Obtention and Products Distribution of Bioliquid from Catalytic Pyrolysis of Tomato Plant Waste
Abstract
1. Introduction
2. Results
2.1. Catalyst Characterization
2.2. Biomass Characterization
2.2.1. Proximate Analysis, Moisture, and Ash Content
2.2.2. Preliminary Results by TGA
2.2.3. Efficiency
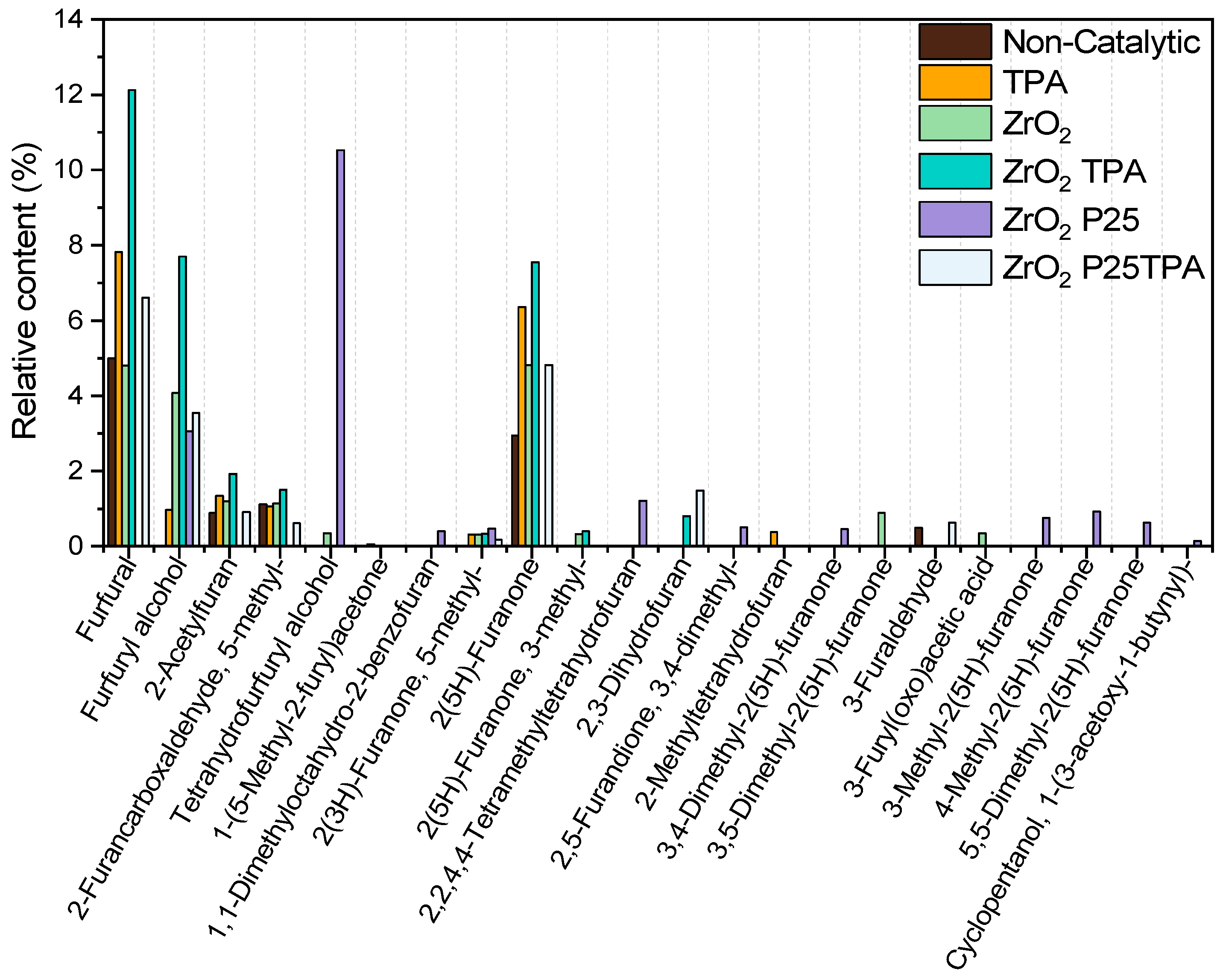
2.2.4. Bioliquid Composition
2.2.5. Correlation Analysis
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Biomass Characterization
3.3.1. Hemicellulose, Cellulose, and Lignin Content
3.3.2. Thermogravimetric Analysis
3.4. Catalytic Pyrolysis
3.5. Stadistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Yang, K.; Tao, Y.; Yang, Q.; Xu, L.; Liu, C.; Ma, L.; Xiao, R. Biomass Directional Pyrolysis Based on Element Economy to Produce High-Quality Fuels, Chemicals, Carbon Materials—A Review. Biotechnol. Adv. 2023, 69, 108262. [Google Scholar] [CrossRef]
- Panchasara, H.; Ashwath, N. Effects of Pyrolysis Bio-Oils on Fuel Atomisation—A Review. Energies 2021, 14, 794. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and Composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Poornima Devi, T.; Sivashanmugam, P.; Kavitha, S.; Yukesh Kannah, R.; Varjani, S.; AdishKumar, S.; Kumar, G.; Rajesh Banu, J. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of Non-Catalytic and Catalytic Fast Pyrolysis of Corncob in a Fluidized Bed Reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Song, M.; Xie, X.; Wei, J.; Xu, D.; Ding, K.; Huang, Y.; Zhang, S.; Hu, X.; Zhang, S.; et al. Oxidative Fast Pyrolysis of Biomass in a Quartz Tube Fluidized Bed Reactor: Effect of Oxygen Equivalence Ratio. Energy 2023, 270, 126987. [Google Scholar] [CrossRef]
- Shao, S.; Liu, C.; Xiang, X.; Li, X.; Zhang, H.; Xiao, R. Insight into the Selective Production of Aldehydes and Ketones by Catalytic Upgrading of Biomass Pyrolysis Vapor over ZrO2: Cellulose and Xylan. Biomass Bioenergy 2022, 162, 106473. [Google Scholar] [CrossRef]
- Onay, Ö.; Beis, S.H.; Koçkar, Ö.M. Fast Pyrolysis of Rape Seed in a Well-Swept Fixed-Bed Reactor. J. Anal. Appl. Pyrolysis 2001, 58–59, 995–1007. [Google Scholar] [CrossRef]
- Behrens, M.; Cross, J.S.; Akasaka, H.; Ohtake, N. A Study of Guaiacol, Cellulose, and Hinoki Wood Pyrolysis with Silica, ZrO2&TiO2 and ZSM-5 Catalysts. J. Anal. Appl. Pyrolysis 2017, 125, 178–184. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Zhang, D.; Wu, Y.; Xie, H.; Brindhadevi, K.; Pugazhendhi, A.; Xia, C. A Review of Biomass Pyrolysis Gas: Forming Mechanisms, Influencing Parameters, and Product Application Upgrades. Fuel 2023, 347, 128461. [Google Scholar] [CrossRef]
- Montaña, M.; Navas, M.B.; Bideberripe, H.P.; Barbelli, M.L.; Lick, I.D.; Casella, M.L. A Heterogeneous Catalytic Process to Mitigate the Acidity of Bio-Oils Caused by the Presence of Volatile Organic Acids. Fuel 2021, 299, 120919. [Google Scholar] [CrossRef]
- Leguizamón Aparicio, M.; Ocsachoque, M.; Gazzoli, D.; Botto, I.; Lick, I. Total Oxidation of Naphthalene with Zirconia-Supported Cobalt, Copper and Nickel Catalysts. Catalysts 2017, 7, 293. [Google Scholar] [CrossRef]
- Grau, J.M.; Vera, C.R.; Parera, J.M. Alternatives for a Better Performance of Pt in SO42−ZrO2 Catalysts for n-Octane Hydroisomerization-Cracking. Selective Adsorption of Pt over Composites of SO42−ZrO2 Mixed or Supported onto Al2O3 and SiO2. Appl. Catal. A Gen. 1998, 172, 311–326. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, C.H.; Suh, Y.W. Higher Brönsted Acidity of WOx/ZrO2 Catalysts Prepared Using a High-Surface-Area Zirconium Oxyhydroxide. Mol. Catal. 2017, 438, 272–279. [Google Scholar] [CrossRef]
- Rivera, T.S.; Sosa, A.; Romanelli, G.P.; Blanco, M.N.; Pizzio, L.R. Tungstophosphoric Acid/Zirconia Composites Prepared by the Sol–Gel Method: An Efficient and Recyclable Green Catalyst for the One-Pot Synthesis of 14-Aryl-14H-Dibenzo[a,j]Xanthenes. Appl. Catal. A General. J. 2012, 443–444, 207–213. [Google Scholar] [CrossRef]
- Gorsd, M.N.; Sosa, A.A.; Frenzel, R.A.; Pizzio, L.R. Synthesis and Characterization of Tungstophosphoric Acid-Modified Mesoporous Sponge-like TUD-1 Materials. J. Solgel Sci. Technol. 2018, 87, 204–215. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Frenzel, R.A.; Soto, E.L.; Pizzio, L.R. Mesoporous Nanospheres of Titania Directly Modified with Tungstosilicic Acid as Catalysts in the Photodegradation of 4-Chlorophenol. In Comprehensive Guide for Mesoporous Materials. Volume 2: Analysis and Functionalization; Aliofkhazraei, M., Ed.; Nova Publishers: New York, NY, USA, 2015; Volume 2, pp. 55–72. [Google Scholar]
- Frenzel, R.; Morales, D.; Romanelli, G.; Sathicq, G.; Blanco, M.; Pizzio, L. Synthesis, Characterization and Catalytic Evaluation of H3PW12O40 Included in Acrylic Acid/Acrylamide Polymer for the Selective Oxidation of Sulfides. J. Mol. Catal. A Chem. 2016, 420, 124–133. [Google Scholar] [CrossRef]
- Fernández-García, M.; Martínez-Arias, A.; Hanson, J.C.; Rodriguez, J.A. Nanostructured Oxides in Chemistry: Characterization and Properties. Chem. Rev. 2004, 104, 4063–4104. [Google Scholar] [CrossRef]
- Morales, D.M.; Frenzel, R.A.; Romanelli, G.P.; Pizzio, L.R. Synthesis and Characterization of Nanoparticulate Silica with Organized Multimodal Porous Structure Impregnated with 12-Phosphotungstic Acid for Its Use in Heterogeneous Catalysis. Mol. Catal. 2020, 481, 110210. [Google Scholar] [CrossRef]
- Kumar Tiwari, J.; Behera, T.K.; Rai, N.; Reddy Yerasu, S.; Singh, M.K.; Mohan Singh, P. Tomato Breeding for Processing in India: Current Status and Prospects. Veg. Sci. 2022, 49, 123–132. [Google Scholar] [CrossRef]
- Zotarelli, L.; Scholberg, J.M.; Dukes, M.D.; Muñoz-Carpena, R.; Icerman, J. Tomato Yield, Biomass Accumulation, Root Distribution and Irrigation Water Use Efficiency on a Sandy Soil, as Affected by Nitrogen Rate and Irrigation Scheduling. Agric. Water Manag. 2009, 96, 23–34. [Google Scholar] [CrossRef]
- Almeida, P.V.; Rodrigues, R.P.; Gaspar, M.C.; Braga, M.E.M.; Quina, M.J. Integrated Management of Residues from Tomato Production: Recovery of Value-Added Compounds and Biogas Production in the Biorefinery Context. J. Environ. Manag. 2021, 299, 113505. [Google Scholar] [CrossRef]
- Producción de Tomate en Argentina. In Evolución del Cultivo Hasta la Temporada 2021/22; Ministerio de Economia Argentina: Buenos Aires, Argentina, 2023.
- Greses, S.; Tomás-Pejó, E.; Gónzalez-Fernández, C. Agroindustrial Waste as a Resource for Volatile Fatty Acids Production via Anaerobic Fermentation. Bioresour. Technol. 2020, 297, 122486. [Google Scholar] [CrossRef]
- Zanón, M.J.; Font, M.I.; Jordá, C. Use of Tomato Crop Residues into Soil for Control of Bacterial Wilt Caused by Ralstonia Solanacearum. Crop Prot. 2011, 30, 1138–1143. [Google Scholar] [CrossRef]
- Zanón, M.J.; Jordá, C. Eradication of Clavibacter michiganensis Subsp. michiganensis by Incorporating Fresh Crop Debris into Soil: Preliminary Evaluations under Controlled Conditions. Crop Prot. 2008, 27, 1511–1518. [Google Scholar] [CrossRef]
- Camargo, F.P.; Rabelo, C.A.B.S.; Duarte, I.C.S.; Silva, E.L.; Varesche, M.B.A. Biogas from Lignocellulosic Feedstock: A Review on the Main Pretreatments, Inocula and Operational Variables Involved in Anaerobic Reactor Efficiency. Int. J. Hydrogen Energy 2023, 48, 20613–20632. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Mariyam, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G.; Parthasarathy, P. Pyrolysis Valorization of Vegetable Wastes: Thermal, Kinetic, Thermodynamics, and Pyrogas Analyses. Energies 2022, 15, 6277. [Google Scholar] [CrossRef]
- Saffari, N.; Hajabbasi, M.A.; Shirani, H.; Mosaddeghi, M.R.; Mamedov, A.I. Biochar Type and Pyrolysis Temperature Effects on Soil Quality Indicators and Structural Stability. J. Environ. Manag. 2020, 261, 110190. [Google Scholar] [CrossRef]
- Hassan, M.; Wang, B.; Wu, P.; Wang, S. Engineered Biochar for In-Situ and Ex-Situ Remediation of Contaminants from Soil and Water. Sci. Total Environ. 2024, 957, 177384. [Google Scholar] [CrossRef]
- Gasim, M.F.; Choong, Z.Y.; Koo, P.L.; Low, S.C.; Abdurahman, M.H.; Ho, Y.C.; Mohamad, M.; Suryawan, I.W.K.; Lim, J.W.; Oh, W.-D. Application of Biochar as Functional Material for Remediation of Organic Pollutants in Water: An Overview. Catalysts 2022, 12, 210. [Google Scholar] [CrossRef]
- Font, R.; Moltó, J.; Gá Lvez, A.; Rey, M.D. Kinetic Study of the Pyrolysis and Combustion of Tomato Plant. J. Anal. Appl. Pyrolysis 2008, 85, 268–275. [Google Scholar] [CrossRef]
- Prasad, K.M.; Murugavelh, S. Experimental Investigation and Kinetics of Tomato Peel Pyrolysis: Performance, Combustion and Emission Characteristics of Bio-Oil Blends in Diesel Engine. J. Clean. Prod. 2020, 254, 120115. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Li, B.; Zhang, L.; Zhang, S.; Huang, Y.; Li, B.; Wang, S.; Li, X.; Hu, X. Volatiles from Pyrolysis of Wet or Dry Tomato Leaves Make a Drastic Difference in Activation of Sawdust-Derived Biochar. Renew. Energy 2024, 223, 120052. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Elhassan, O.; Parthasarathy, P.; Mackey, H.; Al-Ansari, T.; McKay, G. Thermogravimetric Analysis of Individual Food Waste Items and Their Blends for Biochar Production. Comput. Aided Chem. Eng. 2020, 48, 1543–1548. [Google Scholar]
- Ozbay, N.; Yargic, A.S.; Yarbay Sahin, R.Z. Tailoring Cu/Al2O3 Catalysts for the Catalytic Pyrolysis of Tomato Waste. J. Energy Inst. 2018, 91, 424–433. [Google Scholar] [CrossRef]
- Özbay, N.; Yargiç, A.S.; Yarbay Şahin, R.Z.; Yaman, E. Research on the Pyrolysis Characteristics of Tomato Waste with Fe-Al2O3 Catalyst. In Exergetic, Energetic and Environmental Dimensions; Academic Press: Cambridge, MA, USA, 2018; pp. 815–828. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. IUPAC Technical Report Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution. Pure Appl. Chem. 2015, 87, 9–10. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Cáceres, C.V.; Blanco, M.N. Adsorption of Tungstophosphoric or Tungstosilicic Acids from Ethanol–Water Solutions on Carbon. J. Colloid. Interface Sci. 1997, 190, 318–326. [Google Scholar] [CrossRef]
- Patel, S.; Purohit, N.; Patel, A. Synthesis, Characterization and Catalytic Activity of New Solid Acid Catalysts, H3PW12O40 Supported on to Hydrous Zirconia. J. Mol. Catal. A Chem. 2003, 192, 195–202. [Google Scholar] [CrossRef]
- Rubio, J.; Oteo, J.L.; Villegas, M.; Duran, P. Characterization and Sintering Behaviour of Submicrometre Titanium Dioxide Spherical Particles Obtained by Gas-Phase Hydrolysis of Titanium Tetrabutoxide. J. Mater. Sci. 1997, 32, 643–652. [Google Scholar] [CrossRef]
- Van Veen, J.A.R.; Veltmaat, F.T.G.; Jonkers, G. A Method for the Quantitative Determination of the Basic, Acidic, and Total Surface Hydroxy Content of TiO2. J. Chem. Soc. Chem. Commun. 1985, 23, 1656–1658. [Google Scholar] [CrossRef]
- Su, Y.L.; Wang, J.; Liu, H.Z. FTIR Spectroscopic Study on Effects of Temperature and Polymer Composition on the Structural Properties of PEO-PPO-PEO Block Copolymer Micelles. Langmuir 2002, 18, 5370–5374. [Google Scholar] [CrossRef]
- Fuchs, V.; Méndez, L.; Blanco, M.; Pizzio, L. Mesoporous Titania Directly Modified with Tungstophosphoric Acid: Synthesis, Characterization and Catalytic Evaluation. Appl. Catal. A Gen. 2009, 358, 73–78. [Google Scholar] [CrossRef]
- Pérez-Obando, J.; Marín-Silva, D.A.; Pinotti, A.N.; Pizzio, L.R.; Osorio-Vargas, P.; Rengifo-Herrera, J.A. Degradation Study of Malachite Green on Chitosan Films Containing Heterojunctions of Melon/TiO2 Absorbing Visible-Light in Solid-Gas Interfaces. Appl. Catal. B 2019, 244, 773–785. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Caceres, C.V.; Blanco, M.N. Equilibrium Adsorption of 11-Tungstophosphate Anion on Different Supports. Appl. Surf. Sci. 1999, 151, 91–101. [Google Scholar] [CrossRef]
- Gorsd, M.; Pizzio, L.; Blanco, M. Trifluoromethanesulfonic Acid Immobilized on Zirconium Oxide Obtained by the Sol-Gel Method as Catalyst in Paraben Synthesis. Appl. Catal. A Gen. 2011, 400, 91–98. [Google Scholar] [CrossRef]
- Pope, M.T. 1933-Heteropoly and Isopoly Oxometalates, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1983; ISBN 9783662120064. [Google Scholar]
- Fuchs, V.M.; Pizzio, L.R.; Blanco, M.N. Synthesis and Characterization of Aluminum or Copper Tungstophosphate and Tungstosilicate Immobilized in a Polymeric Blend. Eur. Polym. J. 2007, 44, 801–807. [Google Scholar] [CrossRef]
- Cid, R.; Pecchi, G. Potentiometric Method for Determining the Number and Relative Strength of Acid Sites in Colored Catalysts. Appl. Catal. 1985, 14, 15–21. [Google Scholar] [CrossRef]
- Legagneux, N.; Basset, J.M.; Thomas, A.; Lefebvre, F.; Goguet, A.; Sá, J.; Hardacre, C. Characterization of Silica-Supported Dodecatungstic Heteropolyacids as a Function of Their Dehydroxylation Temperature. Dalton Trans. 2009, 12, 2235–2240. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Tsekos, C.; Retschitzegger, S.; Zimbardi, F.; Funke, A.; Banks, S.; Kraia, T.; Marques, P.; Scharler, R.; de Jong, W.; et al. Biomass Pyrolysis TGA Assessment with an International Round Robin. Fuel 2020, 276, 118002. [Google Scholar] [CrossRef]
- Teixeira, M.G.; de Paiva Silva Pereira, S.; Fernandes, S.A.; José Da Silva, M. Enhancement of Levoglucosan Production via Fast Pyrolysis of Sugarcane Bagasse by Pretreatment with Keggin Heteropolyacids. Ind. Crops Prod. 2020, 154, 112680. [Google Scholar] [CrossRef]
- Saynik, P.B.; Moholkar, V.S. Investigations in Influence of Different Pretreatments on A. Donax Pyrolysis: Trends in Product Yield, Distribution and Chemical Composition. J. Anal. Appl. Pyrolysis 2021, 158, 105276. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. Kinetics and Evolved Gas Analysis for Pyrolysis of Food Processing Wastes Using TGA/MS/FT-IR. Waste Manag. 2017, 64, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zolghadr, A.; Kulas, D.; Shonnard, D. Evaluation of Pyrolysis Wax as a Solvent in Polyolefin Pyrolysis Processing. Ind. Eng. Chem. Res. 2022, 61, 11080–11088. [Google Scholar] [CrossRef]
- Hernando, H.; Fermoso, J.; Ochoa-Hernández, C.; Opanasenko, M.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Performance of MCM-22 Zeolite for the Catalytic Fast-Pyrolysis of Acid-Washed Wheat Straw. Catal. Today 2018, 304, 30–38. [Google Scholar] [CrossRef]
- Li, Y.; Hu, B.; Naqvi, S.R.; Zhang, Z.-X.; Li, K.; Lu, Q. Selective Preparation of 5-Hydroxymethylfurfural by Catalytic Fast Pyrolysis of Cellulose over Zirconium-Tin Mixed Metal Oxides. J. Anal. Appl. Pyrolysis 2021, 155, 105103. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Xiang, S.; Wang, Y. Effect of Pd Loading on ZrO2 Support Resulting from Pyrolysis of UiO-66: Application to CO Oxidation. J. Colloid. Interface Sci. 2020, 573, 11–20. [Google Scholar] [CrossRef]
- Zou, Q.; He, H.; Xie, J.; Han, S.; Lin, W.; Mondal, A.K.; Huang, F. Study on the Mechanism of Acid Modified H-Beta Zeolite Acidic Sites on the Catalytic Pyrolysis of Kraft Lignin. Chem. Eng. J. 2023, 462, 142029. [Google Scholar] [CrossRef]
- Inayat, A.; Inayat, A.; Schwieger, W.; Sokolova, B.; Lestinsky, P. Enhancing Aromatics and Olefins Yields in Thermo-Catalytic Pyrolysis of LDPE over Zeolites: Role of Staged Catalysis and Acid Site Density of HZSM-5. Fuel 2022, 314, 123071. [Google Scholar] [CrossRef]
- Yang, H.; Han, T.; Yang, W.; Sandström, L.; Jönsson, P.G. Influence of the Porosity and Acidic Properties of Aluminosilicate Catalysts on Coke Formation during the Catalytic Pyrolysis of Lignin. J. Anal. Appl. Pyrolysis 2022, 165, 105536. [Google Scholar] [CrossRef]
- de Rezende Locatel, W. Valorization of Waste Biomass by Catalytic Pyrolysis. Ph.D. Thesis, Université de Lyon, Lyon, France, 2022. [Google Scholar]
- Musci, J.J.; Casoni, A.I.; Gutiérrez, V.S.; Ocsachoque, M.A.; Merlo, A.B.; Volpe, M.A.; Lick, I.D.; Casella, M.L. Upgrading of Tall Fescue Grass Pyrolytic Bioliquid and Catalytic Valorization of The Biofurfural Obtained. ChemistrySelect 2022, 7, e202202233. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Gao, A.; Ding, K.; Xing, X.; Wei, J.; Huang, Y.; Chun-Ho Lam, J.; Subramanian, K.A.; Zhang, S. Volatile-Char Interactions during Biomass Pyrolysis: Effect of Biomass Acid-Washing Pretreatment. Fuel 2023, 340, 127496. [Google Scholar] [CrossRef]
- Oh, S.-J.; Choi, G.-G.; Kim, J.-S. Production of Acetic Acid-Rich Bio-Oils from the Fast Pyrolysis of Biomass and Synthesis of Calcium Magnesium Acetate Deicer. J. Anal. Appl. Pyrolysis 2017, 124, 122–129. [Google Scholar] [CrossRef]
- Silveira Junior, E.G.; Silveira, T.d.C.; Perez, V.H.; Justo, O.R.; David, G.F.; Fernandes, S.A. Fast Pyrolysis of Elephant Grass: Intensification of Levoglucosan Yield and Other Value-Added Pyrolytic by-Products. J. Energy Inst. 2022, 101, 254–264. [Google Scholar] [CrossRef]
- Casoni, A.I.; Nievas, M.L.; Moyano, E.L.; Álvarez, M.; Diez, A.; Dennehy, M.; Volpe, M.A. Catalytic Pyrolysis of Cellulose Using MCM-41 Type Catalysts. Appl. Catal. A Gen. 2016, 514, 235–240. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of Lignin for Phenols with Alkaline Additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Jeguirim, M.; Khiari, B. Thermochemical Conversion of Tomato Wastes. In Tomato Processing by-Products: Sustainable Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 285–332. [Google Scholar]
- Wang, Q.; Song, H.; Pan, S.; Dong, N.; Wang, X.; Sun, S. Initial Pyrolysis Mechanism and Product Formation of Cellulose: An Experimental and Density Functional Theory (DFT) Study. Sci. Rep. 2020, 10, 3626. [Google Scholar] [CrossRef]
- Arora, J.S.; Ansari, K.B.; Chew, J.W.; Dauenhauer, P.J.; Mushrif, S.H. Unravelling the Catalytic Influence of Naturally Occurring Salts on Biomass Pyrolysis Chemistry Using Glucose as a Model Compound: A Combined Experimental and DFT Study. Catal. Sci. Technol. 2019, 9, 3504–3524. [Google Scholar] [CrossRef]
- Moldoveanu, S.C.; David, V. Chemical Degradation of Polymers and Pyrolysis. In Sample Preparation in Chromatography; Elsevier: Amsterdam, The Netherlands, 2002; Volume 65, pp. 847–917. [Google Scholar]
- Vinu, R.; Broadbelt, L.J. A Mechanistic Model of Fast Pyrolysis of Glucose-Based Carbohydrates to Predict Bio-Oil Composition. Energy Environ. Sci. 2012, 5, 9808–9826. [Google Scholar] [CrossRef]
- Wang, S.; Ru, B.; Lin, H.; Luo, Z. Degradation Mechanism of Monosaccharides and Xylan under Pyrolytic Conditions with Theoretic Modeling on the Energy Profiles. Bioresour. Technol. 2013, 143, 378–383. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S. The Mechanism for Thermal Decomposition of Cellulose and Its Main Products. Bioresour. Technol. 2009, 100, 6496–6504. [Google Scholar] [CrossRef] [PubMed]
- Mante, O.D.; Rodriguez, J.A.; Senanayake, S.D.; Babu, S.P. Catalytic Conversion of Biomass Pyrolysis Vapors into Hydrocarbon Fuel Precursors. Green. Chem. 2015, 17, 2362–2368. [Google Scholar] [CrossRef]
- Banerjee, A.; Mushrif, S.H. Reaction Pathways for the Deoxygenation of Biomass-Pyrolysis-Derived Bio-Oil on Ru: A DFT Study Using Furfural as a Model Compound. ChemCatChem 2017, 9, 2828–2838. [Google Scholar] [CrossRef]
- Norouzi, O.; Taghavi, S.; Arku, P.; Jafarian, S.; Signoretto, M.; Dutta, A. What Is the Best Catalyst for Biomass Pyrolysis? J. Anal. Appl. Pyrolysis 2021, 158, 105280. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Fang, Z.; Zhao, Z.L.; Zheng, A.Q.; Wang, X.B.; Li, H. Bin Levoglucosan and Its Hydrolysates via Fast Pyrolysis of Lignocellulose for Microbial Biofuels: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2019, 105, 215–229. [Google Scholar] [CrossRef]
- Chang, S.; Zhao, Z.; Zheng, A.; Li, X.; Wang, X.; Huang, Z.; He, F.; Li, H. Effect of Hydrothermal Pretreatment on Properties of Bio-Oil Produced from Fast Pyrolysis of Eucalyptus Wood in a Fluidized Bed Reactor. Bioresour. Technol. 2013, 138, 321–328. [Google Scholar] [CrossRef]
- Chen, Z.F.; Zhao, J.M.; Li, H.L.; Chen, H.Y.; Xi, G.L.; Zhang, L.K.; Wang, Q.F.; Wang, Q.L.; Zhao, Y.Z.; Yang, K. Highly Efficient Multigram Synthesis of 1,4:3,6-Dianhydro-α-D-Glucopyranose by Chemical Synthesis Methods: Development and Scale-up Study of Synthetic Routes. Carbohydr. Res. 2025, 551, 109402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ning, Y.; Li, L.; Cao, X.; Xi, G.; Cheng, D.; Wang, Q.; Lu, C.; Yang, K. Production of 1,4:3,6-Dianhydro-α-Dglucopyranose from Methyl 3,6-Anhydro-α-Dglucopyranoside and Its Taste Identification. RSC Adv. 2025, 15, 4481–4486. [Google Scholar] [CrossRef]
- Hu, B.; Lu, Q.; Jiang, X.; Dong, X.; Cui, M.; Dong, C.; Yang, Y. Pyrolysis Mechanism of Glucose and Mannose: The Formation of 5-Hydroxymethyl Furfural and Furfural. J. Energy Chem. 2018, 27, 486–501. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Wang, S.; Wu, Y. Recent Advances in Aqueous-Phase Catalytic Conversions of Biomass Platform Chemicals Over Heterogeneous Catalysts. Front. Chem. 2020, 7, 948. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C 2009, 113, 20097–20107. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L. A Review on Selective Production of Value-Added Chemicals via Catalytic Pyrolysis of Lignocellulosic Biomass. Sci. Total Environ. 2020, 749, 142386. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.B.T.L.; Wu, T.Y. A Review on Solvent Systems for Furfural Production from Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2021, 137, 110172. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Lv, P.; Bie, N.; Cao, P.; Bai, Y.; Song, X.; Yu, G. Capture of Released Alkali Metals and Its Simultaneously Catalytic Performance on Secondary Reactions of Volatiles during Biomass Pyrolysis. Fuel 2022, 317, 123557. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S. Analytical Pyrolysis Studies of Corn Stalk and Its Three Main Components by TG-MS and Py-GC/MS. J. Anal. Appl. Pyrolysis 2012, 97, 11–18. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Lu, M.; Hu, X.; Lu, L.; Tian, X.; Ji, J. Role of Brønsted Acid in Selective Production of Furfural in Biomass Pyrolysis. Bioresour. Technol. 2014, 169, 800–803. [Google Scholar] [CrossRef]
- Paine, J.B.; Pithawalla, Y.B.; Naworal, J.D. Carbohydrate Pyrolysis Mechanisms from Isotopic Labeling. Part 4. The Pyrolysis of d-Glucose: The Formation of Furans. J. Anal. Appl. Pyrolysis 2008, 83, 37–63. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Rodríguez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Guo, H.; Qiu, M.; Qi, X. Efficient Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Zr-Doped Ordered Mesoporous Carbon Synthesized by Zr-Arbutin Coordinated Self-Assembly. Fuel 2023, 331, 125834. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Yang, S.; Wei, J.; He, L.; Peng, L.; Tang, X.; Ni, Y. Highly Selective Conversion of Furfural to Furfural Alcohol or Levulinate Ester in One Pot over ZrO2@SBA-15 and Its Kinetic Behavior. ACS Sustain. Chem. Eng. 2020, 8, 5584–5594. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, L.; Ke, C.; Fan, G.; Yang, L.; Li, F. Highly Efficient Catalytic Transfer Hydrogenation of Furfural over Defect-Rich Amphoteric ZrO2 with Abundant Surface Acid–Base Sites. Dalton Trans. 2021, 50, 2616–2626. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, S.; Alagarasan, J.K.; Yadav, A.K.; Ali, W.; Lee, M.; Khan, M.E.; Ali, S.K.; Bashiri, A.H.; Zakri, W.; Balu, K. Highly Selective Catalytic Transfer Hydrogenation of Biomass Derived Furfural to Furfural Alcohol over Zr/SBA-15 Catalysts. J. Phys. Chem. Solids 2024, 186, 111831. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Y.; Zhang, Y.; Liu, C.; Wang, Y.; Xie, Y.; Ji, G.; Li, A. Fast Pyrolysis of Biomass with Diverse Properties to Produce Liquid Hydrogen Storage Molecules. Carbon. Capture Sci. Technol. 2024, 12, 100230. [Google Scholar] [CrossRef]
- Razzak, S.A.; Khan, M.; Irfan, F.; Shah, M.A.; Nawaz, A.; Hossain, M.M. Catalytic Co-Pyrolysis and Kinetic Study of Microalgae Biomass with Solid Waste Feedstock for Sustainable Biofuel Production. J. Anal. Appl. Pyrolysis 2024, 183, 106755. [Google Scholar] [CrossRef]
- Hu, B.; Lu, Q.; Jiang, X.Y.; Liu, J.; Cui, M.S.; Dong, C.Q.; Yang, Y.P. Formation Mechanism of Hydroxyacetone in Glucose Pyrolysis: A Combined Experimental and Theoretical Study. Proc. Combust. Inst. 2019, 37, 2741–2748. [Google Scholar] [CrossRef]
- Batista, R.R.; Gomes, M.M. Effects of Chemical Composition and Pyrolysis Process Variables on Biochar Yields: Correlation and Principal Component Analysis. Floresta E Ambiente 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Guo, G.; Huang, Q.; Jin, F.; Lin, L.; Wang, Q.; Fu, Q.; Liu, Y.; Sajjad, M.; Wang, J.; Liao, Z.; et al. Exploration of the Interrelationship within Biomass Pyrolysis Liquid Composition Based on Multivariate Analysis. Molecules 2022, 27, 5656. [Google Scholar] [CrossRef]
- Holtzapple, M.T. CELLULOSE. In Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003; pp. 998–1007. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis: Apparatus, Reagents, Procedures and Some Applications; Agriculture handbook; U.S.D.A. Agricultural Research Service: Washington, DC, USA, 1970; Volume 379.
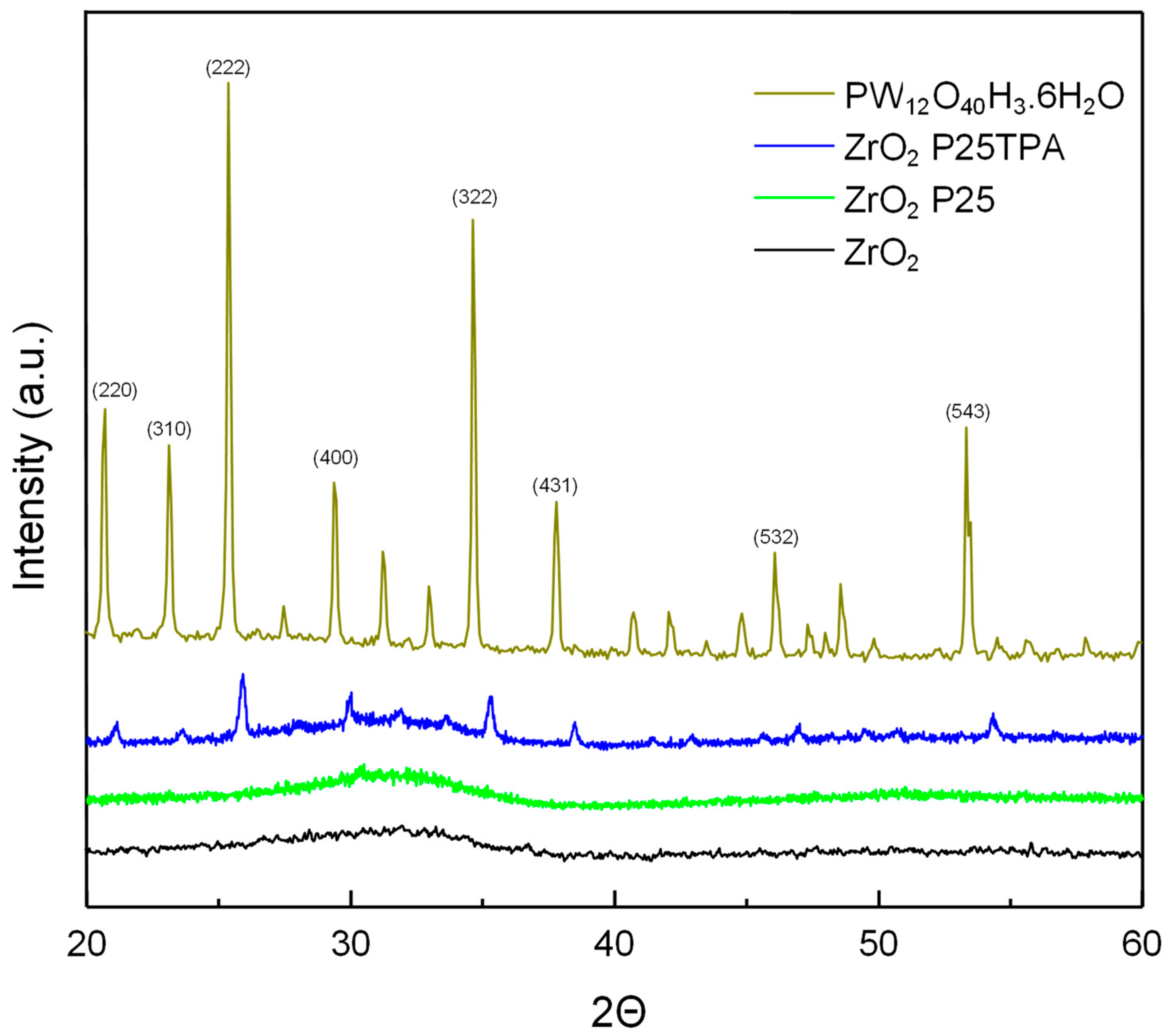
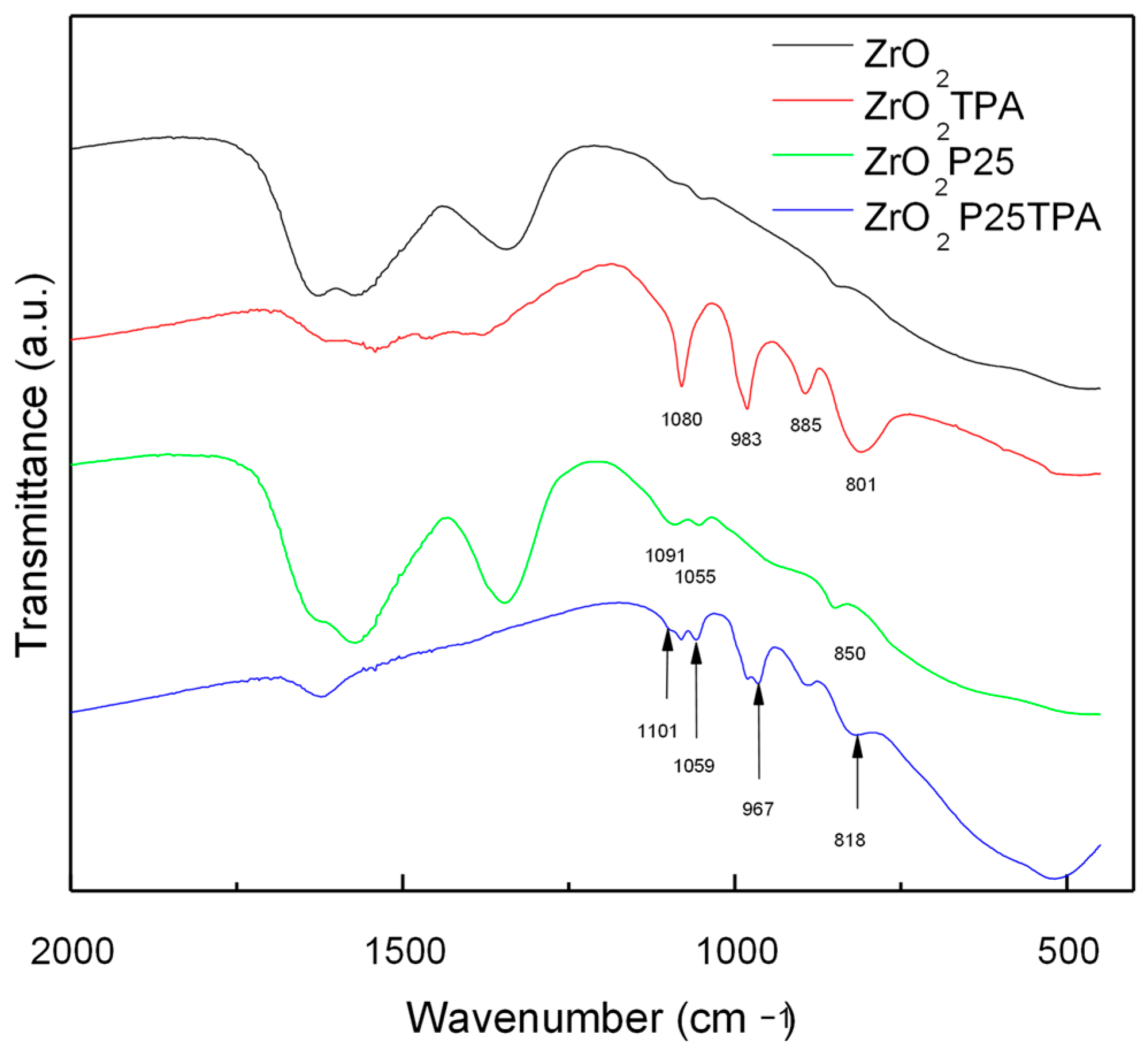

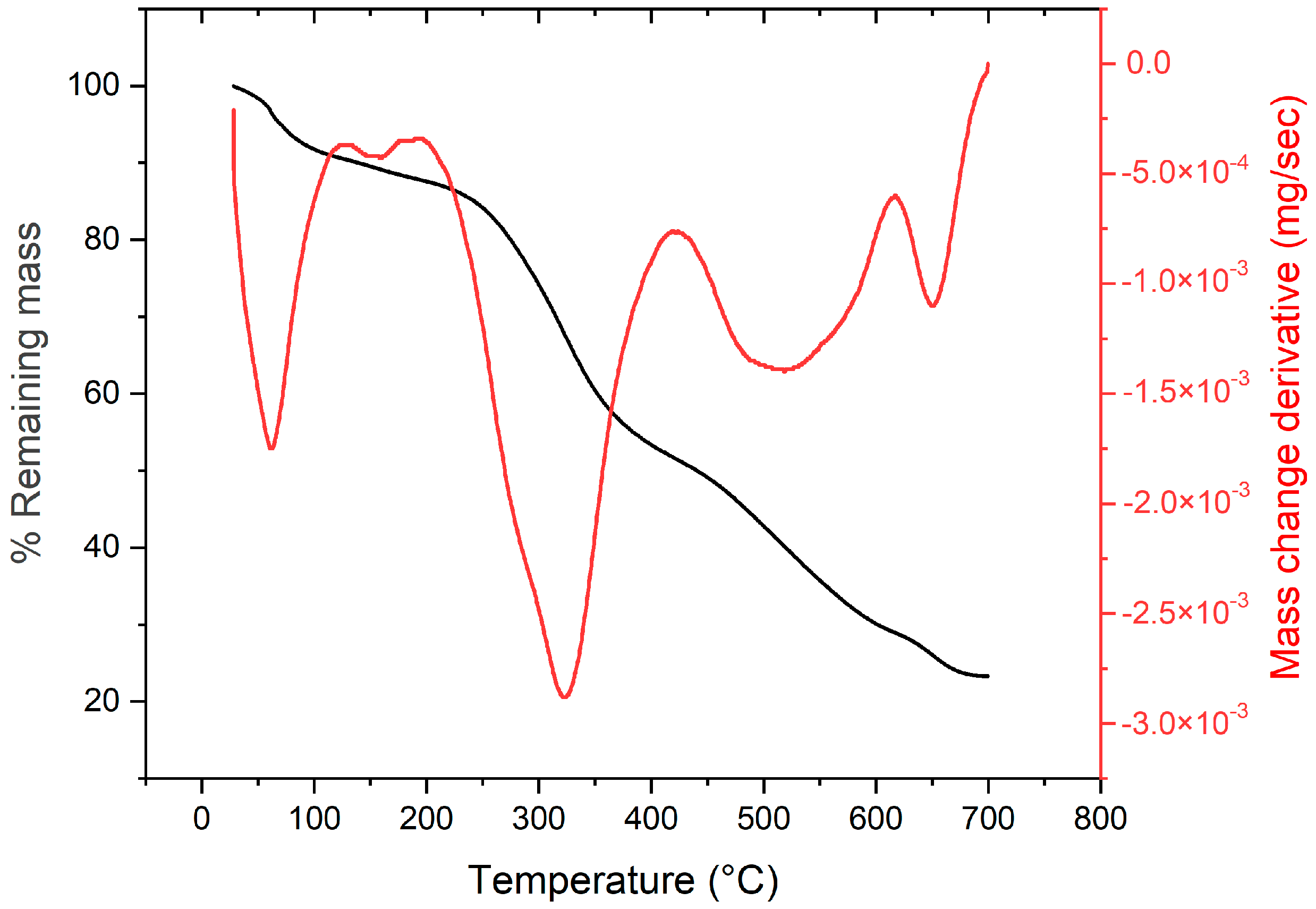


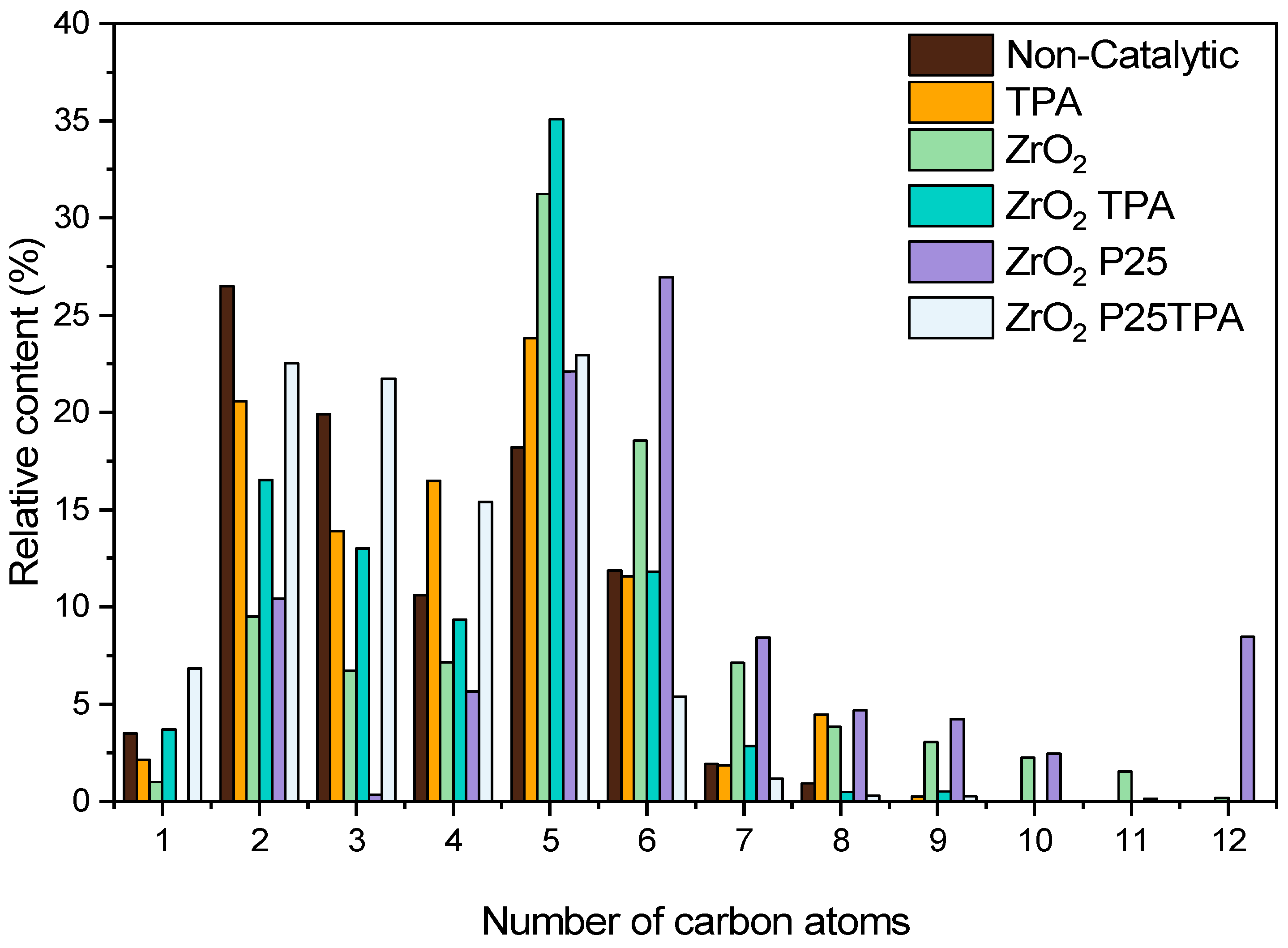


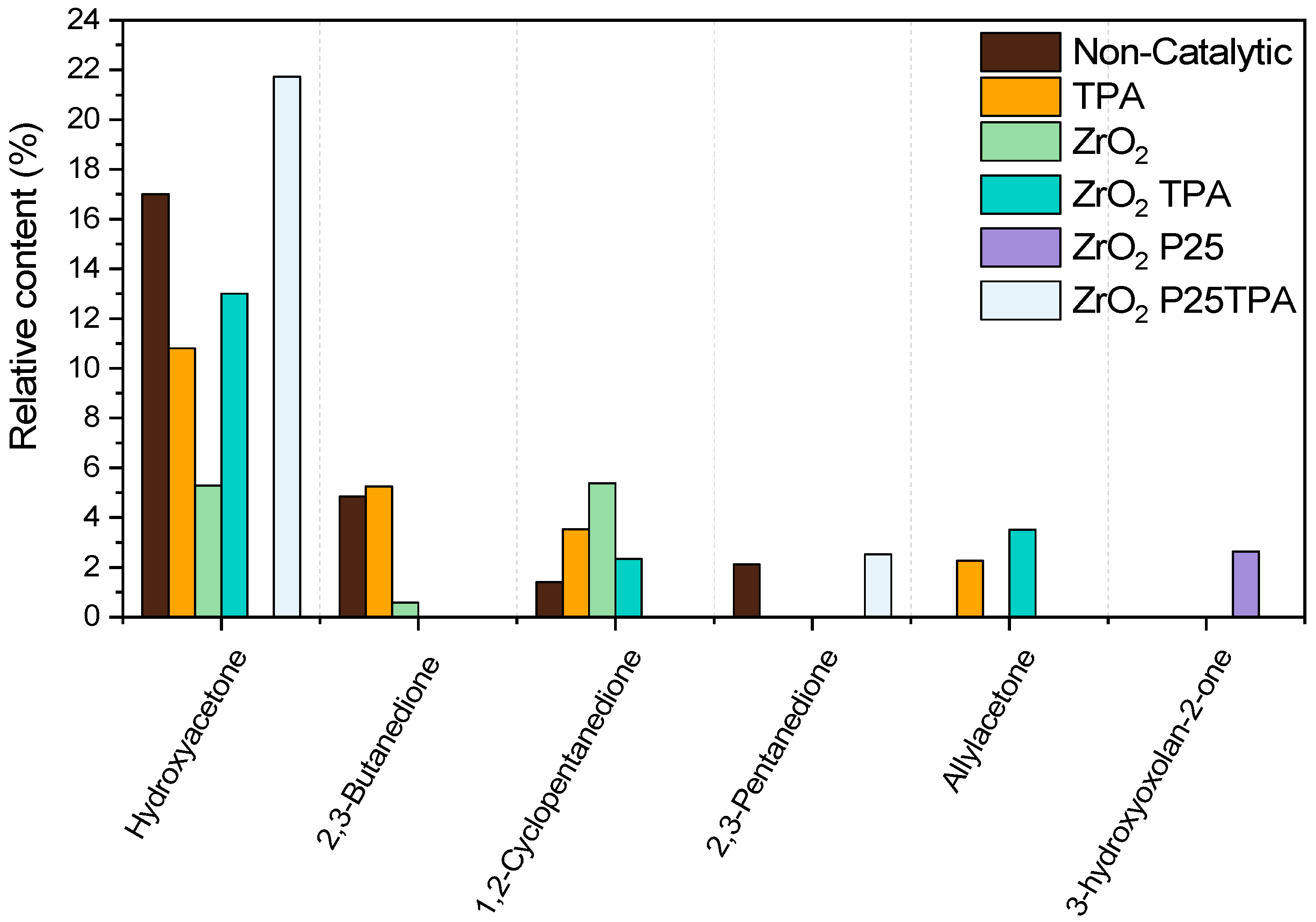


| Samples | SBET (m2/g) | SMIC (m2/g) | SMESO (m2/g) | Dp (nm) | Vp * (cm3/g) |
|---|---|---|---|---|---|
| ZrO2 | 213 | 162 | 51 | 5.4 | 0.136 |
| ZrO2TPA | 87 | 25 | 62 | 6.9 | 0.094 |
| ZrO2P25 * | 192 | 15 | 177 | 3.6 | 0.178 |
| ZrO2P25TPA * | 90 | 6 | 84 | 5.0 | 0.088 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buitrago, J.L.; Méndez, L.J.; Musci, J.J.; Cecilia, J.A.; Ballesteros-Plata, D.; Rodríguez-Castellón, E.; Casella, M.L.; Pizzio, L.R.; Lick, I.D. Obtention and Products Distribution of Bioliquid from Catalytic Pyrolysis of Tomato Plant Waste. Catalysts 2025, 15, 388. https://doi.org/10.3390/catal15040388
Buitrago JL, Méndez LJ, Musci JJ, Cecilia JA, Ballesteros-Plata D, Rodríguez-Castellón E, Casella ML, Pizzio LR, Lick ID. Obtention and Products Distribution of Bioliquid from Catalytic Pyrolysis of Tomato Plant Waste. Catalysts. 2025; 15(4):388. https://doi.org/10.3390/catal15040388
Chicago/Turabian StyleBuitrago, José L., Leticia J. Méndez, Juan J. Musci, Juan A. Cecilia, Daniel Ballesteros-Plata, Enrique Rodríguez-Castellón, Mónica L. Casella, Luis R. Pizzio, and Ileana D. Lick. 2025. "Obtention and Products Distribution of Bioliquid from Catalytic Pyrolysis of Tomato Plant Waste" Catalysts 15, no. 4: 388. https://doi.org/10.3390/catal15040388
APA StyleBuitrago, J. L., Méndez, L. J., Musci, J. J., Cecilia, J. A., Ballesteros-Plata, D., Rodríguez-Castellón, E., Casella, M. L., Pizzio, L. R., & Lick, I. D. (2025). Obtention and Products Distribution of Bioliquid from Catalytic Pyrolysis of Tomato Plant Waste. Catalysts, 15(4), 388. https://doi.org/10.3390/catal15040388











