Inorganic Bismuth Catalysts for Photocatalytic Organic Reactions
Abstract
1. Introduction
2. Application of Bi2O3 (or Bi2S3) in Photocatalytic Organic Reactions
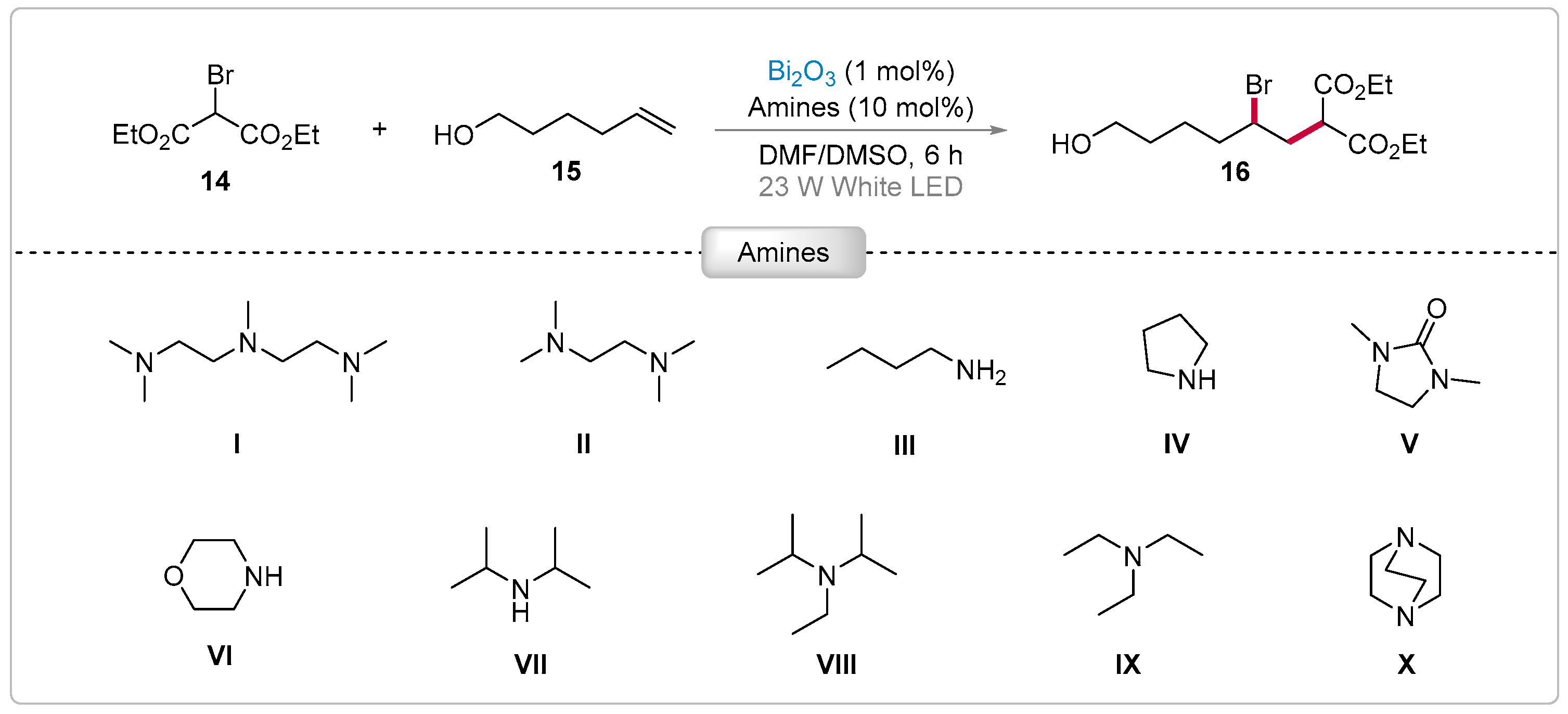
2.1. α-Alkylation of Aldehydes
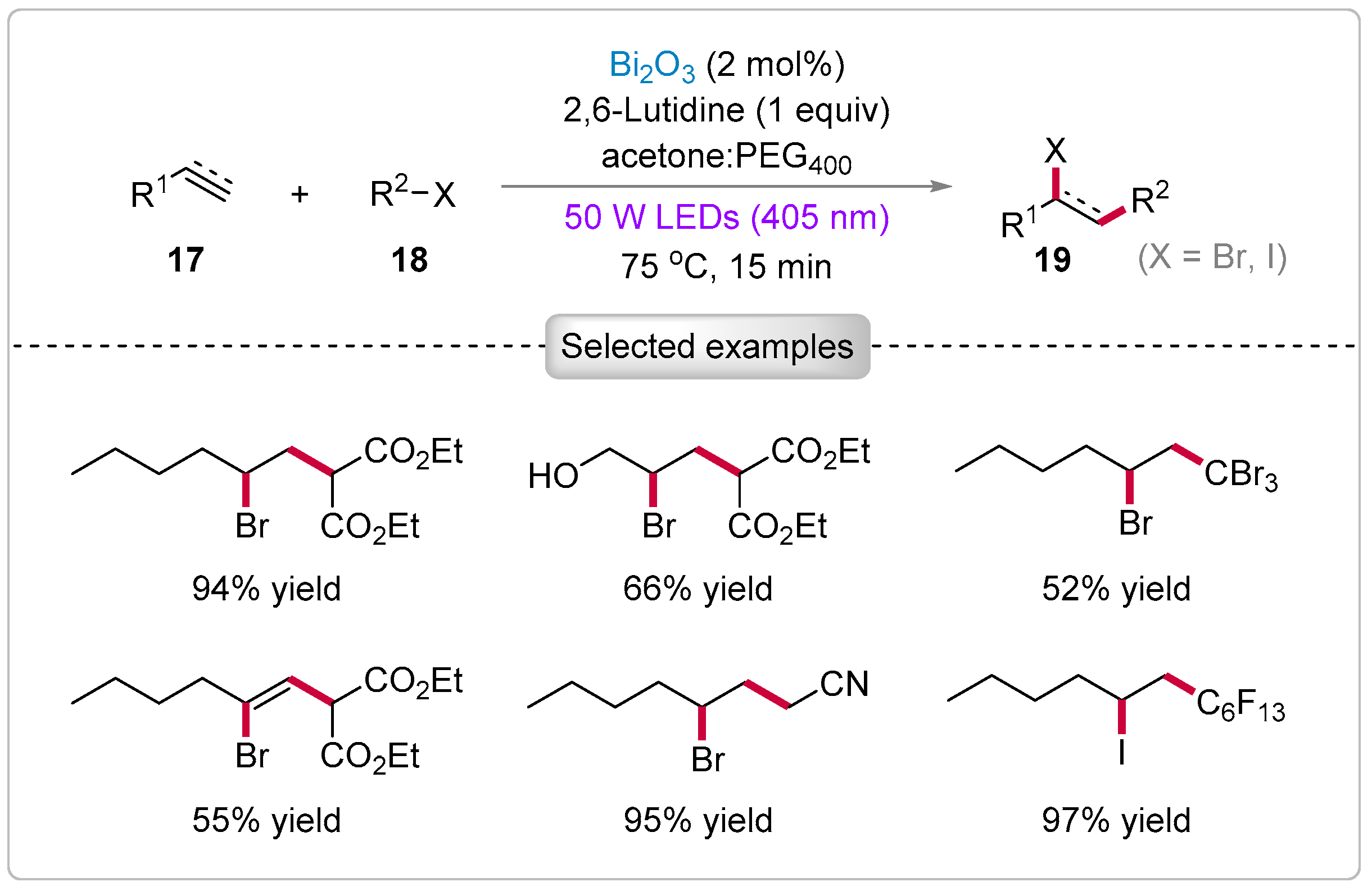
2.2. ATRA Reactions of Olefins
3. Application of BiCl3 in Photocatalytic Organic Reactions
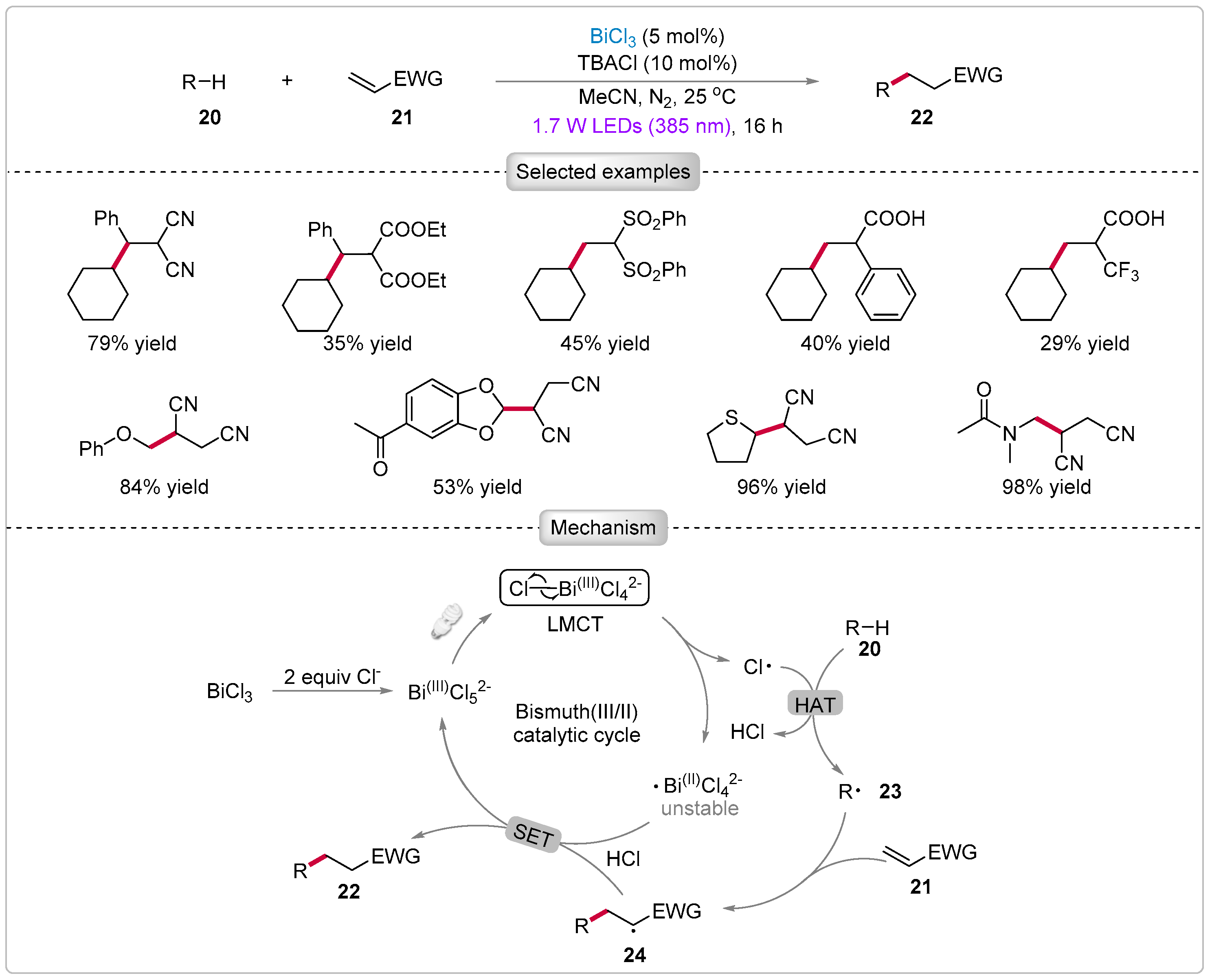
3.1. Alkylation of C(sp3)-H
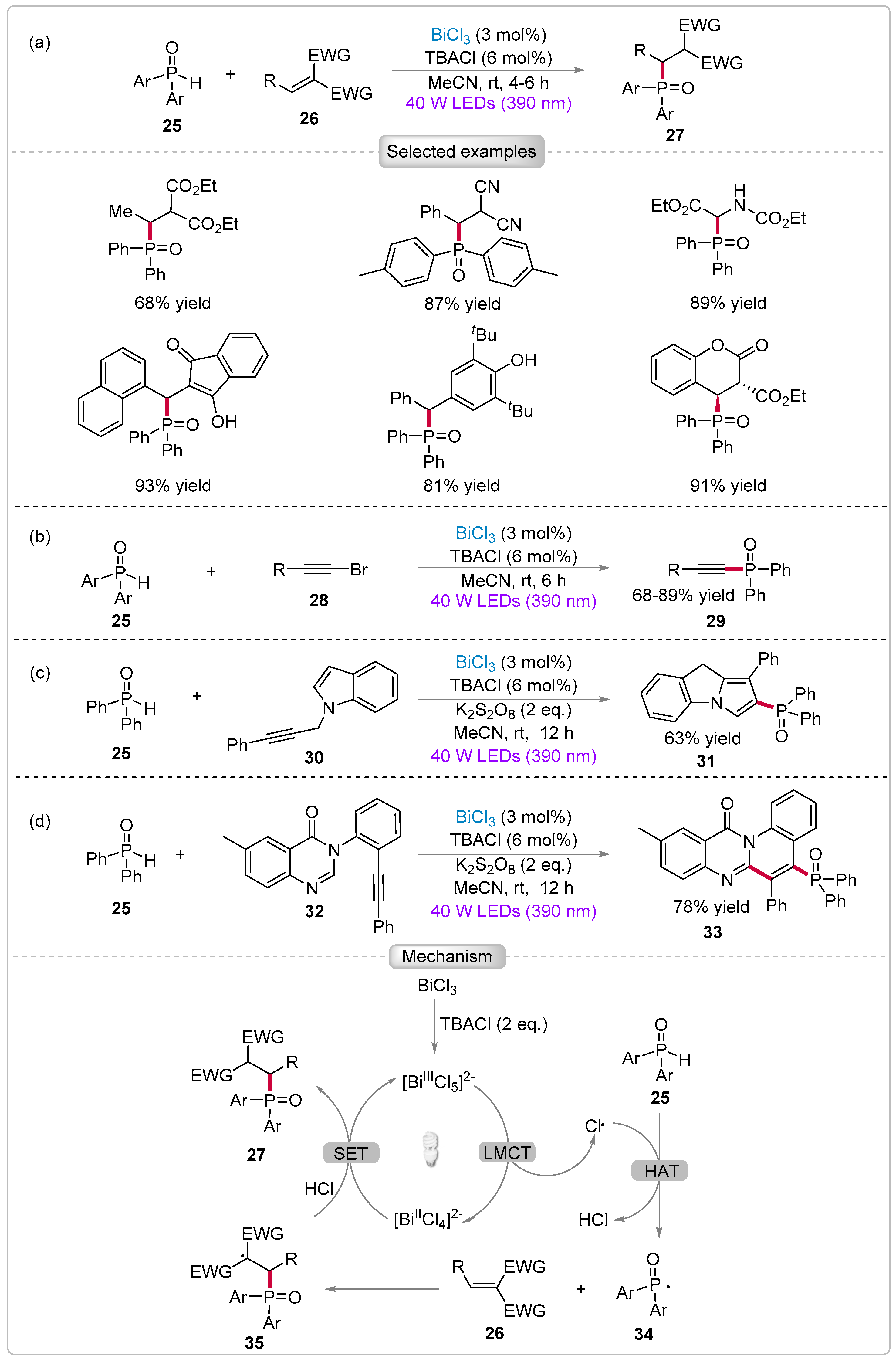
3.2. Functionalization of Diarylphosphine Oxides
4. Application of Bi4NbO8Cl in Photocatalytic Organic Reactions
Trifluoromethylation of Olefins and (Hetero)Aromatics
5. Application of BiVO4 in Photocatalytic Organic Reactions
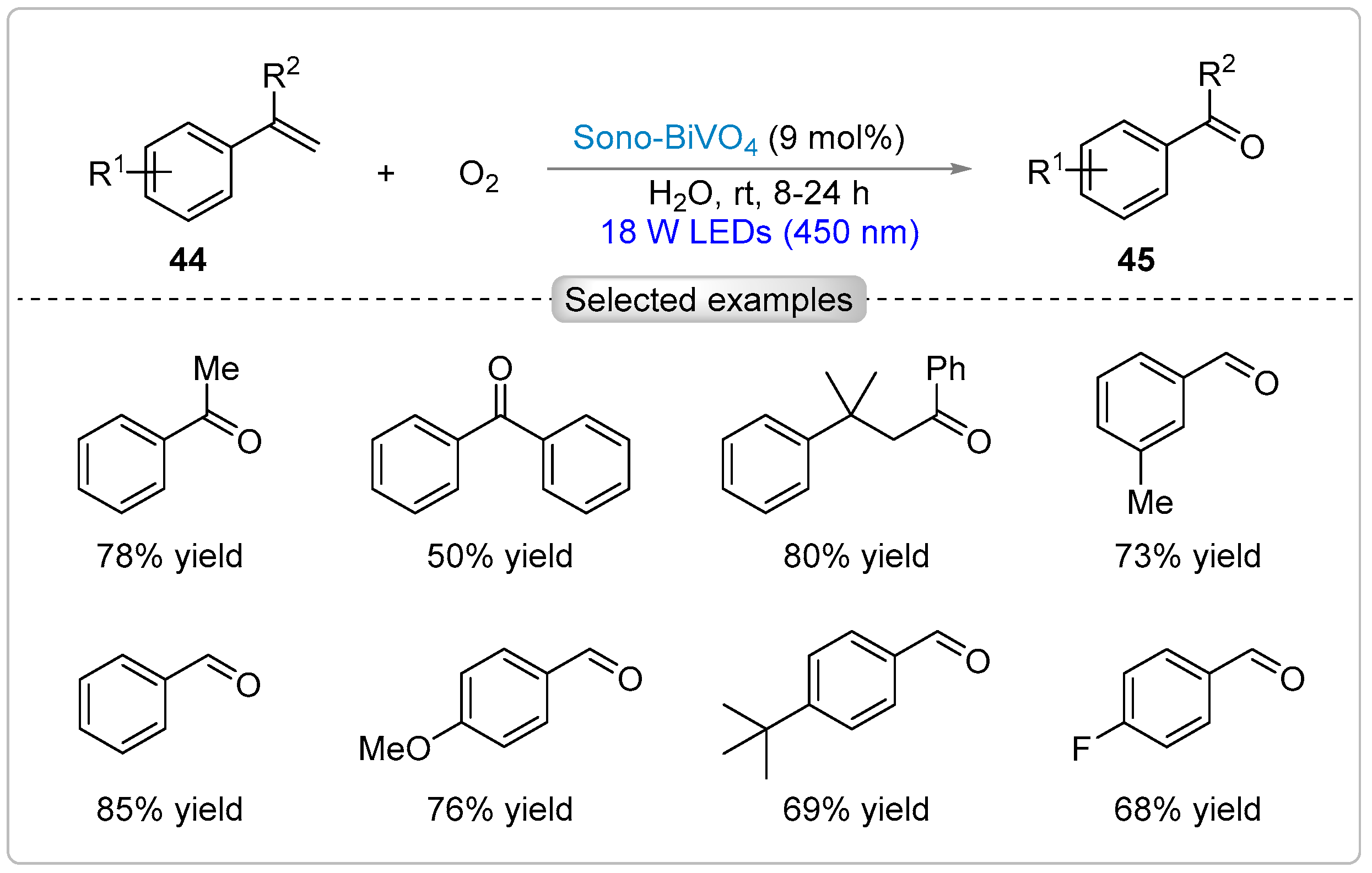
5.1. Oxidation of C(sp2)-H
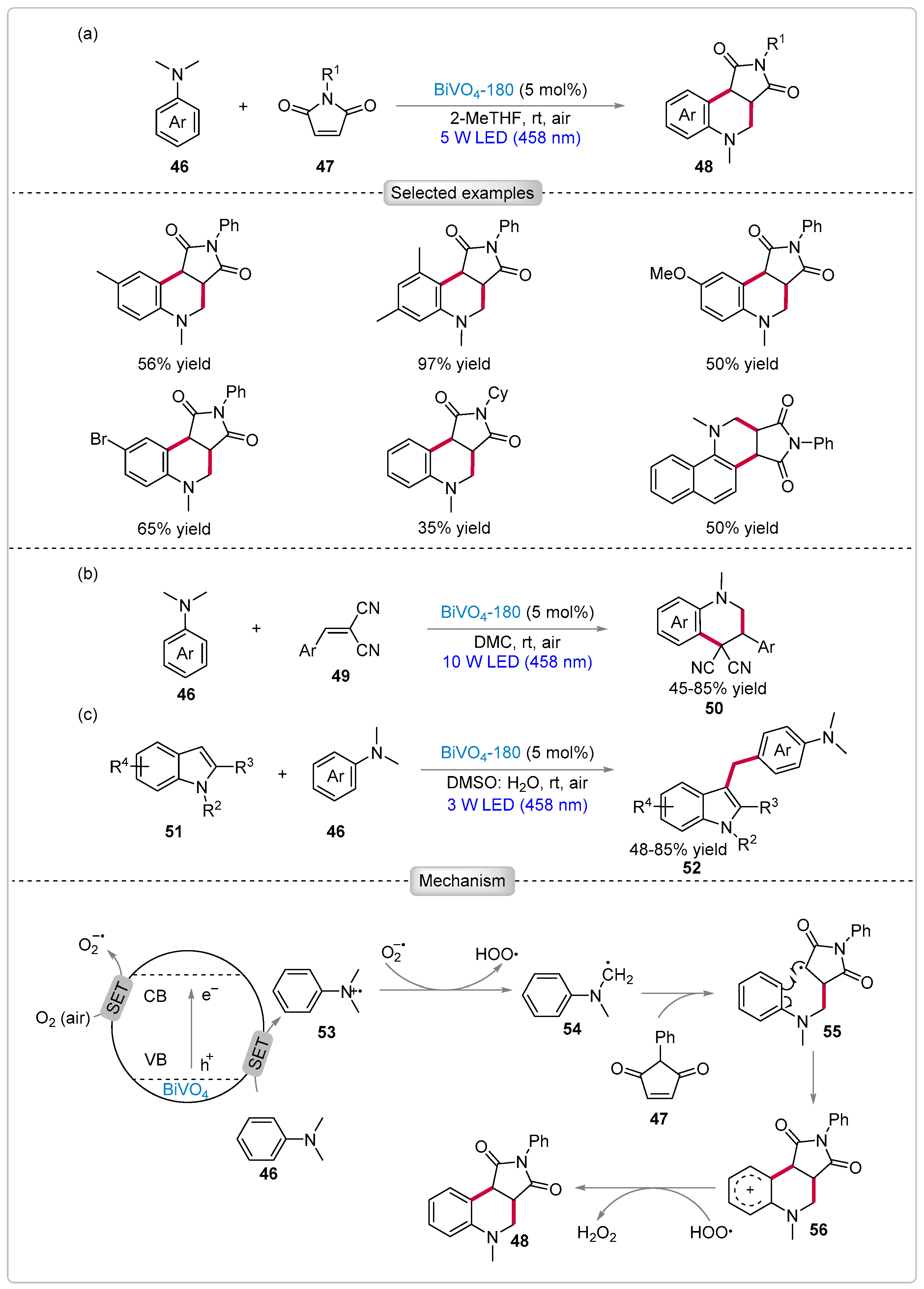
5.2. Functionalization of N,N-Dimethylanilines
6. Application of Bi2WO6 in Photocatalytic Organic Reactions
Selective Oxidation of Benzyl Alcohols
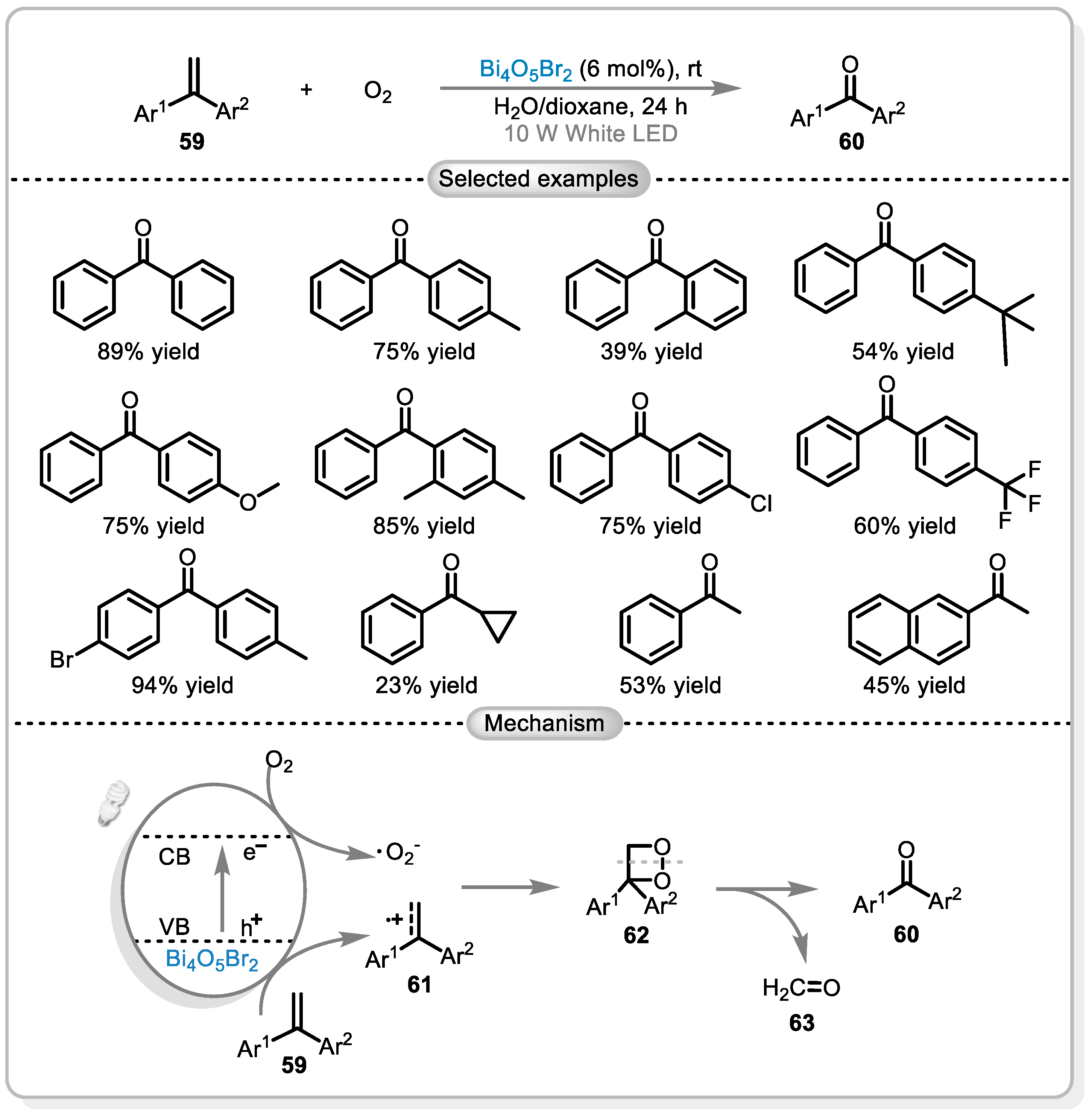
7. Application of Bi4O5Br2 in Photocatalytic Oxidation of C(sp2)-H
8. Application of Bi2MoO6 in Photocatalytic Organic Reactions
Biomass Alcohols Oxidation to Aldehydes
9. Performance of Bismuth-based Photocatalysts
10. Conclusions
- (1)
- Expanding the range of reaction types and developing novel photo-induced reactions to broaden their applications;
- (2)
- Optimizing the structure–performance relationship of the catalysts and exploring hierarchical catalysts to enhance selectivity and activity; designing multifunctional catalysts to achieve highly selective synthesis;
- (3)
- Optimizing reaction conditions to improve product yields and environmental compatibility;
- (4)
- Enhancing the stability and recyclability of the catalysts, exploring reuse strategies, and investigating the potential of bismuth-based catalysts in pharmaceutical synthesis and the development of novel materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marzo, L.; Pagire, S.K.; Reiser, O.; Konig, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xuan, J. Photochemical Synthesis of Aroylated Heterocycles under Catalyst and Additive Free Conditions. Chin. J. Org. Chem. 2022, 42, 923–924. [Google Scholar] [CrossRef]
- Yi, R.; He, W. Visible-Light-Induced Radical Arylation Reactions via Electron Donor-Acceptor Complex. Chin. J. Org. Chem. 2022, 42, 2590–2592. [Google Scholar] [CrossRef]
- Pan, J.; Li, H.; Sun, K.; Tang, S.; Yu, B. Visible-Light-Induced Decarboxylation of Dioxazolones to Phosphinimidic Amides and Ureas. Molecules 2022, 27, 3648. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-M.; Song, L.; Pan, J.; Zeng, F.; Xie, Y.; Wei, W.; Yi, D. Visible-light-induced four-component difunctionalization of alkenes to construct phosphorodithioate-containing quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2024, 35, 110112. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, H.; You, J.; Wei, W.; Yi, D. Additive-free synthesis of S-substituted isothioureas via visible-light-induced four-component reaction of α-diazoesters, aryl isothiocyanates, amines and cyclic ethers. Chin. Chem. Lett. 2024, 35, 109107. [Google Scholar] [CrossRef]
- Yuan, X.; Fan, H.-B.; Liu, J.; Qin, L.-Z.; Wang, J.; Duan, X.; Qiu, J.-K.; Guo, K. Recent advances in photoredox catalytic transformations by using continuous-flow technology. Chin. J. Catal. 2023, 50, 175–194. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Li, Q.-Q.; Studer, A.; Xuan, J. Emerging progress: Photochemical transformation of nitroso compounds. Sci. China Chem. 2025, 68, 118–133. [Google Scholar] [CrossRef]
- Song, H.-Y.; Jiang, J.; Wu, C.; Hou, J.-C.; Lu, Y.-H.; Wang, K.-L.; Yang, T.-B.; He, W.-M. Semi-heterogeneous g-C3N4/NaI dual catalytic C–C bond formation under visible light. Green Chem. 2023, 25, 3292–3296. [Google Scholar] [CrossRef]
- Yi, R.-N.; He, W.-M. Photoinduced bismuth vanadate-catalyzed C(sp2)-H functionalization. Chin. Chem. Lett. 2024, 35, 109253. [Google Scholar] [CrossRef]
- Chen, L.; Tan, K.-X.; Zheng, Z.-J.; Lin, X.-L.; Ming, B.-M.; Zhang, Z.-B.; Yang, G.-P. Uranyl-Organic Framework as Hydrogen Atom Transfer Catalyst for Visible-Light-Driven Heterogeneous Hydroacylation of Azodicarboxylates. Eur. J. Org. Chem. 2024, 27, e202400001. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Lv, Q.; Yu, B. Embracing heterogeneous photocatalysis: Evolution of photocatalysts in annulation of dimethylanilines and maleimides. Chem. Commun. 2024, 60, 8645–8657. [Google Scholar] [CrossRef]
- Wang, R.-N.; Zeng, F.-L.; Chen, X.-L.; Zhu, H.-L.; Qu, L.-B.; Huang, X.-Q.; Tang, S.; Zhao, Y.-F.; Yu, B. Recyclable ZnIn2S4 Microspheres for Photocatalytic Azolation of N-Heterocycles. ACS Sustain. Chem. Eng. 2022, 10, 14212–14219. [Google Scholar] [CrossRef]
- Shaikh, M.; Rubalcaba, K.; Yan, Y. Halide Perovskite Induces Halogen/Hydrogen Atom Transfer (XAT/HAT) for Allylic C-H Amination. Angew. Chem. Int. Ed. 2024, 63, e202413012. [Google Scholar] [CrossRef]
- Yang, M.; Lian, R.; Zhang, X.; Wang, C.; Cheng, J.; Wang, X. Photocatalytic cyclization of nitrogen-centered radicals with carbon nitride through promoting substrate/catalyst interaction. Nat. Commun. 2022, 13, 4900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-H.; Kang, S.-L.; Cui, Z.-S.; Liu, Y.-H.; Zhang, M.; Zhang, Z.-H. Visible light-driven C–H arylation of heteroarenes with aryl diazonium salts in water catalyzed by a Z-scheme CuInS2/K-C3N4 heterojunction. Green Chem. 2024, 26, 4803–4810. [Google Scholar] [CrossRef]
- Mohan, R. Green Bismuth. Nat. Chem. 2010, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.W.; Cornella, J. Bismuth Redox Catalysis: An Emerging Main-Group Platform for Organic Synthesis. ACS Catal. 2022, 12, 1382–1393. [Google Scholar] [CrossRef]
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Applications of Bismuth(III) Compounds in Organic Synthesis. Tetrahedron 2002, 58, 8373–8397. [Google Scholar] [CrossRef]
- Briand, G.G.; Burford, N.T. Bismuth Compounds and Preparations with Biological or Medicinal Relevance. Chem. Rev. 1999, 99, 2601–2658. [Google Scholar] [CrossRef]
- Liu, D.L.; Xu, H.Y.; Hang, Y.; Lu, H.F. 1,6-Addition of Nitrogen Nucleophile to para-Quinone Methides Catalyzed by Recyclable Bismuth Complex: Facile Access to N-Heterocyclic Substituted Unsymmetric Triarylmethane Derivatives. Chin. J. Org. Chem. 2022, 42, 796–802. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Hu, C.; Huang, H. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Catal. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Song, S.; Xing, Z.; Zhao, H.; Li, Z.; Zhou, W. Recent advances in bismuth-based photocatalysts: Environment and energy applications. Green Energy Environ. 2023, 8, 1232–1264. [Google Scholar] [CrossRef]
- Boochakiat, S.; Inceesungvorn, B.; Nattestad, A.; Chen, J. Bismuth-Based Oxide Photocatalysts for Selective Oxidation Transformations of Organic Compounds. ChemNanoMat 2023, 9, e202300140. [Google Scholar] [CrossRef]
- Long, D.; Yu, X.; Li, W.; Ma, S. One bismuth three benefits: An overview of bismuth-based photocatalysts for energy conversion, environmental treatment and biomedical applications. Mater. Adv. 2025, 6, 508–526. [Google Scholar] [CrossRef]
- Riente, P.; Matas Adams, A.; Albero, J.; Palomares, E.; Pericàs, M.A. Light-Driven Organocatalysis Using Inexpensive, Nontoxic Bi2O3 as the Photocatalyst. Angew. Chem. Int. Ed. 2014, 53, 9613–9616. [Google Scholar] [CrossRef] [PubMed]
- Riente, P.; Pericàs, M.A. Visible Light-Driven Atom Transfer Radical Addition to Olefins using Bi2O3 as Photocatalyst. ChemSusChem 2015, 8, 1841–1844. [Google Scholar] [CrossRef]
- Zahid, A.H.; Han, Q. A review on the preparation, microstructure, and photocatalytic performance of Bi2O3 in polymorphs. Nanoscale 2021, 13, 17687–17724. [Google Scholar] [CrossRef] [PubMed]
- Riente, P.; Fianchini, M.; Llanes, P.; Pericàs, M.A.; Noël, T. Shedding light on the nature of the catalytically active species in photocatalytic reactions using Bi2O3 semiconductor. Nat. Commun. 2021, 12, 625. [Google Scholar] [CrossRef]
- Riente, P.; Fianchini, M.; Pericàs, M.A.; Noël, T. Accelerating the Photocatalytic Atom Transfer Radical Addition Reaction Induced by Bi2O3 with Amines: Experiment and Computation. ChemCatChem 2022, 14, e202200319. [Google Scholar] [CrossRef]
- Bianchi, P.; Williams, J.D.; Kappe, C.O. Continuous flow processing of bismuth-photocatalyzed atom transfer radical addition reactions using an oscillatory flow reactor. Green Chem. 2021, 23, 2685–2693. [Google Scholar] [CrossRef]
- Birnthaler, D.; Narobe, R.; Lopez-Berguno, E.; Haag, C.; König, B. Synthetic Application of Bismuth LMCT Photocatalysis in Radical Coupling Reactions. ACS Catal. 2023, 13, 1125–1132. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Wang, C.-C.; Yu, B. Recent advances in FeCl3-photocatalyzed organic reactions via hydrogen-atom transfer. Chin. Chem. Lett. 2024, 35, 109517. [Google Scholar] [CrossRef]
- Dou, Q.; Wang, T.M.; Fang, L.J.; Zhai, H.B.; Cheng, B. Recent Development of Photoinduced Iron-Catalysis in Organic Synthesis. Chin. J. Org. Chem. 2023, 43, 1386–1415. [Google Scholar] [CrossRef]
- Li, H.-C.; Li, G.-N.; Sun, K.; Chen, X.-L.; Jiang, M.-X.; Qu, L.-B.; Yu, B. Ce(III)/Photoassisted Synthesis of Amides from Carboxylic Acids and Isocyanates. Org. Lett. 2022, 24, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.; Nair, A.M.; Volla, C.M.R. Expedient radical phosphonylations via ligand to metal charge transfer on bismuth. Chem. Sci. 2024, 15, 7136–7143. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-C.; Zhang, M.; Lv, Q.; Sun, K.; Chen, X.-L.; Qu, L.; Yu, B. Homogeneous catalysis and heterogeneous separation: Ionic liquids as recyclable photocatalysts for hydroacylation of olefins. Chin. Chem. Lett. 2025, 36, 110579. [Google Scholar] [CrossRef]
- Lin, X.; Huang, T.; Huang, F.; Wang, W.; Shi, J. Photocatalytic activity of a Bi-based oxychloride Bi4NbO8Cl. J. Mater. Chem. 2007, 17, 2145–2150. [Google Scholar] [CrossRef]
- Ogawa, K.; Nakada, A.; Suzuki, H.; Tomita, O.; Higashi, M.; Saeki, A.; Kageyama, H.; Abe, R. Flux Synthesis of Layered Oxyhalide Bi4NbO8Cl Photocatalyst for Efficient Z-Scheme Water Splitting Under Visible Light. ACS Appl. Mater. Inter. 2019, 11, 5642–5650. [Google Scholar] [CrossRef]
- Qu, X.; Liu, M.; Zhai, H.; Zhao, X.; Shi, L.; Du, F. Plasmonic Ag-promoted layered perovskite oxyhalide Bi4NbO8Cl for enhanced photocatalytic performance towards water decontamination. J. Alloy. Compd. 2019, 810, 151919. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Sun, K.; Huang, X.; Chen, X.; Qu, L.; Yu, B. Innovations in photocatalytic trifluoromethylation: Role of heterogeneous BBi4NbO8Cl in radical generation. J. Catal. 2024, 439, 115775. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Han, S.S.; Park, J.Y.; Hwang, H.S.; Choe, H.R.; Nam, K.M.; Cho, E.J. Facile Synthesis of BiVO4 for Visible-Light-Induced C-C Bond Cleavage of Alkenes to Generate Carbonyls. ChemSusChem 2019, 12, 3018–3022. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-L.; Zhu, H.-L.; Wang, R.-N.; Yuan, X.-Y.; Sun, K.; Qu, L.-B.; Chen, X.-L.; Yu, B. Bismuth Vanadate: A Versatile Heterogeneous Catalyst for Photocatalytic Functionalization of C(sp2)-H Bonds. Chin. J. Catal. 2023, 46, 157–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.-J. Bi2WO6: A highly chemoselective visible light photocatalyst toward aerobic oxidation of benzylic alcohols in water. RSC Adv. 2014, 4, 2904–2910. [Google Scholar] [CrossRef]
- Song, P.; Du, J.; Ma, X.; Shi, Y.; Fang, X.; Liu, D.; Wei, S.; Liu, Z.; Cao, Y.; Lin, B.; et al. Design of Bi4O5Br2/g-C3N4 Heterojunction for Efficient Photocatalytic Removal of Persistent Organic Pollutants From Water. EcoEnergy 2023, 1, 197–206. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) Photocatalytic Nanomaterials: Applications for Fuels and Environmental Management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef]
- Jin, X.; Lv, C.; Zhou, X.; Xie, H.; Sun, S.; Liu, Y.; Meng, Q.; Chen, G. A Bismuth Rich Hollow Bi4O5Br2 Photocatalyst Enables Dramatic CO2 Reduction Activity. Nano Energy 2019, 64, 103955. [Google Scholar] [CrossRef]
- Liu, T.; Xue, F.; Chen, Z.; Cheng, Z.; Cao, W.; Wang, B.; Jin, W.; Xia, Y.; Zhang, Y.; Liu, C. Bi4O5Br2 catalyzed selective oxidative of C=C double bonds to ketones with molecular oxygen under visible-light irradiation. J. Catal. 2022, 414, 76–83. [Google Scholar] [CrossRef]
- Chen, H.; Xu, R.; Chen, D.; Lu, T.; Li, H.; Wang, M. Subsurface Mo Vacancy in Bismuth Molybdate Promotes Photocatalytic Oxidation of Lactate to Pyruvate. ACS Catal. 2024, 14, 1977–1986. [Google Scholar] [CrossRef]
- Wu, X.; Ling Tan, H.; Zhang, C.; Teng, Z.; Liu, Z.; Hau Ng, Y.; Zhang, Q.; Su, C. Recent advances in two-dimensional ultrathin Bi-based photocatalysts. Prog. Mater Sci. 2023, 133, 101047. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Tian, F.; Zhang, J.; Yu, J. Structure Tuning of Bi2MoO6 and Their Enhanced Visible Light Photocatalytic Performances. Crit. Rev. Solid State Mater. Sci. 2017, 42, 347–372. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Cao, R.; Cui, C.; Luo, Y.; Huang, C.; Cui, X.; Wang, Z.; Song, Y. Photocatalytic one-pot alkylation of nitrobenzene with benzyl alcohol for the precise synthesis of N-benzylideneaniline over F-doped Bi2MoO6 nanosheets. Catal. Sci. Technol. 2023, 13, 3916–3926. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Gao, Y.; Chong, R.; Wang, Z.; Guo, L.; Zhang, X.; Li, C. To boost photocatalytic activity in selective oxidation of alcohols on ultrathin Bi2MoO6 nanoplates with Pt nanoparticles as cocatalyst. J. Catal. 2017, 345, 96–103. [Google Scholar] [CrossRef]






| Photocatalyst | Bandgap (eV) | Reactions | Runs of Photocatalyst | Ref. |
|---|---|---|---|---|
| Bi2O3 | 2.83 | α-Alkylation Reaction | 3 | [26] |
| ATRA Reaction | [27] | |||
| BiCl3 | - | C(sp3)-H Alkylation | - | [32] |
| Functionalization of Diarylphosphine Oxides | [36] | |||
| Bi4NbO8Cl | 2.51 | Trifluoromethylation Reaction | 5 | [41] |
| Sono-BiVO4 | 2.40 | C(sp2)-H Oxidation | 5 | [43] |
| BiVO4-180 | 2.40 | Functionalization of N,N-Dimethylanilines | 8 | [44] |
| Bi2WO6 | 2.81 | Selective Oxidation of Benzyl Alcohol | - | [45] |
| Bi4O5Br2 | 2.93 | Oxidation of C=C Bonds | 4 | [49] |
| VM-Bi2MoO6 | 2.74 | Oxidation of Alcohols | 5 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Lv, Q.; Yu, B. Inorganic Bismuth Catalysts for Photocatalytic Organic Reactions. Catalysts 2025, 15, 135. https://doi.org/10.3390/catal15020135
He H, Lv Q, Yu B. Inorganic Bismuth Catalysts for Photocatalytic Organic Reactions. Catalysts. 2025; 15(2):135. https://doi.org/10.3390/catal15020135
Chicago/Turabian StyleHe, Hualei, Qiyan Lv, and Bing Yu. 2025. "Inorganic Bismuth Catalysts for Photocatalytic Organic Reactions" Catalysts 15, no. 2: 135. https://doi.org/10.3390/catal15020135
APA StyleHe, H., Lv, Q., & Yu, B. (2025). Inorganic Bismuth Catalysts for Photocatalytic Organic Reactions. Catalysts, 15(2), 135. https://doi.org/10.3390/catal15020135








