Effect of Sm2O3 Doping of CeO2-Supported Ni Catalysts for H2 Production by Steam Reforming of Ethanol
Abstract
1. Introduction
2. Results and Discussion
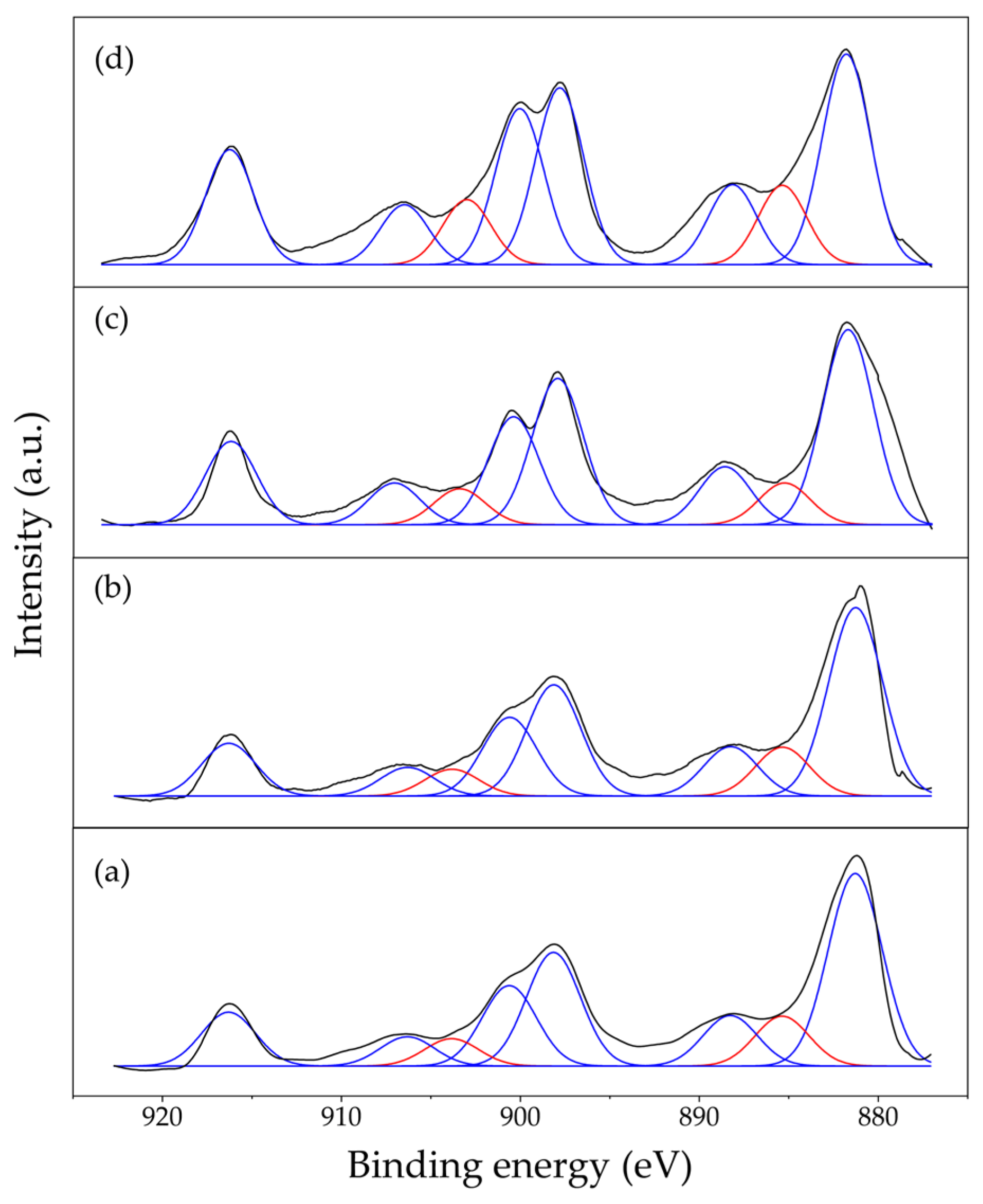
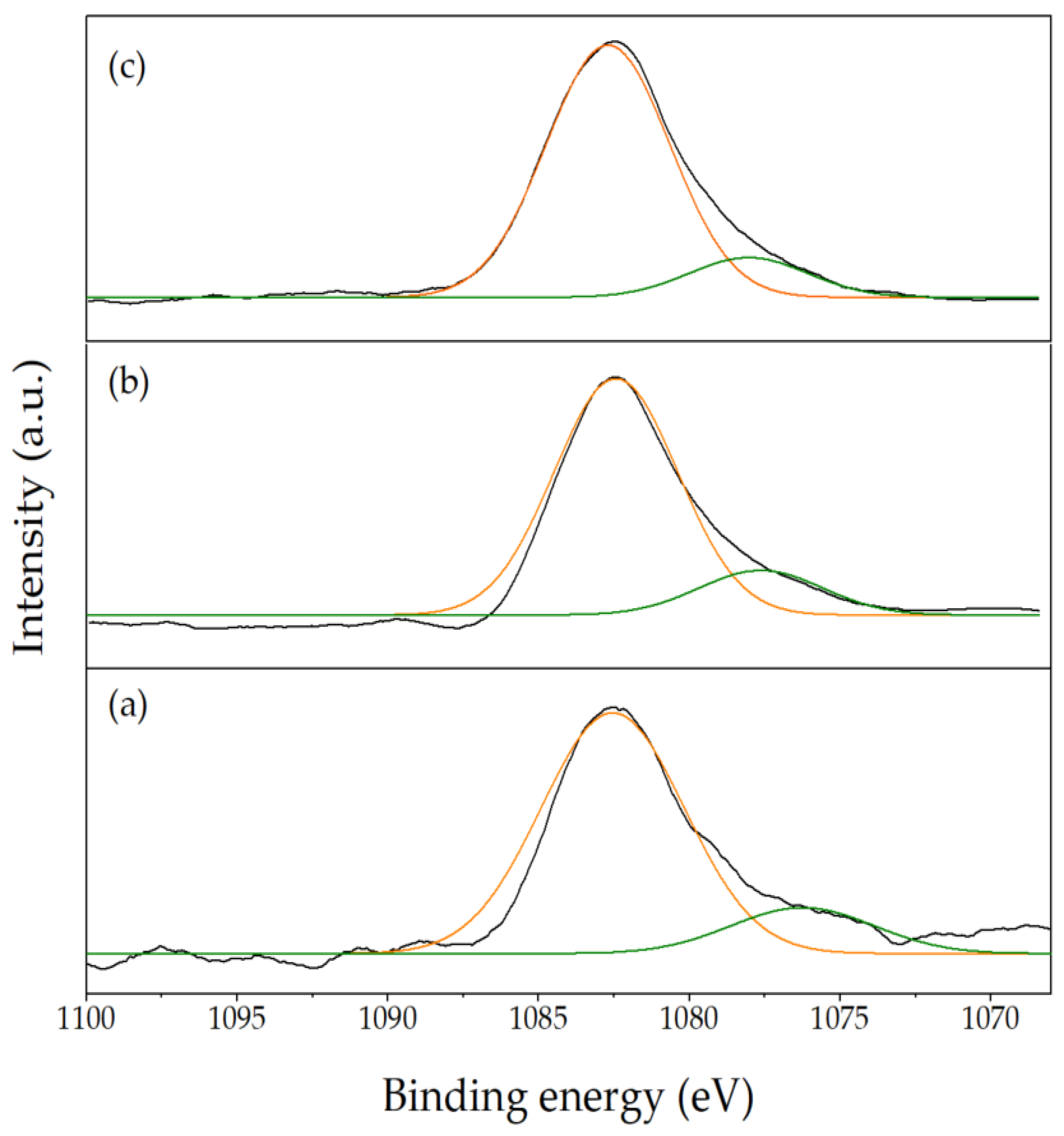
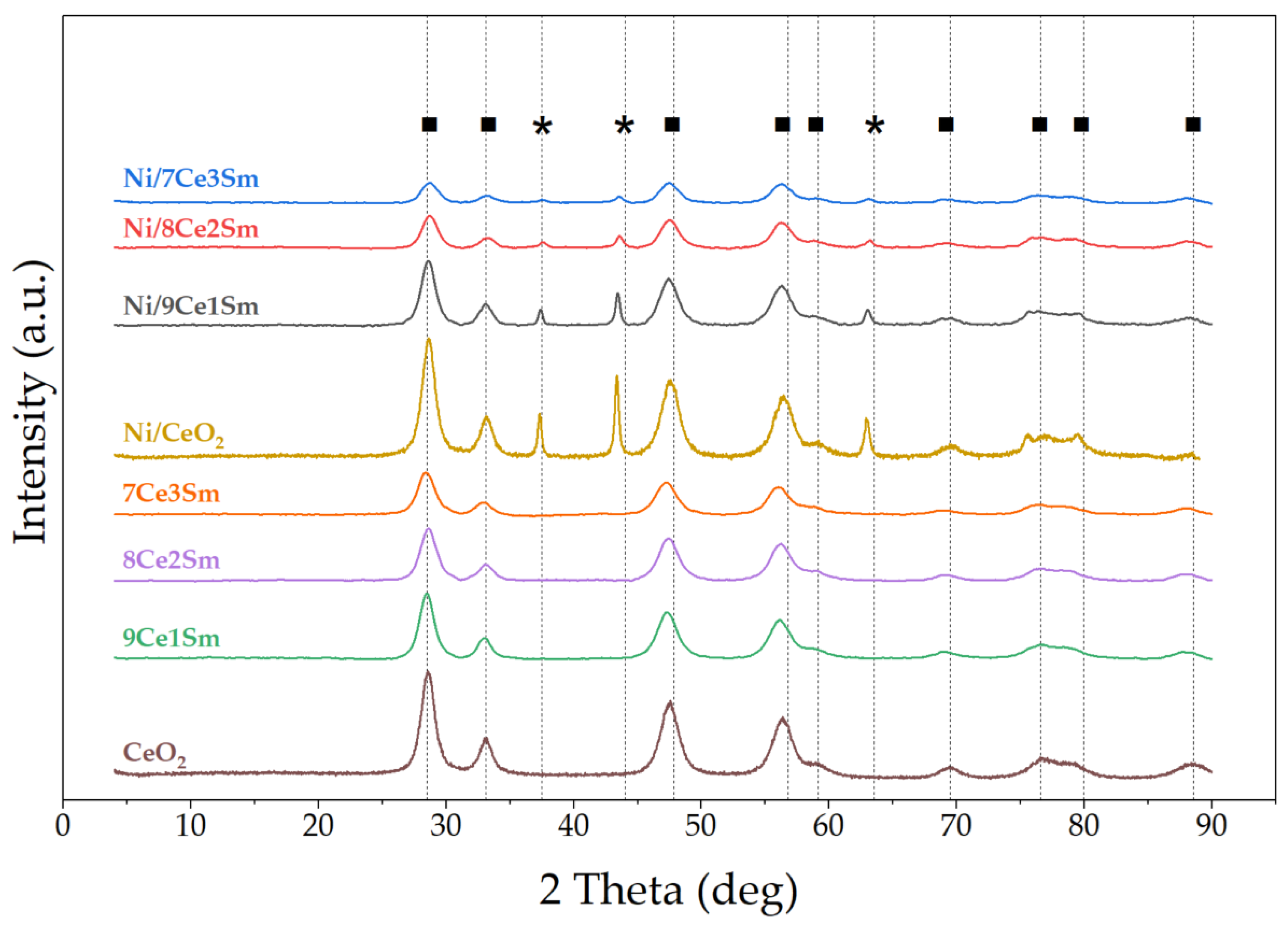
2.1. Characterization Results
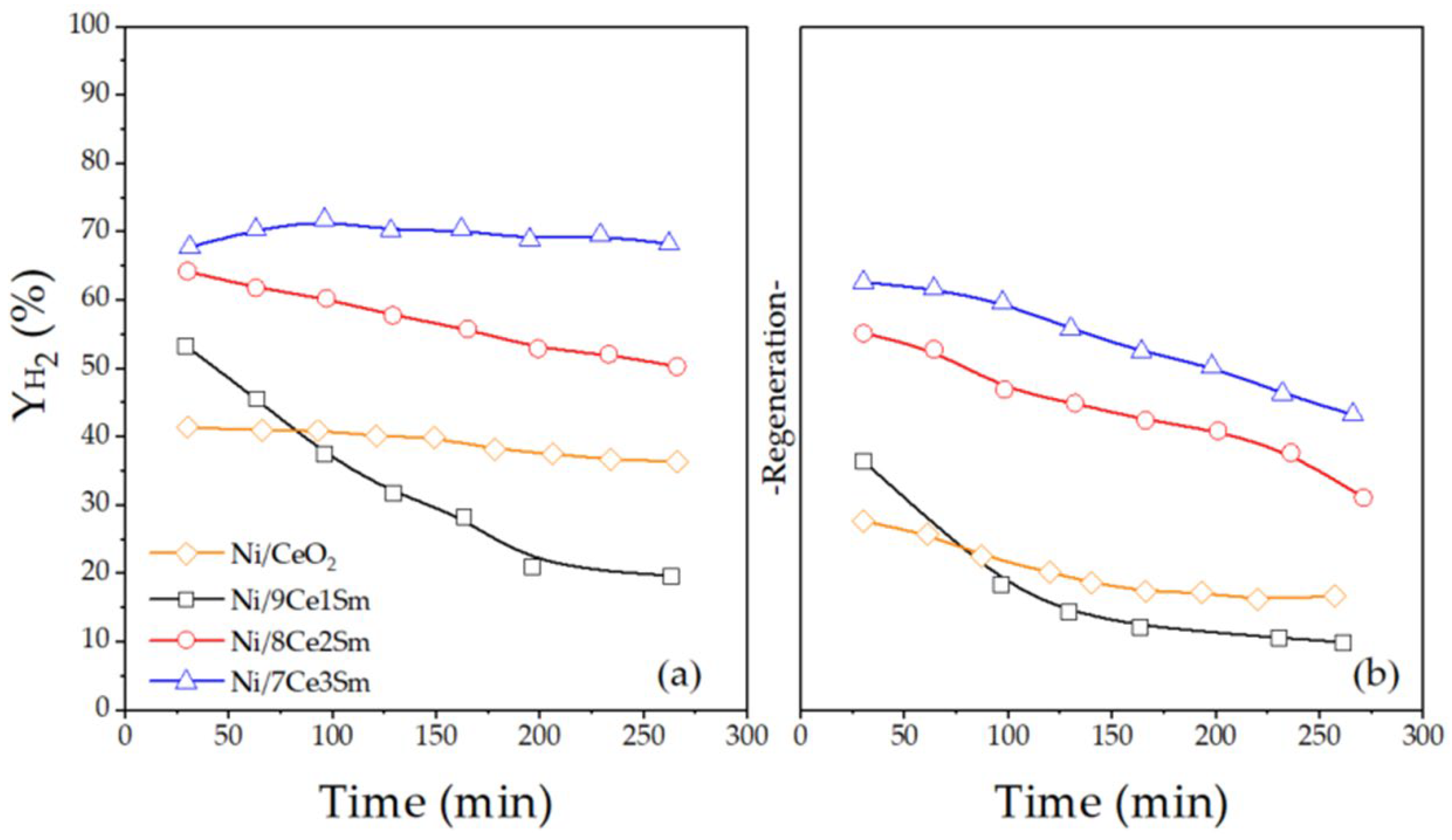
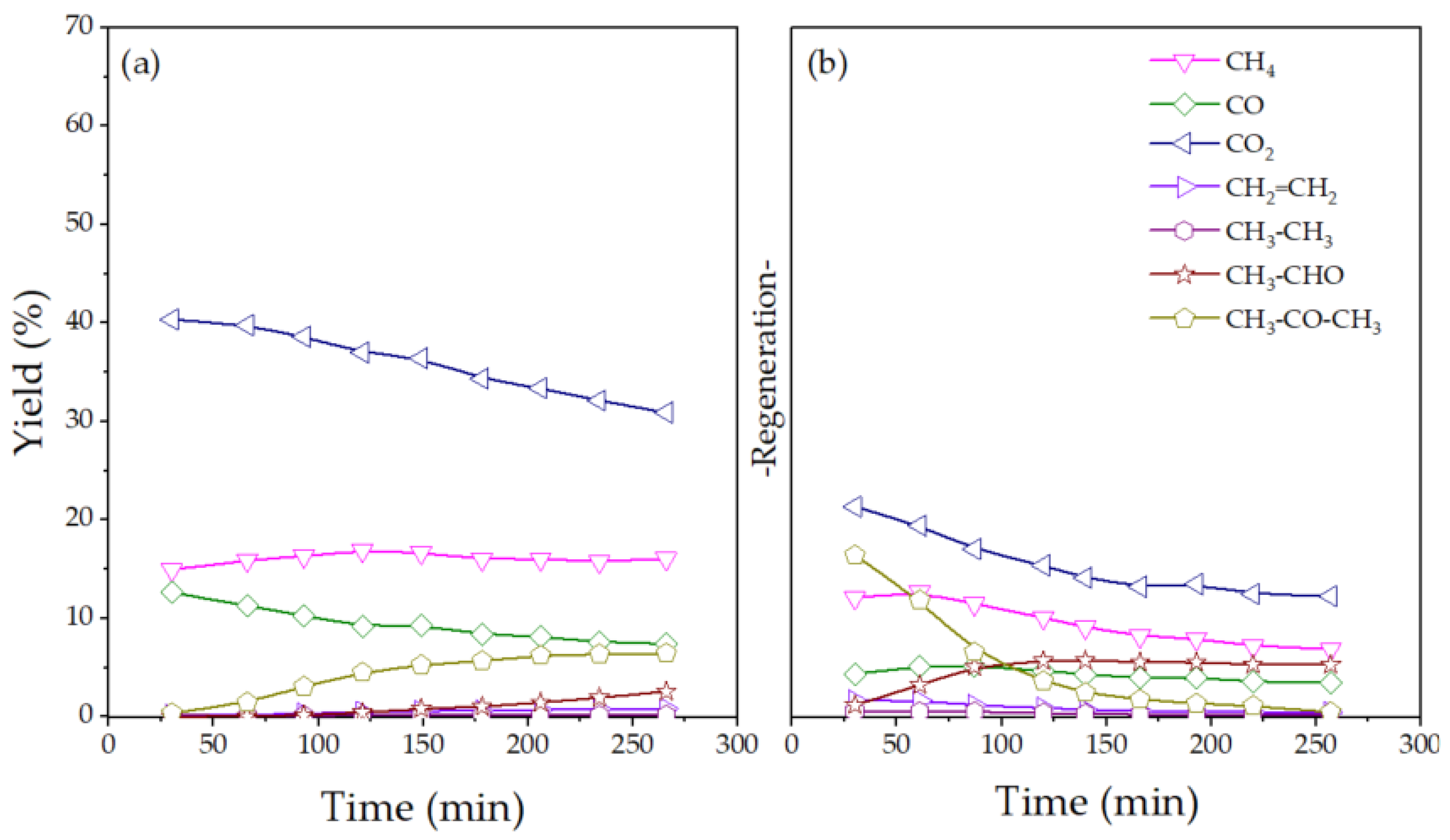
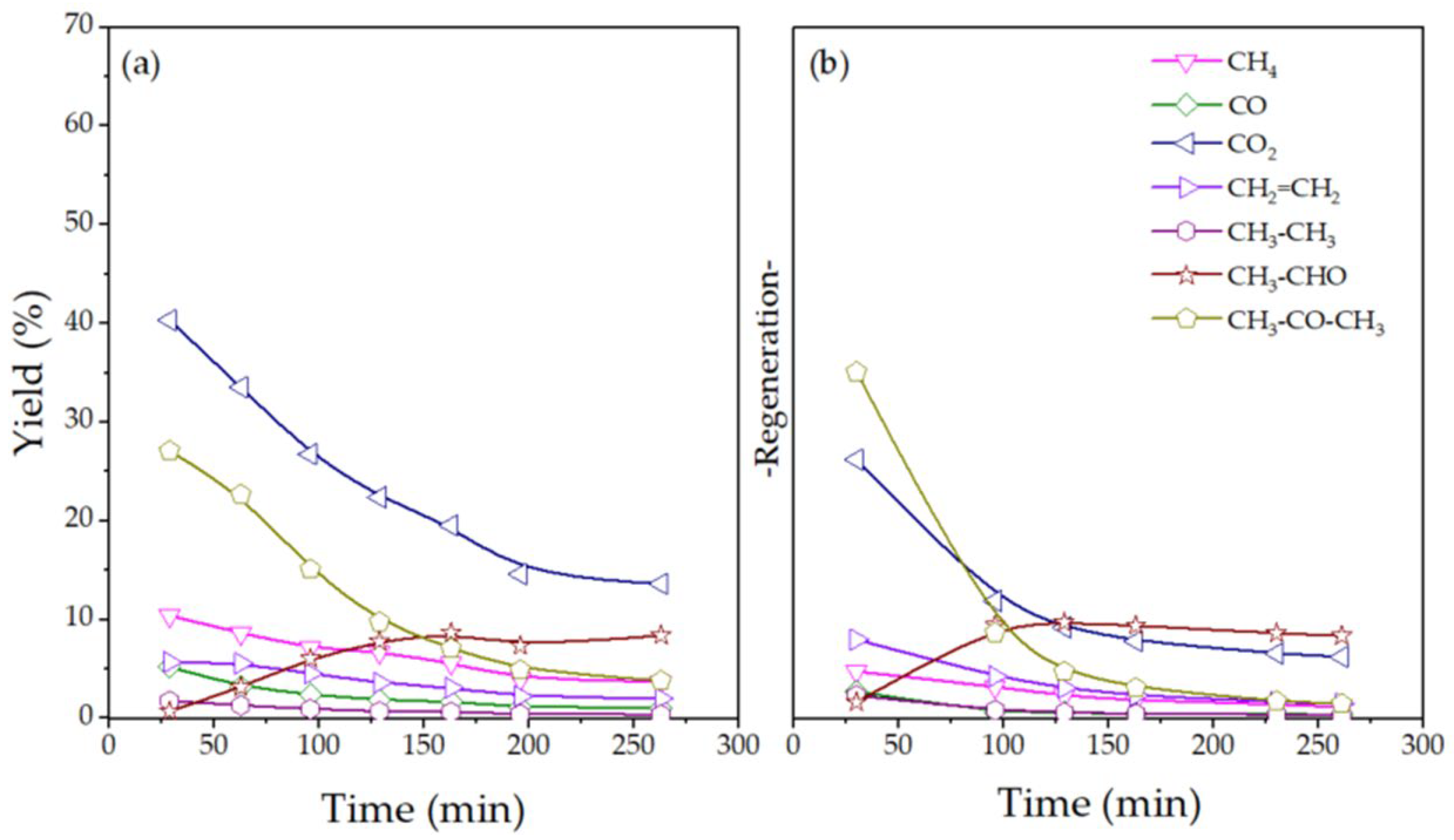
2.2. Catalytic Performance for ESR
3. Materials and Methods
3.1. Catalyts Preparation
3.2. Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Altaf, C.T.; Colak, T.O.; Karagoz, E.; Kurt, M.; Sankir, N.D.; Sankir, M. A Review of the Recent Advances in Composite Membranes for Hydrogen Generation Technologies. ACS Omega 2024, 9, 23138–23154. [Google Scholar] [CrossRef] [PubMed]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J. Deactivation of Copper-Based Catalysts for Fuel Cell Applications. Catal. Lett. 2001, 74, 27–30. [Google Scholar] [CrossRef]
- Hu, G.; Chen, C.; Lu, H.T.; Wu, Y.; Liu, C.; Tao, L.; Men, Y.; He, G.; Li, K.G. A Review of Technical Advances, Barriers, and Solutions in the Power to Hydrogen (P2H) Roadmap. Engineering 2020, 6, 1364–1380. [Google Scholar] [CrossRef]
- Parkhey, P. Biomethanol: Possibilities towards a Bio-Based Economy. Biomass Convers. Biorefin. 2022, 12, 1877–1887. [Google Scholar] [CrossRef]
- Hasegawa, F.; Yokoyama, S.; Imou, K. Methanol or Ethanol Produced from Woody Biomass: Which Is More Advantageous? Bioresour. Technol. 2010, 101, S109–S111. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Rosha, P.; Ali, F.M.; Ibrahim, H. Recent Advances in Hydrogen Production through Catalytic Steam Reforming of Ethanol: Advances in Catalytic Design. Can. J. Chem. Eng. 2023, 101, 5498–5518. [Google Scholar] [CrossRef]
- Boudadi, K.; Bellifa, A.; Márquez-Álvarez, C.; Cortés Corberán, V. Nickel Catalysts Promoted with Lanthanum for Ethanol Steam Reforming: Influence of Support and Treatment on Activity. Appl. Catal. A Gen. 2021, 619, 118141. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G.; Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. Sustainable Hydrogen Production by Catalytic Bio-Ethanol Steam Reforming. In Greenhouse Gases—Capturing, Utilization and Reduction; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef]
- Lin, J.; Chen, L.; Choong, C.K.S.; Zhong, Z.; Huang, L. Molecular Catalysis for the Steam Reforming of Ethanol. Sci. China Chem. 2015, 58, 60–78. [Google Scholar] [CrossRef]
- Hou, T.; Yu, B.; Zhang, S.; Xu, T.; Wang, D.; Cai, W. Hydrogen Production from Ethanol Steam Reforming over Rh/CeO2 Catalyst. Catal. Commun. 2015, 58, 137–140. [Google Scholar] [CrossRef]
- Cai, W.; Wang, F.; Daniel, C.; Van Veen, A.C.; Schuurman, Y.; Descorme, C.; Provendier, H.; Shen, W.; Mirodatos, C. Oxidative Steam Reforming of Ethanol over Ir/CeO2 Catalysts: A Structure Sensitivity Analysis. J. Catal. 2012, 286, 137–152. [Google Scholar] [CrossRef]
- Palma, V.; Castaldo, F.; Ciambelli, P.; Iaquaniello, G. CeO2-Supported Pt/Ni Catalyst for the Renewable and Clean H2 Production via Ethanol Steam Reforming. Appl. Catal. B 2014, 145, 73–84. [Google Scholar] [CrossRef]
- Ramos, I.A.C.; Montini, T.; Lorenzut, B.; Troiani, H.; Gennari, F.C.; Graziani, M.; Fornasiero, P. Hydrogen Production from Ethanol Steam Reforming on M/CeO2/YSZ (M = Ru, Pd, Ag) Nanocomposites. Catal. Today 2012, 180, 96–104. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Rodrigues, A.E. Kinetics of Steam Reforming of Ethanol over a Ru/Al2O3 Catalyst. Ind. Eng. Chem. Res. 2006, 45, 6614–6618. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol Steam Reforming for Hydrogen Production: Latest and Effective Catalyst Modification Strategies to Minimize Carbonaceous Deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Mathure, P.V.; Ganguly, S.; Patwardhan, A.V.; Saha, R.K. Steam Reforming of Ethanol Using a Commercial Nickel-Based Catalyst. Ind. Eng. Chem. Res. 2007, 46, 8471–8479. [Google Scholar] [CrossRef]
- Pájaro, K.C.; Cortés Corberán, V.; Martínez-Arias, A. Influence of the Interaction of Nickel and Copper with Ceria on Ethanol Steam Reforming over Ni-Cu-CeO2 Catalysts. Catalysts 2024, 14, 605. [Google Scholar] [CrossRef]
- Pinton, N.; Vidal, M.V.; Signoretto, M.; Martínez-Arias, A.; Cortés Corberán, V. Ethanol Steam Reforming on Nanostructured Catalysts of Ni, Co and CeO2: Influence of Synthesis Method on Activity, Deactivation and Regenerability. Catal. Today 2017, 296, 135–143. [Google Scholar] [CrossRef]
- Cao, A.N.T.; Ng, K.H.; Ahmed, S.F.; Nguyen, H.T.; Kumar, P.S.; Tan, H.T.; Rajamohan, N.; Yusuf, M.; Show, P.L.; Balakrishnan, A.; et al. Hydrogen Generation by Heterogeneous Catalytic Steam Reforming of Short-Chain Alcohols: A Review. Environ. Chem. Lett. 2023, 22, 561–583. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Zhao, Y.; Wang, H.; Liu, H.; Zhang, Q. A Mini Review on Recent Progress of Steam Reforming of Ethanol. RSC Adv. 2023, 13, 23991–24002. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, H.; Zhang, Y.; Pu, J. Highly Active Ni/CeO2 for the Steam Reforming of Acetic Acid Using CTAB as Surfactant Template. Int. J. Hydrogen Energy 2022, 47, 27493–27507. [Google Scholar] [CrossRef]
- Remiro, A.; Arandia, A.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Stability of a Rh/CeO2-ZrO2 Catalyst in the Oxidative Steam Reforming of Raw Bio-Oil. Energy Fuels 2018, 32, 3588–3598. [Google Scholar] [CrossRef]
- Yadav, A.K.; Vaidya, P.D. Hydrogen Production From Steam Butanol Reforming Over Cobalt Catalyst Supported On Ceria. Catal. Green. Chem. Eng. 2023, 6, 1–14. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam Reforming of Ethanol on Ni/CeO2: Reaction Pathway and Interaction between Ni and the CeO2 Support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Min, P.; Zhang, S.; Xu, Y.; Li, R. Enhanced Oxygen Storage Capacity of CeO2 with Doping-Induced Unstable Crystal Structure. Appl. Surf. Sci. 2018, 448, 435–443. [Google Scholar] [CrossRef]
- Ogo, S.; Sekine, Y. Recent Progress in Ethanol Steam Reforming Using Non-Noble Transition Metal Catalysts: A Review. Fuel Process. Technol. 2020, 199, 106238. [Google Scholar] [CrossRef]
- Kosinski, M.R.; Baker, R.T. Preparation and Property–Performance Relationships in Samarium-Doped Ceria Nanopowders for Solid Oxide Fuel Cell Electrolytes. J. Power Sources 2011, 196, 2498–2512. [Google Scholar] [CrossRef]
- Go, D.; Kim, T.; Li, H.; Garcia, T.J.; Yang, B.C.; Gür, T.M.; Lee, M.H.; An, J. PEALD Ru-Decorated Ni-SDC Cermet Anode for Utilizing Methane Fuel in Low-Temperature SOFCs. Surf. Interfaces 2024, 44, 103657. [Google Scholar] [CrossRef]
- Tepamatr, P.; Laosiripojana, N.; Sesuk, T.; Charojrochkul, S. Effect of Samarium and Praseodymium Addition on Water Gas Shift Performance of Co/CeO2 Catalysts. J. Rare Earths 2020, 38, 1201–1206. [Google Scholar] [CrossRef]
- AlKhoori, A.A.; Polychronopoulou, K.; Belabbes, A.; Jaoude, M.A.; Vega, L.F.; Sebastian, V.; Hinder, S.; Baker, M.A.; Zedan, A.F. Cu, Sm Co-Doping Effect on the CO Oxidation Activity of CeO2. A Combined Experimental and Density Functional Study. Appl. Surf. Sci. 2020, 521, 146305. [Google Scholar] [CrossRef]
- Huang, T.J.; Jhao, S.Y. Ni-Cu/Samaria-Doped Ceria Catalysts for Steam Reforming of Methane in the Presence of Carbon Dioxide. Appl. Catal. A Gen. 2006, 302, 325–332. [Google Scholar] [CrossRef]
- Chien, A.C.; Ye, N.J.; Huang, C.W.; Tseng, I.H. Studies of Nickel/Samarium-Doped Ceria for Catalytic Partial Oxidation of Methane and Effect of Oxygen Vacancy. Catalysts 2021, 11, 731. [Google Scholar] [CrossRef]
- Wang, J.B.; Li, C.H.; Huang, T.J. Study of Partial Oxidative Steam Reforming of Methanol over Cu-ZnO/Samaria-Doped Ceria Catalyst. Catal. Lett. 2005, 103, 239–247. [Google Scholar] [CrossRef]
- Laobuthee, A.; Veranitisagul, C.; Wattanathana, W.; Koonsaeng, N.; Laosiripojana, N. Activity of Fe Supported by Ce1−xSmxO2−δ Derived from Metal Complex Decomposition toward the Steam Reforming of Toluene as Biomass Tar Model Compound. Renew. Energy 2015, 74, 133–138. [Google Scholar] [CrossRef]
- Kosinski, M.R.; Vizcaíno, A.J.; Gómez-Sainero, L.M.; Carrero, A.; Baker, R.T. Methanol Reforming by Nanostructured Pd/Sm-Doped Ceria Catalysts. Appl. Catal. B 2021, 286, 119935. [Google Scholar] [CrossRef]
- Gómez-Sainero, L.M.; Baker, R.T.; Metcalfe, I.S.; Sahibzada, M.; Concepción, P.; López-Nieto, J.M. Investigation of Sm2O3–CeO2-Supported Palladium Catalysts for the Reforming of Methanol: The Role of the Support. Appl. Catal. A Gen. 2005, 294, 177–187. [Google Scholar] [CrossRef]
- Gómez-Sainero, L.M.; Baker, R.T.; Vizcaíno, A.J.; Francis, S.M.; Calles, J.A.; Metcalfe, I.S.; Rodriguez, J.J. Steam Reforming of Methanol with Sm 2O 3-CeO 2-Supported Palladium Catalysts: Influence of the Thermal Treatments of Catalyst and Support. Ind. Eng. Chem. Res. 2009, 48, 8364–8372. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Moura, A.B.L.; e Silva, F.A.; Candido, E.G.; da Silva, A.G.M.; de Oliveira, D.C.; Quiroz, J.; Camargo, P.H.C.; Bergamaschi, V.S.; Ferreira, J.C.; et al. Ni Supported Ce0.9Sm0.1O2−δ Nanowires: An Efficient Catalyst for Ethanol Steam Reforming for Hydrogen Production. Fuel 2019, 237, 1244–1253. [Google Scholar] [CrossRef]
- Rangaswamy, A.; Sudarsanam, P.; Reddy, B.M. Rare Earth Metal Doped CeO2-Based Catalytic Materials for Diesel Soot Oxidation at Lower Temperatures. J. Rare Earths 2015, 33, 1162–1169. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy Database. Available online: https://srdata.nist.gov/xps/EnergyTypeElement (accessed on 15 January 2025).
- Golubina, E.V.; Rostovshchikova, T.N.; Lokteva, E.S.; Maslakov, K.I.; Nikolaev, S.A.; Egorova, T.B.; Gurevich, S.A.; Kozhevin, V.M.; Yavsin, D.A.; Yermakov, A.Y. Chlorobenzene Hydrodechlorination on Bimetallic Catalysts Prepared by Laser Electrodispersion of NiPd Alloy. Pure Appl. Chem. 2018, 90, 1685–1701. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J. Visible-Light Induced Oxo-Bridged ZrIV−O−CeIII Redox Centre in Tetragonal ZrO2–CeO2 Solid Solution for Degradation of Organic Pollutants. Phys. Chem. Chem. Phys. 2011, 13, 3896–3905. [Google Scholar] [CrossRef]
- Cardenas, L.; Molinet-Chinaglia, C.; Loridant, S. Unraveling Ce3+ Detection at the Surface of Ceria Nanopowders by UPS Analysis. Phy. Chem. Chem. Phy. 2022, 24, 22815–22822. [Google Scholar] [CrossRef]
- Zhong, B.; Liang, Z.; Huang, J.; Ye, Y.; Liu, Y.; Wang, T. Effect of CeO2 Morphology in Catalytic Performance via Methanol Oxidative Steam Reforming for Hydrogen Production. Cite This Ind. Eng. Chem. Res. 2024, 63, 6533. [Google Scholar] [CrossRef]
- Yang, C.; Bebensee, F.; Chen, J.; Yu, X.; Nefedov, A.; Wöll, C. Carbon Dioxide Adsorption on CeO2(110): An XPS and NEXAFS Study. ChemPhysChem 2017, 18, 1874–1880. [Google Scholar] [CrossRef]
- Panayotov, D.; Zdravkova, V.; Lagunov, O.; Andonova, S.; Spassova, I.; Nihtianova, D.; Atanasova, G.; Drenchev, N.; Ivanova, E.; Mihaylov, M.; et al. Capturing CO2 by Ceria and Ceria–Zirconia Nanomaterials of Different Origin. Phys. Chem. Chem. Phys. 2023, 25, 17154–17175. [Google Scholar] [CrossRef]
- Phung, T.K.; Pham, T.L.M.; Nguyen, A.N.T.; Vu, K.B.; Giang, H.N.; Nguyen, T.A.; Huynh, T.C.; Pham, H.D. Effect of Supports and Promoters on the Performance of Ni-Based Catalysts in Ethanol Steam Reforming. Chem. Eng. Technol. 2020, 43, 672–688. [Google Scholar] [CrossRef]
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction Pathway for Ethanol Steam Reforming on a Ni/SiO2 Catalyst Including Coke Formation. Int. J. Hydrogen Energy 2014, 39, 18820–18834. [Google Scholar] [CrossRef]






| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| 9Ce1Sm | 180 | 0.13 | 2.8 |
| 8Ce2Sm | 154 | 0.16 | 4.1 |
| 7Ce3Sm | 162 | 0.21 | 5.1 |
| Ni/9Ce1Sm | 122 | 0.11 | 3.5 |
| Ni/8Ce2Sm | 97 | 0.11 | 4.5 |
| Ni/7Ce3Sm | 94 | 0.15 | 6.2 |
| Catalysts | Ni/Ce+Sm | Sm/Ce | Oads/(Olatt + Oads) | ||
|---|---|---|---|---|---|
| Nominal | XPS | Nominal | XPS | ||
| Ni/CeO2 | 0.67 | 14.07 | - | - | 31.7% |
| Ni/9Ce1Sm | 0.67 | 9.73 | 0.22 | 0.49 | 34.2% |
| Ni/8Ce2Sm | 0.67 | 4.96 | 0.50 | 1.03 | 41.9% |
| Ni/7Ce3Sm | 0.67 | 2.11 | 0.86 | 0.50 | 59.0% |
| Sample | DCe2O a | DNiO b |
|---|---|---|
| (nm) | (nm) | |
| CeO2 | 7.3 | - |
| 9Ce1Sm | 6.6 | - |
| 8Ce2Sm | 5.9 | - |
| 7Ce3Sm | 5.4 | - |
| Ni/CeO2 | 6.9 | 47.9 |
| Ni/9Ce1Sm | 6.0 | 23.6 |
| Ni/8Ce2Sm | 5.3 | 18.8 |
| Ni/7Ce3Sm | 4.9 | 13.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirinos, C.A.; Liu, S.; Cortés Corberán, V.; Gómez-Sainero, L.M. Effect of Sm2O3 Doping of CeO2-Supported Ni Catalysts for H2 Production by Steam Reforming of Ethanol. Catalysts 2025, 15, 131. https://doi.org/10.3390/catal15020131
Chirinos CA, Liu S, Cortés Corberán V, Gómez-Sainero LM. Effect of Sm2O3 Doping of CeO2-Supported Ni Catalysts for H2 Production by Steam Reforming of Ethanol. Catalysts. 2025; 15(2):131. https://doi.org/10.3390/catal15020131
Chicago/Turabian StyleChirinos, Carlos Andrés, Sichen Liu, Vicente Cortés Corberán, and Luisa María Gómez-Sainero. 2025. "Effect of Sm2O3 Doping of CeO2-Supported Ni Catalysts for H2 Production by Steam Reforming of Ethanol" Catalysts 15, no. 2: 131. https://doi.org/10.3390/catal15020131
APA StyleChirinos, C. A., Liu, S., Cortés Corberán, V., & Gómez-Sainero, L. M. (2025). Effect of Sm2O3 Doping of CeO2-Supported Ni Catalysts for H2 Production by Steam Reforming of Ethanol. Catalysts, 15(2), 131. https://doi.org/10.3390/catal15020131









