Constructing Pt/Hierarchical HY Bifunctional Catalysts for Selective Hydroisomerization of Phenanthrene to Alkyl-Adamantanes
Abstract
1. Introduction
2. Results and Discussion
2.1. Textural Structure and Morphology of As-Prepared H-MSY-T Zeolites
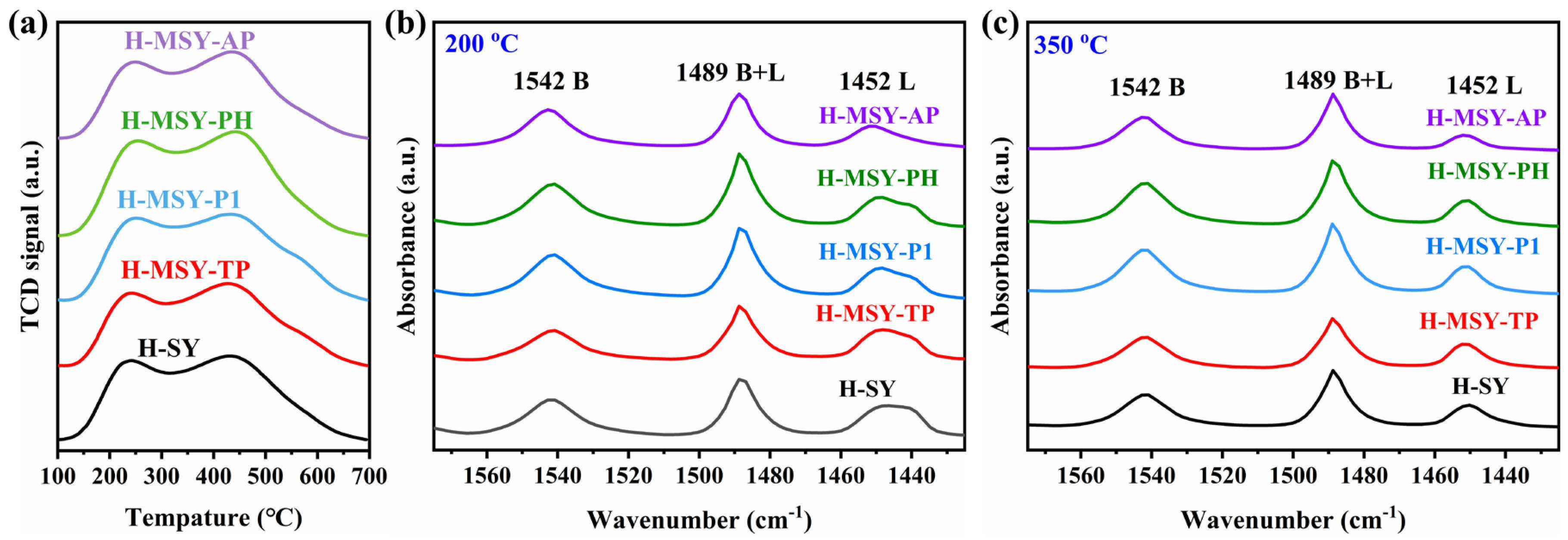
2.2. The Acidity of As-Prepared H-MSY-T Zeolites
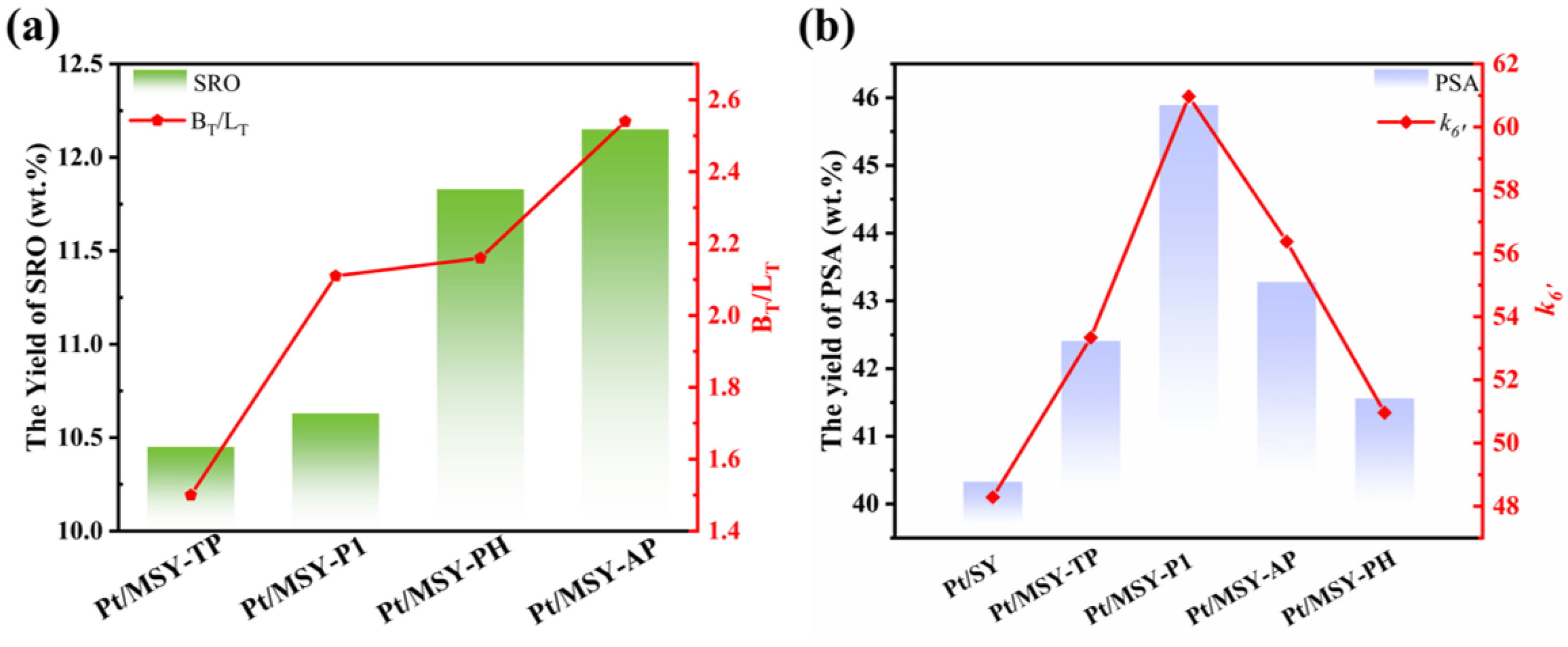
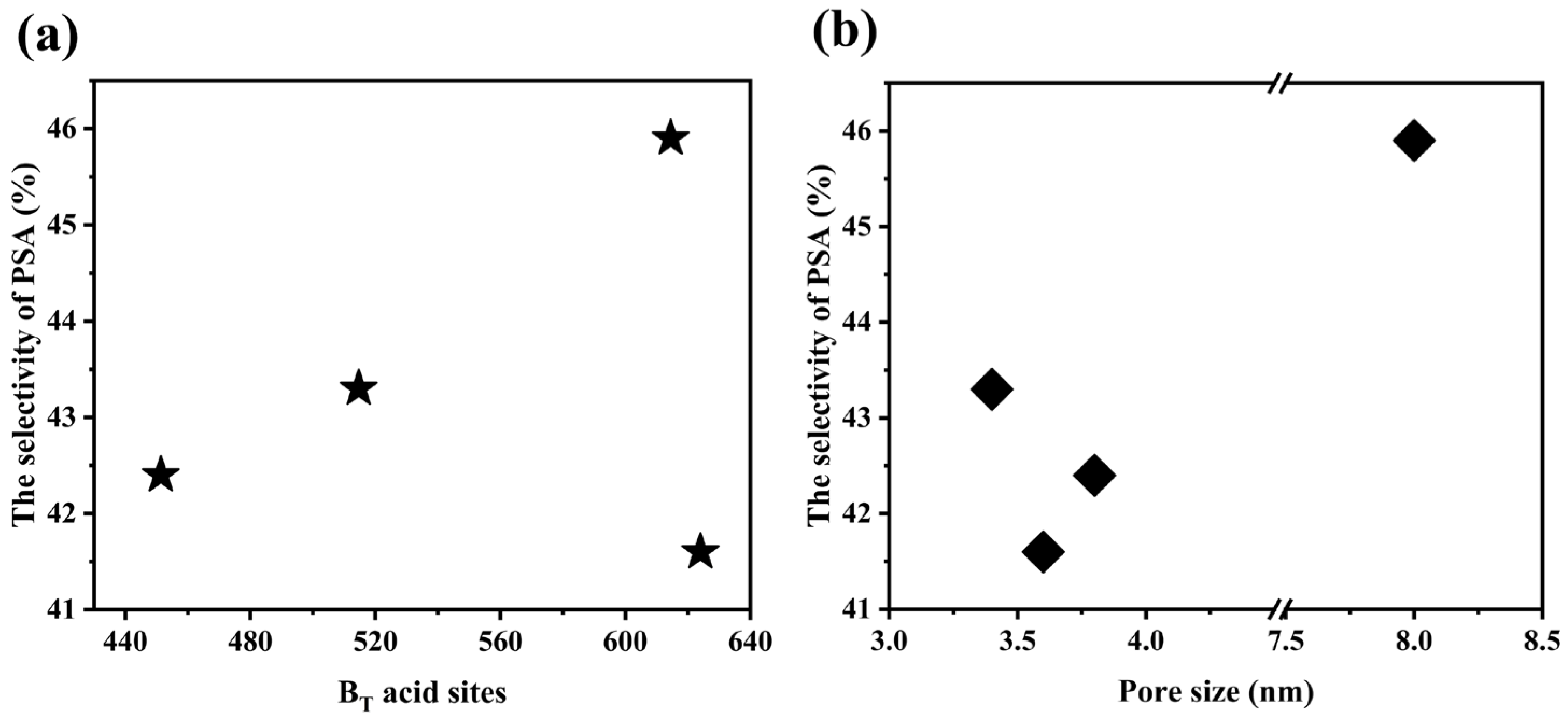
2.3. Catalytic Activity Evaluation
2.4. Possible Reaction Pathways
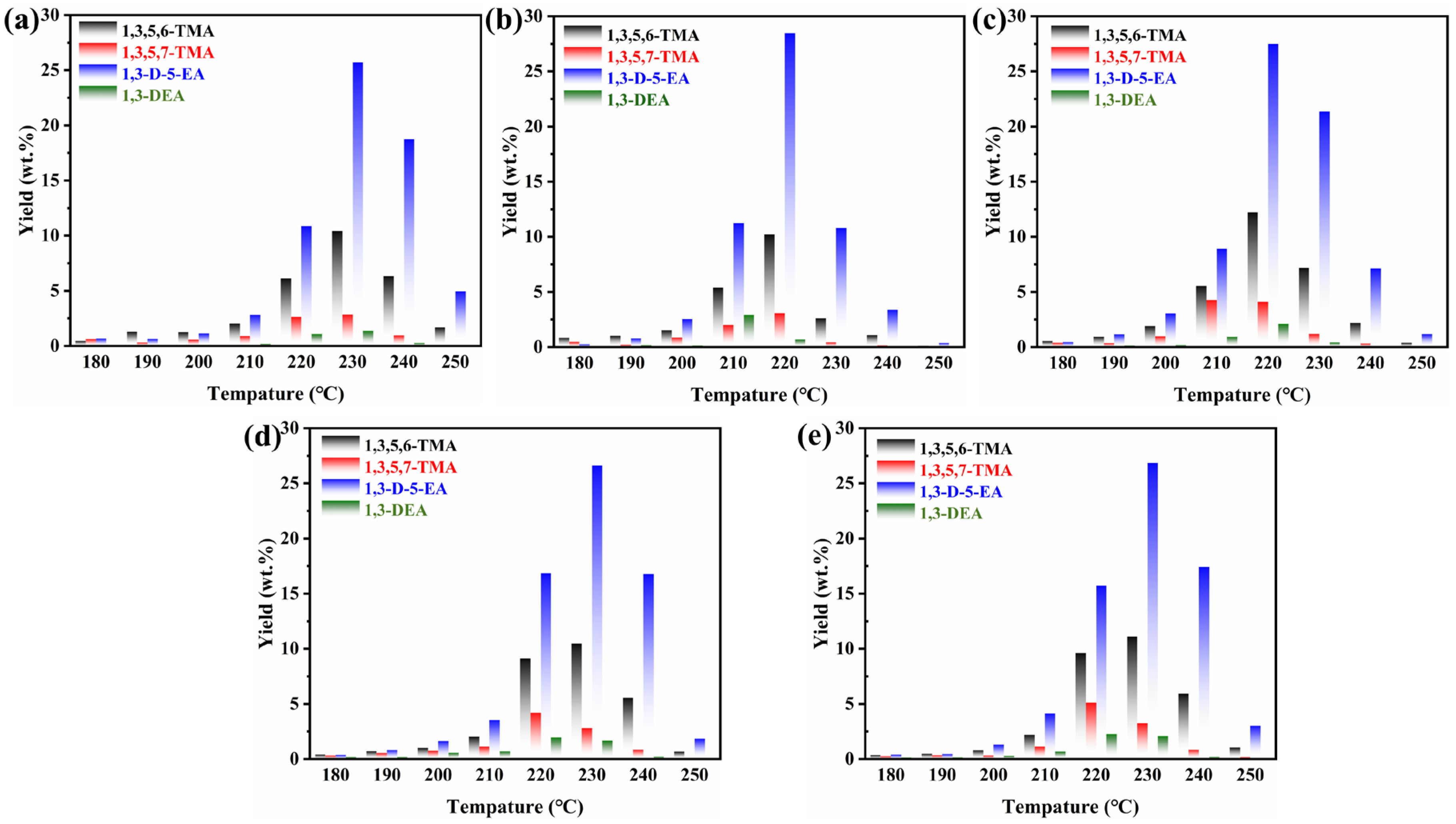
2.5. Distribution of PSA Products
3. Experiments
3.1. Materials
3.2. Preparation of Hierarchical MSY Zeolites
3.3. Preparation of Pt/Zeolite Bifunctional Catalysts
3.4. Catalyst Characterization
3.5. Hydroisomerization Activity of Different Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, C.; Ning, L.; Xiaofeng, Y.; Lin, L.; Guangyi, L.; Shanshan, L.; Wentao, W.; Yancheng, H.; Aiqin, W.; Yu, C. Synthesis of High-Density Aviation Fuel with Cyclopentanol. ACS Sustain. Chem. Eng. 2016, 4, 6160–6166. [Google Scholar]
- Oh, S.K.; Ku, H.; Han, G.B.; Jeong, B.; Jeon, J.-K. Hydrogenation of polycyclic aromatic hydrocarbons over Pt/γ-Al2O3 catalysts in a trickle bed reactor. Catal. Today 2023, 411, 113831. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, W.; Liu, L.; Niu, X.; Wang, Q. Tuning the Structure and Acidity of Pt/Hierarchical SSZ-32 Catalysts to Boost the Selective Hydroisomerization of n-Hexadecane. Catalysts 2023, 13, 702. [Google Scholar] [CrossRef]
- Qie, G.; Zhai, M.; Zhu, K.; Zhu, X. Generating Disk-Shaped MTW Zeolite with Reduced Channel Length Using Polycation Structure-Directing Agent for Hydroisomerization of N-Dodecane. Catalysts 2024, 14, 925. [Google Scholar] [CrossRef]
- Sun, D.; Cai, W.; Li, C.; Lu, J. Experimental study on atomization characteristics of high-energy-density fuels using a fuel slinger. Energy 2021, 234, 121222. [Google Scholar] [CrossRef]
- Jampaiah, D.; Murzin, D.Y.; Lee, A.F.; Schaller, D.; Bhargava, S.K.; Tabulo, B.; Wilson, K. Catalytic selective ring opening of polyaromatics for cleaner transportation fuels. Energy Environ. Sci. 2022, 15, 1760–1804. [Google Scholar] [CrossRef]
- Jia, C.; Xie, S.; Zhang, W.; Intan, N.N.; Sampath, J.; Pfaendtner, J.; Lin, H. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst. Chem Catal. 2021, 1, 437–455. [Google Scholar] [CrossRef]
- Li, T.; Jia, D.; Zhou, S.; Liu, Z.; Chen, J.; Ban, T.; Li, A.; Li, H.; Gao, H. Review on recent advances in supported metal catalysts for synthesis of high energy density fuels. Fuel 2024, 373, 132329. [Google Scholar] [CrossRef]
- Petrukhina, N.; Vinnikova, M.; Maksimov, A. Production of high-density jet and diesel fuels by hydrogenation of highly aromatic fractions. Russ. J. Appl. Chem. 2018, 91, 1223–1254. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Huang, Y.; Li, Y.; Wang, Q.; Li, S.; Qiu, L.; Li, R.; Yan, X. Electron-rich Ni3Zn strongly interacted on ZnO promoter for deep hydrogenation of phenanthrene in dibenzothiophene. Chem. Eng. Sci. 2024, 299, 120404. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Li, Z.; Jing, J.; Li, W. Enhanced phenanthrene hydrogenation saturation performance of Ni-Co/NiAlOX catalysts and its catalytic mechanism. Fuel Process. Technol. 2023, 250, 107902. [Google Scholar] [CrossRef]
- Brito, L.; Pirngruber, G.D.; Guillon, E.; Albrieux, F.; Martens, J.A. Hydroconversion of Perhydrophenanthrene over Bifunctional Pt/H-USY Zeolite Catalyst. ChemCatChem 2020, 12, 3477–3488. [Google Scholar] [CrossRef]
- Qi, J.; Guo, Y.; Jia, H.; Fan, B.; Gao, H.; Qin, B.; Ma, J.; Du, Y.; Li, R. Unpredictable properties of industrial HY zeolite for tetralin hydrocracking. Fuel Process. Technol. 2023, 240, 107586. [Google Scholar] [CrossRef]
- Samoilov, V.; Sultanova, M.; Borisov, R.; Kozhevnikov, A.; Lavrentyev, V.; Utepbergenova, A.S.; Maximov, A. The production and the properties of the liquid organic hydrogen carrier (LOHC) obtained from the light cycle oil (LCO). Fuel Process. Technol. 2023, 240, 107576. [Google Scholar] [CrossRef]
- Harvey, B.G.; Harrison, K.W.; Davis, M.C.; Chafin, A.P.; Baca, J.; Merriman, W.W. Molecular design and characterization of high-cetane alkyl diamondoid fuels. Energy Fuels 2016, 30, 10171–10178. [Google Scholar] [CrossRef]
- Harrison, K.W.; Rosenkoetter, K.E.; Harvey, B.G. High density alkyl diamondoid fuels synthesized by catalytic cracking of alkanes in the presence of adamantane. Energy Fuels 2018, 32, 7786–7791. [Google Scholar] [CrossRef]
- Beltramone, A.; Resasco, D.; Alvarez, W.; Choudhary, T. Simultaneous hydrogenation of multiring aromatic compounds over NiMo catalyst. Ind. Eng. Chem. Res. 2008, 47, 7161–7166. [Google Scholar] [CrossRef]
- Brito, L.; Pirngruber, G.D.; Perez-Pellitero, J.; Guillon, E.; Albrieux, F.; Martens, J.A. Shape selectivity effects in the hydroconversion of perhydrophenanthrene over bifunctional catalysts. Catal. Sci. Technol. 2021, 11, 7667–7682. [Google Scholar] [CrossRef]
- Ro, I.; Qi, J.; Lee, S.; Xu, M.; Yan, X.; Xie, Z.; Zakem, G.; Morales, A.; Chen, J.G.; Pan, X. Bifunctional hydroformylation on heterogeneous Rh-WO x pair site catalysts. Nature 2022, 609, 287–292. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Gao, G.; Zhao, Z.; Sun, P.; Li, F. Enhanced stability of hierarchical zeolite-metal bifunctional catalysts for conversion of levulinic acid to valeric biofuels. Chem. Eng. J. 2024, 483, 149405. [Google Scholar] [CrossRef]
- Li, K.; Cui, T.; Zhao, J.; Liang, C.; Li, C. Regulate the mesopores and acidic structure of Pt/USY zeolite catalyst for improved performance in the hydroisomerization of phenanthrene to alkyl-adamantane. Chem. Eng. Sci. 2023, 279, 118957. [Google Scholar] [CrossRef]
- Tarach, K.A.; Martinez-Triguero, J.; Valencia, S.; Wojciechowska, K.; Rey, F.; Góra-Marek, K. Hierarchical zeolites TNU-9 and IM-5 as the catalysts for cracking processes. Appl. Catal. B 2023, 338, 123066. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, J.; Guan, B.; Yu, J. Structural Engineering of Hierarchical Zeolite-Based Catalysts. Acc. Mater. Res. 2024, 5, 857–871. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef]
- Liu, K.; Çağlayan, M.; Dikhtiarenko, A.; Zhang, X.; Sayidov, O.; Abou-Hamad, E.; Gascon, J.; Chowdhury, A.D. Are hierarchical zeolites good catalysts for Methane Dehydroaromatization? A critical analysis. Catal. Today 2023, 408, 22–35. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Choi, M. Cooperative Interplay of Micropores/Mesopores of Hierarchical Zeolite in Chemical Production. ACS Catal. 2024, 14, 2031–2048. [Google Scholar] [CrossRef]
- Xueming, W.; Xiaohong, L.; Wenying, L. Effect of support acidity on hydrogenation of phenanthrene to alkyl adamantane over Pt/USY catalysts. CIESC J. 2021, 72, 5196. [Google Scholar]
- Tian, H.; Chen, Z.; Yang, X.; Gao, P.; Jiao, C.; Zha, F.; Chang, Y.; Chen, H. Catalytic performance of methanol to aromatics over hierarchical ZSM-5 zeolite synthesized by a soft template method. Energy Fuels 2023, 37, 14180–14191. [Google Scholar] [CrossRef]
- Liu, B.; Chen, F.; Zheng, L.; Ge, J.; Xi, H.; Qian, Y. Synthesis and structural properties of hierarchically structured aluminosilicates with zeolite Y (FAU) frameworks. RSC Adv. 2013, 3, 15075–15084. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.-H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, Y.; Li, Y.; Chen, W.; Liu, B. Synthesis and characterization of mesoporous zeolite Y by using block copolymers as templates. Chem. Eng. J. 2016, 284, 405–411. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, G.; Qin, L.; Li, H.; Chen, Y.; Liu, B. Synthesis and catalytic cracking performance of mesoporous zeolite Y. Catal. Commun. 2016, 73, 98–102. [Google Scholar] [CrossRef]
- Serrano, D.; Aguado, J.; Escola, J.; Rodriguez, J.; Peral, A. Effect of the organic moiety nature on the synthesis of hierarchical ZSM-5 from silanized protozeolitic units. J. Mater. Chem. 2008, 18, 4210–4218. [Google Scholar] [CrossRef]
- Wen, C.; Lu, L.; Zhang, X.; Jin, K.; Chen, L.; Zhang, Q.; Ma, L.; Wang, C. Optimizing zeolitic hierarchical pore structure to boost the direct conversion of aromatics from syngas over the iron-based/zeolite bifunctional catalysts. Fuel 2024, 357, 129791. [Google Scholar] [CrossRef]
- Choo, M.-Y.; Oi, L.E.; Lin, Y.-C.; Ling, T.C.; Ng, E.-P.; Chen, W.-H.; Cheng, C.K.; Juan, J.C. Uniform mesoporous hierarchical nanosized zeolite Y for production of Hydrocarbon-like biofuel under H2-Free deoxygenation. Fuel 2022, 322, 124208. [Google Scholar] [CrossRef]
- Zhang, K.; Fernandez, S.; Converse, E.S.; Kobaslija, S. Exploring the impact of synthetic strategies on catalytic cracking in hierarchical beta zeolites via hydrothermal desilication and organosilane-templated synthesis. Catal. Sci. Technol. 2020, 10, 4602–4611. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Fan, D.; Yan, N.; Huang, S.; Xu, S.; Guo, P.; Yang, M.; Zhang, J.; Tian, P. A bottom-up strategy for the synthesis of highly siliceous faujasite-type zeolite. Adv. Mater. 2020, 32, 2000272. [Google Scholar] [CrossRef]
- Cui, W.; Zhu, D.; Tan, J.; Chen, N.; Fan, D.; Wang, J.; Han, J.; Wang, L.; Tian, P.; Liu, Z. Synthesis of mesoporous high-silica zeolite Y and their catalytic cracking performance. Chin. J. Catal. 2022, 43, 1945–1954. [Google Scholar] [CrossRef]
- Du, Y.; Kong, Q.; Gao, Z.; Wang, Z.; Zheng, J.; Qin, B.; Pan, M.; Li, W.; Li, R. Flowerlike hierarchical Y with dramatically increased external surface: A potential catalyst contributing to improving precracking for Bulky reactant molecules. Ind. Eng. Chem. Res. 2018, 57, 7395–7403. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Daud, W.M.A.W. Synthesis and characterization of hierarchical nanoporous HY zeolites from acid-activated kaolin. Chin. J. Catal. 2015, 36, 1846–1851. [Google Scholar] [CrossRef]
- Alonso-Doncel, M.; Peral, A.; Shamzhy, M.; Čejka, J.; Sanz, R.; Serrano, D. Untangling the role of the organosilane functional groups in the synthesis of hierarchical ZSM-5 zeolite by crystallization of silanized protozeolitic units. Catal. Today 2020, 345, 27–38. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Dong, D.; Li, D.; Hill, M.R.; Hill, A.J.; Wang, H. Synthesis of hierarchical porous zeolite NaY particles with controllable particle sizes. Microporous Mesoporous Mater. 2010, 127, 167–175. [Google Scholar] [CrossRef]
- Sang, S.; Chang, F.; Liu, Z.; He, C.; He, Y.; Xu, L. Difference of ZSM-5 zeolites synthesized with various templates. Catal. Today 2004, 93, 729–734. [Google Scholar] [CrossRef]
- Wang, E.; Hu, D.; Xiao, C.; Song, S.; Zou, Y.; Fu, S.; Chi, K.; Liu, J.; Duan, A.; Wang, X. Highly dispersed Pt on core–shell micro-mesoporous composites assembled by mordenite nanocrystals for selective hydrogenation of polycyclic aromatics. Fuel 2023, 331, 125852. [Google Scholar] [CrossRef]
- Biriaei, R.; Nohair, B.; Kaliaguine, S. A facile route to synthesize mesoporous ZSM-5 with hexagonal arrays using P123 triblock copolymer. Microporous Mesoporous Mater. 2020, 298, 110067. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Yuan, J.; Liu, X.; Yang, Y.; Yu, F. The regulating strategy of hierarchical structure and acidity in zeolites and application of gas adsorption application: A review. Chin. Chem. Lett. 2024, 35, 109693. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Xu, D.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Precisely regulating Brønsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation. Appl. Catal. B 2021, 283, 119648. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, Y.; Zhang, P.; Liu, X.; Zhao, Y.; Zhuang, J.; Zhao, Q.; Wang, Y.; Qian, Y.; Zhu, X. Identifying the location of real active sites in ZSM-5 zeolites for tetralin conversion into light aromatics. Energy Fuels 2023, 37, 3011–3022. [Google Scholar] [CrossRef]
- Saginayev, A.; Kursina, M.; Kalauova, A. Syntheses, Geometrical and Electronic Structure of Alkyladamantanes and Their Thermodynamic Characteristic According to the Density Functional Theory. Sci. J. Chem. 2018, 6, 50–55. [Google Scholar] [CrossRef]
- Berenguer, A.; Bennett, J.A.; Hunns, J.; Moreno, I.; Coronado, J.M.; Lee, A.F.; Pizarro, P.; Wilson, K.; Serrano, D.P. Catalytic hydrodeoxygenation of m-cresol over Ni2P/hierarchical ZSM-5. Catal. Today 2018, 304, 72–79. [Google Scholar] [CrossRef]
- Suárez, N.; Arribas, M.A.; Moreno, A.; Martínez, A. High-performing Ir-and Pt-containing catalysts based on mesoporous beta zeolite for the selective ring opening of decalin. Catal. Sci. Technol. 2020, 10, 1073–1085. [Google Scholar] [CrossRef]
- McKervey, M.A. Adamantane rearrangements. Chem. Soc. Rev. 1974, 3, 479–512. [Google Scholar] [CrossRef]
- Leite, L.; Benazzi, E.; Marchal-George, N. Hydrocracking of phenanthrene over bifunctional Pt catalysts. Catal. Today 2001, 65, 241–247. [Google Scholar] [CrossRef]
- Qi, L.; Peng, C.; Cheng, Z.; Zhou, Z. Selective hydrocracking of poly-aromatics to mono-aromatics in a catalyst grading system of NiMo/Al2O3-HY and NiMo/Beta. Fuel 2023, 351, 128941. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Q.; Qin, Y.; Liu, H.; Liu, H.; Cao, G.; Gao, X.; Song, L.; Sun, Z. Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Microporous Mesoporous Mater. 2023, 359, 112627. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, T. Pore engineering for high performance porous materials. ACS Cent. Sci. 2023, 9, 1499–1503. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite catalysts with tunable hierarchy factor by pore-growth moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Shamzhy, M.; Opanasenko, M.; Concepción, P.; Martínez, A. New trends in tailoring active sites in zeolite-based catalysts. Chem. Soc. Rev. 2019, 48, 1095–1149. [Google Scholar] [CrossRef]
- Zheng, J.; Yi, Y.; Wang, W.; Guo, K.; Ma, J.; Li, R. Synthesis of bi-phases composite zeolites MFZ and its hierarchical effects in isopropylbenzene catalytic cracking. Microporous Mesoporous Mater. 2013, 171, 44–52. [Google Scholar] [CrossRef]
- Schreier, M.; Teren, S.; Belcher, L.; Regalbuto, J.R.; Miller, J.T. The nature of ‘overexchanged’copper and platinum on zeolites. Nanotechnology 2005, 16, S582. [Google Scholar] [CrossRef]
- Eskandari, S.; Tate, G.; Leaphart, N.R.; Regalbuto, J.R. Nanoparticle synthesis via electrostatic adsorption using incipient wetness impregnation. ACS Catal. 2018, 8, 10383–10391. [Google Scholar] [CrossRef]
- Emeis, C. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
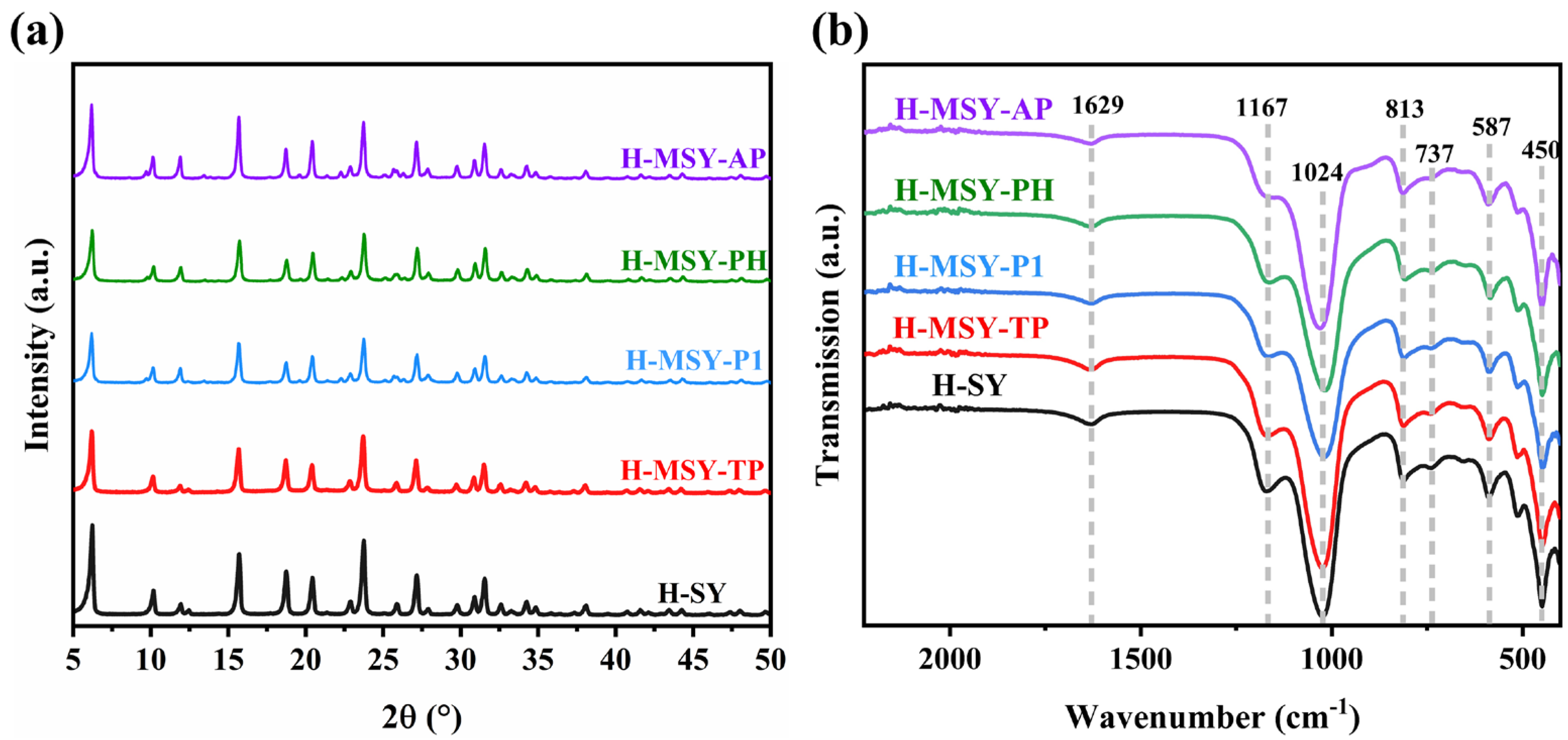
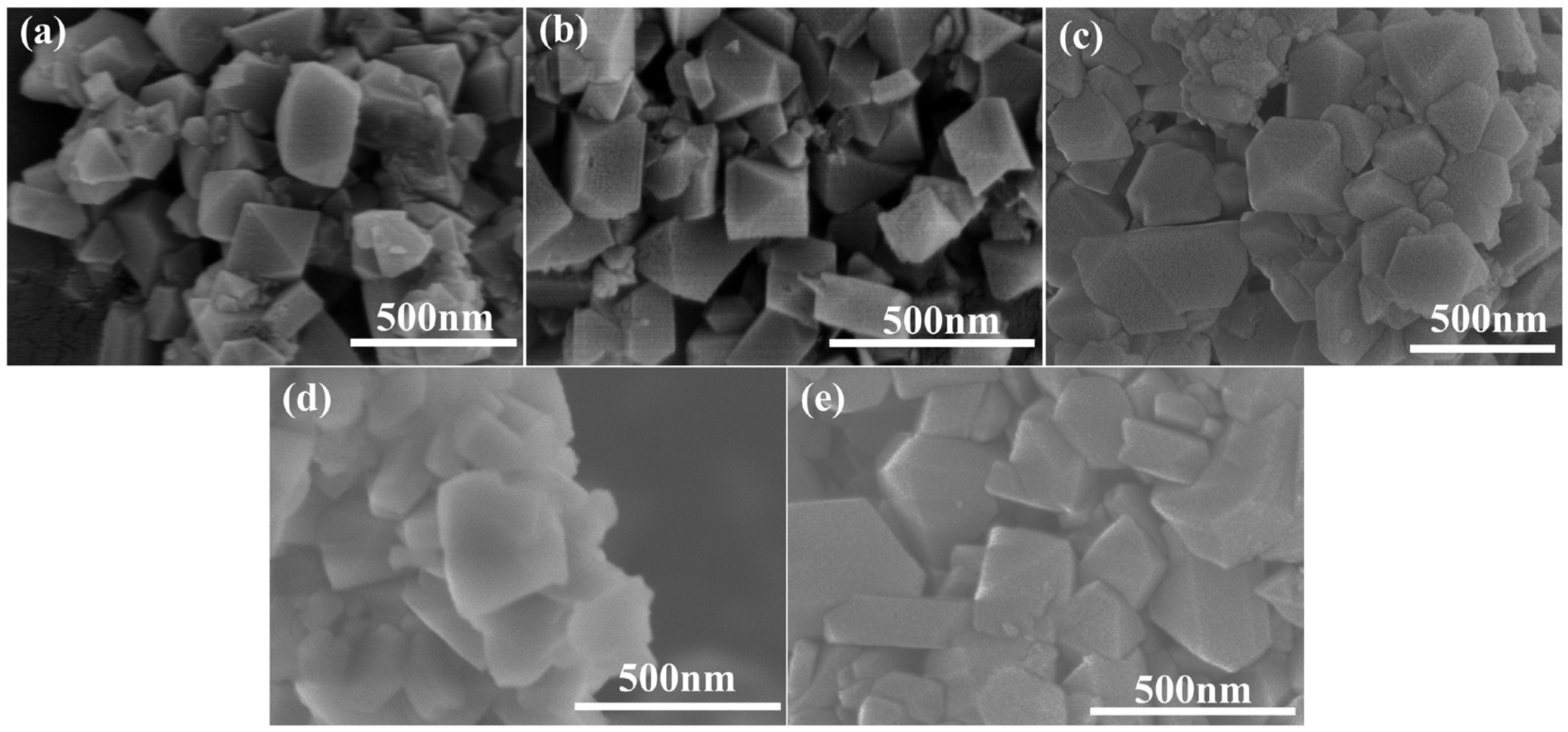
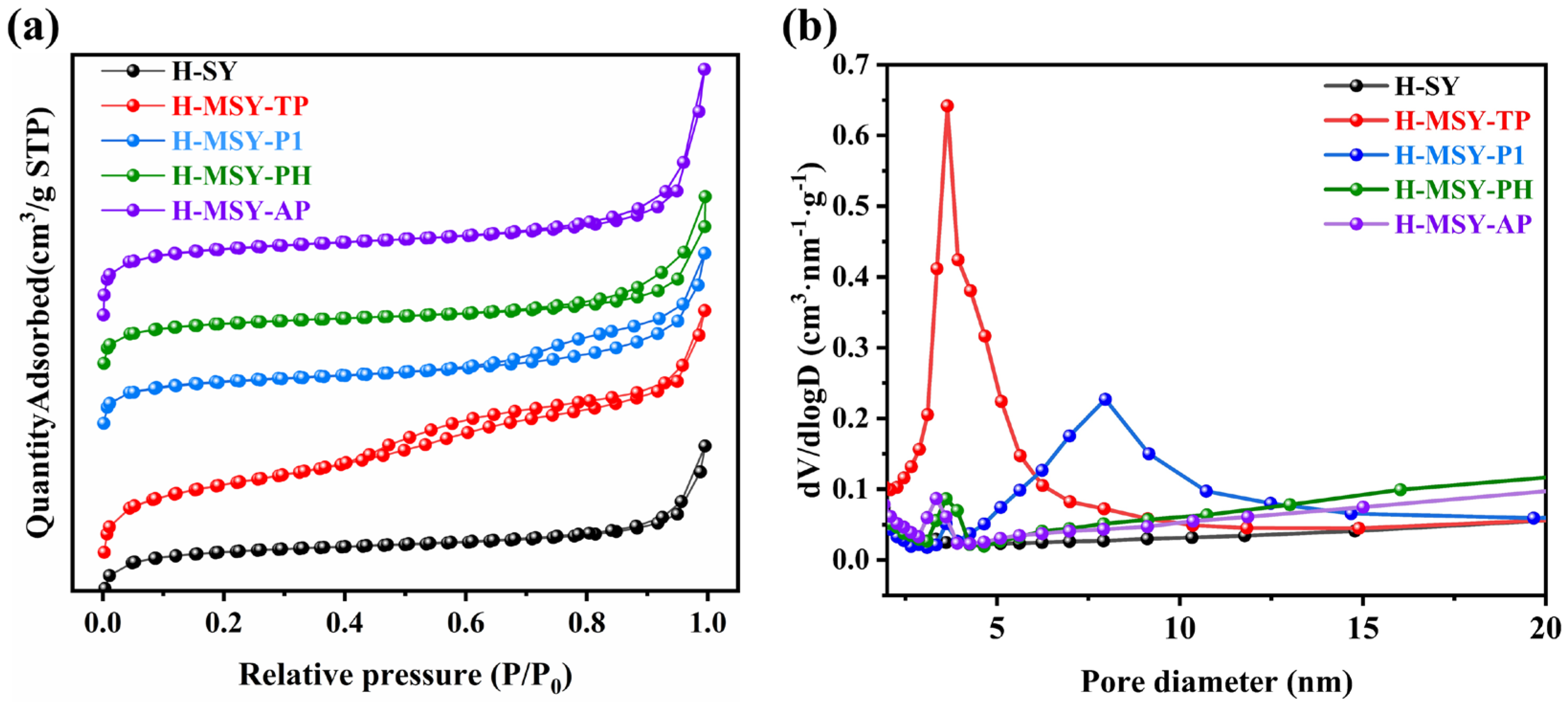
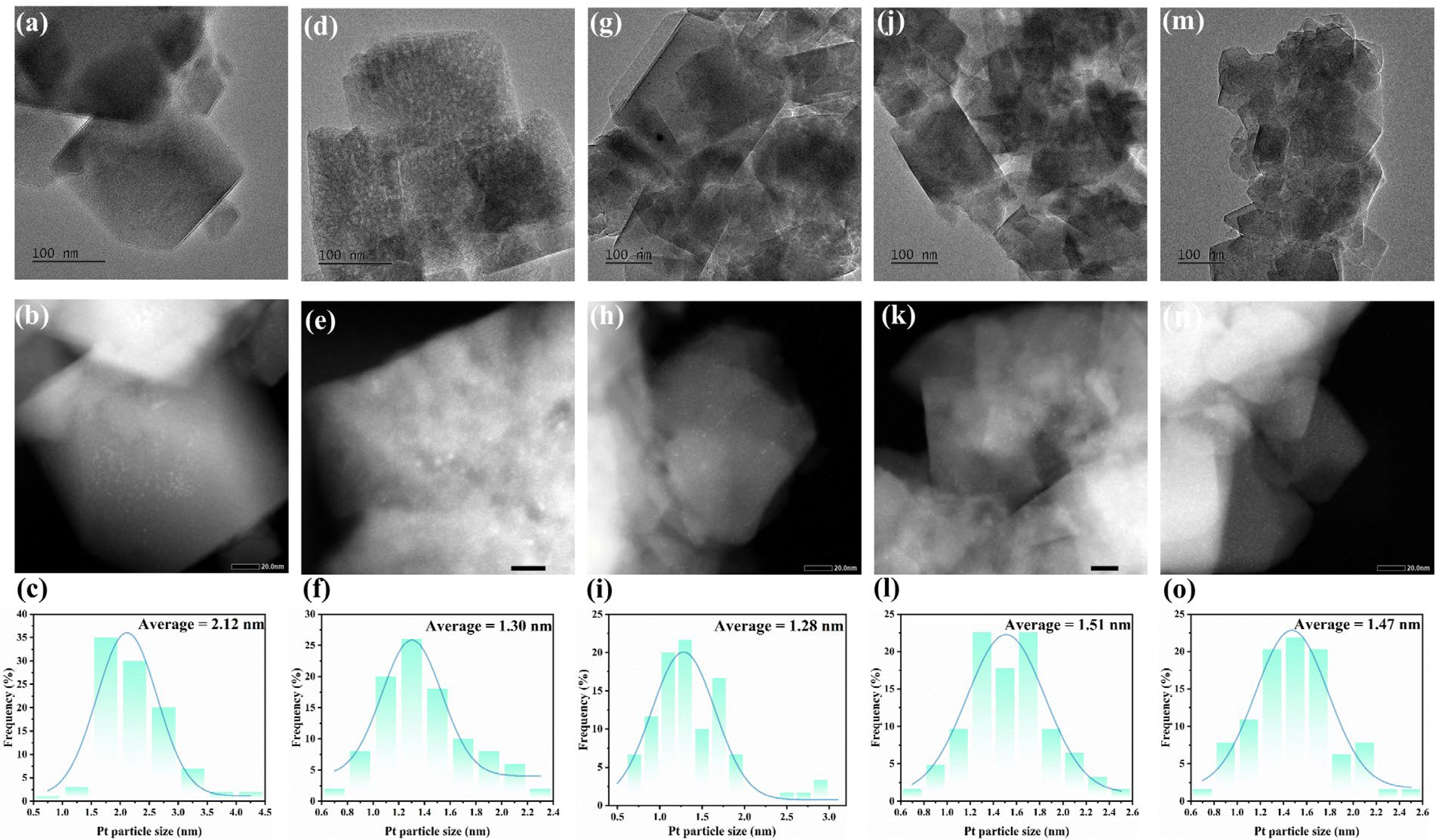


| Samples | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Sext/Smicro | ||||
|---|---|---|---|---|---|---|---|
| SBET | Smicro | Sext | Vtotal | Vmicro | Vext | ||
| H-SY | 878 | 851 | 27 | 0.3869 | 0.3297 | 0.0572 | 0.03 |
| H-MSY-TP | 865 | 619 | 246 | 0.5535 | 0.2416 | 0.3120 | 0.39 |
| H-MSY-P1 | 802 | 614 | 188 | 0.4131 | 0.2356 | 0.1775 | 0.31 |
| H-MSY-PH | 798 | 623 | 175 | 0.4311 | 0.2386 | 0.1925 | 0.29 |
| H-MSY-AP | 928 | 791 | 137 | 0.4882 | 0.3002 | 0.1880 | 0.17 |
| Catalysts | Pt/SY | Pt/MSY-TP | Pt/MSY-P1 | Pt/MSY-PH | Pt/MSY-AP |
|---|---|---|---|---|---|
| Si/Al ratio | 6.65 | 6.78 | 6.37 | 6.33 | 6.84 |
| Pt loading (wt%) | 1.78 | 1.79 | 1.75 | 1.58 | 1.79 |
| Samples | Peak Temperature (°C) | Acidic Sites (mmol·g−1) | |||
|---|---|---|---|---|---|
| LTP | HTP | Weak | Strong | Total | |
| H-SY | 243 | 429 | 0.575 | 1.696 | 2.271 |
| H-MSY-TP | 242 | 427 | 0.451 | 1.703 | 2.154 |
| H-MSY-P1 | 250 | 433 | 0.647 | 1.762 | 2.409 |
| H-MSY-PH | 255 | 443 | 0.822 | 1.888 | 2.710 |
| H-MSY-AP | 249 | 434 | 0.580 | 1.618 | 2.198 |
| Catalysts | Bronsted Acid Sites (μmol·g−1) | Lewis Acid Sites (μmol·g−1) | BS/LS c | BT/LT c | ||||
|---|---|---|---|---|---|---|---|---|
| Total a | Weak | Strong b | Total a | Weak | Strong b | |||
| H-SY | 522.29 | 128.38 | 393.91 | 327.95 | 185.00 | 142.95 | 2.76 | 1.59 |
| H-MSY-TP | 451.37 | 59.49 | 391.88 | 300.59 | 146.04 | 154.55 | 2.54 | 1.50 |
| H-MSY-P1 | 614.45 | 108.65 | 505.80 | 291.27 | 141.13 | 150.14 | 3.37 | 2.11 |
| H-MSY-PH | 624.03 | 110.39 | 513.65 | 288.90 | 123.37 | 165.52 | 3.10 | 2.16 |
| H-MSY-AP | 514.71 | 131.41 | 383.30 | 202.78 | 116.54 | 86.24 | 4.44 | 2.54 |
| Catalysts | ΔPSA | ΔPSI | ΔPHP | ΔCP | k6′ a |
|---|---|---|---|---|---|
| Pt/SY | 19.65 | −32.42 | −8.28 | 24.39 | 0.48 |
| Pt/MSY-TP | 20.88 | −30.36 | −8.74 | 26.57 | 0.53 |
| Pt/MSY-P1 | 26.24 | −32.20 | −10.84 | 19.13 | 0.61 |
| Pt/MSY-PH | 34.18 | −34.19 | −32.88 | 39.79 | 0.51 |
| Pt/MSY-AP | 35.16 | −31.53 | −30.84 | 34.33 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Niu, X.; Liu, D.; Zhang, K.; Guo, Z.; Qin, Y.; Zhao, W.; Zhang, X.; Wang, Q. Constructing Pt/Hierarchical HY Bifunctional Catalysts for Selective Hydroisomerization of Phenanthrene to Alkyl-Adamantanes. Catalysts 2025, 15, 413. https://doi.org/10.3390/catal15050413
Jiang N, Niu X, Liu D, Zhang K, Guo Z, Qin Y, Zhao W, Zhang X, Wang Q. Constructing Pt/Hierarchical HY Bifunctional Catalysts for Selective Hydroisomerization of Phenanthrene to Alkyl-Adamantanes. Catalysts. 2025; 15(5):413. https://doi.org/10.3390/catal15050413
Chicago/Turabian StyleJiang, Nan, Xiaopo Niu, Danni Liu, Kaige Zhang, Zhen Guo, Yue Qin, Wenli Zhao, Xiangwen Zhang, and Qingfa Wang. 2025. "Constructing Pt/Hierarchical HY Bifunctional Catalysts for Selective Hydroisomerization of Phenanthrene to Alkyl-Adamantanes" Catalysts 15, no. 5: 413. https://doi.org/10.3390/catal15050413
APA StyleJiang, N., Niu, X., Liu, D., Zhang, K., Guo, Z., Qin, Y., Zhao, W., Zhang, X., & Wang, Q. (2025). Constructing Pt/Hierarchical HY Bifunctional Catalysts for Selective Hydroisomerization of Phenanthrene to Alkyl-Adamantanes. Catalysts, 15(5), 413. https://doi.org/10.3390/catal15050413







