Hydrogen Storage Technology, and Its Challenges: A Review
Abstract
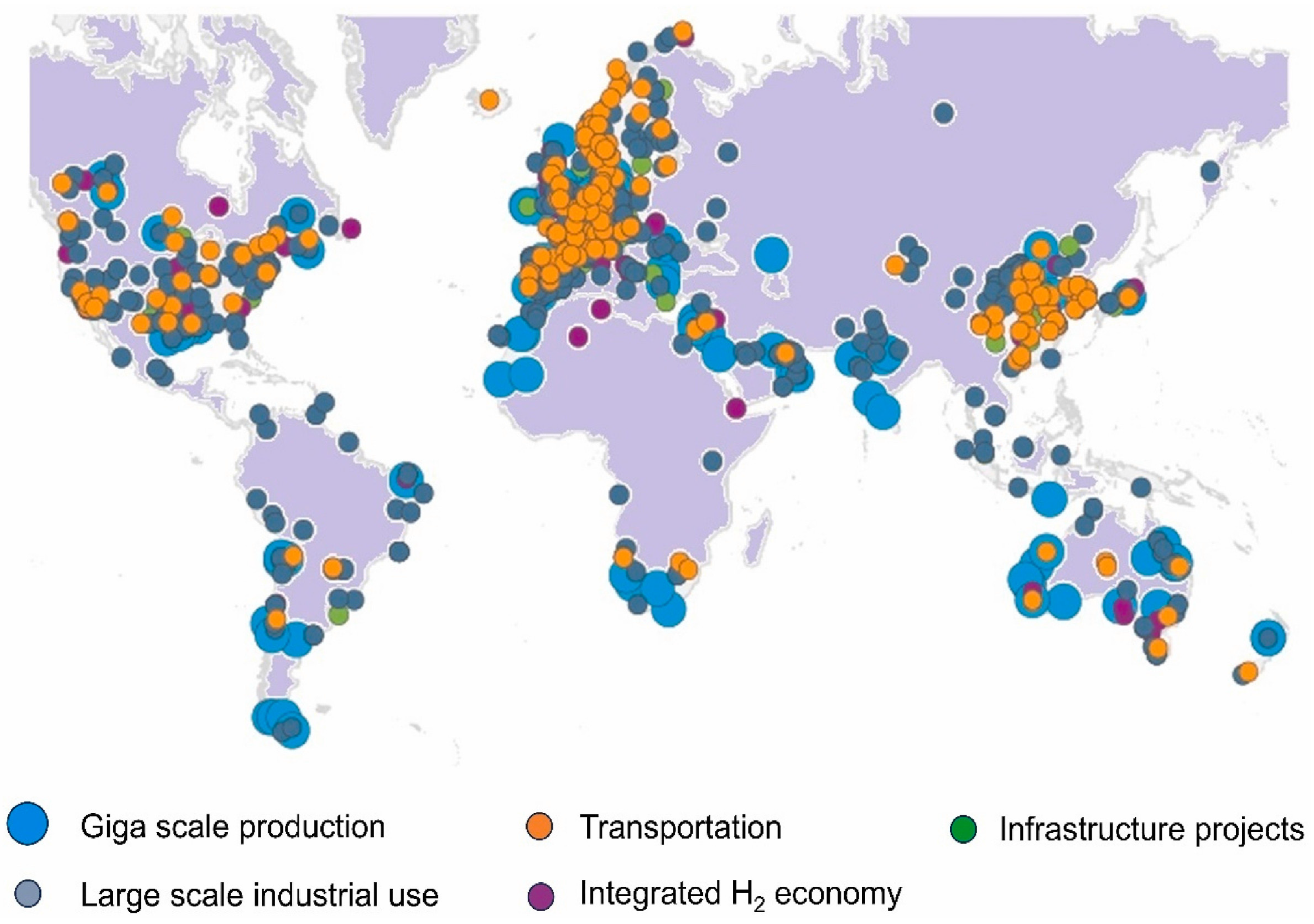
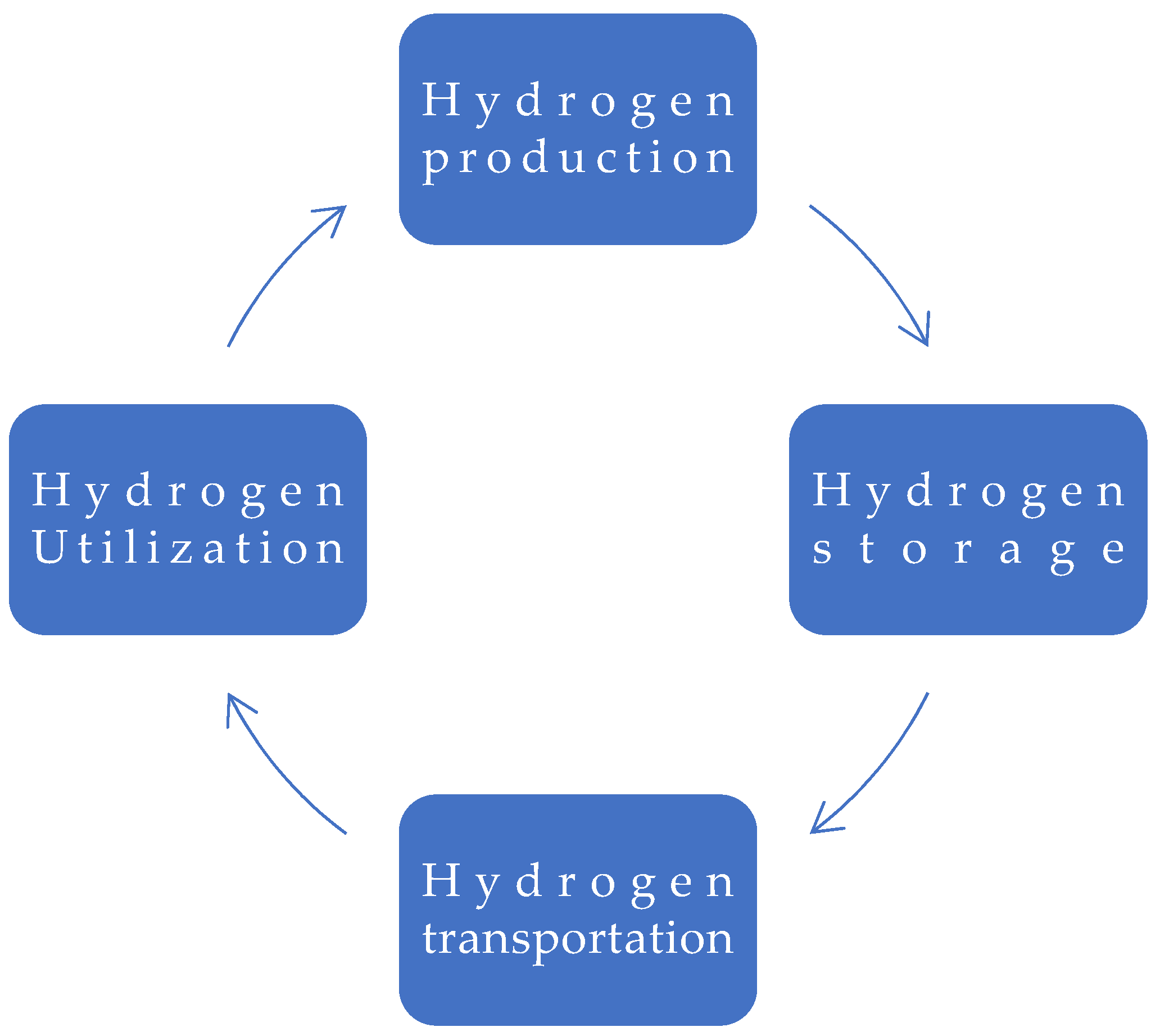
1. Introduction
| Application of Hydrogen Energy | Description | Advantages | Challenges |
|---|---|---|---|
| Transportation | Used in fuel cell vehicles (FCVs) for cars, buses, and trains trucks. Explored as a fuel for rockets and aircraft. | Zero emissions, fast refueling, high energy efficiency. | Storage challenges High production costs, limited refueling infrastructure |
| Industrial Processes | Utilized in refining, ammonia production, and methanol synthesis, steel manufacturing, fertilizer production. | Reduces carbon footprint in industries, essential for chemical production. | Dependency on fossil fuels for grey hydrogen, high energy requirements. |
| Energy Storage | Stores excess renewable energy as hydrogen via electrolysis. | Enables long-term storage, balances grid intermittency. | Low round-trip efficiency, high costs for electrolyzers and storage systems. |
| Heating | Blended with natural gas for residential and commercial heating. | Reduces carbon emissions in heating systems. | Infrastructure modifications needed. |
| Power Generation | Used in gas turbines or fuel cells for electricity generation. | Clean energy production, compatible with existing infrastructure. | High costs, requires pure hydrogen to avoid emissions. |
2. Physical-Based Hydrogen Storage
2.1. Compressed Hydrogen Storage
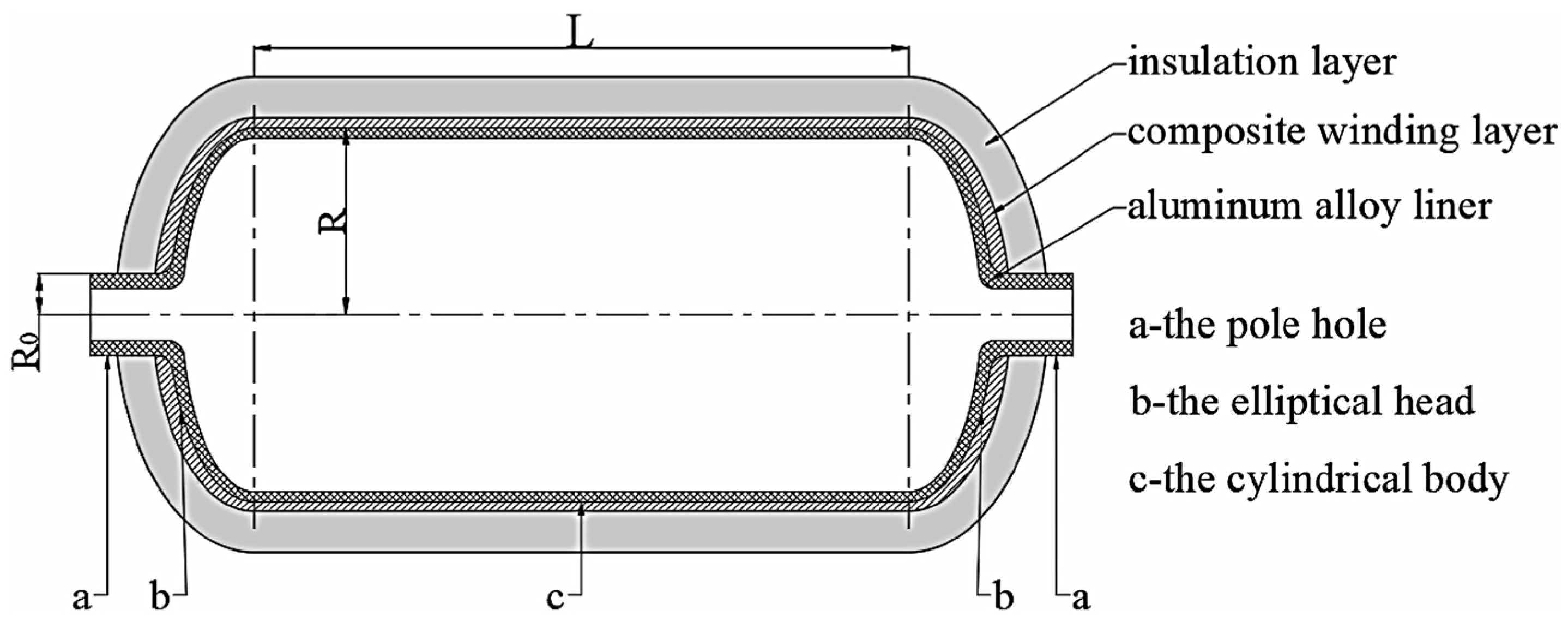
2.1.1. Type I Vessels
2.1.2. Type II Vessels
2.1.3. Type III Vessels
2.1.4. Type IV Vessels
2.1.5. Type V Vessels
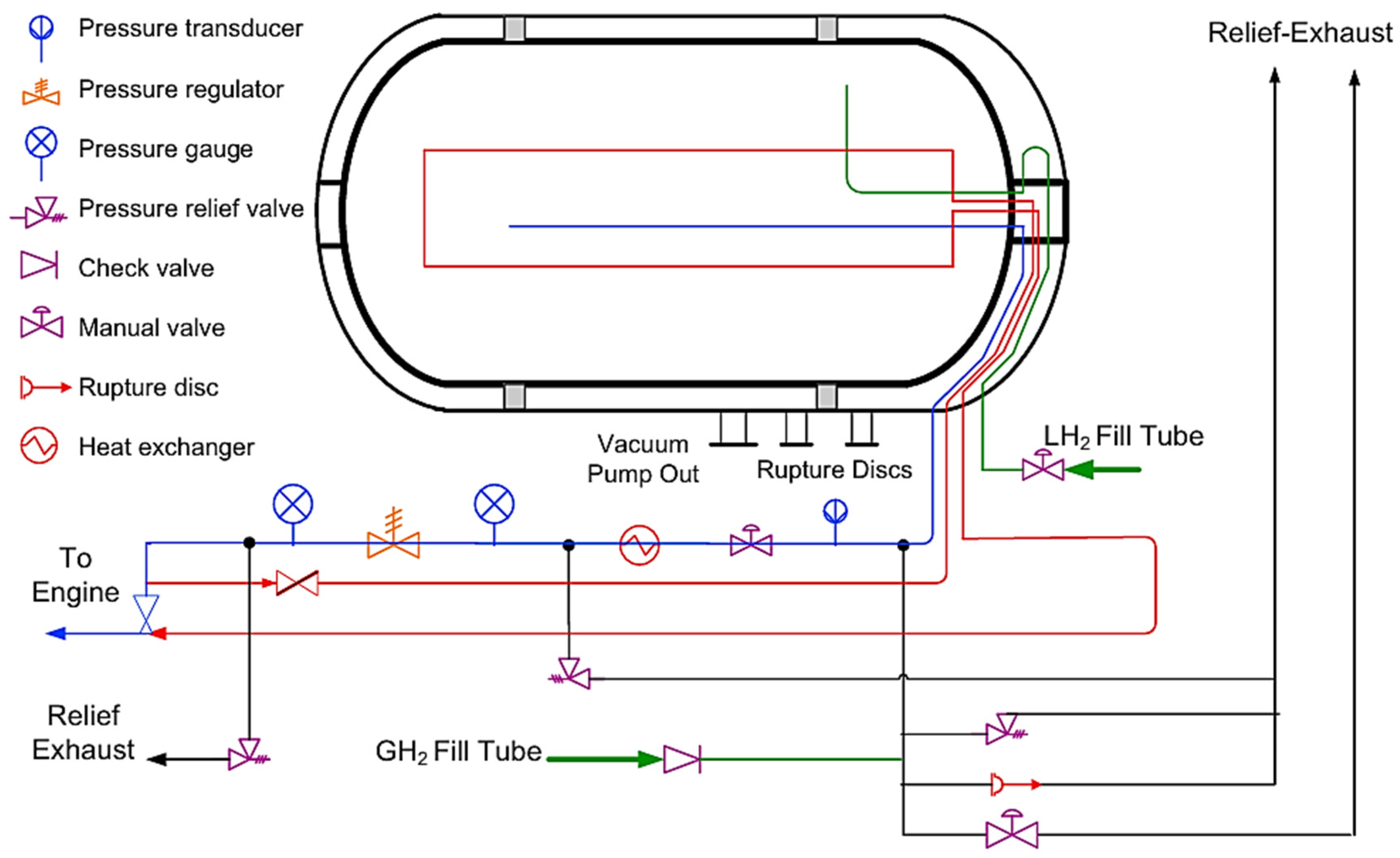
2.2. Liquefied Hydrogen Storage
2.3. Cryo-Compressed Hydrogen Storage
3. Solid-State Hydrogen Storage (SSHS)
3.1. Chemical Storage
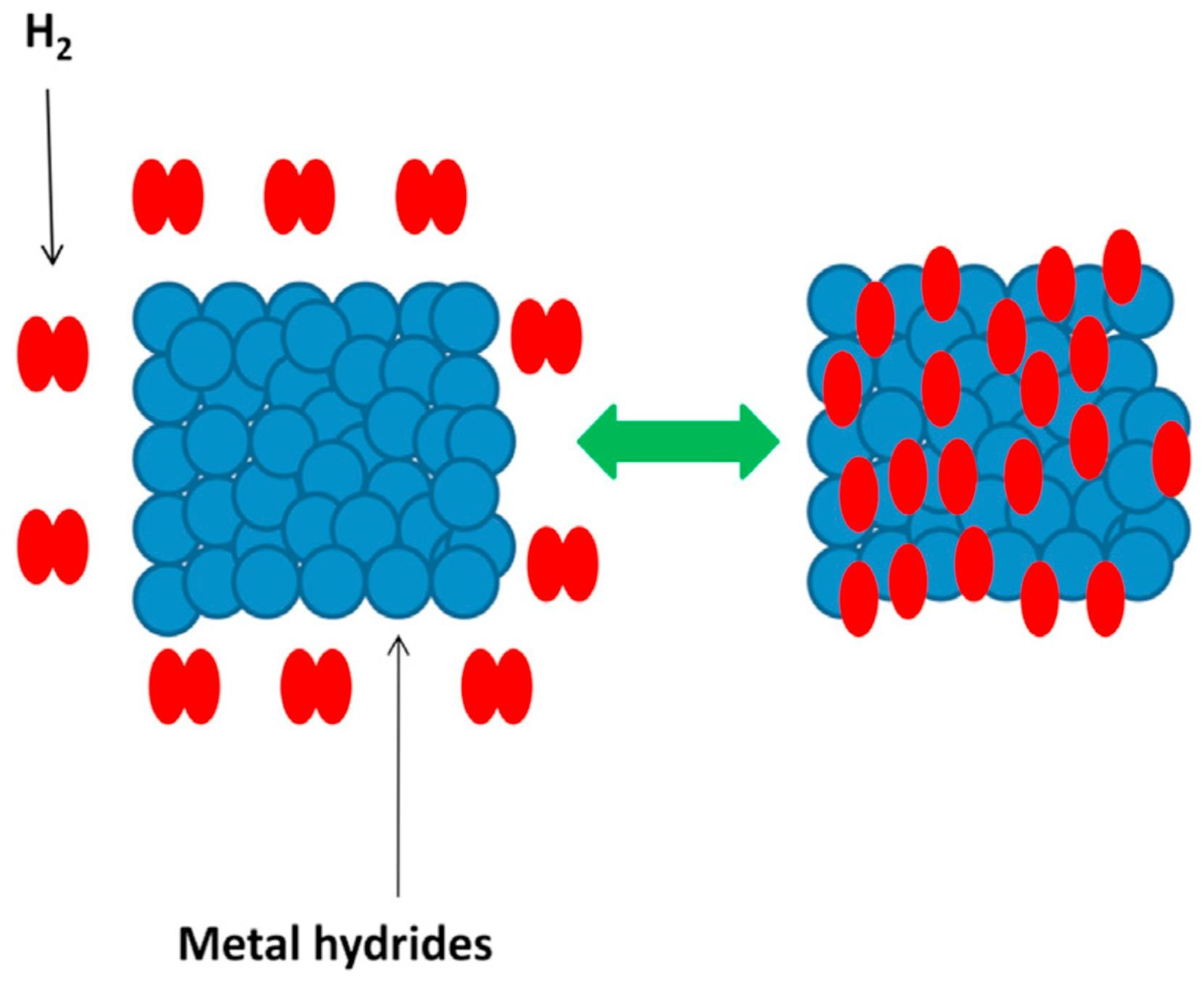
3.1.1. Metal Hydrides Hydrogen Storage
- Excellent safety
- Good reversible cycling performance
- High hydrogen storage capacity (compared to physical-based storage)
- High hydrogen density
- High purity of stored hydrogen
- Low operational, maintenance, and energy costs.
3.1.2. Ammonia (NH3)
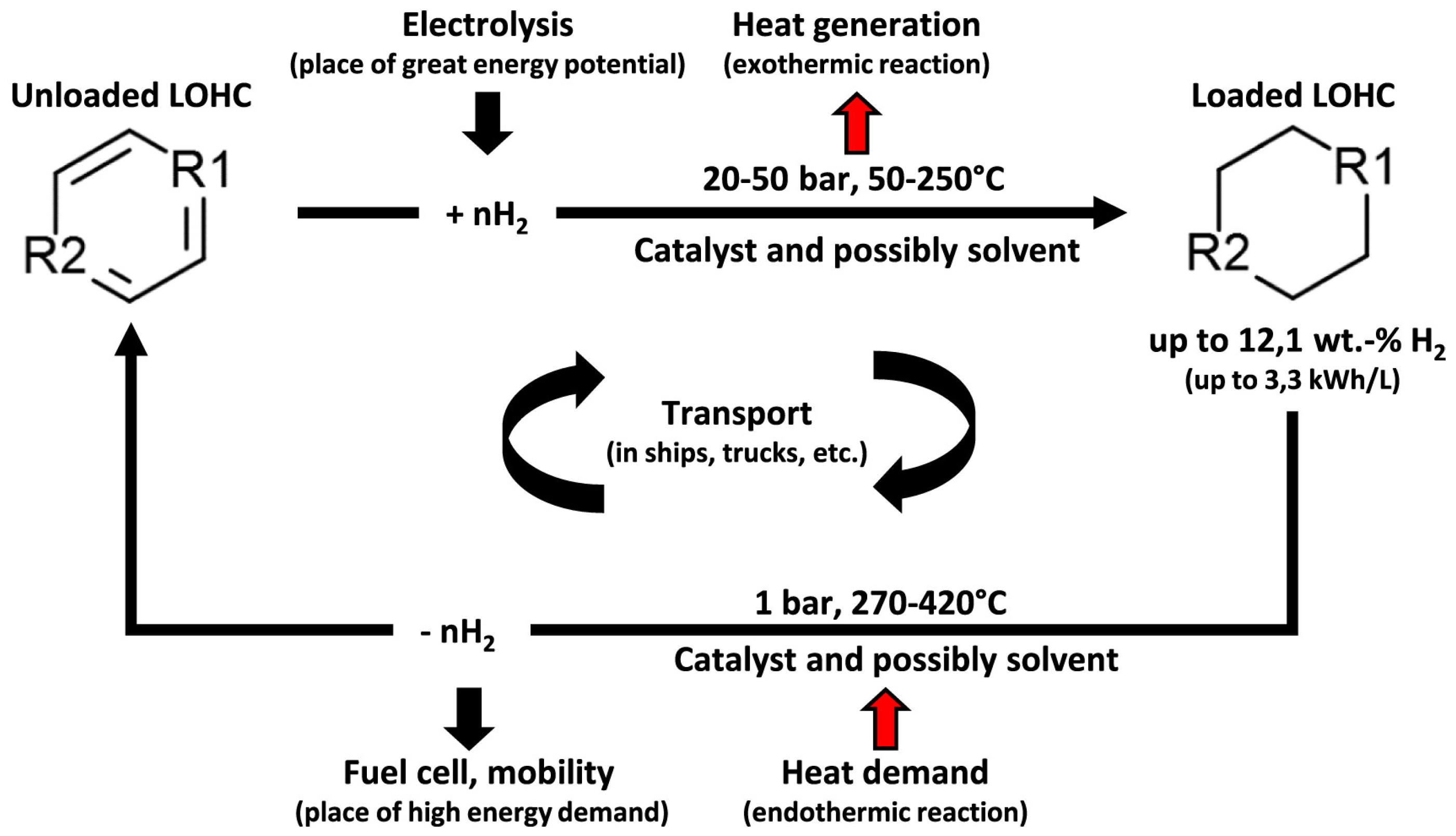
3.1.3. Liquid Organic Hydrogen Carrier (LOHCs)
3.2. Physisorption
3.2.1. Metal Organic Frameworks (MOFs)
Advantages and Challenges of Metal-Organic Frameworks (MOFs) for Hydrogen Storage
3.2.2. Carbon Nanotubes (CNs)
4. Underground Hydrogen Storage
4.1. Salt Caverns
4.2. Saline Aquifer Storage
4.3. Depleted Gas Reservoirs
| Hydrogen Storage Agents | Hydrogen Carrier | Hydrogen Storage Capacity (wt%)/(kg/m3) | Dehydrogenation Temperature/°C |
|---|---|---|---|
| N-ethycarbazole [164,165,176] | Dodecahydro-N-ethylcarbazole | 5.8/- | 170–200 |
| Toluene [177] | Methylcyclohexane | 6.2/47.4 | 300–350 |
| Dibenzytoluene [178,179] | Perhydro-dibenzytoluene | 6.2/57 | 260/310 |
| Benzene [173,180] | Cyclohexane | 7.2/55.9 | 300–320 |
| Biphenyl [180] | Bicyclohexyl | 7.27/- | 310–330 |
| Carbazole [87] | Dodecahydro-carbazole | 6.7/- | 150–170 |
5. Advantages and Challenges of Hydrogen as an Energy Vector
5.1. Advantages
- Hydrogen offers an alternative to fossil fuels and serves as a fuel in various vehicles, replacing conventional energy sources in automobiles trucks, ships, and rockets.
- Hydrogen energy vector bridges the gap with renewable energy sources, which are often intermittent and lack sustainability, and fossil fuels, which contribute to climate change on a global scale and have negative impacts on health.
- Hydrogen possesses significant potential to expedite the transition toward more environmentally friendly and sustainable energy solutions.
- Hydrogen finds utility in powering vehicles, generating electricity, and serving various industrial purposes. These include the production of methanol and ammonia, steel manufacturing, metal treatment process, and fertilizer production.
- Hydrogen generated from renewable energy sources provides a sustainable option for reducing greenhouse gas emissions due to its high energy content, surpassing other energy sources like ethanol, methanol, diesel, gasoline, and propane.
5.2. Challenges
- There is a significant challenge in ensuring safe, economical, robust, compact, and reliable hydrogen storage methods. This is primarily attributed to hydrogen’s distinctive physical properties and the requirement to store substantial quantities to manage energy demand and supply.
- The storage of hydrogen at its utilization site could potentially result in energy inefficiency due to the fact that hydrogen’s low volumetric energy density does not currently meet the required standards set by the United States Department of Energy (DOE) 2030.
- Currently, most hydrogen is produced from natural gas, coal, and other fossil fuels, contributing to increased carbon dioxide levels in the atmosphere. Therefore, it is essential to generate hydrogen using electricity from renewable sources to reduce environmental harm.
- Utilizing hydrogen for onboard vehicles poses substantial challenges, primarily because of the large volume, weight, extremely low temperature, high pressures, and cost of hydrogen (especially for storage methods providing high gravimetric and volumetric density, like liquefied, and cryo-compressed hydrogen storage). These factors severely limit the feasibility of hydrogen-powered vehicles. Addressing these issues requires the development of new and innovative materials capable of tackling these challenges.
- The main challenge in developing material-based hydrogen storage is to create cost-effective options that offer high hydrogen density both by volume and mass. This is due to the characteristic properties of hydrogen, such as its low molecular size, low volumetric energy density, high flammability, low molecular density, high diffusivity, and reactivity. Among the materials-based storage, liquid organic hydrogen carriers and metal hydrides are two hydrogen storage reliant on materials technologies that offer exciting qualities, making them suitable for certain applications, even in storage at a large scale. (LOHCs) provide the most promising means for long-duration and safe hydrogen storage using reversible chemical reactions driven by appropriate catalysts. It has been demonstrated that liquid organic hydrogen carriers offer the most affordable choice for large-volume, long-distance transport. However, LOHC systems face significant challenges, particularly their limited cycle life due to the degradation of carrier molecules and catalysts over repeated hydrogenation and dehydrogenation cycles. This degradation reduces long-term efficiency and increases maintenance costs. In addition, LOHCs need significant energy input during dehydrogenation since the reaction is endothermic, which further affects overall efficiency. These problems need to be addressed through continued research that can enhance the performance, sustainability, and economic feasibility of LOHC-based hydrogen storage systems.
6. Conclusions and Outlook
- Hydrogen has become extensively recognized as a highly promising clean energy source due to its potential as an efficient energy carrier, renewable nature, environmental friendliness, ease of production, abundance, cleanliness, high utilization rate, and sustainability. It is viewed as a pivotal solution for securing future energy needs and fostering global economic stability. The increasing global demand for hydrogen as an energy carrier, driven by the vision of a robust hydrogen economy, is essential for expediting the transition to a carbon dioxide-free global economy and achieving net-zero carbon emissions by 2050. However, a significant challenge lies in hydrogen storage methods. Presently, there is a lack of effective and efficient techniques applicable across all sectors, including transportation and industries.
- Hydrogen has significant potential to expedite the transition to a carbon-neutral, cleaner, and greener economy with a goal to achieve net-zero carbon dioxide emissions by 2050. That can be made possible only if utmost priority is given to producing green hydrogen from water electrolysis by renewable sources, and that makes it an adaptable and versatile carbon-free energy carrier.
- Currently, compressed gaseous cylinders, particularly Type III and Type IV cylinders, are the most widely accepted hydrogen storage technology for onboard applications compared to other methods. It is also, used for stationary storage of hydrogen energy.
- Liquid hydrogen storage faces challenges in maintaining cryogenic temperatures, minimizing boil-off losses, and preventing heat transfer. Effective insulation and vacuum maintenance are difficult over long periods. Materials need to maintain their mechanical properties at low temperatures, resist corrosion, and handle pressure variations. Common materials like stainless steel, aluminum, and nickel alloys come with tradeoffs in terms of weight, cost, and strength. A significant challenge lies in developing cost-effective, high-performance materials. Active thermal protection systems for zero boil-off storage are expensive and primarily used in space missions. The development of large-scale liquid hydrogen (LH2) infrastructure is still limited, especially in the civilian sector. However, innovations in insulation materials, materials science, and thermal protection technologies, such as cryogenic heat pipes and heat exchangers, offer promising solutions to enhance storage efficiency and feasibility.
- Advancements in liquefied hydrogen storage and cryo-compressed hydrogen storage are underway to facilitate global medium-scale hydrogen storage by addressing slow refueling, evaporation, and high energy consumption issues. Resolving technical challenges associated with storing liquefied hydrogen (which consumes a significant amount of energy) and cryo-compressed hydrogen storage (a tank with elevated pressure) could lead to the broad implementation of this technology in the future.
- Underground hydrogen storage has been found to be a promising solution for large-scale hydrogen storage. However, successful implementation and utilization of them require suitable geological storage sites, specific geological structures, and ample space to address safety risks, economic considerations, legal requirements, and various technical challenges. The selection of an appropriate site is critical for the successful operation of underground hydrogen storage (UHS) at geological sites. It is considered the foremost challenge that must be tackled at the outset of any UHS design project.
- All hydrogen storage methods come with their limitations, hindering the wider adoption of hydrogen energy as a fossil fuel alternative and impeding progress toward global carbon emission reduction goals. Therefore, considering the demands of modern society and the emerging challenges, developing a novel, environmentally friendly, and cost-effective hydrogen storage system is crucial for the future hydrogen economy.
- Furthermore, the study should focus on the development of new materials that can store hydrogen at high volumetric and gravimetric densities, resist microcracks, avoid volatile component loss, and retain both stiffness and ductility in every sector condition used for tank construction in order to overcome challenges and barriers with physical-based hydrogen storage systems and pave the way for more efficient and sustainable energy solutions.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANSI | American National Standards Institute |
| Cc-H2 | Cryo-compressed hydrogen |
| CFRPs | Carbon fiber reinforced polymers |
| CG-H2 | Compressed gas hydrogen |
| CNG | Compressed Natural Gas |
| CO2 | Carbon dioxide |
| DOE | Department of Energy |
| EU | Europa Union |
| FCEVs | Fuel cell electric vehicles |
| HDPE | High-density polyethylene |
| HE | Hydrogen Energy |
| HGV | Hydrogen gas vehicle |
| LH2 | Liquefied hydrogen |
| LOHCs | Liquid Organic Hydrogen Carriers |
| MOF | Metal-organic framework’s |
| P | Pressure |
| PTFE | Polytetrafluoroethylene |
| SSHS | Solid-state hydrogen storage |
References
- Chavan, G.T.; Dubal, D.P.; Cho, E.C.; Patil, D.R.; Gwag, J.S.; Mishra, R.K.; Mishra, Y.K.; An, J.; Yi, J. A Roadmap of Sustainable Hydrogen Production and Storage: Innovations and Challenges. Small 2025, 2411444. [Google Scholar] [CrossRef] [PubMed]
- Adu, D.; Jianguo, D.; Asomani, S.N.; Abbey, A. Energy generation and carbon dioxide emission—The role of renewable energy for green development. Energy Rep. 2024, 12, 1420–1430. [Google Scholar] [CrossRef]
- Tang, D.; Tan, G.-L.; Li, G.-W.; Liang, J.-G.; Ahmad, S.M.; Bahadur, A.; Humayun, M.; Ullah, H.; Khan, A.; Bououdina, M. State-of-the-art hydrogen generation techniques and storage methods: A critical review. J. Energy Storage 2023, 64, 107196. [Google Scholar] [CrossRef]
- Mlilo, N.; Brown, J.; Ahfock, T. Impact of intermittent renewable energy generation penetration on the power system networks—A review. Technol. Econ. Smart Grids Sustain. Energy 2021, 6, 25. [Google Scholar] [CrossRef]
- Kamran, M.; Turzyński, M. Exploring hydrogen energy systems: A comprehensive review of technologies, applications, prevailing trends, and associated challenges. J. Energy Storage 2024, 96, 112601. [Google Scholar] [CrossRef]
- Usman, M.; Zeb, Z.; Ullah, H.; Suliman, M.H.; Humayun, M.; Ullah, L.; Shah, S.N.A.; Ahmed, U.; Saeed, M. A review of metal-organic frameworks/graphitic carbon nitride composites for solar-driven green H2 production, CO2 reduction, and water purification. J. Environ. Chem. Eng. 2022, 10, 107548. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cheng, Z.-E.; Tahir, A.A.; Luo, W.; Wang, C. Au surface plasmon resonance promoted charge transfer in Z-scheme system enables exceptional photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2022, 310, 121322. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Stępień, Z. A comprehensive overview of hydrogen-fueled internal combustion engines: Achievements and future challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.; Ares, J.-R.; Baricco, M.; Bourgeois, N.; Buckley, C.; Von Colbe, J.B. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrogen Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Taiwo, G.O.; Tomomewo, O.S.; Oni, B.A. A comprehensive review of underground hydrogen storage: Insight into geological sites (mechanisms), economics, barriers, and future outlook. J. Energy Storage 2024, 90, 111844. [Google Scholar] [CrossRef]
- Marouani, I.; Guesmi, T.; Alshammari, B.M.; Alqunun, K.; Alzamil, A.; Alturki, M.; Hadj Abdallah, H. Integration of renewable-energy-based green hydrogen into the energy future. Processes 2023, 11, 2685. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Sirohi, R.; Tran, M.H.; Truong, T.H.; Duong, M.T.; Pham, M.T.; Cao, D.N. Renewable energy role in low-carbon economy and net-zero goal: Perspectives and prospects. Energy Environ. 2024. [Google Scholar] [CrossRef]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756. [Google Scholar] [CrossRef]
- Le, T.T.; Sharma, P.; Bora, B.J.; Tran, V.D.; Truong, T.H.; Le, H.C.; Nguyen, P.Q.P. Fueling the future: A comprehensive review of hydrogen energy systems and their challenges. Int. J. Hydrogen Energy 2024, 54, 791–816. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Zhuo, S.; Ullah, H.; Zhou, X.; Liu, Z.; Zhao, S.; Xu, A.; Li, X.; Khan, A. Role of–OH groups on Mn3O4 heterogeneous catalyst surfaces in peroxymonosulfate activation: Degradation of levofloxacin via radical/nonradical pathways. Appl. Surf. Sci. 2025, 685, 162007. [Google Scholar] [CrossRef]
- Nsang, G.E.O.; Ullah, B.; Hua, S.; Shah, S.; Ullah, N.; Ullah, N.; Dike, F.; Yaseen, W.; Yuan, A.; Ullah, H. Nis Nanoparticle-MoS2 Nanosheet Core-Shell Spheres: Pvp-Assisted Synthesis and Efficient Electrocatalyst for Hydrogen Evolution Reaction. Energy Mater. 2025, 5, 500047. [Google Scholar] [CrossRef]
- Humayun, M.; Wang, C.; Luo, W. Recent progress in the synthesis and applications of composite photocatalysts: A critical review. Small Methods 2022, 6, 2101395. [Google Scholar] [CrossRef]
- Rahman, U.U.; Humayun, M.; Ghani, U.; Usman, M.; Ullah, H.; Khan, A.; El-Metwaly, N.M.; Khan, A. MXenes as emerging materials: Synthesis, properties, and applications. Molecules 2022, 27, 4909. [Google Scholar] [CrossRef]
- Humayun, M.; Fu, Q.; Zheng, Z.; Li, H.; Luo, W. Improved visible-light catalytic activities of novel Au/P-doped g-C3N4 photocatalyst for solar fuel production and mechanism. Appl. Catal. A Gen. 2018, 568, 139–147. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cao, J.; Pi, W.; Yuan, Y.; Ali, S.; Tahir, A.A.; Yue, P.; Khan, A.; Zheng, Z. Experimental and DFT studies of Au deposition over WO3/gC3N4 Z-scheme heterojunction. Nano-Micro Lett. 2020, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Reda, B.; Elzamar, A.A.; AlFazzani, S.; Ezzat, S.M. Green hydrogen as a source of renewable energy: A step towards sustainability, an overview. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Jia, Z.-C.; Yuan, Z.-M.; Yang, T.; Qi, Y.; Zhao, D.-L. Development and application of hydrogen storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Feng, S.; Lazkano, I. Energy storage and clean energy transitions. Energy Policy 2025, 198, 114447. [Google Scholar] [CrossRef]
- Choi, S.K.; Piao, G.; Choi, W.; Park, H. Highly efficient hydrogen production using p-Si wire arrays and NiMoZn heterojunction photocathodes. Appl. Catal. B Environ. 2017, 217, 615–621. [Google Scholar] [CrossRef]
- Rolo, I.; Costa, V.A.; Brito, F.P. Hydrogen-based energy systems: Current technology development status, opportunities and challenges. Energies 2023, 17, 180. [Google Scholar] [CrossRef]
- Rasul, M.; Hazrat, M.; Sattar, M.; Jahirul, M.; Shearer, M. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Jayachandran, M.; Gatla, R.K.; Flah, A.; Milyani, A.H.; Milyani, H.M.; Blazek, V.; Prokop, L.; Kraiem, H. Challenges and opportunities in green hydrogen adoption for decarbonizing hard-to-abate industries: A comprehensive review. IEEE Access 2024, 12, 23363–23388. [Google Scholar] [CrossRef]
- Hua, S.; Shah, S.A.; Nsang, G.E.O.; Sayyar, R.; Ullah, B.; Ullah, N.; Khan, N.; Yuan, A.; bin Mohd Yusoff, A.R.; Ullah, H. Unveiling active sites in FeOOH nanorods@ NiOOH nanosheets heterojunction for superior OER and HER electrocatalysis in water splitting. J. Colloid Interface Sci. 2025, 679, 487–495. [Google Scholar] [CrossRef]
- Xu, X.; Ullah, H.; Humayun, M.; Li, L.; Zhang, X.; Bououdina, M.; Debecker, D.P.; Huo, K.; Wang, D.; Wang, C. Fluorinated Ni-O-C heterogeneous catalyst for efficient urea-assisted hydrogen production. Adv. Funct. Mater. 2023, 33, 2303986. [Google Scholar] [CrossRef]
- Usman, M.; Humayun, M.; Shah, S.S.; Ullah, H.; Tahir, A.A.; Khan, A.; Ullah, H. Bismuth-graphene nanohybrids: Synthesis, reaction mechanisms, and photocatalytic applications—A review. Energies 2021, 14, 2281. [Google Scholar] [CrossRef]
- Humayun, M.; Israr, M.; Khan, A.; Bououdina, M. State-of-the-art single-atom catalysts in electrocatalysis: From fundamentals to applications. Nano Energy 2023, 113, 108570. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen production technologies: From fossil fuels toward renewable sources. A mini review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Chen, H.; Sun, M.; Huang, J.; Zhao, J.; Ullah, H.; Humayun, M.; Xu, Y.; Bououdina, M.; Li, J. Engineering sulfur doped flower-like BiOBrxI1-x solid solutions for strengthened photocatalytic activities. J. Alloys Compd. 2025, 1010, 177224. [Google Scholar] [CrossRef]
- Shahriar, M.F.; Khanal, A.; Khan, M.I.; Pandey, R. Current status of underground hydrogen storage: Perspective from storage loss, infrastructure, economic aspects, and hydrogen economy targets. J. Energy Storage 2024, 97, 112773. [Google Scholar] [CrossRef]
- Saha, P.; Akash, F.A.; Shovon, S.M.; Monir, M.U.; Ahmed, M.T.; Khan, M.F.H.; Sarkar, S.M.; Islam, M.K.; Hasan, M.M.; Vo, D.-V.N. Grey, blue, and green hydrogen: A comprehensive review of production methods and prospects for zero-emission energy. Int. J. Green Energy 2024, 21, 1383–1397. [Google Scholar] [CrossRef]
- Naseem, K.; Qin, F.; Khalid, F.; Suo, G.; Zahra, T.; Chen, Z.; Javed, Z. Essential parts of hydrogen economy: Hydrogen production, storage, transportation and application. Renew. Sustain. Energy Rev. 2025, 210, 115196. [Google Scholar] [CrossRef]
- Boretti, A.; Pollet, B.G. Hydrogen economy: Paving the path to a sustainable, low-carbon future. Int. J. Hydrogen Energy 2024, 93, 307–319. [Google Scholar] [CrossRef]
- Jiang, Z.; Pan, Q.; Xu, J.; Fang, T. Current situation and prospect of hydrogen storage technology with new organic liquid. Int. J. Hydrogen Energy 2014, 39, 17442–17451. [Google Scholar] [CrossRef]
- Mulky, L.; Srivastava, S.; Lakshmi, T.; Sandadi, E.R.; Gour, S.; Thomas, N.A.; Shanmuga Priya, S.; Sudhakar, K. An overview of hydrogen storage technologies—Key challenges and opportunities. Mater. Chem. Phys. 2024, 325, 129710. [Google Scholar] [CrossRef]
- Kumar, N.; Lee, S.-Y.; Park, S.-J. Advancements in hydrogen storage technologies: A comprehensive review of materials, methods, and economic policy. Nano Today 2024, 56, 102302. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Z.; Meng, Q.; White, S. Advancements in hydrogen storage technologies: Enhancing efficiency, safety, and economic viability for sustainable energy transition. Int. J. Hydrogen Energy 2025, 105, 10–22. [Google Scholar] [CrossRef]
- Alasali, F.; Abuashour, M.I.; Hammad, W.; Almomani, D.; Obeidat, A.M.; Holderbaum, W. A review of hydrogen production and storage materials for efficient integrated hydrogen energy systems. Energy Sci. Eng. 2024, 12, 1934–1968. [Google Scholar] [CrossRef]
- Jayabal, R. Hydrogen energy storage in maritime operations: A pathway to decarbonization and sustainability. Int. J. Hydrogen Energy 2025, 109, 1133–1144. [Google Scholar] [CrossRef]
- Elberry, A.M.; Thakur, J.; Santasalo-Aarnio, A.; Larmi, M. Large-scale compressed hydrogen storage as part of renewable electricity storage systems. Int. J. Hydrogen Energy 2021, 46, 15671–15690. [Google Scholar] [CrossRef]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Magliano, A.; Perez Carrera, C.; Pappalardo, C.M.; Guida, D.; Berardi, V.P. A Comprehensive Literature Review on Hydrogen Tanks: Storage, Safety, and Structural Integrity. Appl. Sci. 2024, 14, 9348. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Hydrogen gas compression for efficient storage: Balancing energy and increasing density. Hydrogen 2024, 5, 293–311. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 44, 16833–16840. [Google Scholar] [CrossRef]
- Muthukumar, P.; Kumar, A.; Afzal, M.; Bhogilla, S.; Sharma, P.; Parida, A.; Jana, S.; Kumar, E.A.; Pai, R.K.; Jain, I. Review on large-scale hydrogen storage systems for better sustainability. Int. J. Hydrogen Energy 2023, 48, 33223–33259. [Google Scholar] [CrossRef]
- Li, J.-Q.; Li, J.-C.; Park, K.; Jang, S.-J.; Kwon, J.-T. An analysis on the compressed hydrogen storage system for the fast-filling process of hydrogen gas at the pressure of 82 MPa. Energies 2021, 14, 2635. [Google Scholar] [CrossRef]
- Li, J.-Q.; Li, J.-C.L.; Park, K.; Kwon, J.-T. Investigation on the changes of pressure and temperature in high pressure filling of hydrogen storage tank. Case Stud. Therm. Eng. 2022, 37, 102143. [Google Scholar] [CrossRef]
- Barthélémy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Sarkar, P.; Bhattacharyya, S.P. Understanding Properties of Atoms, Molecules and Materials; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, R.; Shi, Z.; Lin, J. Review of common hydrogen storage tanks and current manufacturing methods for aluminium alloy tank liners. Int. J. Lightweight Mater. Manuf. 2024, 7, 269–284. [Google Scholar] [CrossRef]
- Akram, W.; Farhan Rafique, A.; Maqsood, N.; Khan, A.; Badshah, S.; Khan, R.U. Characterization of PTFE film on 316L stainless steel deposited through spin coating and its anticorrosion performance in multi acidic mediums. Materials 2020, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Malik, G.; Tiwari, P.; Choudhary, N.; Hirpara, J.; Kumar, A.; Chandra, R. Investigation of wear and corrosion characteristics of PTFE/TiN composite coating on SS-304 fabricated by two-step sputtering technique. Surf. Coat. Technol. 2023, 466, 129660. [Google Scholar] [CrossRef]
- Li, K.; Dong, X.; Wang, H.; Gong, M. Study on the influence factors of gravimetric hydrogen storage density of type III cryo-compressed hydrogen storage vessel. Int. J. Hydrogen Energy 2024, 96, 680–691. [Google Scholar] [CrossRef]
- Fu, H.; Lv, C.; Meng, J.; He, M.; Wu, J.; Gong, L. Strength and fatigue study of on-board type III cryo-compressed hydrogen storage cylinder. Int. J. Hydrogen Energy 2024, 57, 1081–1088. [Google Scholar] [CrossRef]
- Siddiqui, M.J.; Balguri, P.K.; Haripriya, K.; Rajendran, A.R.; Patil, I.V. Analysis of type IV hydrogen pressure vessel with S-glass, Carbon fiber T700 and Kevlar composite materials. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- UK government in £20m boost to develop hydrogen economy. Fuel Cells Bull. 2018, 2018, 10. [CrossRef]
- Li, J.; Chai, X.; Gu, Y.; Zhang, P.; Yang, X.; Wen, Y.; Xu, Z.; Jiang, B.; Wang, J.; Jin, G.; et al. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials 2024, 17, 721. [Google Scholar] [CrossRef]
- Gómez, J.A.; Santos, D.M.F. The Status of On-Board Hydrogen Storage in Fuel Cell Electric Vehicles. Designs 2023, 7, 97. [Google Scholar] [CrossRef]
- von Helmolt, R.; Eberle, U. Fuel cell vehicles: Status 2007. J. Power Sources 2007, 165, 833–843. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Li, G.; Zhou, W.; Ni, Z. Review on linerless type V cryo-compressed hydrogen storage vessels: Resin toughening and hydrogen-barrier properties control. Renew. Sustain. Energy Rev. 2024, 189, 114009. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Air, A.; Shamsuddoha, M.; Gangadhara Prusty, B. A review of Type V composite pressure vessels and automated fibre placement based manufacturing. Compos. Part B Eng. 2023, 253, 110573. [Google Scholar] [CrossRef]
- Mikroni, M.; Koutsoukis, G.; Vlachos, D.; Kostopoulos, V.; Vavouliotis, A.; Trakakis, G.; Athinaios, D.; Nikolakea, C.; Zacharakis, D. Design, Analysis, and Testing of a Type V Composite Pressure Vessel for Hydrogen Storage. Polymers 2024, 16, 3576. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, H. Hydrogen storage—Industrial prospectives. Int. J. Hydrogen Energy 2012, 37, 17364–17372. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Z.; Zhang, Y.; Li, W.; Zhang, W. Analysis of rapid decompression failure in polymer liner of Type IV hydrogen storage vessels using a novel fluid–solid coupling model. Compos. Part A Appl. Sci. Manuf. 2025, 188, 108531. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Xie, Z.; Jin, Q.; Su, G.; Lu, W. A Review of Hydrogen Storage and Transportation: Progresses and Challenges. Energies 2024, 17, 4070. [Google Scholar] [CrossRef]
- Chu, C.; Wu, K.; Luo, B.; Cao, Q.; Zhang, H. Hydrogen storage by liquid organic hydrogen carriers: Catalyst, renewable carrier, and technology—A review. Carbon Resour. Convers. 2023, 6, 334–351. [Google Scholar] [CrossRef]
- Kurtz, J.; Sprik, S.; Bradley, T.H. Review of transportation hydrogen infrastructure performance and reliability. Int. J. Hydrogen Energy 2019, 44, 12010–12023. [Google Scholar] [CrossRef]
- Naquash, A.; Agarwal, N.; Lee, M. A Review on Liquid Hydrogen Storage: Current Status, Challenges and Future Directions. Sustainability 2024, 16, 8270. [Google Scholar] [CrossRef]
- Massaro, M.C.; Biga, R.; Kolisnichenko, A.; Marocco, P.; Monteverde, A.H.A.; Santarelli, M. Potential and technical challenges of on-board hydrogen storage technologies coupled with fuel cell systems for aircraft electrification. J. Power Sources 2023, 555, 232397. [Google Scholar] [CrossRef]
- Petitpas, G.; Bénard, P.; Klebanoff, L.E.; Xiao, J.; Aceves, S. A comparative analysis of the cryo-compression and cryo-adsorption hydrogen storage methods. Int. J. Hydrogen Energy 2014, 39, 10564–10584. [Google Scholar] [CrossRef]
- Brunner, T.; Kircher, O. Cryo-compressed Hydrogen Storage. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 711–732. [Google Scholar] [CrossRef]
- Agnolucci, P.; McDowall, W. Designing future hydrogen infrastructure: Insights from analysis at different spatial scales. Int. J. Hydrogen Energy 2013, 38, 5181–5191. [Google Scholar] [CrossRef]
- Xiao, R.; Tian, G.; Hou, Y.; Chen, S.; Cheng, C.; Chen, L. Effects of cooling-recovery venting on the performance of cryo-compressed hydrogen storage for automotive applications. Appl. Energy 2020, 269, 115143. [Google Scholar] [CrossRef]
- Li, S.; Han, F.; Liu, Y.; Xu, Z.; Yan, Y.; Ni, Z. Evaluation criterion for filling process of cryo-compressed hydrogen storage vessel. Int. J. Hydrogen Energy 2024, 59, 1459–1470. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Hua, T.Q.; Peng, J.K.; Lasher, S.; McKenney, K.; Sinha, J.; Gardiner, M. Technical assessment of cryo-compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2010, 35, 4171–4184. [Google Scholar] [CrossRef]
- Didi, Y.; Bahhar, S.; Tahiri, A.; Naji, M.; Rjeb, A. A Computational Study of Metal Hydrides Based on Rubidium for Developing Solid-State Hydrogen Storage. ChemistrySelect 2024, 9, e202401444. [Google Scholar] [CrossRef]
- Abdechafik, E.H.; Ait Ousaleh, H.; Mehmood, S.; Filali Baba, Y.; Bürger, I.; Linder, M.; Faik, A. An analytical review of recent advancements on solid-state hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 1182–1193. [Google Scholar] [CrossRef]
- Shi, T.; Xu, H. Integration of hydrogen storage and heat storage in thermochemical reactors enhanced with optimized topological structures: Charging process. Appl. Energy 2022, 327, 120138. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Yang, X.; Bulushev, D.A.; Yang, J.; Zhang, Q. New Liquid Chemical Hydrogen Storage Technology. Energies 2022, 15, 6360. [Google Scholar] [CrossRef]
- Drawer, C.; Lange, J.; Kaltschmitt, M. Metal hydrides for hydrogen storage—Identification and evaluation of stationary and transportation applications. J. Energy Storage 2024, 77, 109988. [Google Scholar] [CrossRef]
- He, T.; Pei, Q.; Chen, P. Liquid organic hydrogen carriers. J. Energy Chem. 2015, 24, 587–594. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Liquid-phase chemical hydrogen storage materials. Energy Environ. Sci. 2012, 5, 9698–9725. [Google Scholar] [CrossRef]
- Saad Salman, M.; Rambhujun, N.; Pratthana, C.; Lai, Q.; Sapkota, P.; Aguey-Zinsou, K.-F. Chapter 12—Solid-state hydrogen storage as a future renewable energy technology. In Nano Tools and Devices for Enhanced Renewable Energy; Devasahayam, S., Hussain, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 263–287. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tarasov, B.P.; Yartys, V.A. Gas-phase applications of metal hydrides. J. Energy Storage 2023, 72, 108165. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.W.; Huang, Z. Editorial: Metal Hydride-Based Energy Storage and Conversion Materials. Front. Chem. 2020, 8, 675. [Google Scholar] [CrossRef]
- Lu, L.-W.; Luo, H.; Li, G.-X.; Li, Y.; Wang, X.-H.; Huang, C.-K.; Lan, Z.-Q.; Zhou, W.-Z.; Guo, J.; Ismail, M. Layered niobium carbide enabling excellent kinetics and cycling stability of Li-Mg-BH hydrogen storage material: Layered niobium carbide enabling excellent kinetics. Rare Met. 2024, 43, 1153–1166. [Google Scholar] [CrossRef]
- Liu, H.; Lu, L.; Luo, H.; Deng, J.; Li, G.; Ning, H.; Fan, Y.; Huang, C.; Lan, Z.; Zhou, W. Hybrid of bulk NbC and layered Nb4C3 MXene for tailoring the hydrogen storage kinetics and reversibility of Li–Mg–B–H composite: An experimental and theoretical study. J. Mater. Sci. Technol. 2024, 194, 225–235. [Google Scholar] [CrossRef]
- Baetcke, L.; Kaltschmitt, M. Chapter 5—Hydrogen Storage for Mobile Application: Technologies and Their Assessment. In Hydrogen Supply Chains; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 167–206. [Google Scholar] [CrossRef]
- Jain, I.P.; Lal, C.; Jain, A. Hydrogen storage in Mg: A most promising material. Int. J. Hydrogen Energy 2010, 35, 5133–5144. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J.O. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Amdisen, M.B.; Autrey, T.; Barale, J.; Bowden, M.E.; Buckley, C.E.; Cho, Y.W.; Deledda, S.; Dornheim, M.; de Jongh, P.; et al. Hydrogen storage in complex hydrides: Past activities and new trends. Prog. Energy 2022, 4, 032009. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.-W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Sato, T.; Blomqvist, H.; Noréus, D. Attempts to improve Mg2Ni hydrogen storage by aluminium addition. J. Alloys Compd. 2003, 356–357, 494–496. [Google Scholar] [CrossRef]
- Klopčič, N.; Grimmer, I.; Winkler, F.; Sartory, M.; Trattner, A. A review on metal hydride materials for hydrogen storage. J. Energy Storage 2023, 72, 108456. [Google Scholar] [CrossRef]
- Harrison, D.B. Improving Existing and Discovering New Hydrogen Storage Materials Using Computational Materials Modeling. Ph.D. Thesis, Wake Forest University, Winston-Salem, NC, USA, 2017. [Google Scholar]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y. Complex hydrides for hydrogen storage–new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Asif, M.; Bibi, S.S.; Ahmed, S.; Irshad, M.; Hussain, M.S.; Zeb, H.; Khan, M.K.; Kim, J. Recent advances in green hydrogen production, storage and commercial-scale use via catalytic ammonia cracking. Chem. Eng. J. 2023, 473, 145381. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Jiao, F.; Xu, B. Electrochemical ammonia synthesis and ammonia fuel cells. Adv. Mater. 2019, 31, 1805173. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, P.; Witman, M.; Stavila, V.; Allendorf, M.; Breunig, H. Technoeconomic Insights into Metal Hydrides for Stationary Hydrogen Storage. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen storage technologies for future energy systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef]
- Ali, A.; Shaikh, M.N. Recent developments in catalyst design for liquid organic hydrogen carriers: Bridging the gap to affordable hydrogen storage. Int. J. Hydrogen Energy 2024, 78, 1–21. [Google Scholar] [CrossRef]
- Ramadhani, S.; Dao, Q.N.; Imanuel, Y.; Ridwan, M.; Sohn, H.; Jeong, H.; Kim, K.; Yoon, C.W.; Song, K.H.; Kim, Y. Advances in Catalytic Hydrogenation of Liquid Organic Hydrogen Carriers (LOHCs) Using High-Purity and Low-Purity Hydrogen. ChemCatChem 2024, 16, e202401278. [Google Scholar] [CrossRef]
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renew. Sustain. Energy Rev. 2021, 135, 110171. [Google Scholar] [CrossRef]
- Spatolisano, E.; Restelli, F.; Matichecchia, A.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Assessing opportunities and weaknesses of green hydrogen transport via LOHC through a detailed techno-economic analysis. Int. J. Hydrogen Energy 2024, 52, 703–717. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Tsang, S.E. Structure-reactivity relationship in catalytic hydrogenation of heterocyclic compounds over ruthenium black-Part A: Effect of substitution of pyrrole ring and side chain in N-heterocycles. Appl. Catal. B Environ. 2014, 160, 22–34. [Google Scholar] [CrossRef]
- Uijthof, E.; Chavan, B.; Sluijer, M.; Komath, V.; van der Ham, A.; van den Berg, H.; Lange, J.P.; Higler, A.; Wijnans, S. Liquid organic hydrogen carriers: Process design and economic analysis for manufacturing N-ethylcarbazole. J. Adv. Manuf. Process. 2024, 6, e10173. [Google Scholar] [CrossRef]
- Safronov, S.P.; Vostrikov, S.V.; Samarov, A.A.; Wasserscheid, P.; Müller, K.; Verevkin, S.P. Comprehensive thermodynamic study of substituted indoles/perhydro indoles as potential liquid organic hydrogen carrier system. Fuel 2023, 331, 125764. [Google Scholar] [CrossRef]
- Pedrazzi, S.; Zucchi, M.; Muscio, A.; Kaya, A.F. Liquid organic hydrogen carriers applied on methane–hydrogen-fueled internal combustion engines: A preliminary analysis of process heat balance. Appl. Sci. 2023, 13, 4424. [Google Scholar] [CrossRef]
- Ramesh Kumar, K.; Makhmutov, A.; Spiers, C.J.; Hajibeygi, H. Geomechanical simulation of energy storage in salt formations. Sci. Rep. 2021, 11, 19640. [Google Scholar] [CrossRef]
- Huang, Y.; Si, Y.; Xiang, Y.; Yao, S.; Cheng, Y. Integration of hydrogenation and dehydrogenation based on N-ethylcarbazole as a liquid organic hydrogen carrier. Ind. Eng. Chem. Res. 2023, 62, 6953–6962. [Google Scholar] [CrossRef]
- Shukla, A.; Karmakar, S.; Biniwale, R.B. Hydrogen delivery through liquid organic hydrides: Considerations for a potential technology. Int. J. Hydrogen Energy 2012, 37, 3719–3726. [Google Scholar] [CrossRef]
- Uhrig, F.; Kadar, J.; Müller, K. Reliability of liquid organic hydrogen carrier-based energy storage in a mobility application. Energy Sci. Eng. 2020, 8, 2044–2053. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Khan, M.M.A. Hydrogen Storage by Physisorption: An Overview. Adv. Mater. Res. 2015, 1116, 157–172. [Google Scholar] [CrossRef]
- Saeed, M.; Marwani, H.M.; Shahzad, U.; Asiri, A.M.; Hussain, I.; Rahman, M.M. Utilizing nanostructured materials for hydrogen generation, storage, and diverse applications. Chem.–Asian J. 2024, 19, e202300593. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.; Sun, Y. Enhanced storage of hydrogen at the temperature of liquid nitrogen. Int. J. Hydrogen Energy 2004, 29, 319–322. [Google Scholar] [CrossRef]
- Shet, S.P.; Priya, S.S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen storage in metal–organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Deng, S. Hydrogen adsorption on metal-organic framework MOF-177. Tsinghua Sci. Technol. 2010, 15, 363–376. [Google Scholar] [CrossRef]
- Güleç, F.; Oakley, W.; Liu, X.; Nayebossadri, S.; Wang, F.; Smith, E.K.; Barakat, S.M.; Lester, E.H. Status and Progress of Nanomaterials Application in Hydrogen Storage. In Nanomaterials for Sustainable Hydrogen Production and Storage; CRC Press: Boca Raton, FL, USA, 2024; pp. 136–165. [Google Scholar]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Langmi, H.W.; Ren, J.; North, B.; Mathe, M.; Bessarabov, D. Hydrogen storage in metal-organic frameworks: A review. Electrochim. Acta 2014, 128, 368–392. [Google Scholar] [CrossRef]
- Umar, A.A.; Hossain, M.M. Hydrogen storage via adsorption: A review of recent advances and challenges. Fuel 2025, 387, 134273. [Google Scholar] [CrossRef]
- Yuvaraj, A.; Jayarama, A.; Sharma, D.; Nagarkar, S.S.; Duttagupta, S.P.; Pinto, R. Role of metal-organic framework in hydrogen gas storage: A critical review. Int. J. Hydrogen Energy 2024, 59, 1434–1458. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. A review on contemporary metal–organic framework materials. Inorg. Chim. Acta 2016, 446, 61–74. [Google Scholar] [CrossRef]
- Meskher, H. A critical review about metal organic framework-based composites: Potential applications and future perspective. J. Compos. Compd. 2023, 5, 25–37. [Google Scholar] [CrossRef]
- Tatar, D.; Ullah, H.; Yadav, M.; Kojčinović, J.; Šarić, S.; Szenti, I.; Skalar, T.; Finšgar, M.; Tian, M.; Kukovecz, Á.; et al. High-Entropy Oxides: A New Frontier in Photocatalytic CO2 Hydrogenation. ACS Appl. Mater. Interfaces 2024, 16, 29946–29962. [Google Scholar] [CrossRef]
- Khan, N.A.; Humayun, M.; Usman, M.; Ghazi, Z.A.; Naeem, A.; Khan, A.; Khan, A.L.; Tahir, A.A.; Ullah, H. Structural characteristics and environmental applications of covalent organic frameworks. Energies 2021, 14, 2267. [Google Scholar] [CrossRef]
- Bobbitt, N.S.; Chen, J.; Snurr, R.Q. High-throughput screening of metal–organic frameworks for hydrogen storage at cryogenic temperature. J. Phys. Chem. C 2016, 120, 27328–27341. [Google Scholar] [CrossRef]
- Jia, T.; Gu, Y.; Li, F. Progress and potential of metal-organic frameworks (MOFs) for gas storage and separation: A review. J. Environ. Chem. Eng. 2022, 10, 108300. [Google Scholar] [CrossRef]
- Farha, O.K.; Özgür Yazaydın, A.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Dai, H. Carbon nanotubes: Opportunities and challenges. Surf. Sci. 2002, 500, 218–241. [Google Scholar] [CrossRef]
- Zafar, M.; Iqbal, T.; Fatima, S.; Sanaullah, Q.; Aman, S. Carbon nanotubes for production and storage of hydrogen: Challenges and development. In Chemical Papers; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–17. [Google Scholar]
- Muhammed, N.S.; Haq, B.; Al Shehri, D.A. Hydrogen storage in depleted gas reservoirs using nitrogen cushion gas: A contact angle and surface tension study. Int. J. Hydrogen Energy 2023, 48, 38782–38807. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground hydrogen storage: Characteristics and prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Quintos Fuentes, J.E.; Santos, D.M. Technical and economic viability of underground hydrogen storage. Hydrogen 2023, 4, 975–1000. [Google Scholar] [CrossRef]
- Epelle, E.I.; Obande, W.; Udourioh, G.A.; Afolabi, I.C.; Desongu, K.S.; Orivri, U.; Gunes, B.; Okolie, J.A. Perspectives and prospects of underground hydrogen storage and natural hydrogen. Sustain. Energy Fuels 2022, 6, 3324–3343. [Google Scholar] [CrossRef]
- Zivar, D.; Kumar, S.; Foroozesh, J. Underground hydrogen storage: A comprehensive review. Int. J. Hydrogen Energy 2021, 46, 23436–23462. [Google Scholar] [CrossRef]
- Lemieux, A.; Shkarupin, A.; Sharp, K. Geologic feasibility of underground hydrogen storage in Canada. Int. J. Hydrogen Energy 2020, 45, 32243–32259. [Google Scholar] [CrossRef]
- Alms, K.; Ahrens, B.; Graf, M.; Nehler, M. Linking geological and infrastructural requirements for large-scale underground hydrogen storage in Germany. Front. Energy Res. 2023, 11, 1172003. [Google Scholar] [CrossRef]
- Scafidi, J.; Wilkinson, M.; Gilfillan, S.M.; Heinemann, N.; Haszeldine, R.S. A quantitative assessment of the hydrogen storage capacity of the UK continental shelf. Int. J. Hydrogen Energy 2021, 46, 8629–8639. [Google Scholar] [CrossRef]
- Aslannezhad, M.; Ali, M.; Kalantariasl, A.; Sayyafzadeh, M.; You, Z.; Iglauer, S.; Keshavarz, A. A review of hydrogen/rock/brine interaction: Implications for Hydrogen Geo-storage. Prog. Energy Combust. Sci. 2023, 95, 101066. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Wang, J.; Chou, J.; Fang, Y.; Pan, G.; Gu, W. Feasibility analysis of utilising underground hydrogen storage facilities in integrated energy system: Case studies in China. Appl. Energy 2020, 269, 115140. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Abu-Mahfouz, I.S.; Yekeen, N.; Wolff-Boenisch, D. Organic-rich source rock/H2/brine interactions: Implications for underground hydrogen storage and methane production. J. Energy Storage 2023, 63, 106986. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Chen, C. Numerical simulation of the impact of different cushion gases on underground hydrogen storage in aquifers based on an experimentally-benchmarked equation-of-state. Int. J. Hydrogen Energy 2024, 50, 495–511. [Google Scholar] [CrossRef]
- Thiyagarajan, S.R.; Emadi, H.; Hussain, A.; Patange, P.; Watson, M. A comprehensive review of the mechanisms and efficiency of underground hydrogen storage. J. Energy Storage 2022, 51, 104490. [Google Scholar] [CrossRef]
- Chabab, S.; Théveneau, P.; Coquelet, C.; Corvisier, J.; Paricaud, P. Measurements and predictive models of high-pressure H2 solubility in brine (H2O+NaCl) for underground hydrogen storage application. Int. J. Hydrogen Energy 2020, 45, 32206–32220. [Google Scholar] [CrossRef]
- Tarkowski, R.; Uliasz-Misiak, B.; Tarkowski, P. Storage of hydrogen, natural gas, and carbon dioxide—Geological and legal conditions. Int. J. Hydrogen Energy 2021, 46, 20010–20022. [Google Scholar] [CrossRef]
- Zamehrian, M.; Sedaee, B. Underground hydrogen storage in a partially depleted gas condensate reservoir: Influence of cushion gas. J. Pet. Sci. Eng. 2022, 212, 110304. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Yang, C.; Shi, X.; Wan, J.; Jurado, M.J.; Li, Y.; Jiang, D.; Chen, J.; Qiao, W.; et al. The role of underground salt caverns for large-scale energy storage: A review and prospects. Energy Storage Mater. 2023, 63, 103045. [Google Scholar] [CrossRef]
- Oni, B.A.; Bade, S.O.; Sanni, S.E.; Orodu, O.D. Underground hydrogen storage in salt caverns: Recent advances, modeling approaches, barriers, and future outlook. J. Energy Storage 2025, 107, 114951. [Google Scholar] [CrossRef]
- Song, R.; Yue-ming, B.; Jing-Peng, Z.; De-yi, J.; Chun-he, Y. Experimental investigation of the fatigue properties of salt rock. Int. J. Rock Mech. Min. Sci. 2013, 64, 68–72. [Google Scholar] [CrossRef]
- Caglayan, D.G.; Weber, N.; Heinrichs, H.U.; Linßen, J.; Robinius, M.; Kukla, P.A.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrogen Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Lankof, L.; Tarkowski, R. Assessment of the potential for underground hydrogen storage in bedded salt formation. Int. J. Hydrogen Energy 2020, 45, 19479–19492. [Google Scholar] [CrossRef]
- Jahanbakhsh, A.; Potapov-Crighton, A.L.; Mosallanezhad, A.; Kaloorazi, N.T.; Maroto-Valer, M.M. Underground hydrogen storage: A UK perspective. Renew. Sustain. Energy Rev. 2024, 189, 114001. [Google Scholar] [CrossRef]
- Sadkhan, R.A.; Al-Mudhafar, W.J. Key aspects of underground hydrogen storage in depleted hydrocarbon reservoirs and saline aquifers: A fundamental review and understanding. Energy Geosci. 2024, 5, 100339. [Google Scholar] [CrossRef]
- Bade, S.O.; Taiwo, K.; Ndulue, U.F.; Tomomewo, O.S.; Oni, B.A. A review of underground hydrogen storage systems: Current status, modeling approaches, challenges, and future prospective. Int. J. Hydrogen Energy 2024, 80, 449–474. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Z.; Nasrabadi, H.; Chen, B.; Mehana, M.Z.S.; Van Wijk, J. Capacity assessment and cost analysis of geologic storage of hydrogen: A case study in Intermountain-West Region USA. Int. J. Hydrogen Energy 2023, 48, 9008–9022. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Haq, M.B.; Al Shehri, D.A.; Al-Ahmed, A.; Rahman, M.M.; Zaman, E.; Iglauer, S. Hydrogen storage in depleted gas reservoirs: A comprehensive review. Fuel 2023, 337, 127032. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, X.; You, H.; Gao, M. Research on the design of hydrogen supply system of 70 MPa hydrogen storage cylinder for vehicles. Int. J. Hydrogen Energy 2018, 43, 19189–19195. [Google Scholar] [CrossRef]
- Valenti, G. Hydrogen liquefaction and liquid hydrogen storage. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 27–51. [Google Scholar]
- Ding, Z.; Li, Y.; Jiang, H.; Zhou, Y.; Wan, H.; Qiu, J.; Jiang, F.; Tan, J.; Du, W.; Chen, Y.A. The integral role of high-entropy alloys in advancing solid-state hydrogen storage. Interdiscip. Mater. 2025, 4, 75–108. [Google Scholar] [CrossRef]










| Property | Hydrogen | Methane | Gasoline | Diesel |
|---|---|---|---|---|
| Molecular weight | 2.016 | 16.043 | 110 | 170 |
| Auto-ignition temperature (K) | 853 | 813 | 623 | 523 |
| Carbon content (mass%) | 0 | 75 | 84 | 86 |
| Boiling point (K) | 20.3 | 111 | 298–488 | 453–633 |
| HHV (MJ/kg) | 141.9 | 55.5 | 47.3 | 44.8 |
| LHV (MJ/kg) | 119.9 | 50 | 44.5 | 42.5 |
| Density (at 1 bar and 273 K; kg/m3) | 0.089 | 0.72 | 730–780 | 830 |
| Adiabatic flame temperature (at 1 bar and 298 K; at stoichiometry; K) | 2480 | 2214 | 2580 | 2300 |
| Volumetric energy content (at 1 bar and 273 K; MJ/m3) | 10.7 | 33 | 33,000 | 35,000 |
| Stoichiometry air/fuel mass ratio | 34.4 | 17.2 | 14.7 | 14.5 |
| Types | Material/Description | Advantages | Challenges | Ref. |
|---|---|---|---|---|
| Type I | Fully metallic pressure vessel (usually 4130 steels, stainless steel, high-strength carbon steel), aluminum. Used in industrial gas storage, low-pressure applications. | Cheapest option and widely available. | Heavy, hydrogen embrittlement, internal corrosion, limiting operating pressure, low gravimetric density, not applicable for onboard application. | [69] |
| Type II | Metallic pressure vessel hoop-wrapped with glass fiber composite, used in CNG storage and transport, moderate pressure. | Lighter weight compared to type I, highest pressure tolerance | Serious hydrogen embrittlement problem, more expensive than type I, short lifetime. | [76] |
| Type III | Full composite wrap with metal liners such as aluminum, stainless steel. Used in hydrogen refueling stations, heavy-duty vehicles, aerospace. | High strength-to-weight ratio, reducing weight no permeation. | Linear fatigue, high burst pressure, more expensive compared to steel tank | [61] |
| Type IV | Fully composite (high-density polyethylene (HDPE) inner with carbon glass or carbon fiber), used in FCEVs, portable hydrogen storage, high-pressure transport. | Lightweight, ideal for mobile applications, longer life, lower burst pressure, and permeation through liner | Permeation, cost is still comparatively high, linear collapse, embrittlement. | [77] |
| Type V | Fully composite materials, such as carbon fiber-reinforced polymer (CFRP), with no metal liner. Used in FCEVs, aerospace, and high-pressure storage. | Lighter than other types of tanks, designed to store hydrogen at very high pressures up to 100 MPa, eliminates the risk of hydrogen embrittlement. | High manufacturing cost due to the use of advanced composite materials. | [72] |
| Hydrogen Storage Method (Source) | Advantages | Disadvantages/Challenges | Application Area |
|---|---|---|---|
| Compressed hydrogen storage [178] | Mature technology, fast and reliable refueling process, technology simplicity, low energy consumption compared to liquefied hydrogen storage methods, simple. | High-pressure requirements, volumetrically and gravimetrically inefficient, space inefficiency, gas leakage (safety risks), small quantity storage, energy consumption. | Common cylinders, stationary, and mobile applications, aircraft, lightweight, high pressure hydrogen storage tanks. |
| Liquefied hydrogen storage [179] | High volumetric densities, lower storage pressure, fast kinetics, fast refueling process, reliable, safe. | High energy consumption in the liquefaction process boil-off phenomena, tank cost, complex equipment, ultra-low temperatures, high vessel insulation requirements. | Space exploration, long-distance transport, rocket cryogenic propulsion. |
| Cryo-compressed hydrogen gas storage [87] | Fast filling, long dormancy, offer medium to high gravimetric and volumetric capacities, a long non-emission time, exhibit excellent heat resistance, high safety factor. | Temperature rise during the filling process causes risks such as diffusion, deflagration, cost, detonation. | Heavy-duty vehicles, onboard (fuel cell driven bus), stationary storage solutions backup power systems. |
| Solid-state hydrogen storage [180] | Simple and safe process, high reliability, Low pressure, volumetric efficiency, ideal storage density | Immature technology, high energy requirements during adsorption, slow hydrogen sorption and desorption kinetics, high operating temperatures, high materials requirement, small quantity storage, cost | Used in hydrogen fuel cell vehicles (FCVs), small-scale transportation, stationary power systems |
| Underground hydrogen storage [169] | Exhibited the lowest storage cost than any storage methods, large storage capacity, enables higher storage pressures, enhances safety protocols, stability | Keeping purity, site selection | Long-term storage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekonnin, A.S.; Wacławiak, K.; Humayun, M.; Zhang, S.; Ullah, H. Hydrogen Storage Technology, and Its Challenges: A Review. Catalysts 2025, 15, 260. https://doi.org/10.3390/catal15030260
Mekonnin AS, Wacławiak K, Humayun M, Zhang S, Ullah H. Hydrogen Storage Technology, and Its Challenges: A Review. Catalysts. 2025; 15(3):260. https://doi.org/10.3390/catal15030260
Chicago/Turabian StyleMekonnin, Abdisa Sisay, Krzysztof Wacławiak, Muhammad Humayun, Shaowei Zhang, and Habib Ullah. 2025. "Hydrogen Storage Technology, and Its Challenges: A Review" Catalysts 15, no. 3: 260. https://doi.org/10.3390/catal15030260
APA StyleMekonnin, A. S., Wacławiak, K., Humayun, M., Zhang, S., & Ullah, H. (2025). Hydrogen Storage Technology, and Its Challenges: A Review. Catalysts, 15(3), 260. https://doi.org/10.3390/catal15030260








