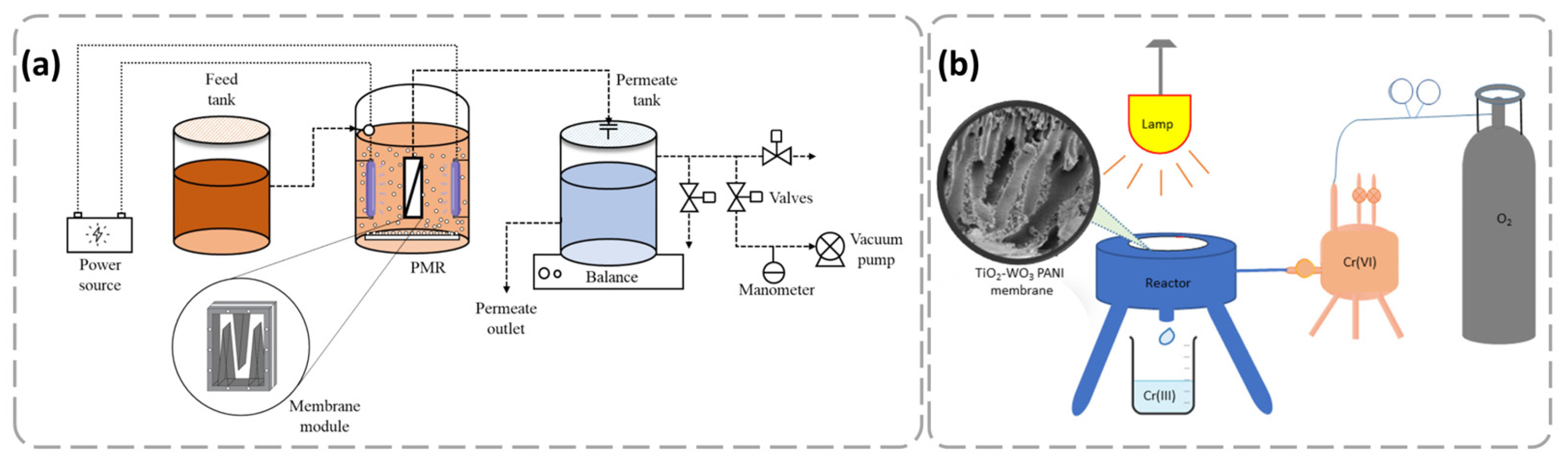
4.3. Heavy Metal Ion Removal
4.4. CO2 Reduction
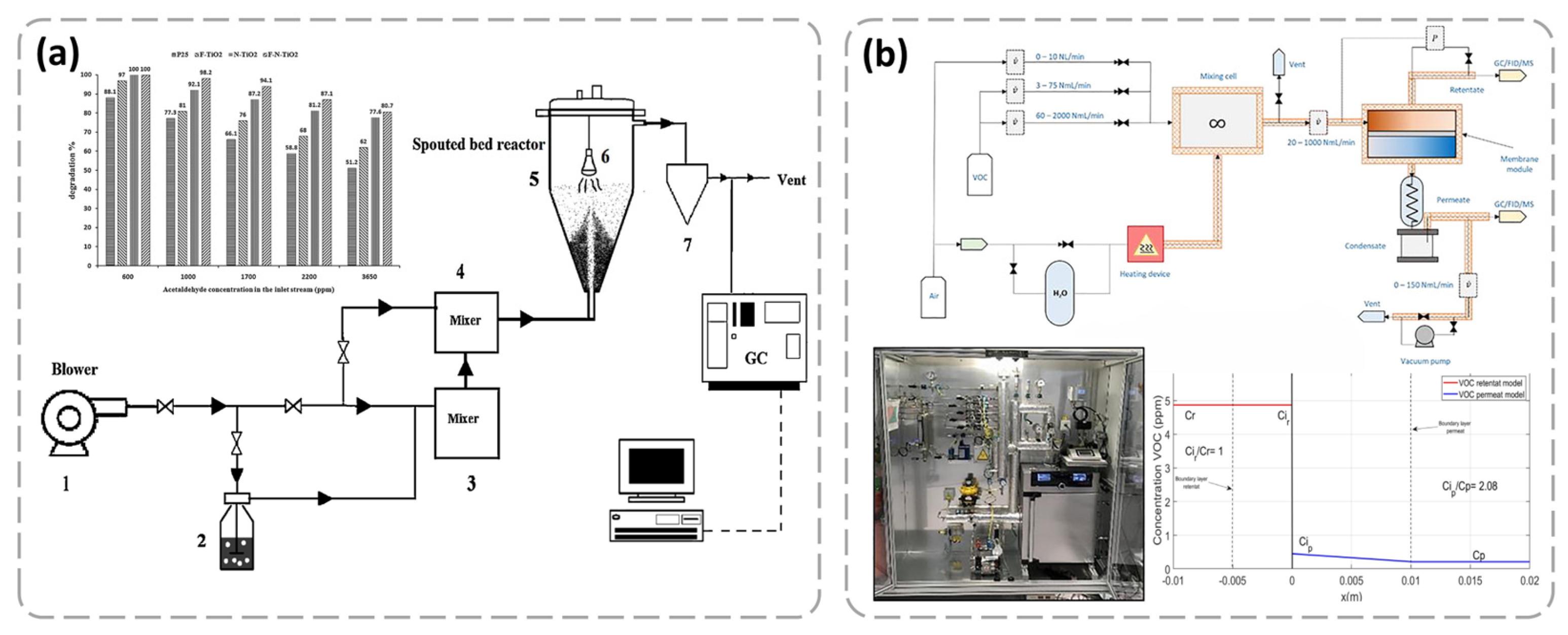
4.5. VOC Removal
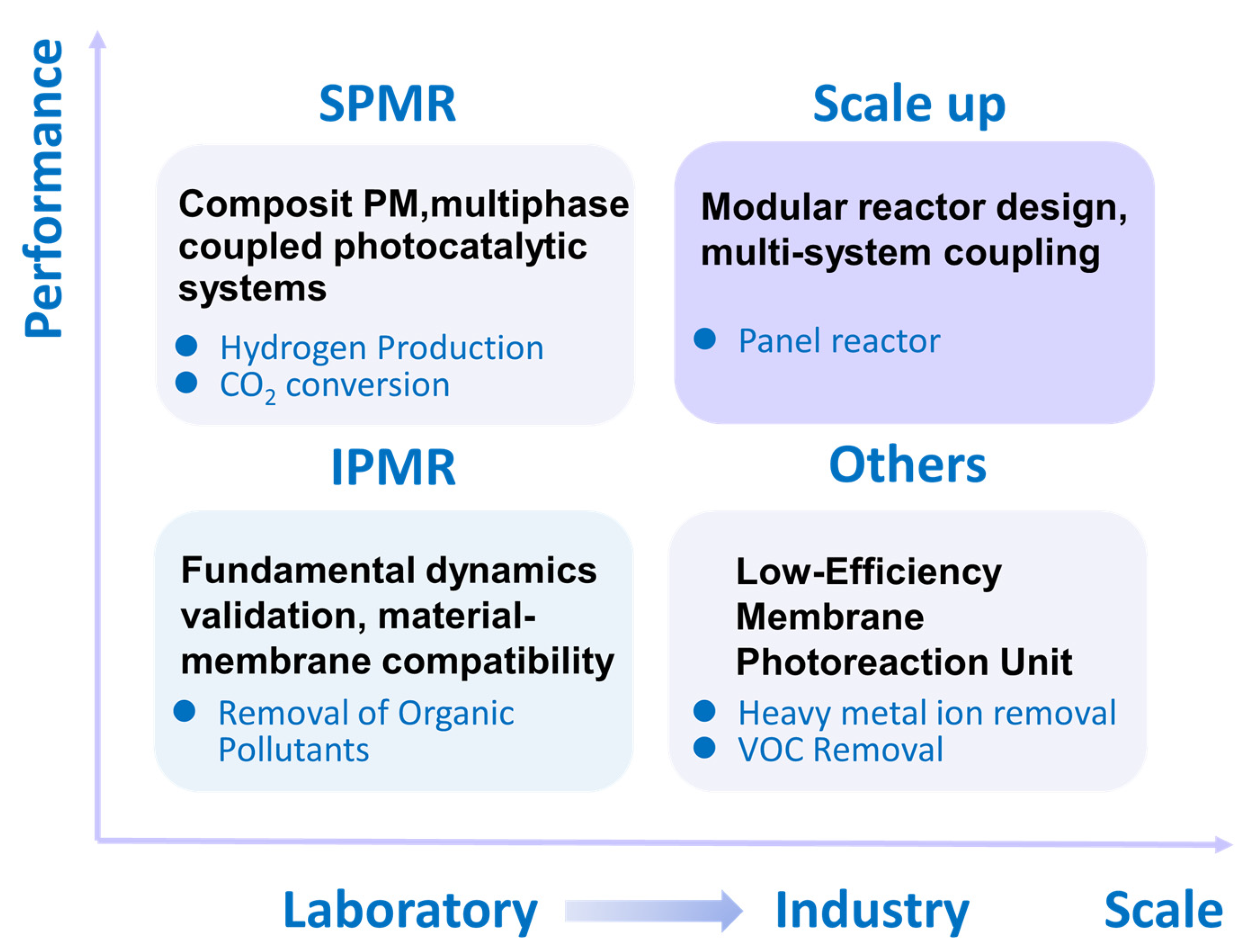
4.6. Amplification of Photocatalytic Reactors
5. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, L.; Zhang, J.; Lu, T.; Zhou, G.; Ren, Y.; Zheng, Z.; Yuan, X.; Wang, S.; He, Z. High-strength TiO2/TPU composite fiber based textiles for organic pollutant removal. npj Clean Water 2024, 7, 98. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, H.; Lu, T.; Zheng, Z.; Tian, S.; Xiang, L.; Zhao, S.; Wang, S.; He, Z. Synergistic Cu single-atoms and clusters on tubular carbon nitride for efficient photocatalytic performances. Rare Met. 2024, 43, 5891–5904. [Google Scholar] [CrossRef]
- Zheng, Z.Y.; Tian, S.; Feng, Y.X.; Zhao, S.; Li, X.; Wang, S.G.; He, Z.L. Recent advances of photocatalytic coupling technologies for wastewater treatment. Chin. J. Catal. 2023, 54, 88–136. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, S.; Chen, J.; Sun, X.; Zhao, S.; Chang, J.; He, Z. Ti3C2@g-C3N4/TiO2 ternary heterogeneous photocatalyst for promoted photocatalytic degradation activities. Coatings 2023, 13, 655. [Google Scholar] [CrossRef]
- Qu, K.; Li, H.; Sun, J.; Wang, S.; He, Z.; Wang, S. Rationally designed assembled FeV3O8/g-C3N4 heterojunction improve carrier transport separation and efficiently promote the degradation of 2,4,6-TCP by photo-activated peroxymonosulfate. Mater. Sci. Semicond. Process. 2025, 194, 109541. [Google Scholar] [CrossRef]
- Qin, S.; Xu, R.; Jin, Q.; Wang, S.; Ren, Y.; Huang, Y.; Zheng, Z.; Xiao, L.; Zhai, D.; Wang, S.; et al. Efficient photocatalytic reduction of hexavalent chromium by NiCo2S4/BiOBr heterogeneous photocatalysts. Coatings 2024, 14, 1492. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Guo, J.; Zhou, G.H.; Xiang, L.; Wang, S.G.; He, Z.L. Novel TiO2/TPU composite fiber-based smart textiles for photocatalytic applications. Mater. Adv. 2022, 3, 1518–1526. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Lin, L.H.; Jeon, T.H.; Lin, S.; Wang, X.C.; Choi, W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy 2017, 42, 58–68. [Google Scholar] [CrossRef]
- Zhang, S.; He, Z.; Li, X.; Zhang, J.; Zang, Q.; Wang, S. Building heterogeneous nanostructures for photocatalytic ammonia decomposition. Nanoscale Adv. 2020, 2, 3610–3623. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Zhang, P.; Li, X.; Wang, S. ZnWO4-ZnIn2S4 S-scheme heterojunction for enhanced photocatalytic H2 evolution. J. Mater. Sci. Technol. 2022, 122, 231–242. [Google Scholar] [CrossRef]
- Li, X.; Chang, J.; Zhang, S.; Xiao, L.; Wu, X.; He, Z. Microcystis@TiO2 nanoparticles for photocatalytic reduction reactions: Nitrogen fixation and hydrogen evolution. Catalysts 2021, 11, 1443. [Google Scholar] [CrossRef]
- Xiao, L.H.; Li, X.; Zhang, J.; He, Z.L. MgB4 MXene-like nanosheets for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 12779–12787. [Google Scholar] [CrossRef]
- Dai, M.; He, Z.; Cao, W.; Zhang, J.; Chen, W.; Jin, Q.; Que, W.; Wang, S. Rational construction of S-scheme BN/MXene/ZnIn2S4 heterojunction with interface engineering for efficient photocatalytic hydrogen production and chlorophenols degradation. Sep. Purif. Technol. 2023, 309, 123004. [Google Scholar] [CrossRef]
- Cao, W.R.; He, Z.L.; Dai, M.; Wang, G.Z.; Huang, G.H.; Wang, S.G. Electronic structure modulation of bimetallic sulfides for efficient sacrificial-agent-free photocatalytic H2 evolution. ACS Appl. Energy Mater. 2023, 6, 4715–4723. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Dai, M.; Yu, H.; Chen, W.; Qu, K.; Zhai, D.; Liu, C.; Zhao, S.; Wang, S.; He, Z. Boosting photocatalytic activity of CdLa2S4/ZnIn2S4 S-scheme heterojunctions with spatial separation of photoexcited carries. Chem. Eng. J. 2023, 470, 144240. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, H.; Ma, J.; Hu, M.; Li, Q.; Chen, S.; Ning, T.; Ge, C.; Liu, X.; Xiao, L.; et al. Cone shaped surface array structure on an alkaline polymer electrolyte membrane improves fuel cell performance. Acta Phys.-Chim. Sin. 2023, 39, 2111037. [Google Scholar] [CrossRef]
- Sun, J.; Ren, Y.; Dai, M.; Li, H.; Yu, H.; Wang, S.; He, Z.; Wang, S. Efficient photocatalytic degradation of sulfamethazine by carboxylic CNT-decorated Bi2O3/Bi2WO6 heterojunction catalysts. Chem. Res. Chin. Univ. 2025. [Google Scholar] [CrossRef]
- Hong, F.; Jing, T.; Wang, S.; He, Z. Defect engineering of ZnIn2S4 photocatalysts for enhanced hydrogen evolution reaction. Coatings 2025, 15, 1061. [Google Scholar] [CrossRef]
- Chen, W.; Dai, M.; Xiang, L.; Zhao, S.; Wang, S.; He, Z. Assembling S-scheme heterojunction between basic bismuth nitrate and bismuth tungstate with promoting charges’ separation for accelerated photocatalytic sulfamethazine degradation. J. Mater. Sci. Technol. 2024, 171, 185–197. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, H.; Shao, Y.; Wang, P.; Yu, S.; Li, H.; Zhou, Y.; Zhou, Z.; Ma, L.; Tan, C. Silver nanoparticle-modified 2D MOF nanosheets for photothermally enhanced silver ion release antibacterial treatment. Acta Phys.-Chim. Sin. 2023, 39, 2211043. [Google Scholar] [CrossRef]
- Cheng, K.; Liu, C.; Wang, Y.; Jiang, Q.; Zheng, T.; Li, X.; Xia, C. Design of noble metal catalysts and reactors for the electrosynthesis of hydrogen peroxide. Acta Phys.-Chim. Sin. 2025, 41, 100112. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Shi, L.; Wu, Z.; Zhang, S.; Wang, S.; Sun, H. Photocatalysis coupling with membrane technology for sustainable and continuous purification of wastewater. Sep. Purif. Technol. 2024, 329, 125225. [Google Scholar] [CrossRef]
- Romanos, G.E.; Athanasekou, C.P.; Likodimos, V.; Aloupogiannis, P.; Falaras, P. Hybrid ultrafiltration/photocatalytic membranes for efficient water treatment. Ind. Eng. Chem. Res. 2013, 52, 13938–13947. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wan, Y.H.; Luo, J.Q.; Darling, S.B. Drawing on membrane photocatalysis for fouling mitigation. ACS Appl. Mater. Interfaces 2021, 13, 14844–14865. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Y.; He, R.; Xu, N.; Qiao, J. Research advances in electrocatalysts, electrolytes, reactors and membranes for the electrocatalytic carbon dioxide reduction reaction. Acta Phys.-Chim. Sin. 2023, 39, 2302037. [Google Scholar] [CrossRef]
- Wu, C.-J.; Valerie Maggay, I.; Chiang, C.; Chen, W.; Chang, Y.; Hu, C.; Venault, A. Removal of tetracycline by a photocatalytic membrane reactor with MIL-53(Fe)/PVDF mixed-matrix membrane. Chem. Eng. J. 2023, 451, 138990. [Google Scholar] [CrossRef]
- Xu, S.; Wu, Q.; Lu, B.-A.; Tang, T.; Zhang, J.-N.; Hu, J.-S. Recent advances and future prospects on industrial catalysts for green hydrogen production in alkaline media. Acta Phys.-Chim. Sin. 2023, 39, 2209001. [Google Scholar] [CrossRef]
- Jin, H.G.; Zhao, P.C.; Qian, Y.Y.; Xiao, J.D.; Chao, Z.S.; Jiang, H.L. Metal-organic frameworks for organic transformations by photocatalysis and photothermal catalysis. Chem. Soc. Rev. 2024, 53, 9378–9418. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Molinari, R.; Marino, T.; Argurio, P. Photocatalytic membrane reactors for hydrogen production from water. Int. J. Hydrogen Energy 2014, 39, 7247–7261. [Google Scholar] [CrossRef]
- Zhang, W.X.; Ding, L.H.; Luo, J.Q.; Jaffrin, M.Y.; Tang, B. Membrane fouling in photocatalytic membrane reactors (PMRs) for water and wastewater treatment: A critical review. Chem. Eng. J. 2016, 302, 446–458. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Recent progress of photocatalytic membrane reactors in water treatment and in synthesis of organic compounds. A review. Catal. Today 2017, 281, 144–164. [Google Scholar] [CrossRef]
- Hassnain, M.; Ali, A.; Azhar, M.R.; Abutaleb, A.; Mubashir, M. Challenges and perspectives on photocatalytic membrane reactors for volatile organic compounds degradation and nitrogen oxides treatment. Glob. Chall. 2025, 9, 2500035. [Google Scholar] [CrossRef] [PubMed]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic membranes in photocatalytic membrane reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. The evolution of photocatalytic membrane reactors over the last 20 years: A state of the art perspective. Catalysts 2021, 11, 775. [Google Scholar] [CrossRef]
- Visan, A.; van Ommen, J.R.; Kreutzer, M.T.; Lammertink, R.G.H. Photocatalytic reactor design: Guidelines for kinetic investigation. Ind. Eng. Chem. Res. 2019, 58, 5349–5357. [Google Scholar] [CrossRef]
- Gupta, S.; Gomaa, H.; Ray, M.B. Fouling control in a submerged membrane reactor: Aeration vs membrane oscillations. Chem. Eng. J. 2022, 432, 134399. [Google Scholar] [CrossRef]
- Mendret, J.; Brosillon, S. Advances in photocatalytic membrane reactor. Membranes 2023, 13, 541. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, Y.; Huo, Y.; Li, H. Development of photocatalysis-membrane separation reactor systems for aqueous pollutant removal. PhotoMat 2023, 1–21. [Google Scholar] [CrossRef]
- Fu, L.; Chen, X.; Xiang, Y.; Zeng, G. Integrated catalytic membranes for advanced antibiotic wastewater treatment: Synergistic separation-degradation mechanisms and next-generation design. Sep. Purif. Technol. 2025, 372, 133440. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.; Shi, L.; Cheng, R.; Yuan, D. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, Y.; Zhu, Y. Photocatalytic sacrificial H2 evolution dominated by micropore-confined exciton transfer in hydrogen-bonded organic frameworks. Nat. Catal. 2023, 6, 574–584. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Liu, S. Fluorinated TiO2 hollow photocatalysts for photocatalytic applications. Acta Phys.-Chim. Sin. 2021, 37, 2009038. [Google Scholar]
- Le, C.C.; Wismer, M.K.; Shi, Z.C.; Zhang, R.; Conway, D.V.; Li, G.; Vachal, P.; Davies, I.W.; MacMillan, D.W.C. A general small-scale reactor To enable standardization and acceleration of photocatalytic reactions. ACS Cent. Sci. 2017, 3, 647–653. [Google Scholar] [CrossRef]
- Tripathi, A.; Dhanda, A.; Raj, R.; Ghangrekar, M.M.; Surampalli, R.Y. Graphene-based photocatalytic membrane application for the remediation of organic dye pollutants: A review. Groundw. Sustain. Dev. 2024, 26, 101214. [Google Scholar] [CrossRef]
- Tsuru, T.; Kan-no, T.; Yoshioka, T.; Asaeda, M. A photocatalytic membrane reactor for gas-phase reactions using porous titanium oxide membranes. Catal. Today 2003, 82, 41–48. [Google Scholar] [CrossRef]
- Yang, J.; Liu, B.; Zhao, X. A visible-light-active Au-Cu(I)@Na2Ti6O13 nanostructured hybrid pasmonic photocatalytic membrane for acetaldehyde elimination. Chin. J. Catal. 2017, 38, 2048–2055. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Romanos, G.E.; Katsaros, F.K.; Kordatos, K.; Likodimos, V.; Falaras, P. Very efficient composite titania membranes in hybrid ultrafiltration/photocatalysis water treatment processes. J. Membr. Sci. 2012, 392, 192–203. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Gionco, C.; Paganini, M.C.; Calza, P.; Magnacca, G. Control of membrane fouling in organics filtration using Ce-doped zirconia and visible light. Nanomaterials 2019, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of new approaches for fouling mitigation in membrane separation processes in water treatment applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Hazaraimi, M.H.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Hashim, N.; Kerishnan, N.D.K.; Yahaya, N.K.E.M.; Mamat, R.B.R. Advancing photocatalytic membrane for efficient endocrine disrupting compounds removal: Progresses and challenges. J. Water Process Eng. 2024, 68, 106338. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A. Photocatalytic-membrane technology: A critical review for membrane fouling mitigation. J. Ind. Eng. Chem. 2021, 93, 101–116. [Google Scholar] [CrossRef]
- Ping, M.-M.; Qiu, S.-J.; Wei, G.-J.; Liu, J.-X.; Wang, Z.-J.; Wang, S.-T.; An, C.-H. Monolith free-standing plasmonic PAN/Ag/AgX (X = Br, I) nanofiber mat as easily recoverable visible-light-driven photocatalyst. Rare Met. 2019, 38, 361–368. [Google Scholar] [CrossRef]
- Bortot Coelho, F.E.; Deemter, D.; Candelario, V.M.; Boffa, V.; Malato, S.; Magnacca, G. Development of a photocatalytic zirconia-titania ultrafiltration membrane with anti-fouling and self-cleaning properties. J. Environ. Chem. Eng. 2021, 9, 106671. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Wang, H. Photocatalytic membrane reactors for produced water treatment and reuse: Fundamentals, affecting factors, rational design, and evaluation metrics. J. Hazard. Mater. 2022, 424, 127493. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.F.; Liu, N.; Kang, S.F. Understanding the interface properties of photocatalytic reactors for rational engineering applications. Chem. Eng. J. 2023, 472, 145057. [Google Scholar] [CrossRef]
- Rosa, V.; Cameli, F.; Stefanidis, G.D.; Van Geem, K.M. Integrating materials in non-thermal plasma reactors: Challenges and opportunities. Acc. Mater. Res. 2024, 5, 1024–1035. [Google Scholar] [CrossRef]
- Esfandiaribayat, M.; Binazadeh, M.; Sabbaghi, S.; Mohammadi, M.; Ghaedi, S.; Rajabi, H. Tetracycline removal from wastewater via g-CN loaded RSM-CCD-optimised hybrid photocatalytic membrane reactor. Sci. Rep. 2024, 14, 1163. [Google Scholar] [CrossRef]
- Binazadeh, M.; Rasouli, J.; Sabbaghi, S.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. An overview of photocatalytic membrane degradation development. Materials 2023, 16, 3526. [Google Scholar] [CrossRef]
- Ahmed, M.; Mavukkandy, M.O.; Giwa, A.; Elektorowicz, M.; Katsou, E.; Khelifi, O.; Naddeo, V.; Hasan, S.W. Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. npj Clean Water 2022, 5, 12. [Google Scholar] [CrossRef]
- Fei, H.; Zhao, T.; Guo, W.; Wang, X.; Zhang, J.; Fei, Z.; Feng, Z.; Liu, G. Strategies for enhancing activities of typical piezo-photocatalytic material and its applications in environmental remediation: A review. J. Environ. Chem. Eng. 2024, 12, 111650. [Google Scholar] [CrossRef]
- Santana, H.S.; Silva, J.L.; Aghel, B.; Ortega-Casanova, J. Review on microfluidic device applications for fluids separation and water treatment processes. SN Appl. Sci. 2020, 2, 395. [Google Scholar] [CrossRef]
- Romay, M.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Critical issues and guidelines to improve the performance of photocatalytic polymeric membranes. Catalysts 2020, 10, 570. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, L.; Wang, L.; Zhang, J.; Xu, L.; Fan, Z. Photocatalytic membrane reactor used for water and wastewater treatment. Recent Pat. Eng. 2012, 6, 127–136. [Google Scholar] [CrossRef]
- Sabouni, R.; Gomaa, H.G. Comparative analysis of aeration and oscillation in a suspended catalyst photocatalytic membrane reactor. Chem. Eng. Res. Des. 2021, 173, 55–62. [Google Scholar] [CrossRef]
- Huang, X.; Meng, Y.; Liang, P.; Qian, Y. Operational conditions of a membrane filtration reactor coupled with photocatalytic oxidation. Sep. Purif. Technol. 2007, 55, 165–172. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymanski, K.; Darowna, D.; Mozia, S. Overview of photocatalytic membrane reactors in organic synthesis, energy storage and environmental applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef]
- Rehm, T.H.; Gros, S.; Renken, A.; Löb, P. Photocatalysis with visible light–optimization and scale-up for the falling-film microreactor. Chem. Ing. Tech. 2016, 88, 1334–1335. [Google Scholar] [CrossRef]
- Binjhade, R.; Mondal, R.; Mondal, S. Continuous photocatalytic reactor: Critical review on the design and performance. J. Environ. Chem. Eng. 2022, 10, 107746. [Google Scholar] [CrossRef]
- Alvey, J.; Dev, S.; Quiñones, O.; Dickenson, E.; Aggarwal, S.; Dotson, A. Photocatalytic membrane reactor utilizing immobile photocatalytic active layer on membranes for the removal of micropollutants. ACS EST Water 2023, 3, 1050–1059. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Liu, T.; Yang, Y.R.; An, A.K.; Liew, K.M.; Li, W.W. Binder-free immobilization of photocatalyst on membrane surface for efficient photocatalytic H2O2 production and water decontamination. Nanomicro. Lett. 2025, 17, 301. [Google Scholar] [CrossRef] [PubMed]
- Kakhki, R.M. Beyond photosynthesis: Engineering self-healing photocatalytic systems for sustainability. Colloid. Interface Sci. Commun. 2025, 67, 100842. [Google Scholar] [CrossRef]
- Wang, D.W.; Mueses, M.A.; Márquez, J.A.C.; Machuca-Martínez, F.; Grcic, I.; Moreira, R.P.M.; Li Puma, G. Engineering and modeling perspectives on photocatalytic reactors for water treatment. Water Res. 2021, 202, 117421. [Google Scholar] [CrossRef]
- Szymański, K.; Morawski, A.W.; Mozia, S. Humic acids removal in a photocatalytic membrane reactor with a ceramic UF membrane. Chem. Eng. J. 2016, 305, 19–27. [Google Scholar] [CrossRef]
- Feng, X.; Long, R.; Liu, C.; Liu, X. Novel dual-heterojunction photocatalytic membrane reactor based on Ag2S/NH2-MIL-88B(Fe)/poly(aryl ether nitrile) composite with enhanced photocatalytic performance for wastewater purification. Chem. Eng. J. 2023, 454, 139765. [Google Scholar] [CrossRef]
- Heredia Deba, S.A.; Wols, B.A.; Yntema, D.R.; Lammertink, R.G.H. Transport and surface reaction model of a photocatalytic membrane during the radical filtration of methylene blue. Chem. Eng. Sci. 2022, 254, 117617. [Google Scholar] [CrossRef]
- Jung, J.T.; Lee, W.H.; Kim, J.O. Photodegradation and permeability of conventional photocatalytic reactor and two different submerged membrane photocatalytic reactors for the removal of humic acid in water. Desalin. Water Treat. 2016, 57, 26765–26772. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.S. Performance analysis of monolith photoreactor for CO2 reduction with H2. Energy Convers. Manag. 2015, 90, 272–281. [Google Scholar] [CrossRef]
- Allasia, N.; Nevskyi, O.; Marelli, M.; Plazl, I.; Albertazzi, J.; Busini, V.; Castiglione, F.; Rossi, F.; Vilé, G. Bijel-based mesophotoreactor with integrated carbon nitride for continuous-flow photocatalysis. Chem. Eng. J. 2024, 499, 155885. [Google Scholar] [CrossRef]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Destruction of water contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Shen, M.W.; Zhang, G.Q.; Liu, J.Y.; Liu, Y.H.; Zhai, J.Y.; Zhang, H.C.; Yu, H.S. Visible-light-driven photodegradation of xanthate in a continuous fixed-bed photoreactor: Experimental study and modeling. Chem. Eng. J. 2023, 461, 141833. [Google Scholar] [CrossRef]
- Sambiagio, C.; Noël, T. Flow photochemistry: Shine some light on those tubes! Trends Chem. 2020, 2, 92–106. [Google Scholar] [CrossRef]
- Ahmad, R.; Lee, C.S.; Kim, J.H.; Kim, J. Partially coated TiO2 on Al2O3 membrane for high water flux and photodegradation by novel filtration strategy in photocatalytic membrane reactors. Chem. Eng. Res. Des. 2020, 163, 138–148. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Lee, S.A.; Choo, K.H.; Lee, C.H.; Lee, H.I.; Hyeon, T.; Choi, W.; Kwon, H.H. Use of ultrafiltration membranes for the separation of TiO2 photocatalysts in drinking water treatment. Ind. Eng. Chem. Res. 2001, 40, 1712–1719. [Google Scholar] [CrossRef]
- Corredor, J.; Perez-Peña, E.; Rivero, M.J.; Ortiz, I. Performance of rGO/TiO2 photocatalytic membranes for hydrogen production. Membranes 2020, 10, 218. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef]
- Gomaa, H.G. Hydrodynamics and energy analysis in suspended catalyst photocatalytic membrane reactors. Chem. Eng. Process. 2023, 193, 109563. [Google Scholar] [CrossRef]
- Yan, G.; Zuo, B.; Liu, S.; Wang, T.; Wang, R.; Bao, J.; Zhao, Z.; Chu, F.; Li, Z.; Yamauchi, Y.; et al. Opportunities and challenges of capacitive deionization for uranium extraction from seawater. Acta Phys.-Chim. Sin. 2025, 41, 100032. [Google Scholar] [CrossRef]
- Chin, S.S.; Lim, T.M.; Chiang, K.; Fane, A.G. Factors affecting the performance of a low-pressure submerged membrane photocatalytic reactor. Chem. Eng. J. 2007, 130, 53–63. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, X.; Chen, S.; Zhao, H.; Zhao, Y. The removal of sodium dodecylbenzene sulfonate surfactant from water using silica/titania nanorods/nanotubes composite membrane with photocatalytic capability. Appl. Surf. Sci. 2006, 252, 8598–8604. [Google Scholar] [CrossRef]
- Horng, R.Y.; Huang, C.P.; Chang, M.C.; Shao, H.; Shiau, B.L.; Hu, Y.J. Application of TiO2 photocatalytic oxidation and non-woven membrane filtration hybrid system for degradation of 4-chlorophenol. Desalination 2009, 245, 169–182. [Google Scholar] [CrossRef]
- Li, Q.L.; Kong, H.; Jia, R.R.; Shao, J.H.; He, Y.L. Enhanced catalytic degradation of amoxicillin with TiO2-Fe3O4 composites via a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). RSC Adv. 2019, 9, 12538–12546. [Google Scholar] [CrossRef]
- Madadi Avargani, V.; Zendehboudi, S.; Osfouri, S.; Rostami, A. Performance evaluation of a solar nano-photocatalytic reactor for wastewater treatment applications: Reaction kinetics, CFD, and scale-up perspectives. J. Clean. Prod. 2023, 421, 138240. [Google Scholar] [CrossRef]
- Janssens, R.; Hainaut, R.; Gillard, J.; Dailly, H.; Luis, P. Performance of a slurry photocatalytic membrane reactor for the treatment of real secondary wastewater effluent polluted by anticancer drugs. Ind. Eng. Chem. Res. 2021, 60, 2223–2231. [Google Scholar] [CrossRef]
- Shao, J.H.; Hou, J.A.; Song, H.C. Comparison of humic acid rejection and flux decline during filtration with negatively charged and uncharged ultrafiltration membranes. Water Res. 2011, 45, 473–482. [Google Scholar] [CrossRef]
- Sarasidis, V.C.; Plakas, K.V.; Patsios, S.I.; Karabelas, A.J. Investigation of diclofenac degradation in a continuous photo-catalytic membrane reactor. Influence of operating parameters. Chem. Eng. J. 2014, 239, 299–311. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Molinari, R.; Severino, A.; Lavorato, C.; Argurio, P. Which configuration of photocatalytic membrane reactors has a major potential to be used at an industrial level in tertiary sewage wastewater treatment? Catalysts 2023, 13, 1204. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Pisano, D.; Sannino, D.; Ciambelli, P. From the design to the development of a continuous fixed bed photoreactor for photocatalytic degradation of organic pollutants in wastewater. Chem. Eng. Sci. 2015, 137, 152–160. [Google Scholar] [CrossRef]
- Shi, H.B.; Magaye, R.; Castranova, V.; Zhao, J.S. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Biao, W.; Awanis Hashim, N.; Rabuni, M.F.B.; Lide, O.; Ullah, A. An innovative strategy for polyester microplastic fiber elimination from laundry wastewater via coupled separation and degradation using TiO2-based photocatalytic membrane reactor. Sep. Purif. Technol. 2025, 356, 129929. [Google Scholar] [CrossRef]
- Rezaei, M.; Rashidi, F.; Royaee, S.J.; Jafarikojour, M. Performance evaluation of a continuous flow photocatalytic reactor for wastewater treatment. Environ. Sci. Pollut. R. 2014, 21, 12505–12517. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.K.; He, M.T.; Duan, X.G.; Yan, B.B.; Chen, G.Y.; Wang, S.B. Catalytic membrane-based oxidation-filtration systems for organic wastewater purification: A review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef]
- Yang, L.P.; Liu, Z.Y.; Shi, H.W.; Hu, H.; Shangguan, W.F. Design consideration of photocatalytic oxidation reactors using TiO2-coated foam nickels for degrading indoor gaseous formaldehyde. Catal. Today 2007, 126, 359–368. [Google Scholar] [CrossRef]
- Darowna, D.; Wróbel, R.; Morawski, A.W.; Mozia, S. The influence of feed composition on fouling and stability of a polyethersulfone ultrafiltration membrane in a photocatalytic membrane reactor. Chem. Eng. J. 2017, 310, 360–367. [Google Scholar] [CrossRef]
- Gao, B.; Chen, W.P.; Liu, J.D.; An, J.J.; Wang, L.; Zhu, Y.; Sillanpää, M. Continuous removal of tetracycline in a photocatalytic membrane reactor (PMR) with ZnIn2S4 as adsorption and photocatalytic coating layer on PVDF membrane. J. Photochem. Photobiol. A Chem. 2018, 364, 732–739. [Google Scholar] [CrossRef]
- Gupta, S.; Gomaa, H.; Ray, M.B. A novel submerged photocatalytic oscillatory membrane reactor for water polishing. J. Environ. Chem. Eng. 2021, 9, 105562. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Sabouni, R.; Shao, Y.; Gomaa, H.G. Performance of submerged oscillatory membrane photoreactor for water treatment. J. Environ. Chem. Eng. 2017, 5, 3330–3336. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. A new photocatalytic membrane reactor (PMR) for removal of azo-dye Acid Red 18 from water. Appl. Catal. B Environ. Energy 2005, 59, 131–137. [Google Scholar] [CrossRef]
- Vatanpour, V.; Darrudi, N.; Sheydaei, M. A comprehensive investigation of effective parameters in continuous submerged photocatalytic membrane reactors by RSM. Chem. Eng. Process.-Process Intensif. 2020, 157, 108144. [Google Scholar] [CrossRef]
- Trinh, T.H.T.; Samhaber, W.M. The coupling of catalysis with submerged ceramic MF membrane for hybrid water treatment process. Chem. Eng. Trans. 2016, 47, 247–252. [Google Scholar]
- Fu, J.F.; Ji, M.; Wang, Z.; Jin, L.N.; An, D.N. A new submerged membrane photocatalysis reactor (SMPR) for fulvic acid removal using a nano-structured photocatalyst. J. Hazard. Mater. 2006, 131, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Choo, K. Photocatalytic mineralization of secondary effluent organic matter with mitigating fouling propensity in a submerged membrane photoreactor. Chem. Eng. J. 2016, 288, 798–805. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Hu, L.M.; Wang, X.J.; Qu, J.H.; Zhang, G.S. Submerged membrane photocatalytic reactor for advanced treatment of p-nitrophenol wastewater through visible-light-driven photo-Fenton reactions. Sep. Purif. Technol. 2021, 256, 117783. [Google Scholar] [CrossRef]
- Li, Q.L.; Jia, R.R.; Shao, J.H.; He, Y.L. Photocatalytic degradation of amoxicillin via TiO2 nanoparticle coupling with a novel submerged porous ceramic membrane reactor. J. Clean. Prod. 2019, 209, 755–761. [Google Scholar] [CrossRef]
- Kertèsz, S.; Cakl, J.; Jiránková, H. Submerged hollow fiber microfiltration as a part of hybrid photocatalytic process for dye wastewater treatment. Desalination 2014, 343, 106–112. [Google Scholar] [CrossRef]
- Gomaa, H.G. Interaction forces and suspension characteristics in an oscillatory membrane photocatalytic reactor. Chem. Eng. Res. Des. 2022, 185, 301–308. [Google Scholar] [CrossRef]
- Leong, S.W.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.W.; Wang, H.T. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Mesgari, Z.; Saien, J. Pollutant degradation over dye sensitized nitrogen doped titania substances in different configurations of visible light helical flow photoreactor. Sep. Purif. Technol. 2017, 185, 129–139. [Google Scholar] [CrossRef]
- Chen, T.; Xue, X.; Li, J.; Cui, M.; Hao, Y.; Xue, M.; Xiao, H.; Ge, J.; Wang, P. Membrane-anchoring nanoengineered carbon dots as a pyroptosis amplifier for robust tumor photodynamic-immunotherapy. Acta Phys.-Chim. Sin. 2025, 41, 100113. [Google Scholar] [CrossRef]
- Liu, S.Q.; Edara, P.C.; Schäfer, A.I. Influence of organic matter on the photocatalytic degradation of steroid hormones by TiO2-coated polyethersulfone microfiltration membrane. Water Res. 2023, 245, 120438. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.N.; Raota, C.S.; Turshatov, A.; Richards, B.S.; Schäfer, A.I. Collision theory and membrane photocatalysis: Steroid hormones meet singlet oxygen in palladium-porphyrin-coated polytetrafluoroethylene membranes. Chem. Eng. J. 2024, 501, 157582. [Google Scholar] [CrossRef]
- Hairom, N.H.H.; Mohammad, A.W.; Kadhum, A.A.H. Effect of various zinc oxide nanoparticles in membrane photocatalytic reactor for Congo red dye treatment. Sep. Purif. Technol. 2014, 137, 74–81. [Google Scholar] [CrossRef]
- Sakhaie, S.; Taghipour, F. Highly durable zirconium/titanium dioxide-silicon carbide photocatalytic membrane for water and wastewater treatment. J. Environ. Chem. Eng. 2023, 11, 110142. [Google Scholar] [CrossRef]
- Heredia Deba, S.A.; Wols, B.A.; Yntema, D.R.; Lammertink, R.G.H. Advanced ceramics in radical filtration: TiO2 layer thickness effect on the photocatalytic membrane performance. J. Membr. Sci. 2023, 672, 121423. [Google Scholar] [CrossRef]
- Li, W.X.; Zhao, W.X.; Zhu, H.Y.; Li, Z.J.; Wang, W.L. State of the art in the photochemical degradation of (micro)plastics: From fundamental principles to catalysts and applications. J. Mater. Chem. A 2023, 11, 2503–2527. [Google Scholar] [CrossRef]
- Yan, X.J.; Li, J.Y.; Ma, C.; Tang, Y.; Kong, X.J.; Lu, J.F. Study on the lifetime of photocatalyst by photocatalytic membrane reactors (PMR). Water Sci. Technol. 2020, 81, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Astuti, A.R.A.; Saputera, W.H.; Ariono, D.; Wenten, I.G.; Sasongko, D. Membrane contactor-photocatalytic hybrid system for carbon dioxide capture and conversion to formic acid. Results Eng. 2024, 22, 102085. [Google Scholar] [CrossRef]
- Khataee, A.; Honarnezhad, R.; Fathinia, M. Degradation of sodium isopropyl xanthate from aqueous solution using sonocatalytic process in the presence of chalcocite nanoparticles: Insights into the degradation mechanism and phyto-toxicity impacts. J. Environ. Manag. 2018, 211, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Fane, A.G.; Lim, T.T. Evaluation of a submerged membrane vis-LED photoreactor (sMPR) for carbamazepine degradation and TiO2 separation. Chem. Eng. J. 2013, 215, 240–251. [Google Scholar] [CrossRef]
- Xue, X.D.; Fu, J.F.; Zhu, W.F.; Guo, X.C. Separation of ultrafine TiO2 from aqueous suspension and its reuse using cross-flow ultrafiltration (CFU). Desalination 2008, 225, 29–40. [Google Scholar] [CrossRef]
- Grandcolas, M.; Oudin, E. Enhanced photocatalytic activity of electrospun TiO2/polyacrylonitrile membranes in a crossflow reactor using dual lights. Environ. Chem. Lett. 2022, 21, 633–638. [Google Scholar] [CrossRef]
- Le, T.M.H.; Wang, Y.N.; Li, C.; Wang, R.; Sairiam, S. Durable PVDF photocatalytic membranes with TiO2@PDA incorporated into/onto for dye degradation under visible-light. Chem. Eng. J. 2024, 499, 156215. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredob, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.M.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Kim, I.; Yamashita, N.; Tanaka, H. Photodegradation of pharmaceuticals and personal care products during UV and UV/H2O2 treatments. Chemosphere 2009, 77, 518–525. [Google Scholar] [CrossRef]
- Kim, I.; Tanaka, H. Photodegradation characteristics of PPCPs in water with UV treatment. Environ. Int. 2009, 35, 793–802. [Google Scholar] [CrossRef]
- Phan, D.D.; Babick, F.; Nguyen, M.T.; Wessely, B.; Stintz, M. Modelling the influence of mass transfer on fixed-bed photocatalytic membrane reactors. Chem. Eng. Sci. 2017, 173, 242–252. [Google Scholar] [CrossRef]
- Hou, L.B.; Catherine, H.N.; Harada, K.; Yoshida, M.; Chen, Y.L.; Hu, C.C. Reactive seeding growth of cobalt-doped MIL-88B(Fe) on Al2O3 membrane for phenol removal in a photocatalytic membrane reactor. J. Membr. Sci. 2023, 680, 121730. [Google Scholar] [CrossRef]
- Molinari, R.; Palmisano, L.; Drioli, E.; Schiavello, M. Studies on various reactor configurations for coupling photocatalysis and membrane processes in water purification. J. Membr. Sci. 2002, 206, 399–415. [Google Scholar] [CrossRef]
- Petsi, P.N.; Sarasidis, V.C.; Plakas, K.V.; Karabelas, A.J. Reduction of nitrates in a photocatalytic membrane reactor in the presence of organic acids. J. Environ. Manag. 2021, 298, 113526. [Google Scholar] [CrossRef] [PubMed]
- Peñas-Garzón, M.; Sampaio, M.J.; Chávez, A.M.; Silva, A.M.T.; Silva, C.G.; Faria, J.L. Film-immobilized carbon nitride for the degradation of pharmaceuticals: A continuous flow photoreactor performance upheld by excitation-emission matrix fluorescence spectroscopy. Chem. Eng. J. 2024, 500, 157384. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Qian, F.; Cao, M.; Cheng, Y.; Li, J.; Tian, M.; Li, W.; Wang, L. The design of novel swash plate photocatalytic reactor with PAN/BiInOCl membrane photocatalyst for excellent RhB degradation. J. Alloys Compd. 2023, 968, 171894. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, W.F.; Li, X.Y.; Qu, R.X.; Zhang, Q.D.; Wei, Y.; Feng, L.; Jiang, L. Fabrication of robust mesh with anchored Ag nanoparticles for oil removal and in situ catalytic reduction of aromatic dyes. J. Mater. Chem. A 2017, 5, 15822–15827. [Google Scholar] [CrossRef]
- Li, C.C.; Yu, H.W.; Huang, B.; Liu, G.J.; Guo, Y.H.; Zhu, H.L.; Yu, B. Fabrication of anatase TiO2/PVDF composite membrane for oil-in-water emulsion separation and dye photocatalytic degradation. Membranes 2023, 13, 364. [Google Scholar] [CrossRef]
- Yang, C.Y.; Zhang, Z.H.; Wang, P.; Xu, P.; Shen, T.Y.; Xin, Y.J.; Zhang, G.S. A novel vertical immobilized photocatalytic membrane reactor based on Bi2WO6-g-C3N4/PVDF for enhanced removal of atrazine, anti-fouling performance and long-term stability. Chem. Eng. J. 2023, 471, 144672. [Google Scholar] [CrossRef]
- Seger, B.; Kamat, P.V. Fuel cell geared in reverse: Photocatalytic hydrogen production using a TiO2/Nafion/Pt membrane assembly with No applied bias. J. Phys. Chem. C 2009, 113, 18946–18952. [Google Scholar] [CrossRef]
- Tsydenov, D.E.; Parmon, V.N.; Vorontsov, A.V. Toward the design of asymmetric photocatalytic membranes for hydrogen production: Preparation of TiO2-based membranes and their properties. Int. J. Hydrogen Energy 2012, 37, 11046–11060. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Wu, L. GO-assisted supramolecular framework membrane for high-performance separation of nanosized oil-in-water emulsions. Acta Phys.-Chim. Sin. 2024, 40, 2305038. [Google Scholar] [CrossRef]
- Wang, T.H.; Chen, M.J.; Lai, Y.S.; Doong, R.A.; Westerhoff, P.; Rittmann, B. High-efficiency photocatalytic H2O2 production in a dual optical- and membrane-fiber system. ACS Sustain. Chem. Eng. 2023, 11, 6465–6473. [Google Scholar] [CrossRef]
- Hisatomi, T.; Domen, K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019, 2, 387–399. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Han, X.; Qian, A.; Ye, L.; Fan, M.; Yu, J.; Zhang, C.; Zheng, Y.; Yang, Q. Photocatalytic materials and reactors for hydrogen production: A review. Mol. Chem. Eng. 2025, 1, 100001. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Z.; Shen, Y.; Lu, J.; Shi, Y.; Cui, Y.; Guo, F.; Shi, W. Plasmonic coupling-boosted photothermal nanoreactor for efficient solar light-driven photocatalytic water splitting. J. Colloid. Interface Sci. 2023, 652, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Kailasam, K.; Borgmeyer, J.; Neumann, M.; Thomas, A.; Schomäcker, R.; Schwarze, M. Hydrogen Evolution Reaction in a Large-Scale Reactor using a Carbon Nitride Photocatalyst under Natural Sunlight Irradiation. Energy Technol. 2015, 3, 1014–1017. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Jiang, H.B.; Zhang, G.L.; Huang, T.; Chen, J.Y.; Wang, Q.D.; Meng, Q. Photocatalytic membrane reactor for degradation of acid red B wastewater. Chem. Eng. J. 2010, 156, 571–577. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.-S.; Chen, C.-H.; Chen, Y.-R.; Huang, P.-H.; Tung, K.-L. Phosphorus-doped g-C3N4 integrated photocatalytic membrane reactor for wastewater treatment. J. Membr. Sci. 2019, 580, 1–11. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, Z.P.; Li, H.F.; Pei, W.K.; Ding, M.N.; Xie, Z.L.; Huo, Y.N.; Li, H.X. Synergistic removal of organic pollutant and metal ions in photocatalysis-membrane distillation system. Appl. Catal. B Environ. 2020, 264, 118463. [Google Scholar] [CrossRef]
- Ashar, A.; Bhatti, I.A.; Ashraf, M.; Tahir, A.A.; Aziz, H.; Yousuf, M.; Ahmad, M.; Mohsin, M.; Bhutta, Z.A. Fe3+@ ZnO/polyester based solar photocatalytic membrane reactor for abatement of RB5 dye. J. Clean. Prod. 2020, 246, 119010. [Google Scholar] [CrossRef]
- Gao, X.Y.; Meng, X.C. Photocatalysis for heavy metal treatment: A review. Processes 2021, 9, 1729. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, C.; Liu, L.Z.; Zhang, T.S.; Wang, J.; Wang, R.; Du, T.; Yang, C.Y.; Zhang, L.; Xie, L.X.; et al. A conductive network and dipole field for harnessing photogenerated charge kinetics. Adv. Mater. 2021, 33, 2104099. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate biopolymers, lignin based adsorbents for removal of heavy metals (Cd2+, Pb2+, Zn2+) from wastewater, regeneration and reuse for spent adsorbents including latent fingerprint detection: A review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Arshad, F.; Al Momani, D.E.; de Vos, W.M.; Zou, L.D. Nanocomposite membrane for simultaneous removal of dye and heavy metal ions from wastewater. J. Environ. Manag. 2024, 371, 123242. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- dos Anjos Silva, G.R.; do Espírito Santo, A.S.S.; Tomaz, G.S.; Moreira, V.R.; de Oliveira Machado, C.P.; Viana, M.M.; Amaral, M.C.S. Photocatalytic modified membranes with dopamine and GO-TiO2 applied to petroleum refinery wastewater treatment. Sep. Purif. Technol. 2025, 361, 131621. [Google Scholar] [CrossRef]
- Rathna, T.; PonnanEttiyappan, J.; RubenSudhakar, D. Fabrication of visible-light assisted TiO2-WO3-PANI membrane for effective reduction of chromium (VI) in photocatalytic membrane reactor. Environ. Technol. Innov. 2021, 24, 102023. [Google Scholar] [CrossRef]
- Chen, J.J.; Oh, P.C.; Saleh, S.B.M. Recent development on photocatalysts and membrane processes for photoreduction of CO2 into C1 solar fuels. Korean J. Chem. Eng. 2024, 41, 609–637. [Google Scholar] [CrossRef]
- de Richter, R.K.; Ming, T.Z.; Caillol, S. Fighting global warming by photocatalytic reduction of CO2 using giant photocatalytic reactors. Renew. Sustain. Enrergy Rev. 2013, 19, 82–106. [Google Scholar] [CrossRef]
- Mohd Saleh, S.; Oh, P.C.; Zain, M.M.; Quek, V.C.; Zulkifli, A.S.; Chew, T.L. Revolutionizing CO2 reduction with TiO2 photocatalyst immobilized on polytetrafluoroethylene (PTFE) membrane. J. Ind. Eng. Chem. 2024, 131, 230–239. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, R.; Zhu, X.; Liao, Q.; He, X.; Li, S.; Li, L. Optofluidic membrane microreactor for photocatalytic reduction of CO2. Int. J. Hydrogen Energy 2016, 41, 2457–2465. [Google Scholar] [CrossRef]
- Liu, B.; Li, D.; Yao, J.; Sun, H. Enhanced CO2 selectivity of polyimide membranes through dispersion of polyethyleneimine decorated UiO-66 particles. J. Appl. Polym. Sci. 2020, 137, 49068. [Google Scholar] [CrossRef]
- Moulis, F.; Krysa, J. Photocatalytic degradation of several VOCs (-hexane, -butyl acetate and toluene) on TiO2 layer in a closed-loop reactor. Catal. Today 2013, 209, 153–158. [Google Scholar] [CrossRef]
- Debono, O.; Hequet, V.; Le Coq, L.; Locoge, N.; Thevenet, F. VOC ternary mixture effect on ppb level photocatalytic oxidation: Removal kinetic, reaction intermediates and mineralization. Appl. Catal. B Environ. 2017, 218, 359–369. [Google Scholar] [CrossRef]
- Khalilzadeh, A.; Fatemi, S. Spouted bed reactor for VOC removal by modified nano-TiO2 photocatalytic particles. Chem. Eng. Res. Des. 2016, 115, 241–250. [Google Scholar] [CrossRef]
- Gérardin, F.; Cloteaux, A.; Simard, J.; Favre, É. A photodriven energy efficient membrane process for trace VOC removal from air: First step to a smart approach. Chem. Eng. J. 2021, 419, 129566. [Google Scholar] [CrossRef]
- McCullagh, C.; Skillen, N.; Adams, M.; Robertson, P.K.J. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar] [CrossRef]
- Deepracha, S.; Atfane, L.; Ayral, A.; Ogawa, M. Simple and efficient method for functionalizing photocatalytic ceramic membranes and assessment of its applicability for wastewater treatment in up-scalable membrane reactors. Sep. Purif. Technol. 2021, 262, 118307. [Google Scholar] [CrossRef]
- Kant, P.; Liang, S.Z.; Rubin, M.; Ozin, G.A.; Dittmeyer, R. Low-cost photoreactors for highly photon/energy-efficient solar-driven synthesis. Joule 2023, 7, 1347–1362. [Google Scholar] [CrossRef]
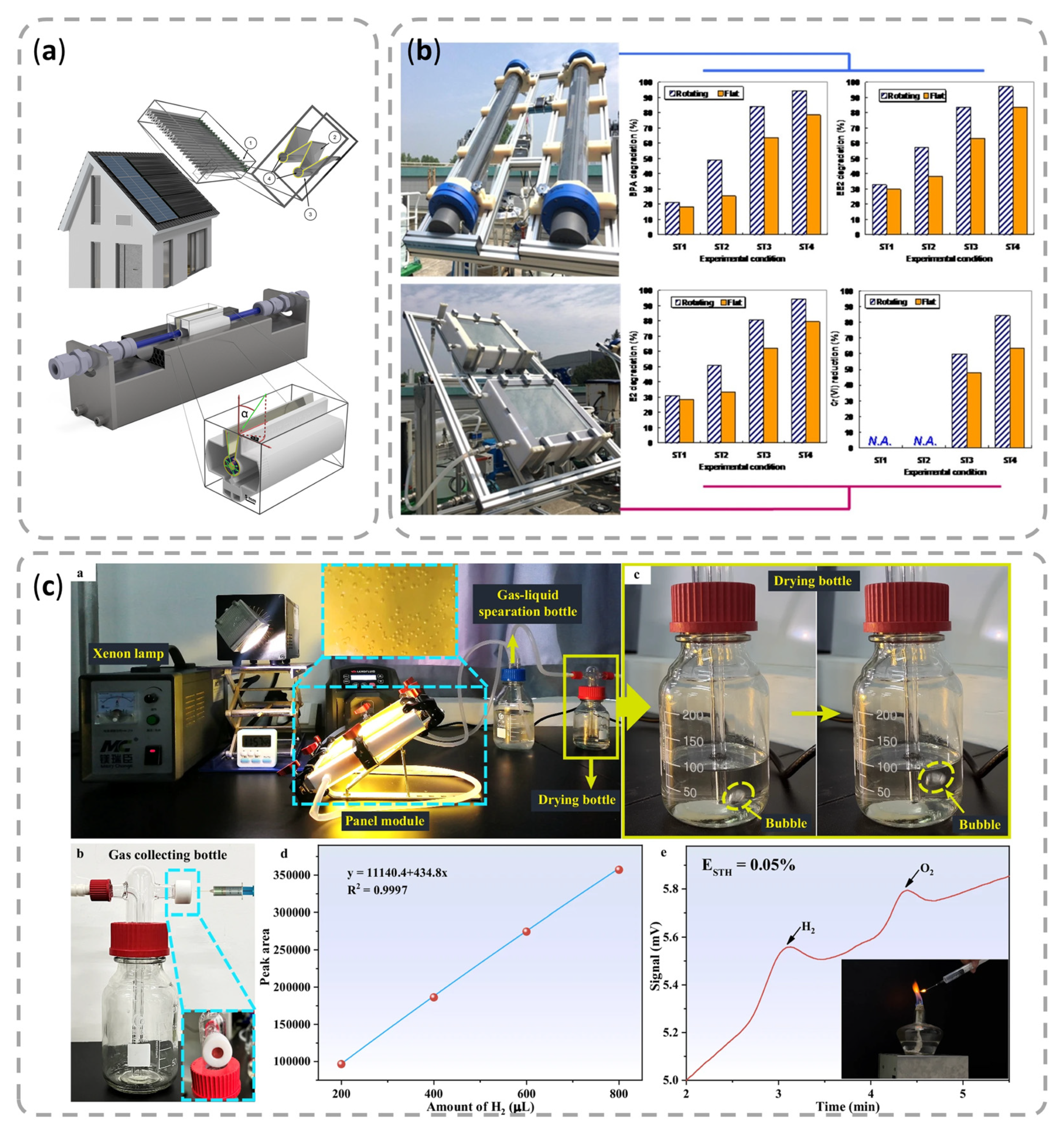
- Kim, S.; Cho, H.; Joo, H.; Her, N.; Han, J.; Yi, K.; Kim, J.O.; Yoon, J. Evaluation of performance with small and scale-up rotating and flat reactors; photocatalytic degradation of bisphenol A, 17β-estradiol, and 17α-ethynyl estradiol under solar irradiation. J. Hazard. Mater. 2017, 336, 21–32. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibanez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
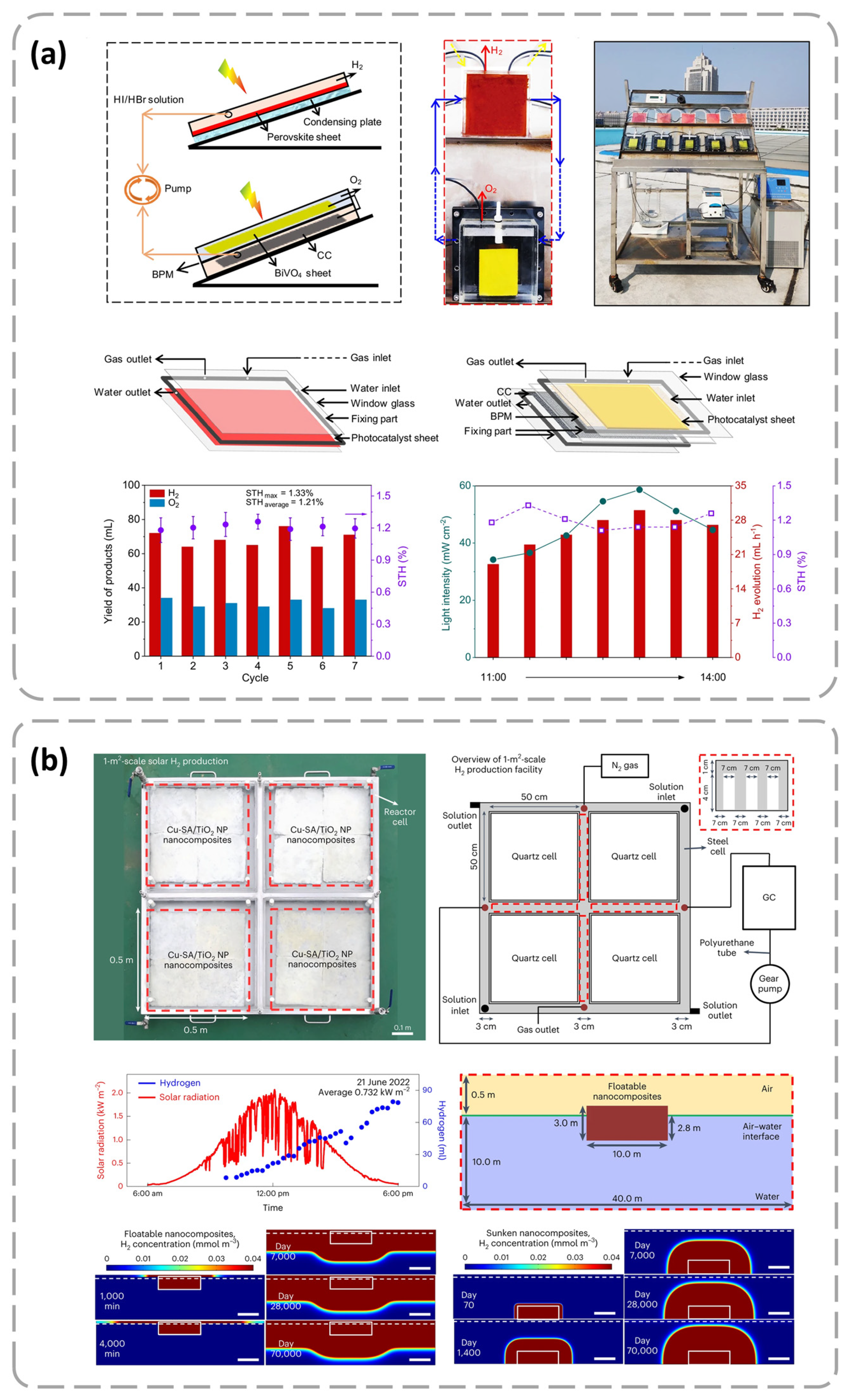
- Lee, W.H.; Lee, C.W.; Cha, G.D.; Lee, B.-H.; Jeong, J.H.; Park, H.; Heo, J.; Bootharaju, M.S.; Sunwoo, S.-H.; Kim, J.H.; et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat. Nanotechnol. 2023, 18, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.C.; Chen, Y.C.; Ajebe, E.G.; Tsai, M.C.; Tsai, H.C.; Lee, K.R.; Lai, J.Y. A study on photocatalytic Janus membrane reactor for direct conversion of low concentration carbon dioxide in air to methane: Effects of combination of two-dimensional carbon nanosheets. Chem. Eng. J. 2023, 478, 147358. [Google Scholar] [CrossRef]
- Li, W.; Duan, W.; Liao, G.; Gao, F.; Wang, Y.; Cui, R.; Zhao, J.; Wang, C. 0.68% of solar-to-hydrogen efficiency and high photostability of organic-inorganic membrane catalyst. Nat. Commun. 2024, 15, 6763. [Google Scholar] [CrossRef]
- Fu, H.; Wu, Y.Q.; Guo, Y.H.; Sakurai, T.; Zhang, Q.Q.; Liu, Y.Y.; Zheng, Z.K.; Cheng, H.F.; Wang, Z.Y.; Huang, B.B.; et al. A scalable solar-driven photocatalytic system for separated H2 and O2 production from water. Nat. Commun. 2025, 16, 990. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Qin, S.; Lu, T.; Wang, S.; Chen, J.; He, Z. Engineering Photocatalytic Membrane Reactors for Sustainable Energy and Environmental Applications. Catalysts 2025, 15, 947. https://doi.org/10.3390/catal15100947
Xu R, Qin S, Lu T, Wang S, Chen J, He Z. Engineering Photocatalytic Membrane Reactors for Sustainable Energy and Environmental Applications. Catalysts. 2025; 15(10):947. https://doi.org/10.3390/catal15100947
Chicago/Turabian StyleXu, Ruofan, Shumeng Qin, Tianguang Lu, Sen Wang, Jing Chen, and Zuoli He. 2025. "Engineering Photocatalytic Membrane Reactors for Sustainable Energy and Environmental Applications" Catalysts 15, no. 10: 947. https://doi.org/10.3390/catal15100947
APA StyleXu, R., Qin, S., Lu, T., Wang, S., Chen, J., & He, Z. (2025). Engineering Photocatalytic Membrane Reactors for Sustainable Energy and Environmental Applications. Catalysts, 15(10), 947. https://doi.org/10.3390/catal15100947