Abstract
Biomass, as a renewable carbon resource, holds broad application prospects. Among various bio-based platform molecules, furan derivatives play a significant role in green chemical production. Notably, the conversion of 2-methylfuran (2-MF) to 3-acetyl-1-propanol (3-AP) over bifunctional catalysts has attracted considerable interest. In this study, a Pd@PHZSM-5 catalyst was prepared by encapsulating Pd nanoparticles within P-doped HZSM-5 for 2-MF conversion. The encapsulation improved Pd dispersion and metal–acid synergy, enhancing both catalytic activity and 3-AP selectivity. Additionally, phosphorus doping increased HZSM-5 crystallinity, resulting in excellent stability. This work provides a feasible strategy for optimizing metal–acid cooperation, offering theoretical guidance for bifunctional catalysis and biomass valorization.
1. Introduction
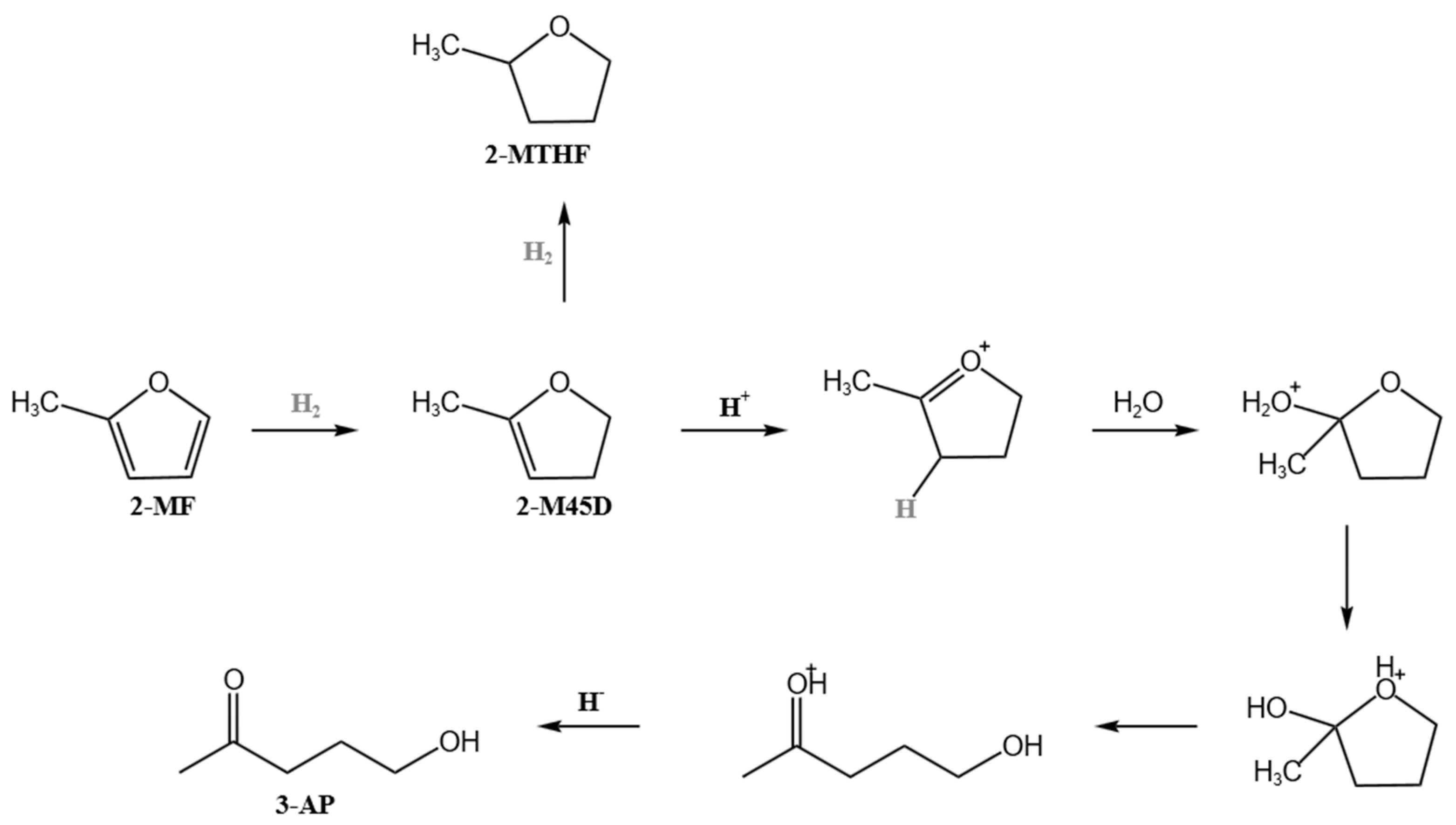
Biomass has attracted worldwide interest as an alternative to fossil fuel-derived energy sources, particularly for liquid fuel production [1]. As well as the production of bioenergy and biofuels, biomass has been employed for the production of specific biomass-derived chemicals, which are called biomass-derived platform chemicals [2,3,4,5,6,7,8]. Biomass-derived platform chemicals, due to their highly functionalized structures, have great potential in catalytic conversion and are considered one of the most effective alternatives for fossil fuel-based energy. Among them, furan and its derivatives, as key platform chemicals derived from hemicellulose and cellulose, have broad application potential [8]. 3-acetyl-1-propanol (referred to as 3-AP) plays a crucial role in the synthesis of drugs, pesticides, and fungicides. It is mainly used to prepare antimalarial drugs, such as chloroquine hydrochloride and chloroquine phosphate, and it can also be used to produce other pharmaceutical products, such as vitamin B1. Industrially, 3-AP can be synthesized via bifunctional catalytic ring-opening and hydrogenation of furfural or 2-methylfuran. Pirmorad et al. [9] employed a Pd-TiO2/CACM catalyst for the continuous production of 3-acetyl-1-propanol from furfural, achieving a conversion rate of 76% and a yield of approximately 39%. Liu et al. [10] used a Ru/C catalyst to convert furfural into 3-acetyl-1-propanol, with a conversion rate for furfural of 67% and a yield of 3-AP of 10%. However, due to the inherent instability of furfural, the ideal yield can only be achieved under low substrate concentration conditions, which restricts its application in the industrial field. As early as the 1930s, some companies began the industrial production of the conversion of 2-methylfuran to 3-AP [11,12]. The reaction proceeds as follows (Scheme 1) [13,14,15,16,17]: first, 2-methylfuran (2-MF) undergoes a hydrogenation reaction, and its chemical structure changes to the form 2-methyl-4,5-dihydrofuran (referred to as 2M45D). This step is a hydrogenation process in which the furan ring section of 2-MF is reduced by hydrogen to form the saturated 2M45D, and Pd/C is often chosen industrially as a hydrogenation catalyst. Then, under acidic conditions, the 2M45D generated in the previous step further undergoes a hydrolysis reaction and is converted into 3-AP. The hydrolysis reaction typically requires HCl, but the acidic conditions lead to Pd/C catalyst deactivation due to metal leaching under hydrothermal conditions.
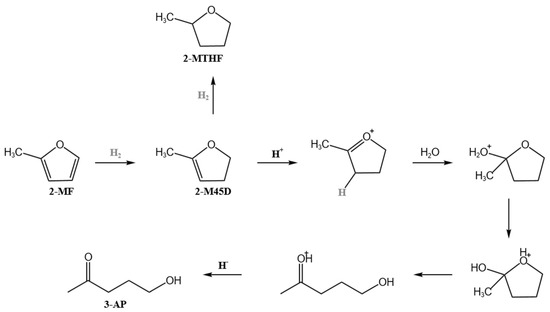
Scheme 1.
The reaction mechanism for the synthesis of 3-AP.
Heterogeneous bifunctional catalysts have been previously used for the conversion of 2-methylfuran to 3-acetyl-1-propanol, mainly Pd supported on acidic supports [17]. The application of acidic support, rather than the liquid inorganic acid used industrially, significantly reduces the corrosiveness of the reaction medium, thereby decreasing equipment requirements [18,19]. Carefully adjusting the matching degree between the metal and the acid sites is important for catalytic conversion [20,21]. However, the effect of acid strength and acid type on the reaction remains unclear due to the difficulties of acid modulation. In addition, the stability of the catalysts is unsatisfactory due to the aggregation of the metal nanoparticles under hydrothermal conditions. Therefore, controlling acid strength and type without changing the type of metal used and improving the recyclability of the catalysts are the main goals in the development of new bifunctional catalysts.
Molecular sieves, as a porous material, contain abundant acid sites. These acid sites mainly come from aluminum atoms or other acidic metal ions in the molecular sieve framework. They significantly influence reactant molecules through adsorption and catalytic effects. In the fields of chemistry and catalysis, the acid sites provided by molecular sieves can promote a series of chemical reactions, such as cracking, isomerization, and alkylation, thereby enabling the effective conversion and selective control of the target product [22,23,24,25,26]. The microporous structure of zeolite can effectively limit the size of metal particles, thereby enhancing their unsaturated coordination environment and significantly enhancing the activity of metal sites, which is of great significance in many application fields [27,28,29,30,31,32].
Compared with the metal/zeolite catalysts prepared by the traditional impregnation method, a metal@zeolite material has been developed to enhance the cooperation of the bifunctional sites and improve the stability of the catalysts by avoiding the agglomeration and sintering of metal particles through a confined effect. Sun et al. successfully encapsulated single-atom rhodium in zeolite using hydrothermal synthesis for efficient hydrogen generation and the shape-selective tandem hydrogenation of nitroaromatics, demonstrating the potential of this synthetic method for the preparation of highly active and selective catalysts [33]. The prepared zeolite-encapsulated sub-nanometer Pt-Zn bimetallic clusters exhibited excellent activity and stability in the propane dehydrogenation reaction, which significantly improved the propylene selectivity [34]. Liu et al. synthesized sub-nanometer Pt@zeolite composites with high stability through the transformation process from two-dimensional zeolite to three-dimensional zeolite, which provides a new way to regulate the dispersion and stability of metals in zeolites [35]. Wang et al. demonstrated that metal@zeolite composites enable direct syngas-to-ethanol conversion, which provides a new direction for the efficient utilization of syngas, and that the process exhibits good catalytic activity and selectivity [36]. Therefore, metal@zeolite catalysts could be effectively used for the conversion of 2-MF into 3-AP with good stability, which would promote the industrial application of 3-AP. In addition, some studies have found that the introduction of P elements into HZSM-5 zeolites can significantly modulate the acidic site distribution and zeolite structure of the catalysts, thus improving the performance of the catalysts [37,38]. Therefore, it would be meaningful to prepare metal@zeolite catalysts with the addition of P for the catalytic conversion of 2-MF into 3-AP.
In this study, a novel bifunctional catalyst (Pd@PxHZSM-5) was prepared using steam-assisted milling, which showed good catalytic performance for the conversion of 2-MF into 3-AP. The surface states and structural properties were studied using various characterization techniques, including X-ray diffraction and infrared spectroscopy, to investigate the effect of encapsulation and P doping on the active site. Additionally, the relationship between the structure and electronic properties of the catalysts and their catalytic performance was studied. Pd@P1/5HZSM-5 showed excellent stability with good crystallinity that was retained even after 15 cycles, which makes it suitable for further industrial use. This study provides a new idea for the design of bifunctional catalysts through the combination of encapsulation and phosphorus doping, which outperforms conventional catalysts in terms of metal dispersion, acidity modulation, and stability.
2. Results
2.1. Morphology and Structure of the Catalysts
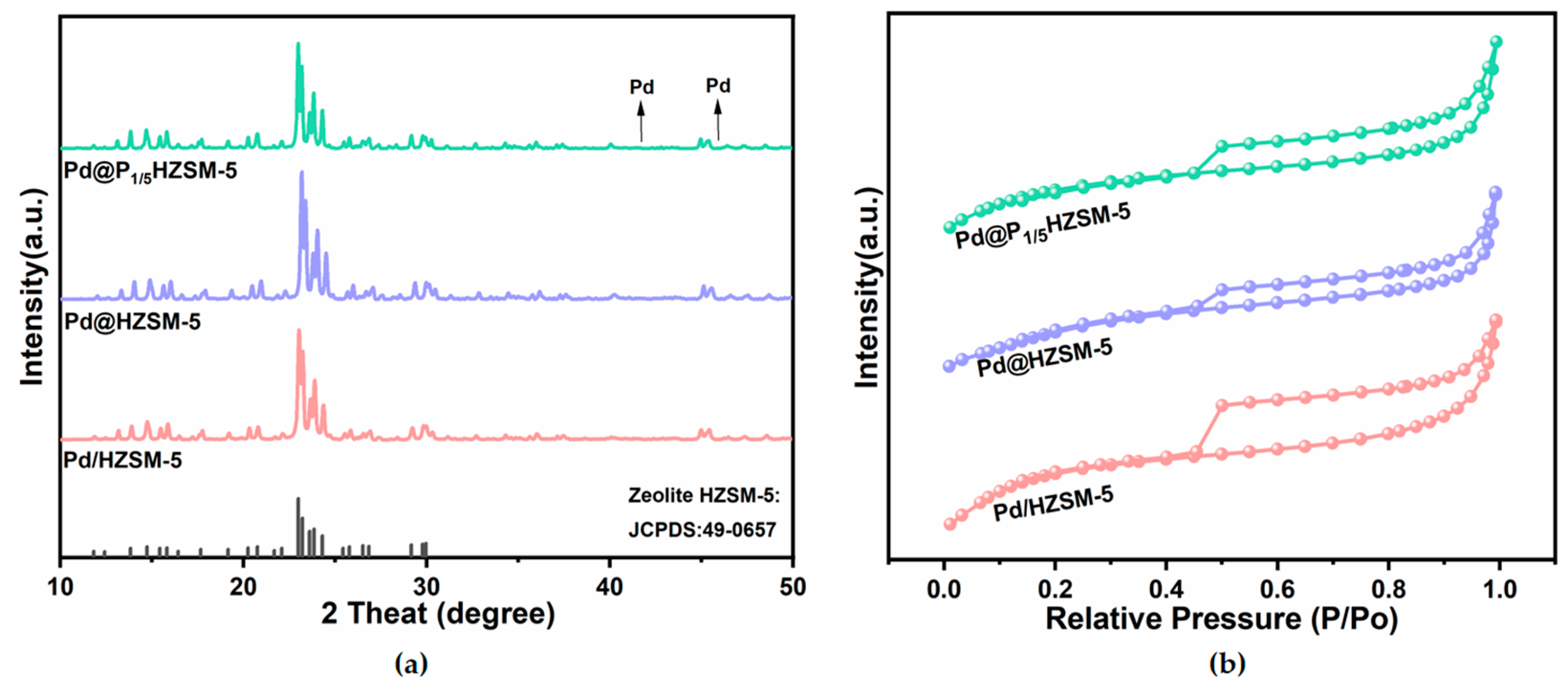
The ICP-AES results are shown in Table 1. The metal palladium loading of the Pd@HZSM-5, Pd@PxHZSM-5, and Pd/HZSM-5 catalysts was close to the theoretical value. This indicates a minimal loss of metal during the preparation of the catalysts. The XRD spectra of Pd/HZSM-5, Pd@HZSM-5, and Pd@PxHZSM-5 catalysts are shown in Figure 1a and Figure S1a. They all show diffraction patterns ascribed to the molecular sieve ZSM-5 ((Joint Committee on Powder Diffraction Standards-Powder Diffraction File) JCPDF: 49-0657), which is consistent with previous reports [18,23]. The prominent diffraction peak indicates the good crystallinity of ZSM-5 for all catalysts. This indicates that the phosphorus modification did not disrupt the main crystal structure of the ZSM-5 molecular sieve. No distinct Pd diffraction peaks were observed, which might be due to the low content of Pd or the high dispersion. The intensity of the diffraction peaks shows a decreasing trend with increasing phosphorus loading. This phenomenon occurs because the introduction of excessive phosphorus species induces the removal of aluminum from the molecular sieve skeleton, a phenomenon closely related to the hydrolytic breaking of Si-O-Al bonds. As the degree of modification deepens, the number of bridging oxygen bonds in molecular sieves gradually decreases, which in turn leads to a decreasing trend in their crystallinity [39].

Table 1.
Physical and chemical properties of various catalysts.
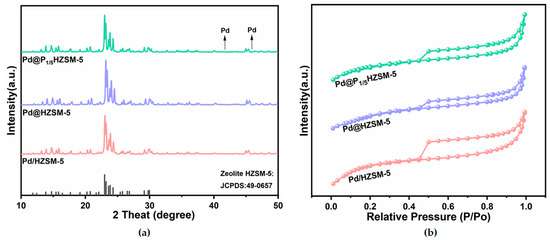
Figure 1.
(a) XRD patterns of Pd@HZSM-5, Pd@P1/5HZSM-5, and Pd/HZSM-5; (b) BET patterns of Pd@HZSM-5, Pd@P1/5HZSM-5, and Pd/HZSM-5.
The physical adsorption of N2 was tested for all catalysts. Moreover, the isothermal adsorption and desorption curves of N2 are shown in Figure 1b and Figure S1b. All catalysts showed type IV isotherms and hysteresis loops, which indicate that the Pd@HZSM-5, Pd@PxHZSM-5, and Pd/HZSM-5 catalysts all have a large number of microporous structures. Compared with the Pd/HZSM-5, the Pd@HZSM-5 andPd@PxHZSM-5 catalysts exhibited significantly smaller specific surface areas. This might have been caused by the encapsulation of Pd NPs within the ZSM-5 framework during preparation. Meanwhile, compared with other catalysts, the adsorption capacities of Pd@P1/3HZSM-5 and Pd@P1/4HZSM-5 in the medium-and high-pressure regions were significantly reduced, accompanied by decreases in specific surface area and pore volume. This can be attributed to the fact that, when the P/Al ratio is less than 5, excessive P doping blocks the pores and damages part of the pore structure. Excessive phosphorus doping not only induces a substantial decline in the specific surface area but also blocks micropores and mesopores, reducing the total pore volume. Ultimately, the diminished specific surface area and pore volume weaken the adsorption capacity of ZSM-5 [40].
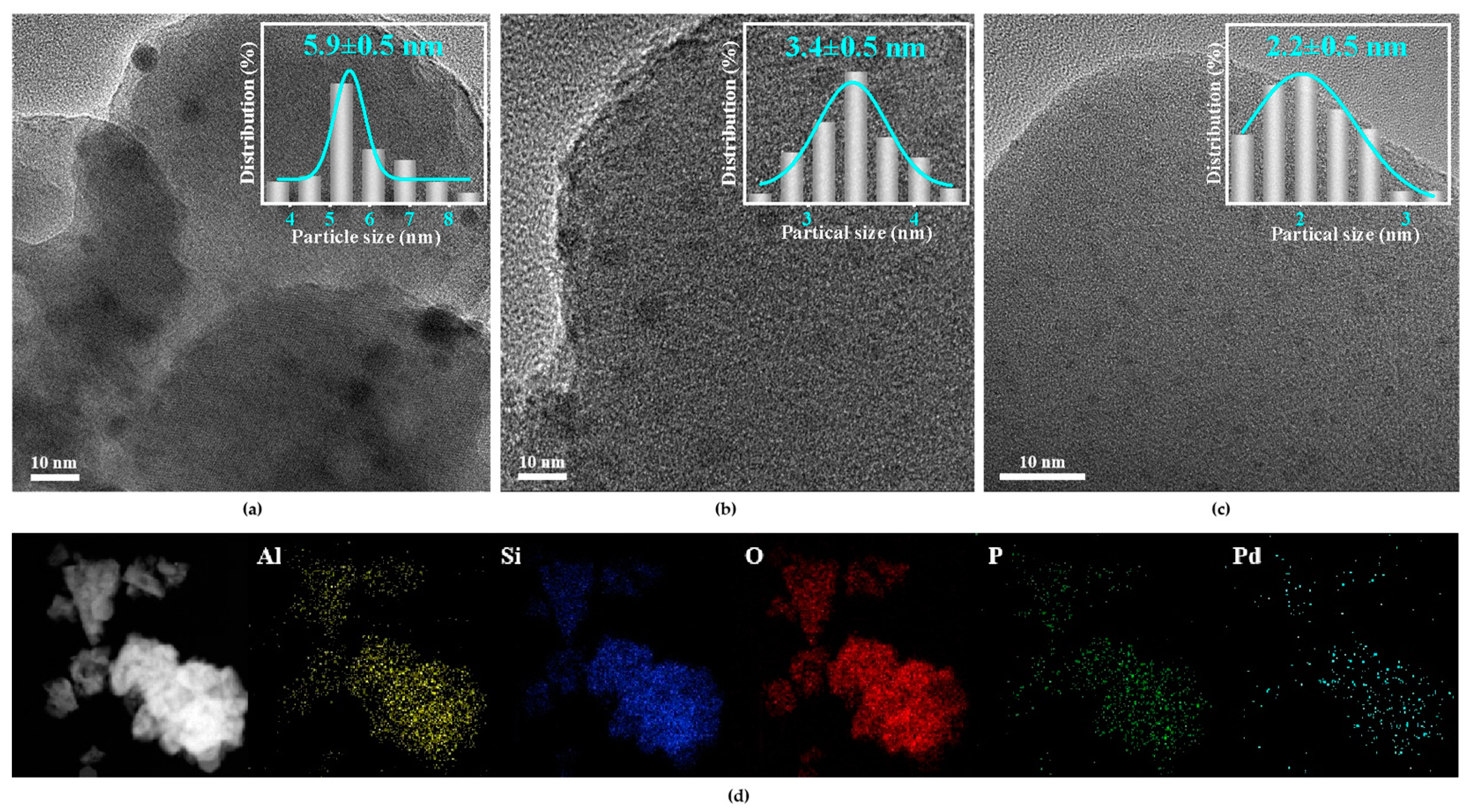
The TEM images of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts are shown in Figure 2. It is clear that Pd nanoparticles are uniformly dispersed. Particle size distributions were statistically analyzed. The average particle size of Pd/HZSM-5 was 5.9 nm, while Pd@HZSM-5 and Pd@P1/5HZSM-5 exhibited 3.4 and 2.2 nm, respectively. TEM analysis demonstrates that encapsulation significantly improves Pd dispersion, which might be caused by the confined effect of zeolite pores [41]. The Energy-Dispersive Spectroscopy (EDS) mapping of Pd@P1/5HZSM-5 showed that all elements are uniformly distributed with good dispersion and no agglomeration of the Pd NPs over Pd@HZSM-5 and Pd@P1/5HZSM-5.
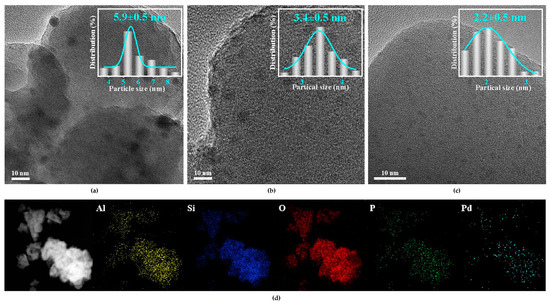
Figure 2.
(a) TEM pattern of Pd/HZSM-5 catalyst; (b) TEM pattern of Pd@HZSM-5 catalyst; (c) TEM pattern of Pd@P1/5HZSM-5 catalyst; (d) EDS pattern of Pd@P1/5HZSM-5 catalyst.
The TEM images of the Pd@PxHZSM-5 catalysts in Figure S2 reveal the uniform dispersion of Pd NPs. Pd@P1/5HZSM-5, Pd@P1/6HZSM-5, and Pd@P1/7HZSM-5 catalysts all exhibit improved dispersion compared with Pd@HZSM-5, indicating that the doping of P further enhanced the dispersion of Pd. The P doping in the HZSM-5 molecular sieves restricts the growth of Pd nanoparticles through the spatial site-blocking effect, which enhances the bonding force between Pd and the carrier and modifies the surface chemistry of the molecular sieves to promote the uniform adsorption and distribution of the Pd precursor [40]. However, the average particle size of Pd NPs in Pd@P1/3HZSM-5 is larger than that in Pd@HZSM-5, which can be primarily ascribed to the fact that excessive P modification may give rise to an overabundance of P species within the molecular sieve pores. The presence of these excessive P species can result in pore clogging or localized structural damage. Such pore blockage restricts the homogeneous distribution of Pd NPs within the molecular sieve pores, thereby promoting the aggregation of Pd NPs outside the pores or on the surface, ultimately leading to the formation of larger particles [40].
2.2. Chemical Properties of Catalysts
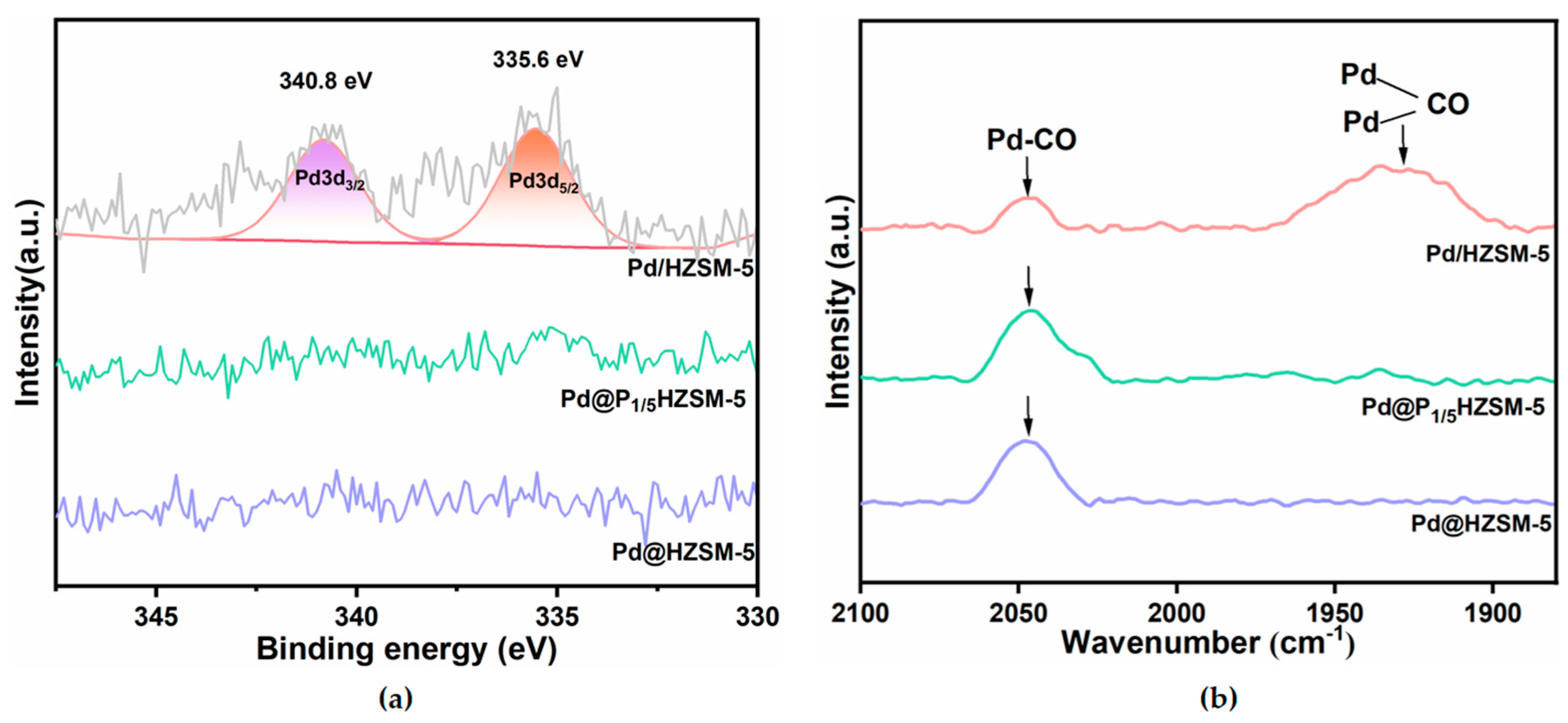
Figure 3a presents the XPS analysis results of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts. These catalysts exhibit distinct electronic state characteristics. Specifically, Pd/HZSM-5 displays two characteristic peaks at 335.6 eV and 340.8 eV, corresponding to the 3d5/2 and 3d3/2 electronic orbitals of Pd [38], respectively. As shown in Figure S3a, no detectable Pd signals were observed in the energy spectra of Pd@HZSM-5 and Pd@PxHZSM-5. The XPS profiles of Pd@PxHZSM-5 (Figure S3a) all showed the absence of Pd signals as shown in Figure S3. This absence of Pd signals suggests that the Pd nanoparticles have been successfully encapsulated within the skeletal structure of the HZSM-5 molecular sieves [34,40].
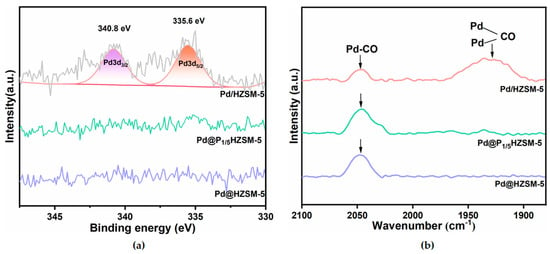
Figure 3.
(a) XPS pattern of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts; (b) CO-DRIFTS of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts.
The results of the CO diffuse reflectance infrared Fourier transform spectroscopy (CO-DRIFTS) of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 are shown in Figure 3b. The adsorption peaks observed at 1930 cm−1 and 2045 cm−1 are assigned to the bridge-bonded and linearly adsorbed CO species on Pd nanoparticles, respectively [41,42]. Notably, the Pd/HZSM-5 catalyst displays a dominant bridge adsorption mode, whereas Pd@HZSM-5 and Pd@P1/5HZSM-5 exhibit preferential linear CO adsorption. The CO-DRIFTS spectra of Pd@PxHZSM-5 (Figure S3b) all showed peaks for linear CO adsorption, lacking bridge-bonded CO features. The absence of peaks for the bridge-bonded CO species might be ascribed to the encapsulation of Pd nanoparticles in Pd@HZSM-5 and Pd@PxHZSM-5 [41,43]. The encapsulation of Pd nanoparticles results in spatial confinement effects, which restricts the bridge adsorption of CO and facilitates the linear adsorption.
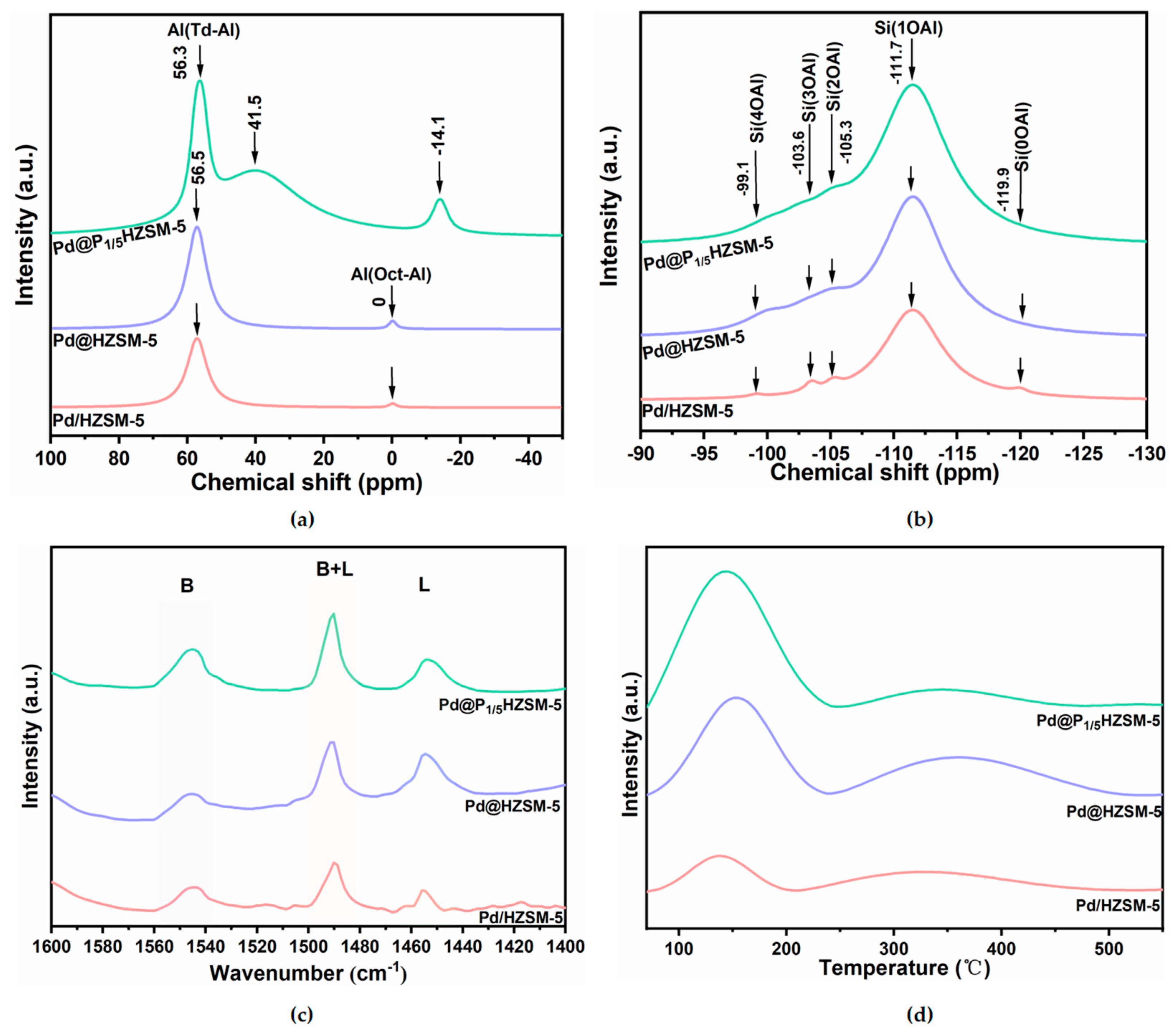
The solid-state 27Al NMR spectra of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts are presented in Figure 4a. Two distinct peaks are observed at 56.5 ppm and 0 ppm, corresponding to tetrahedrally coordinated Al (Td-Al) species and octahedrally coordinated Al (Oct-Al) species for both Pd/HZSM-5 and Pd@HZSM-5, respectively. The peak of Td-Al is ascribed to the Al species located in the molecular sieve skeleton, while the Oct-Al may be ascribed to the amorphous Al2O3. Td-Al is located in the molecular sieve backbone and is the main source of Brønsted acid sites, which can provide protons to participate in the reaction, whereas Oct-Al is usually present in a non-skeletal form and is less acidic. Notably, the peak intensities of the tetrahedrally coordinated Al species in the Pd@HZSM-5 catalysts, synthesized via steam-assisted milling, are significantly higher than those in the Pd/HZSM-5 catalysts prepared by loading Pd onto HZSM-5, which indicated that almost all of Al species were embedded in the skeleton. In contrast to Pd@HZSM-5, the Pd@P1/5HZSM-5 catalyst exhibits three distinct signals at 56.3 ppm, 41.5 ppm, and −14.1 ppm [42]. These signals are attributed to tetrahedrally coordinated framework Al (Td-Al), tetrahedrally coordinated Al in a distorted environment (either within or outside the framework), and octahedrally coordinated extra-framework Al, respectively. The shift for the Oct-Al species and the resonance at 41.5 ppm for Pd@P1/5HZSM-5 confirm the coordination interaction between P species and framework Al. The prominent peak area centered at 41.5 ppm suggests that a significant portion of the framework Al maintains its coordination with P species [43].
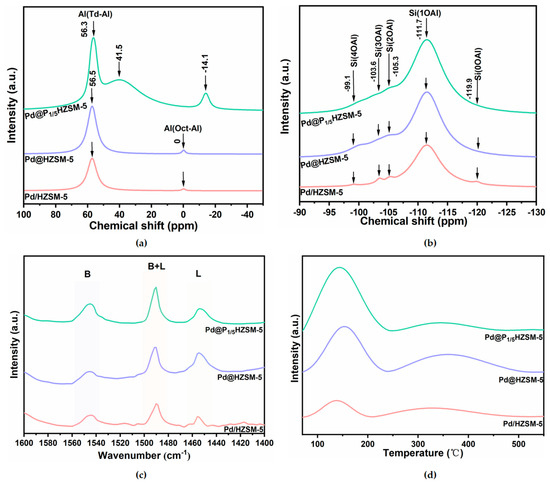
Figure 4.
(a) 27Al MAS NMR spectrum of samples; (b) 29Si MAS NMR spectrum of samples; (c) Py-IR profiles of samples; (d) NH3-TPD profiles of samples.
Figure 4b presents the 29Si solid-state NMR spectra of the Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts. Five distinct characteristic peaks are observed at −119.9 ppm, −111.7 ppm, −105.3 ppm, −103.6 ppm, and −99.1 ppm, corresponding to the chemical environments of Si(0OAl), Si(1OAl), Si(2OAl), Si(3OAl), and Si(4OAl), respectively [18]. Notably, the Si(1OAl) signals in the Pd@HZSM-5 and Pd@P1/5HZSM-5 catalysts are significantly enhanced, while the Si(0OAl) signals are notably weakened. This indicates the improved degree of crystallinity for Pd@HZSM-5 and Pd@P1/5HZSM-5, which is consistent with the spectra of 27Al NMR. The acidic intensities of the different Si(nOAl) (n = 0–4) coordination environments exhibited a consistent trend in the order of Si(1OAl) > Si(2OAl) > Si(3OAl) > Si(4OAl) > Si(0OAl) [44]. Notably, the signal intensity of Si(1OAl) in the encapsulated catalysts, Pd@HZSM-5 and Pd@P1/5HZSM-5, showed a 30% increase compared to that of Pd/HZSM-5. This observation suggests that the Pd@HZSM-5 and Pd@P1/5HZSM-5 catalysts prepared via the steam-assisted milling method possess a greater abundance of acidic sites. This structural distinction may be attributed to the interaction between the active components and the molecular sieve framework during the encapsulation process, which induces significant alterations in the acidic properties of the catalyst surface.
Figure S4 demonstrates the 31P solid-state NMR spectrum of Pd@P1/5HZSM-5 catalysts, which exhibits four broad signals at −7.4 ppm, −30.7 ppm, −39.2 ppm, and −44.6 ppm. The signal at −7.4 ppm is associated with pyrophosphate, terminal groups of short-chain polyphosphates, or similar species, and the signal at −44.6 ppm is assigned to P4O10, which indicates the existence of pyrophosphate. The signal at −30.7 ppm is attributed to amorphous aluminum phosphate, while the signal at approximately −39.2 ppm corresponds to highly condensed polyphosphate species [45]. The signal centered at −30.7 ppm indicates coordination between P and framework Al of the molecular sieve. The main peak at −30.7 ppm suggests that a significant proportion of P is coordinated with Al, which can inhibit the dealumination process and thereby enhance the hydrothermal stability of the Pd@P1/5HZSM-5 catalyst. This stabilization mechanism is critical for maintaining the structural integrity and catalytic performance of the material under hydrothermal conditions.
Figure 4c displays the Py-IR spectra of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5. The peaks at 1540 cm−1 and 1450 cm−1 are assigned to Brønsted acid sites (B) and Lewis acid sites (L), respectively. The acid amounts of the Pd@HZSM-5, Pd@PxHZSM-5, and Pd/HZSM-5 catalysts are summarized in Table 2. Notably, the Pd@HZSM-5 catalyst exhibits a significantly higher number of Brønsted acid and Lewis acid sites, with a higher B/L ratio, than the Pd/HZSM-5 catalyst. Simultaneously, Pd@PxHZSM-5 shows increased acid amounts with almost unchanged or slightly decreased B/L ratios compared with Pd/HZSM-5 by the doping of P (Figure 4c and Figure S5a, Table 2). The amount of Lewis acid of Pd@PxHZSM-5 and Pd@HZSM-5 is close, while the amount of Bronsted acid is different. This is ascribed to the fact that P atoms interact with specific atoms (e.g., silicon, aluminum) or groups (e.g., oxygen bridges, hydroxyl groups) within the molecular sieve framework. The chemical reaction between P atoms and the protons of Brønsted acid centers causes some of these centers to lose protons and transform into other types of acid centers, leading to the generation of non-acidic sites [45,46]. Additionally, with an increased amount of P doping, the decrease in the Bronsted acid is larger, which indicates that excess P doping might block the pore, and is unfavorable for the accessibility of the acid sites.

Table 2.
Calculation Table of the Acid Amount of Various Catalysts.
Figure 4d and Figure S5b present the NH3-TPD curves for Pd/HZSM-5, Pd@HZSM-5, and Pd@PxHZSM-5 catalysts. Two distinct NH3 desorption peaks are observed in the ranges of 70–200 °C and 200–500 °C, corresponding to weak and strong acid sites, respectively. The acid amounts calculated from NH3-TPD for the different catalysts are summarized in Table 2. A significant increase in the total acid amount is observed for Pd@HZSM-5 and Pd@P1/5HZSM-5 compared to Pd/HZSM-5. Furthermore, the amount of weak acid sites increases for the Pd@PxHZSM-5 catalyst relative to Pd@HZSM-5. The formation of new weak acid sites may be attributed to the formation of P-OH groups in silanol nests, Si-OH groups bound to hydrogen bonds, and phosphate species bound to the backbone by P species [46].
In contrast, the amount of strong acid sites in the Pd@P1/5HZSM-5 catalyst decreases. This reduction is mainly because the P doping is the chemical reaction of phosphorus with the Brønsted acid sites in the molecular sieve backbone. Specifically, the phosphoric acid (H3PO4) generated from the hydrolysis of (NH4)2HPO4 reacts with the Brønsted acid sites (Si-OH-Al) in the HZSM-5 molecular sieves to form P-OH groups. These P-OH groups, although still acidic, have much lower acid strength than the original Brønsted acid sites because of the weaker proton dissociation of P-OH. In addition, the introduction of phosphorus alters the charge distribution of the molecular sieve skeleton, further weakening the proton supplying ability of the Brønsted acid sites, which leads to a weakening of the overall acid strength. This mechanism was verified by the results of NH3-TPD and Py-IR experiments, which showed a decrease in the strong acid sites and an increase in the weak acid sites after phosphorus doping [47].
2.3. Reaction Performance of Catalysts
The catalytic performance of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 for the conversion of 2-methylfuran (2-MF) to 3-acetyl-1-propanol (3-AP) was carried out in an aqueous environment, as summarized in Table 3. Compared to the Pd/HZSM-5 catalyst, the Pd@HZSM-5 catalyst demonstrated significantly enhanced catalytic activity. This improvement highlights the feasibility and effectiveness of the steam-assisted milling method for fabricating encapsulated catalysts. The superior performance of Pd@HZSM-5 can be attributed to its unique structure, where acidic sites surround the majority of Pd nanoparticles. Compared with the Pd@PxHZSM-5 catalysts, the Pd@P1/5HZSM-5 catalyst exhibits the best performance. The slightly increased catalytic performance of Pd@P1/5HZSM-5, compared to that of Pd@HZSM-5, indicates that the weak acid and Lewis acid play a key role in the acid catalysis, while the strong acid and Bronsted acid have little effect on the reaction. The encapsulation of Pd NPs resulted in the confinement effect and the close proximity of metal sites to Brønsted acid sites, which inhabit the over-hydrogenation of the semi-hydrogenation intermediates into 2-MTHF [48]. Also, the increased amount of acid around the metal is facilitated for the access of the intermediate, which is more likely to undergo hydrolysis, thus resulting in the improved yield of 3-AP.

Table 3.
Catalytic performance of Pd/HZSM-5, Pd@HZSM-5, and Pd@P1/5HZSM-5 catalysts.
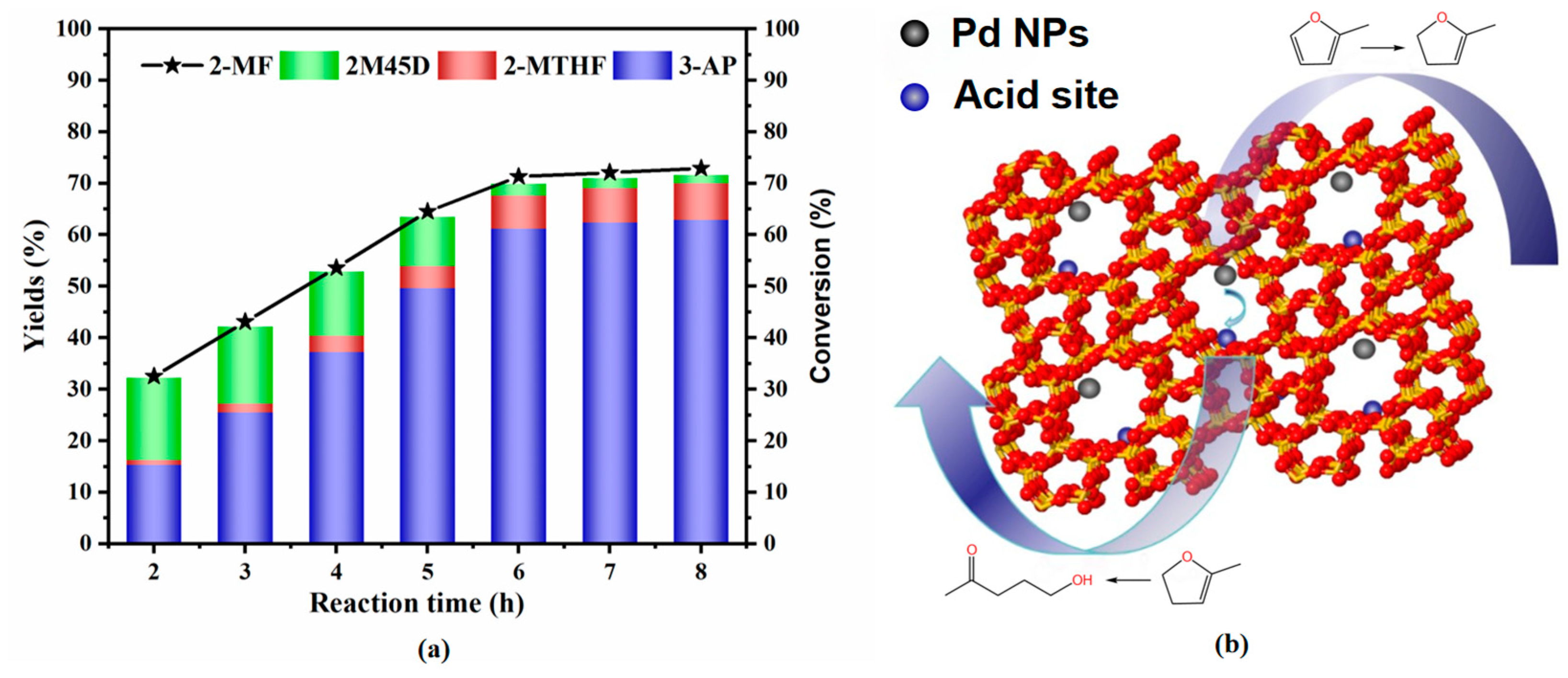
Figure 5a illustrates that the conversion of 2-methylfuran (2-MF) increases rapidly with reaction time. In contrast, the yield of 4,5-dihydro-2-methylfuran (2M45D) decreases as the reaction progresses, while the yield of 3-acetyl-1-propanol (3-AP) rises significantly. After 6 h, the reaction reached equilibrium and the conversion of 2-methylfuran (2-MF) and the yield of 3-acetyl-1-propanol (3-AP) stabilized, and further prolongation of the reaction time did not significantly increase the conversion or yield. This trend indicates that 2-MF undergoes partial hydrogenation followed by ring opening to form 3-AP. Based on these observations, we propose the reaction mechanism depicted in Figure 5b. Initially, 2-MF is hydrogenated at the metal active sites to produce 2-methyltetrahydrofuran (2-MTHF) and 2M45D, with the latter serving as an intermediate. Subsequently, the acidic sites facilitate the decomposition of 2M45D, yielding the target product, 3-AP. This mechanism highlights the synergistic role of metal and acidic sites in the catalytic conversion process.
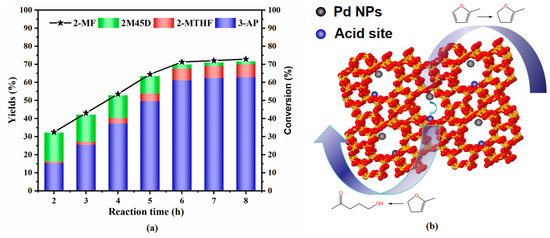
Figure 5.
(a) Conversion of 2-methylfuran; (b) reaction mechanism for the conversion of 2-methylfuran to 3-AP.
2.4. The Stability of the Catalysts
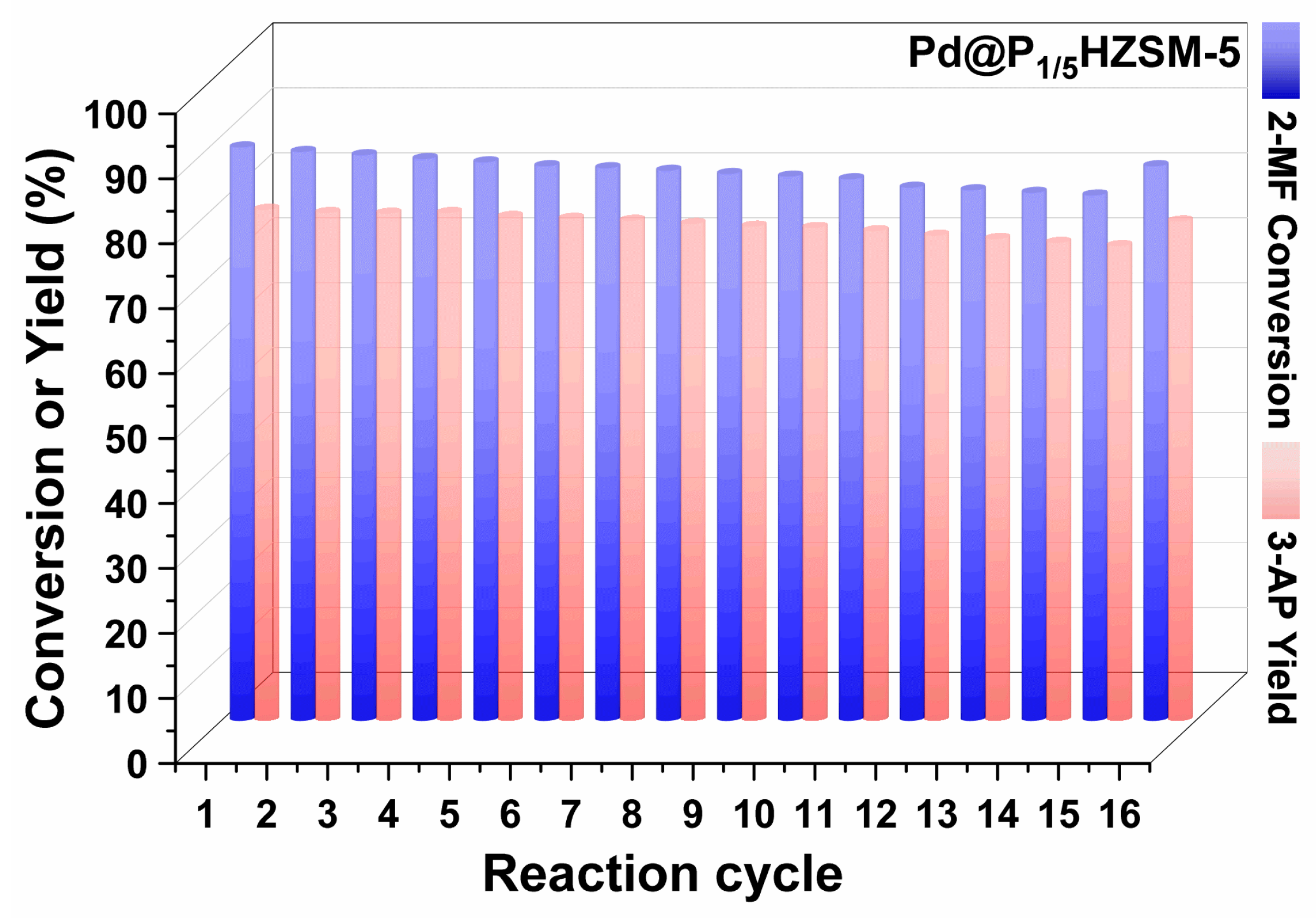
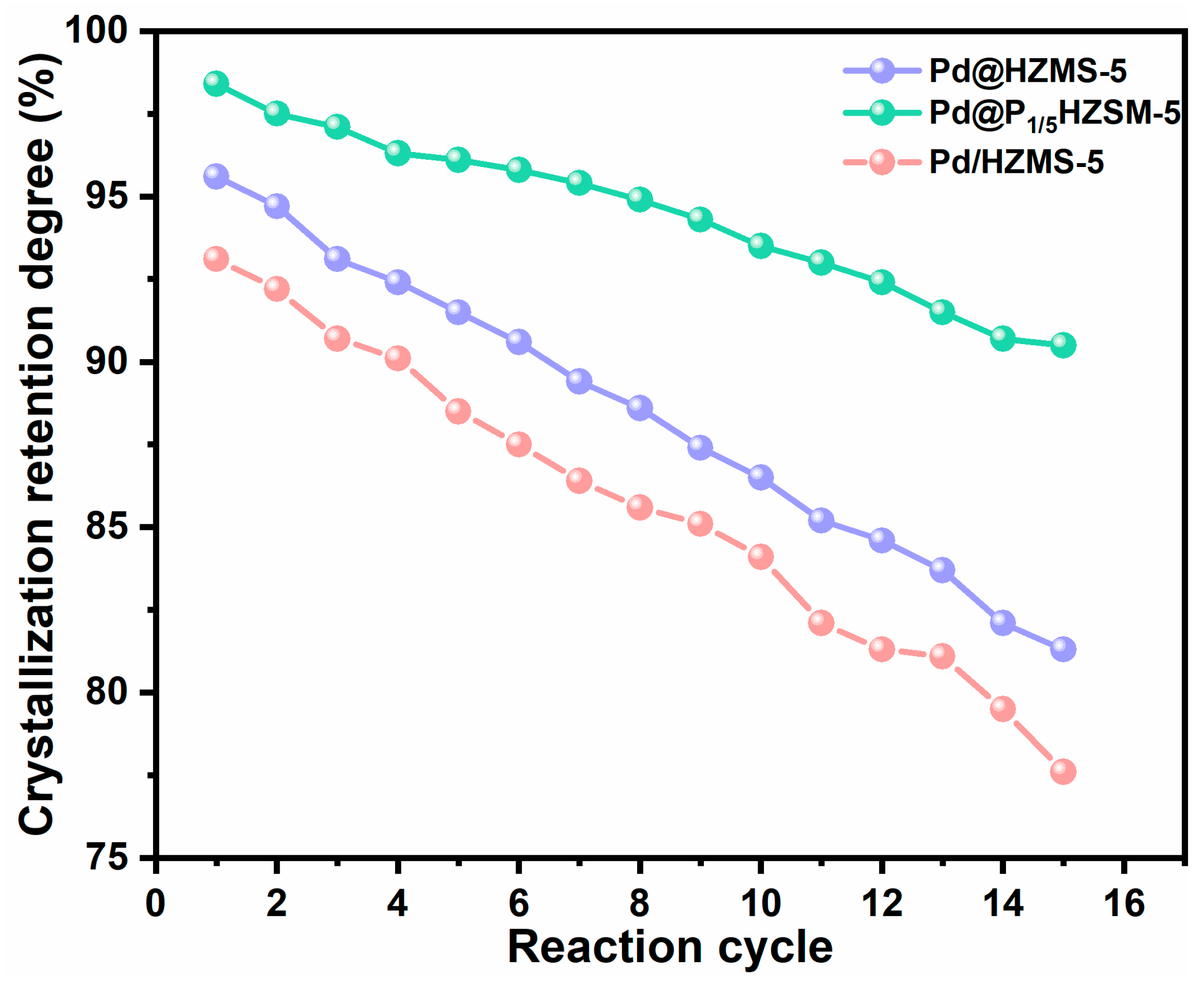
Figures S6 and S7 display the results of the cyclic performance test of Pd/HZSM-5 and Pd@HZSM-5. Pd@HZSM-5 showed better recyclability than Pd/HZSM-5 with a certain degree of loss of activity and selectivity. The cycling performance of catalyst Pd@P1/5HZSM-5 is displayed in Figure 6. Pd@P1/5HZSM-5 displayed good stability as the conversion of 2-methylfuran only went down slightly (from 88.3% to 80.9% after 15 cycles), with a decrease rate of 8.4%. However, after roasting and reducing, it increased again to 85.4%. The yield of 3-acetyl-1-propanol decreased less after the 15th cycle, from 78.7% to 73.1%, with a decrease rate of 7.1%. After roasting and reduction, the selectivity of 3-acetyl-1-propanol increased to 76.9%. This fully demonstrates the significant role of P in maintaining the stability of the catalyst. Figure 7 shows that the crystallinity of Pd@HZSM-5 decreased more quickly than that of Pd@P1/5HZSM-5; after 15 cycles, the crystallinity of Pd@P1/5HZSM-5 stayed above 90%, while it was only 80% for Pd@HZSM-5. This showed that adding P could effectively keep the catalyst’s structure intact.
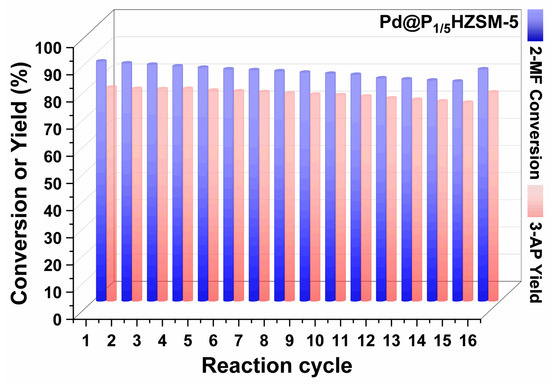
Figure 6.
Cycling performance test: Pd@P1/5HZSM-5 catalyst. Reaction conditions: 3 g 2-methylfuran, 27 mL H2O, 0.2 g catalyst at 90 °C, 1.0 MPa H2 for 6 h.
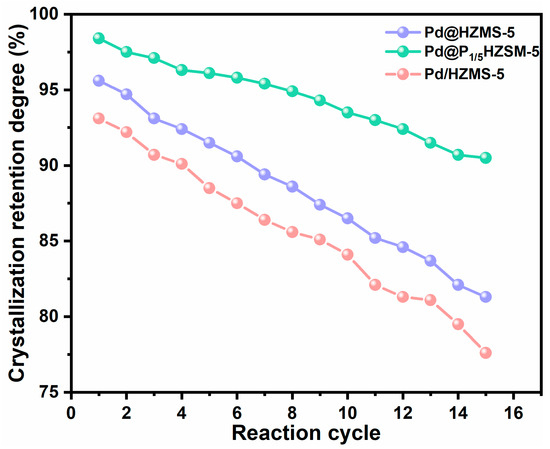
Figure 7.
Crystalline retention.
3. Experimental Section
3.1. Chemical Reagents
The following reagents were used directly without further purification: 2-methylfuran (2-MF, 98%, Macklin, Shanghai, China), 2-methyltetrahydrofuran (2-MTHF, 99%, Aladdin), 3-acetyl-1-propanol (3-AP, 95%, Macklin), 4,5-dihydro-2-methylfuran (2M45D, 97%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China), palladium(II) nitrate dihydrate (39% palladium metal-based, Aladdin), tetraethyl orthosilicate (C8H20O4Si, Macklin), tetrapropylammonium hydroxide solution (TPAOH, 25% in water, Macklin), silica gel (SiO2, 300–400 mesh, Aladdin), diammonium hydrogen phosphate ((NH4)2HPO4, Kemio), ethylenediamine monohydrate (C2H8N2·H2O, 98%, Aladdin), sodium metasilicate nonahydrate (Na2SiO3·9H2O, 96%, Aladdin), aluminum sulfate octa decahydrate (Al2(SO4)3·18H2O, Macklin), ammonium chloride (NH4Cl, Fengchuan, Xingtai, China), and ammonia water (NH3·H2O, Shangyanggong, Zhengzhou, China).
3.2. Preparation of Catalysts
The preparation of the crystal seeds was carried out as follows [49]: The crystal seeds of silicalite-1 with a size of approximately 200 nm were synthesized according to the molar ratio of 4 TPAOH: 25 TEOS: 480 H2O. The specific steps were as follows. First, 9.8 g of TPAOH was added to 15.6 g of TEOS. Then, 9.3 g of deionized water was added, and the mixture was stirred at room temperature for 12 h. Subsequently, the mixture was transferred to a 100 mL autoclave and heat-treated at 98 °C for 20 h. After cooling, the solid product was separated using filtration, washed with deionized water, and then dried at 60 °C overnight. Finally, calcination was carried out at 550 °C for 6 h to remove the templating agent; thus, silicalite-1 crystal seeds with a uniform size of approximately 200 nm were obtained.
The preparation of the metal Pd precursor solution was as follows: In a 50 mL solution containing 10 mL of ethylenediamine (EDA), 1.0 g of Pd(NO3)2·2H2O was added and stirred at room temperature for 6 h. The resulting solution was referred to as Pd-EDA.
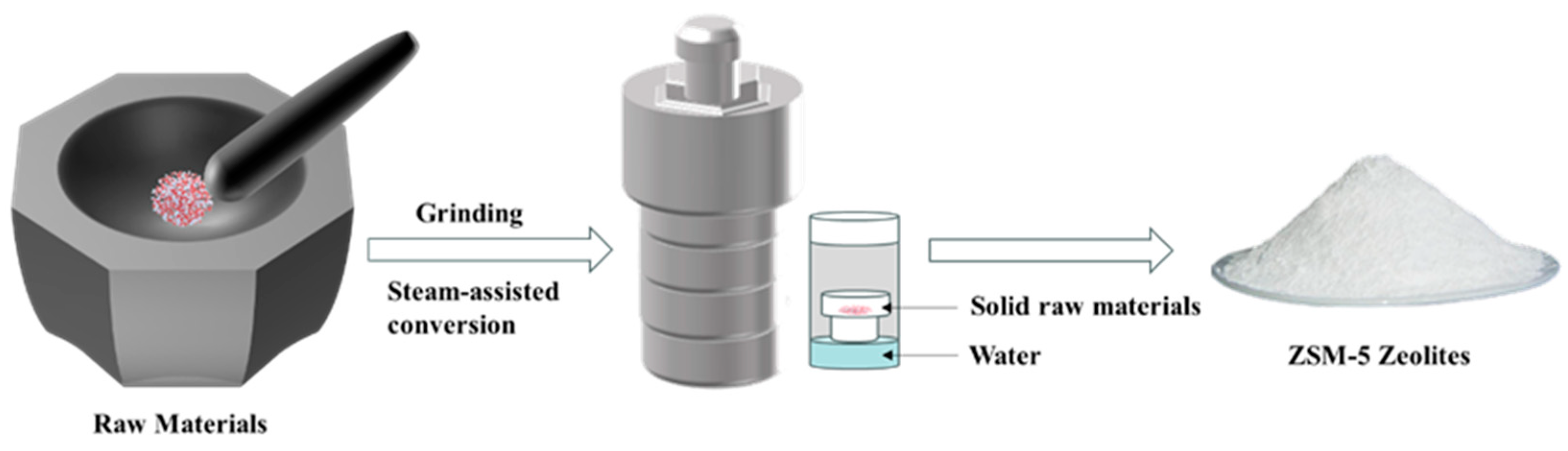
The preparation of a Pd@HZSM-5 zeolite molecular sieve using the in situ solid-phase steam method was carried out as follows [50]: First, 1.5 g of sodium silicate hydrate (Na2SiO3·9H2O), 0.62 g of aluminum sulfate octa decahydrate (Al2(SO4)3·18H2O), 1.34 g of solid silica gel (SiO2), and 0.2 g of zeolite crystal species were added to the agate mortar and mixed well. During the grinding process, 0.504 mL of metal precursor solution was slowly added and fully ground for 30 min. Subsequently, the fully milled feedstock was transferred to a 100 mL (Polytetrafluoroethylene) PTFE liner, and 4 mL of water was added to the bottom of the liner without touching the feedstock. We crystallized the product at 180 °C for 12 h, then washed, dried, and calcined it to prepare a sample known as Pd@ZSM-5. The specific steps are shown in Figure 8. The Pd@ZSM-5 that was collected was mixed with NH4Cl solution that had a solid-to-liquid ratio of 1 g/20 mL and a concentration of 0.05 g/mL, and ammonia was added dropwise until the pH reached 6. The mixture was then stirred at 80 °C for 3 h to exchange ions. It was then washed with deionized water and dried at 80 °C for 12 h. The above steps were repeated three times. Finally, we obtained the Pd@HZSM-5 by calcining it in a muffle furnace at 550 °C for 4 h.
Figure 8.
Preparation of ZSM-5 zeolite using steam-assisted grinding method.
In order to make HZSM-5, 1.5 g of sodium silicate hydrate, 0.62 g of aluminum sulfate hydrate octa decahydrate, 1.34 g of solid silica gel, and 0.2 g of zeolite crystalline species were put into an onyx mortar and mixed well. Subsequently, the fully ground feedstock was transferred to a 100 mL PTFE liner, and 4 mL of water was added to the bottom of the liner without touching the feedstock. It was crystallized at 180 °C for 12 h, then washed, dried, and heated to obtain ZSM-5. This was then ion-exchanged using the same method that was used to make Pd@HZSM-5.
To prepare the Pd/HZSM-5 catalyst, we performed the following steps of the initial wet impregnation method: Firstly, 1.0 g of treated HZSM-5 molecular sieve was placed in the crucible, and its water absorption was tested to be 1mL. Second, 2 g of the HZSM-5 molecular sieve was put into the crucible. Then, a pipette gun was used to measure out 0.504 mL of the ready-made Pd-EDA solution and 1.496 mL of deionized water. These amounts were then slowly added to the crucible. Subsequently, the crucible was placed in an oscillator and shaken and stirred for 30 min so that the solution and the molecular sieve were mixed well. After mixing evenly, the crucible was put into a vacuum drying oven at 80 °C and dried for 12 h. After the sample dried, it was ground into a powder and put into a muffle furnace at 500 °C for 6 h to obtain Pd/ZSM-5. The Pd/ZSM-5 was then ion-exchanged using the same method as that for preparing Pd@HZSM-5. Next, we spread out the catalyst evenly on a porcelain boat and placed it in a tube furnace with 10 vol% H2/Ar. The catalyst underwent a reduction process at 350 °C for four hours. The final gray powder is the Pd/HZSM-5 catalyst, which requires storage in a vacuum environment.
The method we used for preparing Pd@PxZSM-5 zeolite molecular sieves with different P contents using the solid-phase steam grinding method was as follows: Firstly, 1.5 g of non-aqueous sodium silicate (Na2SiO3·9H2O), 0.62 g of aluminum sulfate decahydrate (Al2(SO4)3·18H2O), 1.34 g of solid silica gel (SiO2), y g of diammonium hydrogen phosphate ((NH4)2HPO4, where y = 0.0167, 0.0125, 0.0100, 0.0083, 0.0071), and 2.0 g of zeolite seeds were added to an agate mortar and mixed evenly. During the grinding process, 0.504 mL of the metal precursor solution was slowly added and the mixture was thoroughly ground for 30 min. Then, the well-ground raw materials were transferred into a 100 mL polytetrafluoroethylene liner, and 4 mL of water was added to the bottom of the liner without contacting the raw materials. After crystallization at 180 °C for 12 h, the product was washed, dried, and calcined. The prepared samples were named Pd@PxZSM-5 (x: P/Al, x = 1/3–1/7). The obtained Pd@ZSM-5 was added to an NH4Cl solution with a solid-to-liquid ratio of 1 g/20 mL, and the concentration of the NH4Cl solution was 0.05 g/mL. Ammonia water was added until the pH value reached 6. Then, the mixture was stirred at a constant temperature of 80 °C for 3 h for ion exchange. Subsequently, the product was washed with deionized water and dried at 80 °C for 12 h. The above steps were repeated three times. Finally, it was calcined in a muffle furnace at 550 °C for 4 h to obtain Pd@PxHZSM-5.
3.3. Catalytic Tests
The catalytic conversion of 2-MF was carried out in a high-pressure stainless steel autoclave (Yanzheng Instruments, 100 mL) with a stirring speed of 800 rpm [18]. In a typical experiment, 0.3 g of 2-MF, 27 mL of H2O, and 0.2 g of catalyst were homogeneously mixed in the autoclave. Prior to the reaction, the reactor was purged with hydrogen at room temperature at least five times and then heated to 90 °C. The reaction then occurred by passing hydrogen at 1.0 MPa. At the end of the reaction, the liquid products and substrates were analyzed using gas chromatography (Agilent 8900 GC System, Santa Clara, CA, USA).
The external standard method was used to quantitatively analyze the reaction products, while gas chromatography-mass spectrometry (GCMS Agilent 8890-5977B) was used to qualitatively analyze them.
For the recovery test, the catalyst was centrifuged at 8000 rpm for 3 min, washed with ethanol, and set aside.
Conversion rate, yield, selectivity, and carbon balance were defined as follows:
where mbefore represents the mass of 2-MF before the reaction, and mafter represents the mass of 2-MF after the reaction.
where nproduct represents the amount of substance of 3-AP after the reaction, and nafter represents the amount of substance of 2-MF before the reaction.
where nbefore is the number of moles of carbon in the reactants before the reaction, and nafter is the total number of moles of carbon in all the products after the reaction.
The relative crystallinity of molecular sieves was calculated using the following equation:
where α is the crystallinity of the sample to be tested, and it is related to the relative crystallinity of the zeolite specimen. The ZSM-5 zeolite purchased from Nankai University Catalyst Factory (Tianjin, China) was used as the standard specimen, and its crystallinity was set at 100%. I and I0 are the integral area of the characteristic diffraction peak of 2θ at 22–25° for the sample and commercially available ZSM-5 zeolite, respectively.
3.4. Catalyst Characterization
The samples’ X-ray diffraction (XRD) patterns were recorded on SmartLab SE (Rigaku, Tokyo, Japan) within a scanning range of 10° to 90° at a scanning speed of 10° per minute with a step size of 0.02°. The equipment employed a Cu Kα ray source (λ = 0.15418 nm).
We measured the sample’s N2 adsorption–desorption isotherm at −196 °C using the Micromeritics ASAP 2020 (Norcross, GA, USA). Prior to each measurement, the sample was degassed at 300 °C for 6 h. The surface area was determined using the Brunauer–Emmett–Teller (BET) model. At the same time, the pore size distribution, pore volume, and average pore diameter were obtained via the Barret–Joyner–Halenda (BJH) method.
Inductively coupled plasma atomic emission spectrometry (Optimass 9500, GBC Scientific Equipment Pty Ltd., Melbourne, Australia) was used to determine the palladium content of the samples.
The catalysts’ X-ray photoelectron spectra (XPS) were obtained using a PHI Quantera SXM (ULVAC-PHI USA, Inc., Methuen, MA, USA) spectrometer. The excitation source was Al Ka = 1486.6 eV, and the C1s was set to 284.8 eV to fix the binding energy.
The programmed temperature rise desorption (NH3-TPD) was used to analyze the amount and intensity of acid in the samples. It was measured on a fully automated gas adsorption and desorption instrument, Autosorb-IQ, from Quantachrome, Boynton Beach, FL, USA. Accurately weigh 60 mg of the sample, place it in an atmosphere of helium gas, heat it to 350 °C at a heating rate of 30 °C/min, and keep purging for 40 min. After the sample is cooled to 100 °C, purge the sample with a mixed gas of NH3/He with a volume fraction of 10% until the sample is saturated with adsorption, and then purge it with helium gas for 1 h. After that, heat the sample from 100 °C to 700 °C at a rate of 10 °C/min, and collect the signals during this process.
Transmission electron microscopy (TEM) was used to observe the surface morphology of the samples as well as the metal particle size distribution. The instrument chosen was an FEI Tecnai G2 F20 (FEI USA, Inc., Hillsboro, OR, USA) electron microscope with an electron beam voltage of 200 kV. The sampling procedure was as follows: the sample was dispersed in anhydrous ethanol using ultrasonic dispersion for 30 min, and then the upper layer of the suspension was added dropwise to the copper mesh by pipetting, and dried.
Solid-state magic angle spinning nuclear magnetic resonance (MAS NMR) 27Al, 29Si NMR, and 31P NMR were measured using a Bruker Ascend 400WB spectrometer (Billerica, MA, USA).
CO-diffuse reflectance infrared Fourier transform spectroscopy (CO-DRIFTS) was performed using a Bruker Tensor 27 spectrometer. After emptying the cuvette, we heated the sample to 350 °C in H2/He (5 vol%, 40 mL·min−1) for 60 min. The gas was then changed to N2 (20 mL∙min−1), and the sample was cooled to room temperature. The gas was then switched to 10% CO/He for 30 min. Spectra were then collected. After equilibration, the spectra were further recorded under N2 flow until no significant change was found.
The acidity of the zeolite was determined using pyridine Fourier Transform Infrared Spectroscopy (FTIR) spectroscopy and a Bruker Tensor 27 instrument. The samples were first pressed using a 13 mm diameter mold and weighed as dry weight after pressing, followed by a 1 h treatment at 450 °C under vacuum. After pretreatment, the sample was cooled to 40 °C, and then pyridine was introduced to obtain the pyridine adsorption spectrum. Next, we heated the sample to 250 °C and kept it in a vacuum state for 30 min. After the sample was cooled to room temperature, the spectrum was collected to obtain the desorption spectrum of pyridine. The quantities of acid were calculated using Equations (6) and (7). Equation (6) calculates the number of Brønsted acid sites, while Equation (7) calculates the number of Lewis acid sites, both based on peak areas and extinction coefficients from pyridine adsorption infrared spectroscopy (Py-IR). These equations help us to quantify the distribution of different types of acid sites in the catalyst, thus revealing the synergistic effect of the acidic and metal sites of the catalyst.
where ABronsted and ALewis are peak areas obtained by Gaussian integration, EBronsted and ELewis are extinction coefficients of Bronsted and Lewis acids, which are 1.95 and 1.42 [51], respectively, Ss is the area of the sample after pressing, and Ms is the dry weight of the sample.
4. Conclusions
In this study, Pd@PxHZSM-5 was synthesized using steam-assisted milling with Pd NPs encapsulated by HZSM-5. The Pd@P1/5HZSM-5 catalyst demonstrated the highest 2-MF conversion (88.3%) and 3-AP yield (78.7%) under mild reaction conditions (90 °C, 1.0 MPa H2, 6 h), outperforming both Pd@HZSM-5 (conversion: 83.4%; yield: 72.4%) and Pd/HZSM-5 (conversion: 50.6%; yield: 30.7%). The encapsulation of Pd nanoparticles significantly improved the dispersion of Pd, leading to higher catalytic activity and selectivity compared to traditional impregnated Pd/HZSM-5 catalysts. Moreover, weak acids and Lewis acids play a crucial role in the ring-opening reaction of the furan ring. The doping of phosphorus increases the amount of weak acids and Lewis acids, which further improves the catalytic performance of the catalyst. The hydrothermal stability of the catalyst improved by the addition of P was ascribed to the formation of P-O-Al coordination bonds. In conclusion, this work developed an effective way to change the distribution of metal nanoparticles and the acid strength and amount, thus improving the bifunctional catalysis process. The work also demonstrated the important role of P doping on the stability of Pd@PxHZSM-5. Moreover, this work offers a green and sustainable approach for converting biomass-derived platform molecules into high-value chemicals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15040390/s1, Figure S1: (a) XRD pattern of Pd@PxHZSM-5 (b) BET pattern of Pd@PxHZSM-5; Figure S2: (a) TEM pattern of Pd@P1/7HZSM-5; (b) TEM pattern of Pd@P1/6HZSM-5; (c) TEM pattern of Pd@P1/4HZSM-5. (d) TEM pattern of Pd@P1/3HZSM-5; Figure S3: (a) XPS pattern of Pd@PxHZSM-5; (b) CO-DRIFTS pattern of Pd@PxHZSM-5; Figure S4: 31P MAS NMR spectra of Pd@P1/5HZSM-5; Figure S5: (a) Py-IR pattern of Pd@PxHZSM-5; (b) NH3-TPD pattern of Pd@PxHZSM-5; Figure S6: Cycling performance test: Pd@/HZSM-5 catalyst. reaction conditions: 3 g 2-methylfuran, 27 mL H2O, 0.2 g catalyst at 90 °C, 1.0 MPa H2 for 6h; Figure S7: Cycling performance test: Pd@HZSM-5 catalyst. reaction conditions: 3 g 2-methylfuran, 27 mL H2O, 0.2 g catalyst at 90 °C, 1.0 MPa H2 for 6h.
Author Contributions
Z.B.: conceptualization, investigation, data curation, visualization, and writing—original draft. Z.L.: conceptualization, resources, writing—review and editing, and supervision. Q.L.: conceptualization, validation, investigation, writing—review and editing, visualization, and funding acquisition. Y.G.: investigation. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (Project No. 22108259).
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank the National Natural Science Foundation of China (Project No. 22108259) for funding this study. We also thank the School of Chemistry and the State Key Laboratory of Green Coal Resources Development of Zhengzhou University for providing the experimental platform and technical support. In addition, we thank our laboratory colleagues for their help in experimental design and data analysis, and the reviewers for their valuable comments on this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohammed, A.; Dongmei, L.; Mahtab, N.; Ateeq, S.; Xiao, M.Z.; Donald, L.S. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Cai, J.; Wei, L.; Wang, J.; Lin, N.; Li, Y.; Li, F.; Zha, X.; Li, W. Application of catalysts in the conversion of biomass and its derivatives. Catalysts 2024, 14, 499. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; He, Q.; Chai, Y.; Qin, P.; Wu, Z.; Liu, C.; Gong, X.; Liang, Y. Preparation and application of UiO-66(Zr) and its derivatives as catalysts in lignocellulosic biomass conversion. Chem. Eng. J. 2024, 486, 149971. [Google Scholar] [CrossRef]
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Conversion of biomass and its derivatives to levulinic acid and levulinate esters via ionic liquids. Ind. Eng. Chem. Res. 2018, 57, 4749–4766. [Google Scholar] [CrossRef]
- Hronec, M.; Fulajtarová, K. Selective transformation of furfural to cyclopentanone. Catal. Commun. 2012, 24, 100–104. [Google Scholar] [CrossRef]
- Moravvej, Z.; Farshchi Tabrizi, F.; Rahimpour, M.R.; Behrad Vakylabad, A. Exploiting the potential of cobalt molybdenum catalyst in elevated hydrodeoxygenation of furfural to 2-methyl furan. Fuel 2023, 332, 126193. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, Z.; Guo, W.; Liu, J.; Li, R.; Chen, R.; Huang, J. Hydrogenation and hydrolysis of furfural to furfuryl alcohol, cyclopentanone, and cyclopentanol with a heterogeneous copper catalyst in water. Ind. Eng. Chem. Res. 2019, 58, 3988–3993. [Google Scholar] [CrossRef]
- Fulignati, S. Novel catalytic strategies for the synthesis of furans and their derivatives. Catalysts 2024, 14, 349. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Kastner, J.R. A kinetic model of multi-step furfural hydrogenation over a Pd-TiO2 supported activated carbon catalyst. Chem. Eng. J. 2021, 414, 128693. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Q.; Xu, J.; Li, L.; Cui, Y.-T.; Lang, R.; Li, L.; Su, Y.; Miao, S.; Sun, H.; et al. Catalytic cascade conversion of furfural to 1,4-pentanediol in a single reactor. Green Chem. 2018, 20, 1770–1776. [Google Scholar] [CrossRef]
- Taraban’ko, V.E.; Smirnova, M.A.; Zhizhina, E.G. Methods for the synthesis of γ-acetopropyl alcohol. Catal. Ind. 2022, 14, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Zolotarev, N.S.; Latvis, P.P.; Buimov, A.A.; Sirotenko, V.I.; Lisnyanskii, I.M.; Novikova, K.E.; Bogatyrev, Y.V.; Zhdanovich, E.S. A study of the process of preparing γ-acetopropyl alcohol from furfural. Pharm. Chem. J. 1972, 6, 184–187. [Google Scholar] [CrossRef]
- Swadesh, S.; Smith, S.; Dunlop, A.P. Mechanism of hydrogenation of 2-methylfuran. J. Org. Chem. 1951, 16, 476–479. [Google Scholar] [CrossRef]
- Bel’skii, I.F.; Shuikin, N.I. Catalytic hydrogenation and hydrogenolysis of furan compounds. Russ. Chem. Rev. 1963, 32, 307. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Talsi, V.P.; Gulyaeva, T.I.; Trenikhin, M.V.; Belskaya, O.B. Aqueous-phase hydrogenation of furfural over supported palladium catalysts: Effect of the support on the reaction routes. React. Kinet. Mech. Catal. 2018, 12, 811–827. [Google Scholar] [CrossRef]
- Soós, J. Mechanism of the formation of 5-hydroxy-2-pentanone from 2-methylfuran in alkaline media. React. Kinet. Catal. Lett. 1987, 34, 333–337. [Google Scholar] [CrossRef]
- Fu, X.X.; Li, J.P.; Li, Z.Q.; Liu, Y.; Feng, C.X.; Wang, H.Y.; Zhao, Z.P.; Liu, Q.-Y.; Liu, Z.Y.; Peng, Z.K. Selective conversion of 2-methylfuran to 3-acetyl-1-propanol in water over Pd@HZSM-5 catalyst with balanced metal-acid cooperation. J. Catal. 2022, 413, 648–657. [Google Scholar] [CrossRef]
- Perchenok, M.S.; Shevchenko, V.S.; Komarov, V.M.; Zavel’skii, D.Z. Continuous method of obtaining 3-acetylpropan-1-ol from methylfuran. Pharm. Chem. J. 1976, 10, 222–226. [Google Scholar] [CrossRef]
- Londergan, T.E.; Hause, N.L.; Schmitz, W.R. A new synthesis of the thiazole fragment of vitamin B11. J. Am. Chem. Soc. 1953, 75, 4456–4458. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, S.K. Room-temperature total hydrogenation of biomass-derived furans and furan/acetone aldol adducts over a Ni–Pd Alloy Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 4793–4800. [Google Scholar] [CrossRef]
- Jin, Z.; Yi, X.; Wang, L.; Xu, S.; Wang, C.; Wu, Q.; Wang, L.; Zheng, A.; Xiao, F.-S. Metal-acid interfaces enveloped in zeolite crystals for cascade biomass hydrodeoxygenation. Appl. Catal. B Environ. 2019, 254, 560–568. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Zhang, Y.; Su, Y.; Wang, A.; Zhang, T. Ru/H-beta as an efficient catalyst for the conversion of furfural into 3-acetyl-1-propanol (3-AP) toward one-pot transformation of xylan to 3-AP. Mol. Catal. 2019, 476, 110506. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Wu, Z.; Wang, H.; Wang, C.; Han, S.; Xiao, F.S. Solvent-free synthesis of core–shell Zn/ZSM-5@Silicalite-1 catalyst for selective conversion of methanol to BTX Aromatics. Ind. Eng. Chem. Res. 2019, 58, 15453–15458. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Zhu, H.; Yuan, P.; Yang, C.; Li, C.; Bao, X. Insights into the reaction pathway of n-butane conversion over HZSM-5 zeolite at low temperature. Appl. Catal. A Gen. 2019, 584, 117135. [Google Scholar] [CrossRef]
- Desmurs, L.; Cammarano, C.; Gimello, O.; Galarneau, A.; Hulea, V. Influence of the mesoporosity of hierarchical ZSM-5 in toluene alkylation by methanol. Materials 2023, 16, 6872. [Google Scholar] [CrossRef]
- Tong, Y.; Ke, M. Study on the Acidic Modification of Mesoporous HZSM-5 Zeolite and Its Catalytic Cracking Performance. Catalysts 2024, 14, 713. [Google Scholar] [CrossRef]
- Jokar, F.; Alavi, S.M.; Rezaei, M. Investigating the hydroisomerization of n-pentane using Pt supported on ZSM-5, desilicated ZSM-5, and modified ZSM-5/MCM-41. Fuel 2022, 324, 124511. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xiao, F.-S. Metal@Zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef]
- Chai, Y.; Shang, W.; Li, W.; Wu, G.; Dai, W.; Guan, N.; Li, L. Noble metal particles confined in zeolites: Synthesis, characterization, and applications. Adv. Sci. 2019, 6, 1900299. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.; He, S.; Xiao, F.-S. Rational construction of metal nanoparticles fixed in zeolite crystals as highly efficient heterogeneous catalysts. Nano Today 2018, 20, 74–83. [Google Scholar] [CrossRef]
- Choi, M.; Wu, Z.; Iglesia, E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. J. Am. Chem. Soc. 2010, 132, 9129–9137. [Google Scholar] [CrossRef]
- Otto, T.; Zones, S.I.; Iglesia, E. Challenges and strategies in the encapsulation and stabilization of monodisperse Au clusters within zeolites. J. Catal. 2016, 339, 195–208. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Zhang, T.; Bai, R.; Mayoral, A.; Zhang, P.; Zhang, Q.; Terasaki, O.; Yu, J. Zeolite-Encaged Single-Atom Rhodium Catalysts: Highly-Efficient Hydrogen Generation and Shape-Selective Tandem Hydrogenation of Nitroarenes. Angew. Chem. Int. Ed. Engl. 2019, 58, 18570–18576. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Fan, Q.; Zeng, L.; Mayoral, A.; Miao, S.; Yang, R.; Jiang, Z.; Zhou, W.; Zhang, J.; et al. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. Engl. 2020, 59, 19450–19459. [Google Scholar] [CrossRef]
- Liu, L.; Diaz, U.; Arenal, R.; Agostini, G.; Concepcion, P.; Corma, A. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 2017, 16, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Qin, G.; Wang, L.; Zuidema, E.; Yang, Q.; Dang, S.; Yang, C.; Xiao, J.; Meng, X.; et al. Direct conversion of syngas to ethanol within zeolite crystals. Chem 2020, 6, 646–657. [Google Scholar] [CrossRef]
- Ding, J.; Wang, M.; Peng, L.; Xue, N.; Wang, Y.; He, M.Y. Combined desilication and phosphorus modification for high-silica ZSM-5 zeolite with related study of hydrocarbon cracking performance. Appl. Catal. A Gen. 2015, 503, 147–155. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Sun, J.; Guo, W.; Wang, Q. Effects of phosphorus-modified HZSM-5 on distribution of hydrocarbon compounds from wood–plastic composite pyrolysis using Py-GC/MS. J. Anal. Appl. Pyrolysis 2015, 116, 223–230. [Google Scholar] [CrossRef]
- Ramesh, K.; Jie, C.; Han, Y.F.; Borgna, A. Synthesis, characterization, and catalytic activity of phosphorus modified H-ZSM-5 catalysts in selective ethanol dehydration. Ind. Eng. Chem. Res. 2010, 49, 4080–4090. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Fu, X.; Gao, C.; Huang, J.; Li, B.; Yang, Y.; Gao, J.; Shen, Y.; Peng, Z.; Yang, J.H.; et al. Enhancing the matching of acid/metal balance by engineering an extra Si–Al framework outside the Pd/HBeta catalyst towards benzene hydroalkylation. Catal. Sci. Technol. 2020, 10, 1467–1476. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, J.; Wang, H.; Lewis, J.P.; Xiao, F.S. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. J. Am. Chem. Soc. 2016, 138, 7880–7883. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Yang, S.; Cao, C.; Song, W. Palladium nanoparticles encapsulated in a silicalite-1 zeolite shell for size-selective catalysis in liquid-phase solution. ChemCatChem 2016, 8, 1279–1282. [Google Scholar] [CrossRef]
- Rogers, S.M.; Catlow, C.R.A.; Chan-Thaw, C.E.; Chutia, A.; Jian, N.; Palmer, R.E.; Perdjon, M.; Thetford, A.; Dimitratos, N.; Villa, A.; et al. Tandem site- and size-controlled Pd nanoparticles for the directed hydrogenation of furfural. ACS Catal. 2017, 7, 2266–2274. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Yuan, D.; Leng, L.; Zhang, M.; Di, M.; Horton, J.H.; Wang, J.; Sun, L.; Sun, W. Photoinduction of palladium single atoms supported on defect-containing γ-AlOOH nanoleaf for efficient trans-stilbene epoxidation. Chem. Eng. J. 2022, 429, 132149. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Martínez, T.J. Hydrothermal stabilization of ZSM-5 catalytic-cracking additives by phosphorus addition. J. Catal. 2006, 237, 267–277. [Google Scholar] [CrossRef]
- Lercher, J.A.; Rumplmayr, G. Controlled decrease of acid strength by orthophosphoric acid on ZSM5. Appl. Catal. 1986, 25, 215–222. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q. Enhanced acidity and thermal stability of mesoporous materials with post-treatment with phosphoric acid. Chem. Lett. 1999, 28, 829–830. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Z.; Ding, L.; Huang, K.; Xue, N.; Peng, L.; Ding, W. Platinum nanoparticles encapsulated in HZSM-5 crystals as an efficient catalyst for green production of p-aminophenol. Catal. Commun. 2017, 97, 98–101. [Google Scholar] [CrossRef]
- Mi, X.T.; Li, X.G.; Chang, Y.; Zhang, Y.K.; Li, Y.H.; Cao, H.; Hou, Z.G. Collaborative construction of nanoscale ZSM-5 aggregates by crystal seeds and templates. Inorg. Chem. Ind. 2023, 55, 130–135. [Google Scholar] [CrossRef]
- Xie, H.Y. Synthesis of ZSM-5 Molecular Sieves by Steam-Assisted Method and Its Catalytic Properties. Ph.D. Thesis, Qingdao University of Science and Technology, Qingdao, China, 2023. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J. Am. Chem. Soc. 2014, 136, 15280–15290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).