Recent Advances in Iron Oxide-Based Heterojunction Photo-Fenton Catalysts for the Elimination of Organic Pollutants
Abstract
1. Introduction
2. Classification of Heterojunctions
2.1. Schottky Junction
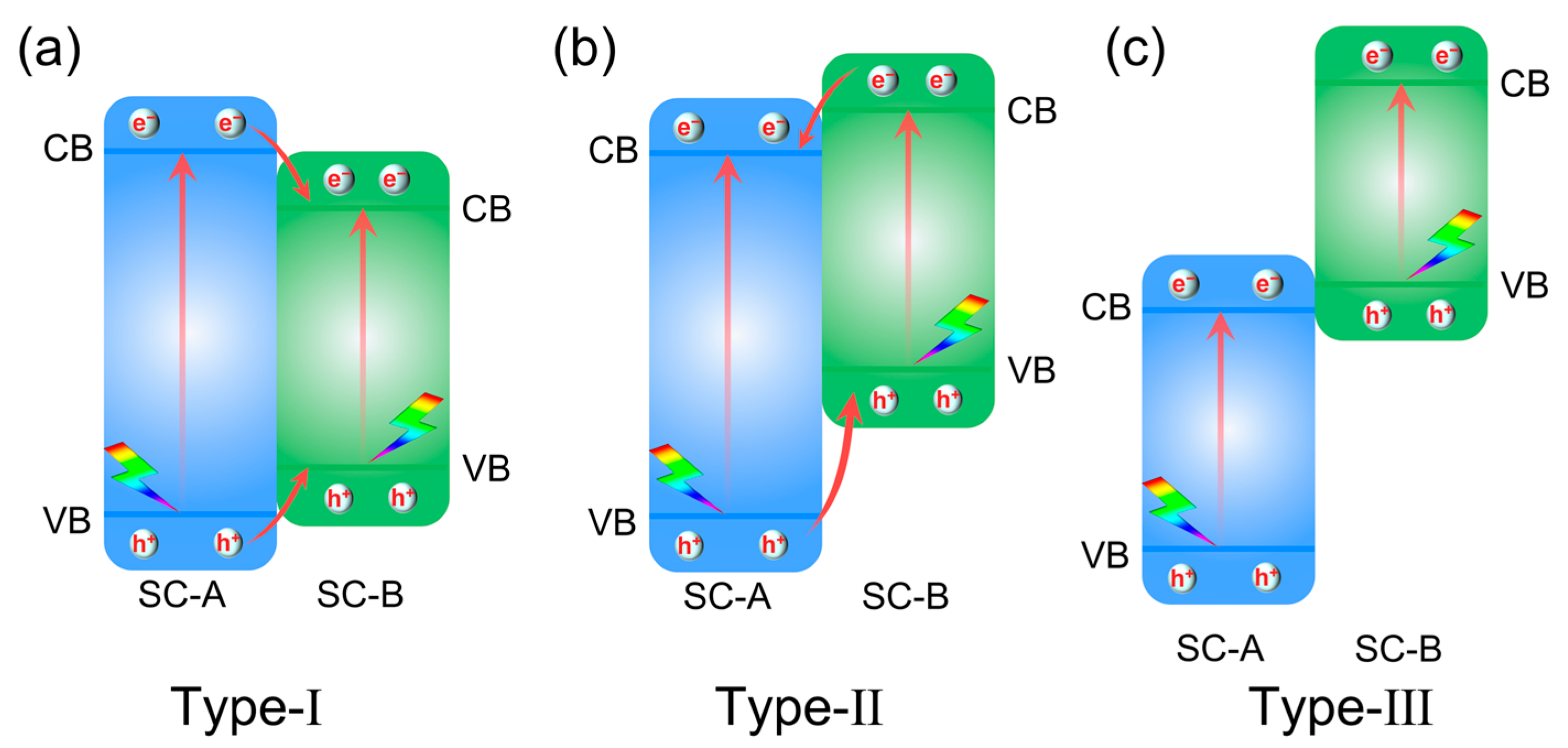
2.2. Type-I, Type-II, and Type-III
2.3. Z-Scheme and S-Scheme
3. Synthesis Methods of Iron Oxide-Based Heterojunction
4. Iron Oxide-Based Heterojunction with Metal Oxides
4.1. Iron Oxide-Based Heterojunction with TiO2
4.2. Iron Oxide-Based Heterojunction with ZnO
5. Iron Oxide-Based Heterojunction with Bismuth-Related Semiconductors
5.1. Iron Oxide-Based Heterojunction with BiOX (X = Cl, Br, I)
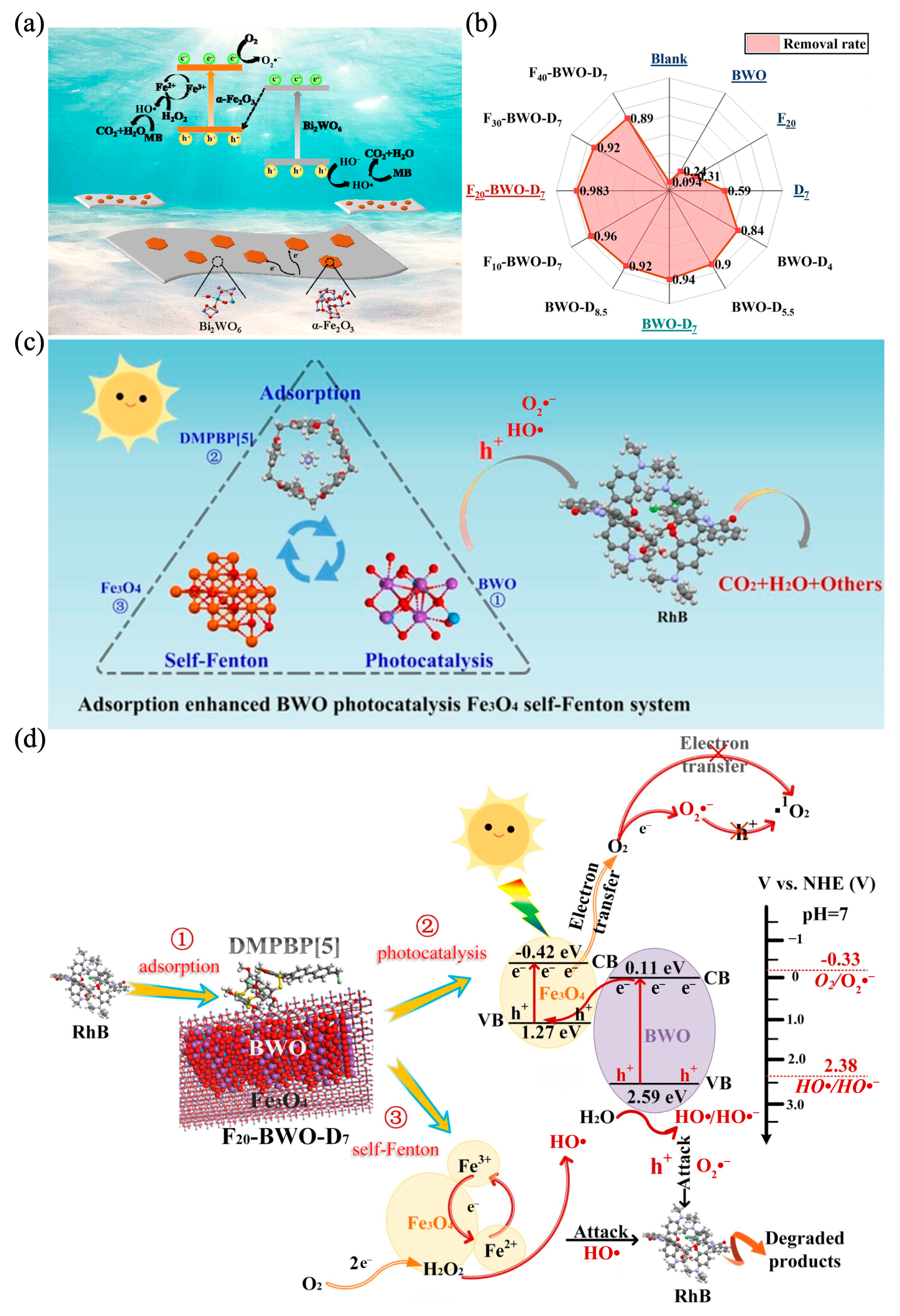
5.2. Iron Oxide-Based Heterojunction with Bi2WO6
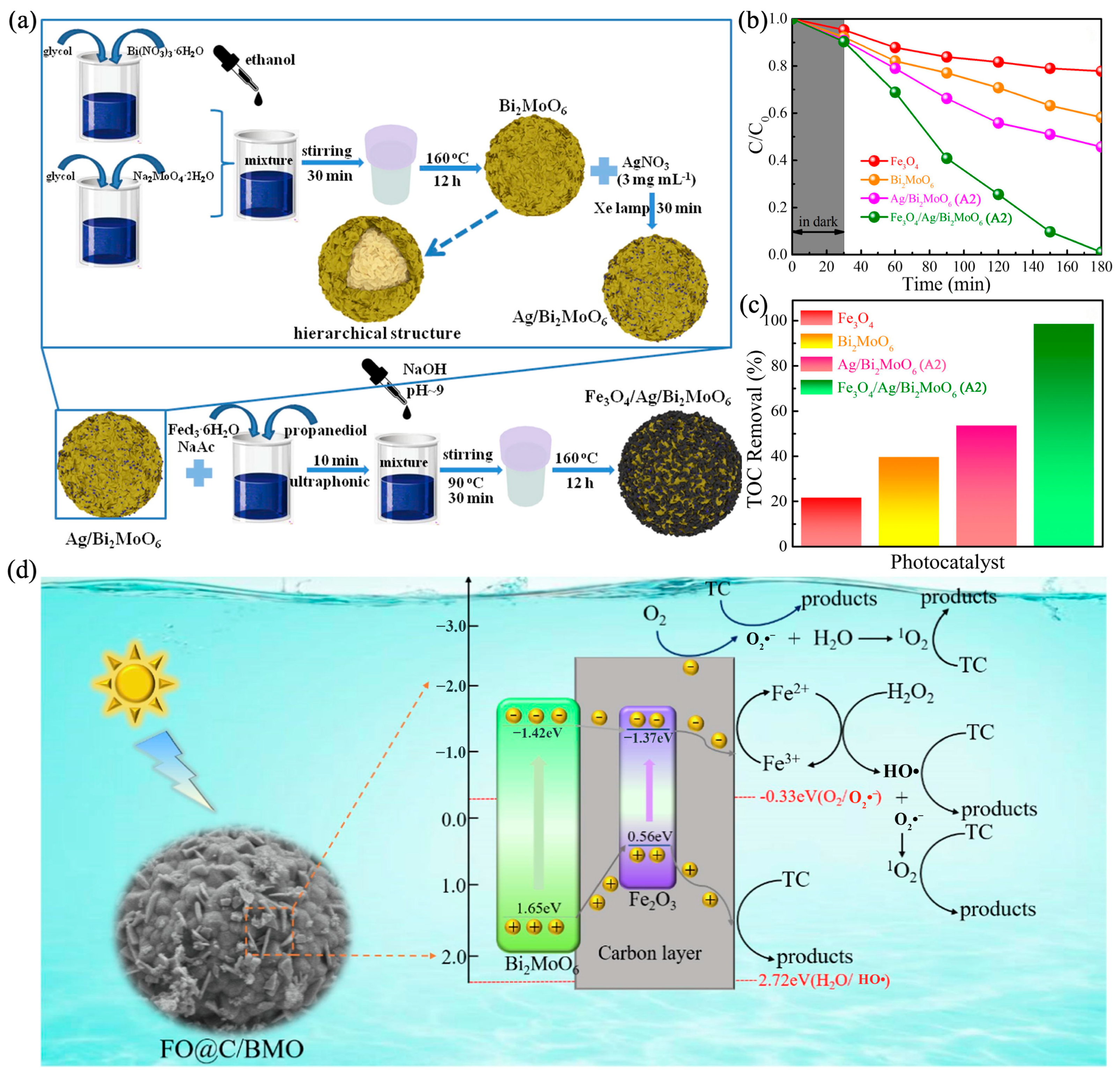
5.3. Iron Oxide-Based Heterojunction with Bi2MoO6
6. Iron Oxide-Based Heterojunction with Carbon-Based Materials
6.1. Iron Oxide-Based Heterojunction with Metal–Organic Frameworks (MOFs)
6.2. Iron Oxide-Based Heterojunction with g-C3N4
6.3. Iron Oxide-Based Heterojunction with Graphene Derivatives (GO/rGO)
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDCs | Endocrine disruptors |
| PPCPs | Pharmaceuticals and personal care products |
| PFCs | Perfluorinated compounds |
| BFRs | Brominated flame retardants |
| AOPs | Advanced oxidation processes |
| ROS | Reactive oxygen species |
| HO• | Hydroxyl radicals |
| O2•− | Superoxide radicals |
| 1O2 | Singlet oxygen |
| SO4•− | Sulfate radicals |
| VB | Valence band |
| CB | Conduction band |
| e− | Electron |
| h+ | Hole |
| –OH | Surface hydroxyl |
| α-Fe2O3 | Hematite |
| γ-Fe2O3 | Maghemite |
| Fe3O4 | Magnetite |
| FeO | Wurtzite |
| TAS | Transient absorption spectroscopy |
| SC | Semiconductor |
| EF | Fermi energy level |
| IEF | Internal electric field |
| TOC | Total organic carbon |
| CFX | Cefuroxime sodium salt |
| MB | Methylene blue |
| UV-vis DRS | UV-vis diffuse reflectance spectra |
| TC-H | Tetracycline hydrochloride |
| VSM | Vibrating sample magnetometer |
| PL | Photoluminescence |
| RhB | Rhodamine B |
| CBZ | Carbamazepine |
| T.E.S.T | Toxicity estimation software tool |
| DFT | Density functional theory |
| BPA | Bisphenol A |
| EPR | Electron paramagnetic resonance |
| MOFs | Metal–organic frameworks |
| LEV | Levofloxacin |
| g-C3N4 | Graphite-phase carbon nitride |
| FTIR | Fourier transform infrared spectrometer |
| FESEM | Field emission scanning electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| CIP | Ciprofloxacin |
| FED | Frontier electron density |
| GO | Graphene oxide |
| rGO | Reduced graphene oxide |
| NOM | Natural organic matter |
References
- Weng, H.; Yang, Y.; Zhang, C.; Cheng, M.; Wang, W.; Song, B.; Luo, H.; Qin, D.; Huang, C.; Qin, F.; et al. Insight into FeOOH-Mediated Advanced Oxidation Processes for the Treatment of Organic Polluted Wastewater. Chem. Eng. J. 2023, 453, 139812. [Google Scholar] [CrossRef]
- Ruan, Y.; Kong, L.; Zhong, Y.; Diao, Z.; Shih, K.; Hou, L.; Wang, S.; Chen, D. Review on the Synthesis and Activity of Iron-Based Catalyst in Catalytic Oxidation of Refractory Organic Pollutants in Wastewater. J. Clean. Prod. 2021, 321, 128924. [Google Scholar] [CrossRef]
- Liu, M.-L.; Li, L.; Sun, Y.-X.; Fu, Z.-J.; Cao, X.-L.; Sun, S.-P. Scalable Conductive Polymer Membranes for Ultrafast Organic Pollutants Removal. J. Membr. Sci. 2021, 617, 118644. [Google Scholar] [CrossRef]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Abu-Nada, A.; Abdala, A.; McKay, G. Removal of Phenols and Dyes from Aqueous Solutions Using Graphene and Graphene Composite Adsorption: A Review. J. Environ. Chem. Eng. 2021, 9, 105858. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent Advancement in Remediation of Synthetic Organic Antibiotics from Environmental Matrices: Challenges and Perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Kamenická, B. Chemical Degradation of Azo Dyes Using Different Reducing Agents: A Review. J. Water Process Eng. 2024, 61, 105350. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F.; et al. Fe-Based Metal Organic Frameworks (Fe-MOFs) for Organic Pollutants Removal via Photo-Fenton: A Review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, X.; Jia, Y.; Xu, H.; Pan, B. Oxidative Polymerization in Water Treatment: Chemical Fundamentals and Future Perspectives. Environ. Sci. Technol. 2025, 59, 1060–1079. [Google Scholar] [CrossRef]
- Kumar, L.; Ragunathan, V.; Chugh, M.; Bharadvaja, N. Nanomaterials for Remediation of Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 3139–3163. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhang, X.; Du, P.; Zhou, B.; Meng, F.; Wei, C.; Zhou, L.; Wen, G.; Wang, Y. Manganese Oxide Catalytic Materials for Degradation of Organic Pollutants in Advanced Oxidation Processes: A Review. J. Water Process Eng. 2024, 66, 106048. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Gul, N.S.; Sabahat, S.; Sun, J.; Tahir, K.; Shah, N.S.; Muhammad, N.; Rahim, A.; Imran, M.; Iqbal, J.; et al. Removal of Organic Pollutants through Hydroxyl Radical-Based Advanced Oxidation Processes. Ecotoxicol. Environ. Saf. 2023, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.V.; Miao, J.; Duan, L.; Yi, J.; He, C.; Jiang, Y.; Chen, Y.; Kakalatsa, S.S.; Duan, Z.; Farooq, U.; et al. Synergy of Adsorption and Fenton Processes in Water Decontamination: A Review. Sep. Purif. Technol. 2024, 348, 127803. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Fan, X.; Zhu, G.; Liu, Y.; Quan, X. Efficient Electro-Fenton Degradation of Organic Pollutants via the Synergistic Effect of 1O2 and •OH Generated on Single Fe—N4 Sites. Sci. Total Environ. 2024, 932, 173042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Zhang, W.; Zhang, Y.; Wang, S.; Zhu, Z.; Xiang, B.; Wang, C. Fabrication of N-Doped Graphitic Carbon Encapsulated Fe3C/Fe Nanoparticles with Dual-Reaction Centers for Highly Effective Fenton-like Degradation of Organic Pollutants. Surf. Interfaces 2024, 49, 104388. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, Y.; Yan, X.; Gan, Y.; Xia, C.; Xu, Y.; Xie, M. Nitrogen-Defect-Modified g-C3N4/BaFe12O19 S-Scheme Heterojunction Photocatalyst with Enhanced Advanced Oxidation Technology Synergistic Photothermal Degradation Ability of Antibiotic: Insights into Performance, Electron Transfer Pathways and Toxicity. Appl. Catal. B-Environ. 2024, 343, 123485. [Google Scholar] [CrossRef]
- Chen, Q.; Lü, F.; Zhang, H.; He, P. Where Should Fenton Go for the Degradation of Refractory Organic Contaminants in Wastewater? Water Res. 2023, 229, 119479. [Google Scholar] [CrossRef]
- Deng, F.; Olvera-Vargas, H.; Zhou, M.; Qiu, S.; Sirés, I.; Brillas, E. Critical Review on the Mechanisms of Fe2+ Regeneration in the Electro-Fenton Process: Fundamentals and Boosting Strategies. Chem. Rev. 2023, 123, 4635–4662. [Google Scholar] [CrossRef]
- Deng, G.; Wang, Z.; Ma, J.; Jiang, J.; He, D.; Li, X.; Szczuka, A.; Zhang, Z. Ferryl Ion in the Photo-Fenton Process at Acidic pH: Occurrence, Fate, and Implications. Environ. Sci. Technol. 2023, 57, 18586–18596. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, Y.; Wang, Y.; Sun, S.-P.; Wu, W.D.; Wu, Z. Nanostructured Semiconductor Supported Iron Catalysts for Heterogeneous Photo-Fenton Oxidation: A Review. J. Mater. Chem. A 2020, 8, 15513–15546. [Google Scholar] [CrossRef]
- Liu, T.; Hu, K.; Li, Y.; Wang, Y.; Han, D.; Wang, Z.; Gu, F. The Z-scheme MIL-88B(Fe)/BiOBr Heterojunction Promotes Fe(III)/Fe(II) Cycling and photocatalytic-Fenton-like Synergistically Enhances the Degradation of Ciprofloxacin. Small 2024, 20, 2309541. [Google Scholar] [CrossRef] [PubMed]
- Oller, I.; Malato, S. Photo-Fenton Applied to the Removal of Pharmaceutical and Other Pollutants of Emerging Concern. Curr. Opin. Green Sustain. Chem. 2021, 29, 100458. [Google Scholar] [CrossRef]
- Choe, Y.J.; Kim, J.; Choi, I.-S.; Kim, S.H. Metal Oxides for Fenton Reactions toward Radical-Assisted Water Treatment: A Review. J. Ind. Eng. Chem. 2025, 142, 127–140. [Google Scholar] [CrossRef]
- Li, J.; You, J.; Wang, Z.; Zhao, Y.; Xu, J.; Li, X.; Zhang, H. Application of α-Fe2O3-Based Heterogeneous Photo-Fenton Catalyst in Wastewater Treatment: A Review of Recent Advances. J. Environ. Chem. Eng. 2022, 10, 108329. [Google Scholar] [CrossRef]
- Xu, G.; Liu, Q.; Mai, Z.; Wang, M.; Zhao, H.; Xu, K. Strategies for Improving Performance of Iron-Based Catalysts in Activating Heterogeneous Fenton-like Oxidation in Pollutants Degradation: From the Perspective of Materials Structure Design. Process Saf. Environ. Prot. 2024, 190, 794–820. [Google Scholar] [CrossRef]
- Bouzayani, B.; Sanromán, M.Á. Polymer-Supported Heterogeneous Fenton Catalysts for the Environmental Remediation of Wastewater. Molecules 2024, 29, 2188. [Google Scholar] [CrossRef]
- Fathabadi, M.; Qorbani, M.; Sabbah, A.; Quadir, S.; Huang, C.-Y.; Chen, K.-H.; Chen, L.-C.; Naseri, N. An Ultrathin Amorphous Defective co-Doped Hematite Passivation Layer Derived via an in Situ Electrochemical Method for Durable Photoelectrochemical Water Oxidation. J. Mater. Chem. A 2022, 10, 16655–16665. [Google Scholar] [CrossRef]
- Park, J.; Kang, J.; Chaule, S.; Jang, J.-H. Recent Progress and Perspectives on Heteroatom Doping of Hematite Photoanodes for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2023, 11, 24551–24565. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite Heterostructures for Photoelectrochemical Water Splitting: Rational Materials Design and Charge Carrier Dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Kronawitter, C.X.; Vayssieres, L.; Shen, S.; Guo, L.; Wheeler, D.A.; Zhang, J.Z.; Antoun, B.R.; Mao, S.S. A Perspective on Solar-Driven Water Splitting with All-Oxide Hetero-Nanostructures. Energy Environ. Sci. 2011, 4, 3889–3899. [Google Scholar] [CrossRef]
- Miao, J.; Yang, Y.; Cui, P.; Ru, C.; Zhang, K. Improving Charge Transfer Beyond Conventional Heterojunction Photoelectrodes: Fundamentals, Strategies and Applications. Adv. Funct. Mater. 2024, 34, 2406443. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and Catalytic Mechanisms of Heterojunction Photocatalysts and the Application of Titanium Dioxide (TiO2)-Based Heterojunctions in Environmental Remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Y.; Song, Y.; Ma, Z.; Gao, Q.; Liu, Y.; Li, J.; Wu, X.; Wang, X.; Mao, W.; et al. Recent Advances in Photocatalytic Hydrogen Evolution of AgIn5S8-based Photocatalysts. Interdiscip. Mater. 2023, 2, 669–688. [Google Scholar] [CrossRef]
- Luo, C.; Yang, C.; Xie, J.; Li, X.; Lin, Y.; Tong, S.; He, S. Role of Polarized and Interfacial Built-in Electric Fields in Photocatalysts for Enhanced Photocatalytic Performance. Chem. Eng. J. 2024, 497, 155514. [Google Scholar] [CrossRef]
- Li, G.; Yang, C.; He, Q.; Liu, J. Ag-Based Photocatalytic Heterostructures: Construction and Photocatalytic Energy Conversion Application. J. Environ. Chem. Eng. 2022, 10, 107374. [Google Scholar] [CrossRef]
- Suresh, R.; Gnanasekaran, L.; Rajendran, S.; Soto-Moscoso, M.; Chen, W.-H.; Show, P.L.; Khoo, K.S. Application of Nanocomposites in Integrated Photocatalytic Techniques for Water Pollution Remediation. Environ. Technol. Innov. 2023, 31, 103149. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Y.; Lu, C.; Lin, X.; Fu, Z.; Shi, W.; Guo, F. Photocatalytic Self-Fenton System of g-C3N4-Based for Degradation of Emerging Contaminants: A Review of Advances and Prospects. Molecules 2023, 28, 5916. [Google Scholar] [CrossRef]
- Dai, C.; Feng, Z.; Hu, Q.; Qiu, J.; You, J.; Guo, R.; Liu, X.; Zhang, H. Recent Progress in Modification and Composite Strategies of Graphitic Carbon Nitride as Catalysts for Heterogeneous Photo-Fenton Reaction. Mater. Sci. Semicond. Process. 2023, 167, 107807. [Google Scholar] [CrossRef]
- Poonia, K.; Hasija, V.; Singh, P.; Parwaz Khan, A.A.; Thakur, S.; Thakur, V.K.; Mukherjee, S.; Ahamad, T.; Alshehri, S.M.; Raizada, P. Photocatalytic Degradation Aspects of Atrazine in Water: Enhancement Strategies and Mechanistic Insights. J. Clean. Prod. 2022, 367, 133087. [Google Scholar] [CrossRef]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A Heterojunction Strategy to Improve the Visible Light Sensitive Water Splitting Performance of Photocatalytic Materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumée, L.F. Engineering Schottky-like and Heterojunction Materials for Enhanced Photocatalysis Performance—A Review. Mater. Adv. 2022, 3, 2309–2323. [Google Scholar] [CrossRef]
- Li, J.; Xu, N.; Zhang, Y.; Dong, H.; Li, C. Research Progress of Heterogeneous Photocatalyst for H2O2 Production: A Mini Review. Chin. Chem. Lett. 2024, 110470. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and Status of Polymeric Graphitic Carbon Nitride Based Z-Scheme Photocatalytic Systems for Sustainable Photocatalytic Water Purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Yi, P.; Li, Y.; Wu, X.-L.; Duan, X. Z-Scheme Single-Atom Photocatalyst for Advanced Oxidation Processes. Curr. Opin. Chem. Eng. 2024, 45, 101028. [Google Scholar] [CrossRef]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton Degradation of Tetracycline over Z-Scheme Fe-g-C3N4/Bi2WO6 Heterojunctions: Mechanism Insight, Degradation Pathways and DFT Calculation. Appl. Catal. B 2022, 310, 121326. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Wang, H.; Fang, Y.; Huang, Y.; Wang, S. Carbon Nitride-Based Z-Scheme Heterojunctions for Solar-Driven Advanced Oxidation Processes. J. Hazard. Mater. 2022, 434, 128866. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Wu, H.; Yang, Y.; Wang, C.; Wang, Q.; Jia, B.; Zheng, J. Research Progress on Zinc Oxide-Based Heterojunction Photocatalysts. J. Mater. Chem. A 2024, 12, 20838–20867. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Wen, X.; Chen, S.; Cheng, M.; Deng, R.; Li, B.; Yang, Y.; Liu, X. Silver-Based Semiconductor Z-Scheme Photocatalytic Systems for Environmental Purification. J. Hazard. Mater. 2020, 390, 122128. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced Photocatalytic Performance of Direct Z-Scheme g-C3N4–TiO2 Photocatalysts for the Decomposition of Formaldehyde in Air. Phys. Chem. Chem. Phys. 2013, 15, 16883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Yu, J.; García, H. Charge-Transfer Dynamics in S-Scheme Photocatalyst. Nat. Rev. Chem. 2025, 2025, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bie, C.; Yu, J. Challenges of Z-Scheme Photocatalytic Mechanisms. Trends Chem. 2022, 4, 973–983. [Google Scholar] [CrossRef]
- Zhu, B.; Sun, J.; Zhao, Y.; Zhang, L.; Yu, J. Construction of 2D S-scheme Heterojunction Photocatalyst. Adv. Mater. 2024, 36, 2310600. [Google Scholar] [CrossRef]
- Nie, C.; Wang, X.; Lu, P.; Zhu, Y.; Li, X.; Tang, H. Advancements in S-Scheme Heterojunction Materials for Photocatalytic Environmental Remediation. J. Mater. Sci. Technol. 2024, 169, 182–198. [Google Scholar] [CrossRef]
- Ye, Y.-Y.; Yang, H.-Q.; Chen, Z.-Y.; Chen, W.; Zhang, J.; Zhang, M.; Wang, L.; Song, Z.; Huang, G.-B. Fabrication of S-Scheme FeIn2S4/Fe2O3 Heterostructures with Improved Photo-Fenton Catalytic Activity for Removing Pharmacologically Active Compounds. J. Alloys Compd. 2025, 1010, 178249. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Zhang, Z.; Zeng, W.; Gao, M.; Lv, Y.; Wei, T.; Ren, Y.; Fan, Z. Pt Enhanced the Photo-Fenton Activity of ZnFe2O4/α-Fe2O3 Heterostructure Synthesized via One-Step Hydrothermal Method. J. Colloid Interface Sci. 2020, 561, 793–800. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, S.; Qu, J.; Li, J.; Liu, Y.; Wang, Q.; Jing, J.; Yuan, Y.; Yao, T.; Wu, J. Transforming Type-II Fe2O3@polypyrrole to Z-Scheme Fe2O3@polypyrrole/Prussian Blue via Prussian Blue as Bridge: Enhanced Activity in Photo-Fenton Reaction and Mechanism Insight. J. Hazard. Mater. 2021, 405, 124668. [Google Scholar] [CrossRef]
- GC, S.S.; Alkanad, K.; Hezam, A.; Alsalme, A.; Al-Zaqri, N.; NK, L. Enhanced Photo-Fenton Activity over a Sunlight-Driven Ignition Synthesized α-Fe2O3-Fe3O4/CeO2 Heterojunction Catalyst Enriched with Oxygen Vacancies. J. Mol. Liq. 2021, 335, 116186. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Zhan, H.; Wen, M.; Guo, Q.; Chen, X.; Li, H. The Construction of S-Scheme Heterojunction between P-CN/NCDs/Fe3O4 to Enhance the Synergistic Degradation of Levofloxacin by Photo-Fenton Oxidation. J. Alloys Compd. 2025, 1010, 178189. [Google Scholar] [CrossRef]
- Li, Y.; Cao, H.; Liu, W.; Liu, P. Effective Degradation of Tetracycline via Recyclable Cellulose Nanofibrils/Polyvinyl Alcohol/Fe3O4 Hybrid Hydrogel as a Photo-Fenton Catalyst. Chemosphere 2022, 307, 135665. [Google Scholar] [CrossRef] [PubMed]
- Subha, N.; Mahalakshmi, M.; Monika, S.; Senthil Kumar, P.; Preethi, V.; Vaishnavi, G.; Rajabhuvaneswari, A. Heterostructured γ-Fe2O3/FeTiO3 Magnetic Nanocomposite: An Efficient Visible-Light-Driven Photocatalyst for the Degradation of Organic Dye. Chemosphere 2022, 306, 135631. [Google Scholar] [CrossRef]
- Sharma, K.; Sudhaik, A.; Sonu; Kumar, R.; Nguyen, V.-H.; Le, Q.V.; Ahamad, T.; Thakur, S.; Kaya, S.; Nguyen, L.H.; et al. Advanced Photo-Fenton Assisted Degradation of Tetracycline Antibiotics Using α-Fe2O3/CdS/SiO2 Based S-Scheme Photocatalyst. J. Water Process Eng. 2024, 59, 105011. [Google Scholar] [CrossRef]
- Zenna, O.; Younis, S.A.; Hamed, S.; Zaki, T.; Makki, S. Establishing an Affordable Solar-Floating Fe2O3@A1-xRx-TiO2 Photo-Fenton Catalytic System through the Cyclic Utilization of Iron Waste to de-Pollute Textile Water Contamination. J. Environ. Manag. 2024, 366, 121863. [Google Scholar] [CrossRef]
- Hassan, M.E.; Chen, Y.; Liu, G.; Zhu, D.; Cai, J. Heterogeneous Photo-Fenton Degradation of Methyl Orange by Fe2O3/TiO2 Nanoparticles under Visible Light. J. Water Process Eng. 2016, 12, 52–57. [Google Scholar] [CrossRef]
- Deng, X.; Yang, Y.; Mei, Y.; Li, J.; Guo, C.; Yao, T.; Guo, Y.; Xin, B.; Wu, J. Construction of Fe3O4@FeS2@C@MoS2 Z-Scheme Heterojunction with Sandwich-like Structure: Enhanced Catalytic Performance in Photo-Fenton Reaction and Mechanism Insight. J. Alloys Compd. 2022, 901, 163437. [Google Scholar] [CrossRef]
- Tian, S.; Cai, X.; Zhou, X.; Luo, Z.; Yu, J.; Ge, J.; Li, Y. S Vacancies and Heterojunction Modulated MoS2/C@Fe3O4 towards Improved Full-Spectrum Photo-Fenton Catalysis: Enhanced Interfacial Charge Transfer and NIR Absorption. Chem. Eng. J. 2024, 498, 155430. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Wang, B.; Chen, M.; Ran, L.; Liu, S.; Zhou, M.; Zhang, L.; Jiang, Y.; Dai, X.; et al. Fabrication of Close-Contact S-Scheme Cr2Bi3O11-Bi2O3/Fe3O4@porous Carbon Microspheres Based on in-Situ Reaction: Enhanced Photo-Fenton Wastewater Treatment. J. Colloid Interface Sci. 2024, 673, 690–699. [Google Scholar] [CrossRef]
- Deng, Y.; Xing, M.; Zhang, J. An Advanced TiO2/Fe2TiO5/Fe2O3 Triple-Heterojunction with Enhanced and Stable Visible-Light-Driven Fenton Reaction for the Removal of Organic Pollutants. Appl. Catal. B 2017, 211, 157–166. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Yue, C.; He, L.; Li, H.; Zhang, H.; Yang, S.; Ma, T. Photocatalytic Degradation by TiO2-Conjugated/Coordination Polymer Heterojunction: Preparation, Mechanisms, and Prospects. Appl. Catal. B 2024, 344, 123605. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, W.; Hou, C.; Wang, L.; Cai, H.; Wang, S.; Dong, G.; Wang, C. Confined Growth of Oxygen-Vacancy Rich TiO2-x Nanoparticles with Heterophase Junction for High-Efficiency Photocatalytic Water Purification. Surf. Interfaces 2024, 48, 104323–104335. [Google Scholar] [CrossRef]
- Padmanabhan, N.T.; Thomas, N.; Louis, J.; Mathew, D.T.; Ganguly, P.; John, H.; Pillai, S.C. Graphene Coupled TiO2 Photocatalysts for Environmental Applications: A Review. Chemosphere 2021, 271, 129506. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Sun, N.; Qiu, Y.; Zhu, L.; Du, Z.; Liu, F. A Direct Z-Scheme 0D α-Fe2O3/TiO2 Heterojunction for Enhanced Photo-Fenton Activity with Low H2O2 Consumption. Chin. Chem. Lett. 2024, 35, 109698. [Google Scholar] [CrossRef]
- Hernández-Coronado, E.E.; Ruiz-Ruiz, E.J.; Hinojosa-Reyes, L.; Beltrán, F.J.; López-Gallego, J.; Gracia-Pinilla, M.Á.; Villanueva-Rodríguez, M. Effective Degradation of Cefuroxime by Heterogeneous Photo-Fenton under Simulated Solar Radiation Using α-Fe2O3-TiO2. J. Environ. Chem. Eng. 2021, 9, 106822. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Liu, S.; Wang, S. A Comparative Study of Reduced Graphene Oxide Modified TiO2, ZnO and Ta2O5 in Visible Light Photocatalytic/Photochemical Oxidation of Methylene Blue. Appl. Catal. B 2014, 146, 162–168. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Liu, L.; Li, J.; Wang, S.; Znad, H.; Liu, S. Magnetic ZnO@Fe3O4 Composite for Self-Generated H2O2 toward Photo-Fenton-like Oxidation of Nitrophenol. Compos. B Eng. 2020, 200, 108345. [Google Scholar] [CrossRef]
- Uma, K.; KrishnaKumar, B.; Pan, G.-T.; Yang, T.C.-K.; Lin, J.-H. Enriched Silver Plasmon Resonance Activity on the Sonochemical Synthesis of ZnO Flowers with α-Fe2O3 as an Efficient Catalyst for Photo-Fenton Reaction and Photo-Oxidation of Ethanol. J. Water Process Eng. 2020, 34, 101089. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, W.; Kang, F.; Peng, H.; Wen, J. Enhanced Photo-Fenton Degradation of Tetracycline Using TiO2-Coated α-Fe2O3 Core–Shell Heterojunction. J. Ind. Eng. Chem. 2018, 68, 14–23. [Google Scholar] [CrossRef]
- Zheng, R.; Yang, D.; Chen, Y.; Bian, Z.; Li, H. Fe2O3/TiO2/Reduced Graphene Oxide-Driven Recycled Visible-Photocatalytic Fenton Reactions to Mineralize Organic Pollutants in a Wide pH Range. J. Environ. Sci. 2023, 134, 11–20. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Aanchal; Basu, S. Synthesis of Fe2O3/TiO2 Monoliths for the Enhanced Degradation of Industrial Dye and Pesticide via Photo-Fenton Catalysis. J. Photochem. Photobiol. A 2019, 376, 32–42. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Xu, K.; Yang, Z.; Dong, Z. The Synergistic Enhancement of Photocatalytic Activity by Fe3O4–TiO2 Nanosheets for Rhodamine B Degradation. Colloids Surf. A 2024, 700, 134803. [Google Scholar] [CrossRef]
- Kumar, U.; Sinha, I. Visible Light Photo-Fenton Degradation of p-Nitrophenol on Ag/Fe3O4/WO3 Nanocomposites: Experimental and Molecular Dynamics Investigations. J. Environ. Chem. Eng. 2023, 11, 111280. [Google Scholar] [CrossRef]
- Abhivyakti; Kaur, P.; Aggarwal, D.; Nitansh; Singhal, S. Defect-Engineered C,N-ZnO/Co3O4/CoFe2O4/Fe3O4 for Ultra-Fast Tetracycline Degradation and Environmental Impact Assessment Using an in Silico Mathematical Model. Adv. Compos. Hybrid Mater. 2025, 8, 156. [Google Scholar] [CrossRef]
- Li, H.; Cheng, B.; Zhang, J.; Zhou, X.; Shi, C.; Zeng, L.; Wang, C. Recent Advances in the Application of Bismuth-Based Catalysts for Degrading Environmental Emerging Organic Contaminants through Photocatalysis: A Review. J. Environ. Chem. Eng. 2023, 11, 110371. [Google Scholar] [CrossRef]
- Chong, D.S.; Foo, J.J.; Tan, X.-Q.; Ling, G.Z.S.; Tan, L.-L.; Chen, X.; Ong, W.-J. Evolutionary Face-to-Face 2D/2D Bismuth-Based Heterojunction: The Quest for Sustainable Photocatalytic Applications. Appl. Mater. Today 2022, 29, 101636. [Google Scholar] [CrossRef]
- Ray, S.K.; Cho, J.; Hur, J. A Critical Review on Strategies for Improving Efficiency of BaTiO3-Based Photocatalysts for Wastewater Treatment. J. Environ. Manag. 2021, 290, 112679. [Google Scholar] [CrossRef]
- Miao, H.; Zeng, G.; Zong, H.; Mo, Z.; Zhu, X.; Dionysiou, D.D.; Xu, H. Enhanced Tetracycline Hydrochloride Degradation at near Neutral Conditions with FeOx Modified Bi4TaO8Cl Catalyst: Coupling the Photocatalytic and Fenton Oxidation. J. Environ. Chem. Eng. 2024, 12, 113496. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Yasin Naz, M.; Ullah, S.; Ali Assiri, M. Designing and Modification of Bismuth Oxyhalides BiOX (X = Cl, Br and I) Photocatalysts for Improved Photocatalytic Performance. J. Ind. Eng. Chem. 2022, 105, 1–33. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Wang, W.-S.; Li, B.; Zhang, L. Mechanism Insights into the Enhanced Photocatatlytic Peroxydisulfate Activation by Fe3O4/BiOI Heterojunction. Mater. Sci. Eng. B 2023, 294, 116509. [Google Scholar] [CrossRef]
- Tao, Y.; Fan, G.; Li, X.; Cao, X.; Du, B.; Li, H.; Luo, J.; Hong, Z.; Xu, K.-Q. Recyclable Magnetic AgBr/BiOBr/Fe3O4 Photocatalytic Activation Peroxymonosulfate for Carbamazepine Degradation: Synergistic Effect and Mechanism. Sep. Purif. Technol. 2024, 330, 125392. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Wu, X.; Lynch, I. Z-Scheme Fe@Fe2O3/BiOBr Heterojunction with Efficient Carrier Separation for Enhanced Heterogeneous Photo-Fenton Activity of Tetracycline Degradation: Fe2+ Regeneration, Mechanism Insight and Toxicity Evaluation. Environ. Res. 2024, 252, 118396. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Wang, W.; Ren, K.; Shi, H. 2D/2D Black-BiOCl/Fe2O3 Heterojunction Photo-Fenton Catalytic System for Enhanced Visible-Light Tetracycline Degradation. Colloids Surf. A 2021, 626, 126953. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, G.; Jiao, Z. Highly Efficient Photo-Fenton Ag/Fe2O3/BiOI Z-Scheme Heterojunction for the Promoted Degradation of Tetracycline. Nanomaterials 2023, 13, 1991. [Google Scholar] [CrossRef]
- Li, B.; Liu, X.; Zhu, H.; Guan, H.; Guo, R. A Review on Bi2WO6-Based Materials for Photocatalytic CO2 Reduction. Small 2024, 20, 2406074. [Google Scholar] [CrossRef]
- Khedr, T.M.; Wang, K.; Kowalski, D.; El-Sheikh, S.M.; Abdeldayem, H.M.; Ohtani, B.; Kowalska, E. Bi2WO6-based Z-Scheme Photocatalysts: Principles, Mechanisms and Photocatalytic Applications. J. Environ. Chem. Eng. 2022, 10, 107838. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Zhu, Z.; Zhang, Y.; Tu, S.; Zhang, Y.; Ma, T.; Chen, F.; Huang, H. Efficient Piezo-Photocatalysis of 0D/2D α-Fe2O3/Bi2WO6: Synergy of Weak Force-Driven Piezoelectric Polarization and Z-Scheme Junction. J. Colloid Interface Sci. 2023, 650, 1536–1549. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.-H. Construction of Heterojunction Photoelectrode via Atomic Layer Deposition of Fe2O3 on Bi2WO6 for Highly Efficient Photoelectrochemical Sensing and Degradation of Tetracycline. Appl. Catal. B 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Jaramillo-Páez, C.; Navío, J.A.; Hidalgo, M.C.; Bouziani, A.; Azzouzi, M.E. Mixed α-Fe2O3/Bi2WO6 Oxides for Photoassisted Hetero-Fenton Degradation of Methyl Orange and Phenol. J. Photochem. Photobiol. A 2017, 332, 521–533. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, W.; Zhang, H.; Dou, X.; Shi, H. 2D/2D Step-Scheme α-Fe2O3/Bi2WO6 Photocatalyst with Efficient Charge Transfer for Enhanced Photo-Fenton Catalytic Activity. Chin. J. Catal. 2021, 42, 97–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, X.; Xu, G.; Liu, W.; Hu, D.; Sun, Q.; Xu, J.; Zhang, G.; Xiong, W.; Ma, Z.; et al. Theoretical Study and Experimental Verification of the DMPBP [5] Adsorption-Enhanced Bi2WO6 Photocatalysis Fe3O4 Self-Fenton System. Sep. Purif. Technol. 2023, 327, 124916. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, J.; Gu, H.; Huang, J.; Lin, J.; Shao, J.; Wang, D.; Li, H. A Review on Research Progress in Photocatalytic Degradation of Organic Pollutants by Bi2MoO6. J. Environ. Chem. Eng. 2023, 11, 110911. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, J.; Zhou, Y.; Liu, D. Peroxymonosulfate-Assisted g-C3N4@Bi2MoO6 Photocatalytic System for Degradation of Nimesulide through Phenyl Ether Bond Cleavage under Visible Light Irradiation. Sep. Purif. Technol. 2021, 264, 118288. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Zhang, H.; Yao, H.; Chai, J.; Wang, J.; Fang, D.; Zhang, Z.; Tie, M. Construction of High-Proportion Dual Bismuth-Based Z-Scheme Bi3O4Cl/Bi2MoO6 Photocatalytic System via in-Situ Growth of Bi2MoO6 on Bi3O4Cl for Enhanced Photocatalytic Degradation of Organic Pollutants. J. Alloys Compd. 2023, 956, 170375. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Cai, M.; Wang, Y.; Zhang, H.; Guo, Y.; Zhao, W.; Wang, Z.; Chen, X. Photocatalytic Degradation of Tetracycline Antibiotic by a Novel Bi2Sn2O7/Bi2MoO6 S-Scheme Heterojunction: Performance, Mechanism Insight and Toxicity Assessment. Chem. Eng. J. 2022, 429, 132519. [Google Scholar] [CrossRef]
- Xiu, Z.; Cao, Y.; Xing, Z.; Zhao, T.; Li, Z.; Zhou, W. Wide Spectral Response Photothermal Catalysis-Fenton Coupling Systems with 3D Hierarchical Fe3O4/Ag/Bi2MoO6 Ternary Hetero-Superstructural Magnetic Microspheres for Efficient High-Toxic Organic Pollutants Removal. J. Colloid Interface Sci. 2019, 533, 24–33. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, H.; Hong, J.; Zhao, L. MOF-Derived Fe2O3@C-Coupled Bi2MoO6 Heterojunctions for Highly Efficient Photo-Fenton Degradation of Tetracycline. J. Mol. Liq. 2023, 383, 122157. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, J.; Li, T.; Chen, Y.; Wu, Q.; Xie, T.; Lin, Y.; Dong, S. Visible-Light-Driven Photo-Fenton Reaction with α-Fe2O3/BiOI at near Neutral pH: Boosted Photogenerated Charge Separation, Optimum Operating Parameters and Mechanism Insight. J. Colloid Interface Sci. 2019, 554, 531–543. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, C.; Yang, L.; Wu, N.; Chen, R. Fabrication of (H2PO4−, Ni2+)—α-Fe2O3/Bi2S3 Heterojunction Photocatalysts with Improved Visible-Light Catalytic Activity. Chem. Eng. Sci. 2024, 289, 119874. [Google Scholar] [CrossRef]
- Naik, V.A.; Thakur, V.A. Hydrothermal Synthesis of Fe3O4@rGO@CdS/Bi2S3 Nanocomposite as an Efficient, Recyclable Magnetic Photocatalyst for Photo-Fenton Dye Degradation and Chromium VI Reduction under Sunlight. Inorg. Chem. Commun. 2024, 160, 111962. [Google Scholar] [CrossRef]
- Shan, Z.; Ma, F.; Xu, M.; Shan, X.; Shan, L.; Cui, C.; Guo, H. Kinetics and Mechanism of Enhanced Norfloxacin Degradation by Fe3O4@La-BiFeO3 Based on Weak Magnetization and Efficient Charge Separation. Chem. Eng. J. 2023, 466, 143229. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhou, X.; Xu, X.; Pan, M. Synergistic Mechanisms of Carbon-Based Materials for VOCs Photocatalytic Degradation: A Critical Review. J. Environ. Manag. 2024, 367, 122087. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, K.; Du, P.; Mehmandoust, M.; Karimi, F.; Erk, N. Carbon-Based Photocatalysts for Hydrogen Production: A Review. Chemosphere 2022, 308, 135998. [Google Scholar] [CrossRef]
- Xie, L.; Du, T.; Wang, J.; Ma, Y.; Ni, Y.; Liu, Z.; Zhang, L.; Yang, C.; Wang, J. Recent Advances on Heterojunction-Based Photocatalysts for the Degradation of Persistent Organic Pollutants. Chem. Eng. J. 2021, 426, 130617. [Google Scholar] [CrossRef]
- Nawaz, F.; Ali, M.; Ahmad, S.; Yong, Y.; Rahman, S.; Naseem, M.; Hussain, S.; Razzaq, A.; Khan, A.; Ali, F.; et al. Carbon Based Nanocomposites, Surface Functionalization as a Promising Material for VOCs (Volatile Organic Compounds) Treatment. Chemosphere 2024, 364, 143014. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Ahmed, B.; Mehnaz, T.; Shafiullah, G.M.; Nguyen, V.N.; Duong, X.Q.; Mofijur, M.; Badruddin, I.A.; Kamangar, S. Carbon-Based Nanomaterials: Characteristics, Dimensions, Advances and Challenges in Enhancing Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 52, 424–442. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, M.; Al-Sehemi, A.G.; Al-Ghamdi, A.A.; Park, J.-W.; Algarni, H.; Anwer, H. Carbon-Integrated Semiconductor Photocatalysts for Removal of Volatile Organic Compounds in Indoor Environments. Chem. Eng. J. 2023, 452, 139436. [Google Scholar] [CrossRef]
- Cao, C.; Guo, Y.; Chen, D.; Cui, J.; Hu, X. A MOF-Derived Fe3O4/Fe0/Fe3C-2-600 Dual-Cathode Electro-Fenton System Construction: Revealing the Mechanism for Active Centers Linked by Fe0 as an Electronic Transfer Station for Enhancing Fenton Reaction. Chem. Eng. J. 2025, 507, 160710. [Google Scholar] [CrossRef]
- Feng, T.; Wang, B.; Li, J.; Wang, T.; Huang, P.; Xu, X. Metal–Organic Framework Based Photocatalytic Membrane for Organic Wastewater Treatment: Preparation, Optimization, and Applications. Sep. Purif. Technol. 2025, 355, 129540. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Yang, S.; Garcia, H. Metal–Organic Framework Heterojunctions for Photocatalysis. Chem. Soc. Rev. 2024, 53, 3002–3035. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, X.; Jiang, Y.; Zheng, X.; Zhang, J.; Li, Y.; Li, D.; Lin, K. Photo-Fenton Degradation of Tetracycline Hydrochloride with Fe2O3-on-ZrO2 Polypods Derived from MIL-88B-on-UiO-66-NH2 within Full pH Range: Kinetics, Degradation Pathway and Mechanism Insight. Appl. Surf. Sci. 2023, 621, 156715. [Google Scholar] [CrossRef]
- Hu, C.; He, J.; Liang, J.; Lin, T.; Liu, Q. Heterogeneous Photo-Fenton Catalyst α-Fe2O3@g-C3N4@NH2-MIL-101(Fe) with Dual Z-Scheme Heterojunction for Degradation of Tetracycline. Environ. Res. 2023, 231, 116313. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, Z.; Lv, S.; Niu, M.; Zhou, W.; Li, J.; Lu, R.; Gao, H.; Pan, C.; Zhang, S. Facile Synthesis of Fe3O4@MIL-100(Fe) towards Enhancing Photo-Fenton like Degradation of Levofloxacin via a Synergistic Effect between Fe3O4 and MIL-100(Fe). Chem. Eng. J. 2021, 409, 128274. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent Advances in g-C3N4-Based Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Mallick, P.; Sahoo, S.K.; Satpathy, S.K. Different Strategies to Improve Photocatalytic Activity of Graphitic Carbon Nitride (g-C3N4) Semiconductor Nanomaterials for Hydrogen Generation. J. Mol. Liq. 2024, 406, 125071. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.S.; Singh, V.K.; Pal, D. g-C3N4 Based Z-Scheme Photocatalysts for Tetracycline Degradation: A Comprehensive Review. J. Hazard. Mater. Lett. 2024, 5, 100116. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent Developments of Doped g-C3N4 Photocatalysts for the Degradation of Organic Pollutants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 751–790. [Google Scholar] [CrossRef]
- Hao, P.; Chen, Z.; Yan, Y.; Shi, W.; Guo, F. Recent Advances, Application and Prospect in g-C3N4-Based S-Scheme Heterojunction Photocatalysts. Sep. Purif. Technol. 2024, 330, 125302. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. g-C3N4-Based 2D/2D Composite Heterojunction Photocatalyst. Small Struct. 2021, 2, 2100086. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, X.; He, T.; Wang, R.; Zhao, Y.; Song, H.; Wang, H. 3D/2D Fe2O3/g-C3N4 Z-Scheme Heterojunction Catalysts for Fast, Effective and Stable Photo Fenton Degradation of Azo Dyes. J. Environ. Chem. Eng. 2021, 9, 105907. [Google Scholar] [CrossRef]
- Xu, F.; Chai, B.; Liu, Y.; Liu, Y.; Fan, G.; Song, G. Superior Photo-Fenton Activity toward Tetracycline Degradation by 2D α-Fe2O3 Anchored on 2D g-C3N4: S-Scheme Heterojunction Mechanism and Accelerated Fe3+/Fe2+ Cycle. Colloids Surf. A 2022, 652, 129854. [Google Scholar] [CrossRef]
- Meng, D.; Hou, J.; Wang, L.; Hu, X.; Gao, D.; Guo, Q. Rational Construction of α-Fe2O3/g-C3N4 Heterojunction for Effective Photo-Fenton-like Degradation of Tetracycline. Mater. Res. Bull. 2023, 168, 112454. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A Novel α-Fe2O3@g-C3N4 Catalyst: Synthesis Derived from Fe-Based MOF and Its Superior Photo-Fenton Performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Sharma, K.; Sudhaik, A.; Raizada, P.; Thakur, P.; Pham, X.M.; Van Le, Q.; Nguyen, V.-H.; Ahamad, T.; Thakur, S.; Singh, P. Constructing α-Fe2O3/g-C3N4/SiO2 S-Scheme-Based Heterostructure for Photo-Fenton like Degradation of Rhodamine B Dye in Aqueous Solution. Environ. Sci. Pollut. Res. 2023, 30, 124902–124920. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, N.; Li, Y.; Zhang, X.; Zhang, L. Step Scheme Fe2O3/S Doped g-C3N4 Heterojunction Photocatalysts for Photo-Fenton Norfloxacin and Tetracycline Degradation. Mater. Sci. Semicond. Process. 2023, 160, 107423. [Google Scholar] [CrossRef]
- Ge, F.; Li, X.; Wu, M.; Ding, H.; Li, X. A Type II Heterojunction α-Fe2O3/g-C3N4 for the Heterogeneous Photo-Fenton Degradation of Phenol. RSC Adv. 2022, 12, 8300–8309. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Oulego, P.; Giannakis, S. Deriving an α-Fe2O3/g-C3N4 Nanocomposite from a Naturally Hematite-Rich Soil, for Dual Photocatalytic and Photo-Fenton Degradation of Acetaminophen under Visible Light. Sep. Purif. Technol. 2022, 299, 121723. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, P.; Zhang, Y.; Li, Y.; Zhang, H.; He, T.; Li, S. Fabrication of 2D TiO2/α-Fe2O3/Fe-g-C3N4 Heterogeneous from Metallurgical Solid Waste for Photo-Fenton Catalysis of Levofloxacin/Cr(VI): Electron Transfer Pathway and DFT Calculation. J. Catal. 2024, 432, 115447. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, S.; Shao, Z.; Xie, L.; Qin, Y.; Zhang, L.; Xu, K.; Chai, X. 0D/2D Dual Fenton α-Fe2O3/Fe-Doped g-C3N4 Photocatalyst and the Synergistic Photo-Fenton Catalytic Mechanism Insight. Chemosphere 2024, 358, 142158. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Zhang, J.; Lei, J.; Liu, Y. Well-Dispersed Fe2O3 Nanoparticles on g-C3N4 for Efficient and Stable Photo-Fenton Photocatalysis under Visible-Light Irradiation. Eur. J. Inorg. Chem. 2016, 2016, 5387–5392. [Google Scholar] [CrossRef]
- Das, K.K.; Mansingh, S.; Mohanty, R.; Sahoo, D.P.; Priyadarshini, N.; Parida, K. 0D–2D Fe2O3/Boron-Doped g-C3N4 S-Scheme Exciton Engineering for Photocatalytic H2O2 Production and Photo-Fenton Recalcitrant-Pollutant Detoxification: Kinetics, Influencing Factors, and Mechanism. J. Phys. Chem. C 2023, 127, 22–40. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, C.; Mei, J.; Yang, S.; Wong, P.K. Faster Electron Injection and Higher Interface Reactivity in g-C3N4/Fe2O3 Nanohybrid for Efficient Photo-Fenton-Like Activity Toward Antibiotics Degradation. Environ. Res. 2021, 195, 110842. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhong, H.; Deng, J.; Li, M.; Tang, C.; Hu, X.; Zhu, M. Z-Scheme γ-Fe2O3/g-C3N4 in Photo-Fenton Reaction for Oxytetracycline Degradation: Mechanism Study and DFT Calculation. Sep. Purif. Technol. 2025, 354, 129185. [Google Scholar] [CrossRef]
- Li, X.; Ge, F.; Ding, H.; Zhou, X.; Li, X. Nitrogen-Doped Carbon Dots as Electron “Bridge” in Heterostructure of Alpha-Fe2O3/NCDs/g-C3N4 for Efficient Degradation of Indole Using Heterogeneous Photo-Fenton. J. Environ. Chem. Eng. 2022, 10, 106824. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, B.; Li, X.; Liu, B.; Wang, S.; Yu, P.; Xu, Y.; Jiang, G. High-Efficiency Removal of Tetracycline by Carbon-Bridge-Doped g-C3N4/Fe3O4 Magnetic Heterogeneous Catalyst through Photo-Fenton Process. J. Hazard. Mater. 2021, 418, 126333. [Google Scholar] [CrossRef]
- He, W.; Jia, H.; Li, Z.; Miao, C.; Lu, R.; Zhang, S.; Zhang, Z. Magnetic Recyclable g-C3N4/Fe3O4@MIL-100(Fe) Ternary Catalyst for Photo-Fenton Degradation of Ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 108698. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Zhang, X.; Wang, S.; Xia, Z.; Zeng, G.; Ding, J.; Ren, N. Construction of Fe3O4@β-CD/g-C3N4 Nanocomposite Catalyst for Degradation of PCBs in Wastewater through Photodegradation and Heterogeneous Fenton Oxidation. Chem. Eng. J. 2022, 429, 132445. [Google Scholar] [CrossRef]
- Permana, M.D.; Takei, T.; Khatun, A.A.; Eddy, D.R.; Saito, N.; Kumada, N. Effect of Wavelength in Light Irradiation for Fe2+/Fe3+ Redox Cycle of Fe3O4/g-C3N4 in Photocatalysis and Photo-Fenton Systems. J. Photochem. Photobiol. A 2024, 457, 115876. [Google Scholar] [CrossRef]
- Cui, K.-P.; Yang, T.-T.; Chen, Y.-H.; Weerasooriya, R.; Li, G.-H.; Zhou, K.; Chen, X. Magnetic Recyclable Heterogeneous Catalyst Fe3O4/g-C3N4 for Tetracycline Hydrochloride Degradation via Photo-Fenton Process under Visible Light. Environ. Technol. 2022, 43, 3341–3354. [Google Scholar] [CrossRef]
- Rai, H.; Ponomarev, I.; Thakur, D.; Balakrishnan, V.; Polcar, T.; Gosvami, N.N. Impact of Wrinkle Orientation on the Nanotribological Properties of Chemical Vapor Deposited TMD Monolayers Using AFM. Appl. Surf. Sci. 2024, 672, 160884. [Google Scholar] [CrossRef]
- Wyss, K.M.; Tour, J.M. Turning Straw into Reduced Graphene Oxide. Nat. Sustain. 2024, 7, 1558–1559. [Google Scholar] [CrossRef]
- Ramachandran, T.; Roy, N.; Hegazy, H.H.; Yahia, I.S.; Kumar, Y.A.; Moniruzzaman, M.; Joo, S.W. From Graphene Aerogels to Efficient Energy Storage: Current Developments and Future Prospects. J. Alloys Compd. 2025, 1010, 177248. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.C.; Xia, D.; Wong, P.K.; Li, Y. Graphene and g-C3N4 Nanosheets Cowrapped Elemental α-Sulfur As a Novel Metal-Free Heterojunction Photocatalyst for Bacterial Inactivation under Visible-Light. Environ. Sci. Technol. 2013, 47, 8724–8732. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Dabur, V.A.; Rachmantyo, R.; Judawisastra, H.; Hu, C.; Wibowo, A. Carbon Nitride- and Graphene-Based Materials for the Photocatalytic Degradation of Emerging Water Pollutants. Mater. Adv. 2024, 5, 2668–2688. [Google Scholar] [CrossRef]
- Wen, J.; Yu, F.; Chen, G.; Wang, C.; Li, S. Photoelectric Properties of Metallic Elements Doping and Adsorption on Graphene/Black Phosphene Heterojunction. Surf. Interfaces 2024, 52, 104884. [Google Scholar] [CrossRef]
- Zhang, Y.; Hawboldt, K.; Zhang, L.; Lu, J.; Chang, L.; Dwyer, A. Carbonaceous Nanomaterial-TiO2 Heterojunctions for Visible-Light-Driven Photocatalytic Degradation of Aqueous Organic Pollutants. Appl. Catal. A 2022, 630, 118460. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to Improve the Adsorption Properties of Graphene-Based Adsorbent towards Heavy Metal Ions and Their Compound Pollutants: A Review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; He, M.; Li, S.; Guo, H.; Kan, D.; Qiu, H.; Chen, L.; Gu, J. Electromagnetic Interference Shielding Films: Structure Design and Prospects. Small Methods 2024, 2401324. [Google Scholar] [CrossRef]
- Wang, F.; Yu, X.; Ge, M.; Wu, S. One-Step Synthesis of TiO2/γ-Fe2O3/GO Nanocomposites for Visible Light-Driven Degradation of Ciprofloxacin. Chem. Eng. J. 2020, 384, 123381. [Google Scholar] [CrossRef]
- Asif, A.H.; Rafique, N.; Hirani, R.A.K.; Shi, L.; Zhang, S.; Wang, S.; Sun, H. Graphitic Carbon Nitride Engineered α-Fe2O3/rGO Heterostructure for Visible-Light-Driven Photochemical Oxidation of Sulfamethoxazole. Chem. Eng. J. 2023, 451, 138630. [Google Scholar] [CrossRef]










| Catalysts | Fabrication Method | Condition | Reference |
|---|---|---|---|
| FeIn2S4/Fe2O3 | Hydrothermal | 90 °C, 6 h | [56] |
| ZnFe2O4/α-Fe2O3/Pt | Hydrothermal | 180 °C, 24 h | [57] |
| Fe2O3@PPy/PB | Reactive template | 90 min | [58] |
| α-Fe2O3-Fe3O4/CeO2 | Solution combustion | Sunlight, 1 h | [59] |
| P-CN/NCDs/Fe3O4 | Co-precipitation | 90 °C, 1 h | [60] |
| PVA/CNF/Fe3O4 | Co-precipitation | 50 °C, 24 h | [61] |
| γ-Fe2O3/FeTiO3 | Hydrothermal | 150 °C, 5 h | [62] |
| α-Fe2O3/CdS/SiO2 | Hydrothermal | 120 °C, 24 h | [63] |
| Fe2O3@A1−xRx-TiO2 | Sol–gel | 100 °C, 5 h | [64] |
| Fe2O3/TiO2 | Sol–gel | 60 °C, 3 h | [65] |
| Fe3O4@FeS2@C@MoS2 | Hydrothermal | 200 °C, 7 h | [66] |
| MoS2/C@Fe3O4 | Hydrothermal | 180 °C, 16 h | [67] |
| Cr2Bi3O11-Bi2O3/Fe3O4@PCs | Hydrothermal | 160 °C, 8 h | [68] |
| TiO2/Fe2TiO5/Fe2O3 | Ion exchange | Roon temperature, 2 h | [69] |
| Catalysts | Pollutants | Condition | Removal Efficiency | Reference |
|---|---|---|---|---|
| α-Fe2O3@TiO2 | TCH (50 mg L−1) | 300 W Xe lamp (λ > 420 nm), pH = 5.45, [H2O2] = 120 μL, [Catalyst] = 0.1 g | 100%, 90 min | [79] |
| TiO2/Fe2TiO5/Fe2O3 | MO (10 mg L−1) | 300 W Xe lamp (λ > 420 nm), pH = 4, [H2O2] = 130 μL, [Catalyst] = 50 mg | 87%, 10 min | [69] |
| α-Fe2O3/TiO2 | 2,4-DCP (10 mg L−1) | 300 W Xe lamp (λ > 420 nm), pH = 3, [H2O2] = 1 mM, [Catalyst] = 50 mg | 100%, 12 min | [73] |
| Fe2O3/TiO2/rGO | MO (20 mg L−1) | 5 W LED lamp (λ = 420 nm), pH = 7.3, [H2O2] = 19.8 mM, [Catalyst] = 1 g L−1 | 97%, 20 min | [80] |
| Fe2O3/TiO2 | RbX (50 mg L−1) | 65 W mercury lamp (λ = 365 nm), pH = 3, [H2O2] = 60 mM, [Catalyst] = 2 g L−1 | 90.57%, 180 min | [81] |
| Fe3O4-TiO2 | RhB (30 mg L−1) | Ultraviolet analyzer (365 nm, 6W), pH = 7, [H2O2] = 485 mM, [Catalyst] = 1 g L−1 | 98.12%, 120 min | [82] |
| Ag-ZnO@α-Fe2O3 | MB (5 ppm) | 350 W mercury lamp (λ > 380 nm), pH = 3, [H2O2] = 0.5 mL, [Catalyst] = 30 mg | 99.3%, 30 min | [78] |
| Ag/Fe3O4/WO3 | p-NP (5 mg L−1) | cool white LED, pH = 3, [H2O2] = 1 M, [Catalyst] = 2 mg | 100%, 90 min | [83] |
| ZnO/Co3O4/CoFe2O4/Fe3O4 | TC (15 mg L−1) | λ = 500 nm, pH = 7, [H2O2] = 4.4 mM, [Catalyst] = 150 mg | 90.9%, 6 min | [84] |
| Catalysts | Pollutants | Condition | Removal Efficiency | Reference |
|---|---|---|---|---|
| FeOx/Bi4TaO8Cl | TC-H (20 mg L−1) | 300 W Xe lamp, (λ ≥ 420 nm), pH = 6, [H2O2] = 99 mM, [Catalyst] = 0.4 g L−1 | 83.5%, 20 min | [88] |
| α-Fe2O3/Bi2WO6 | MB (5 mg L−1) | 300 W Xe lamp, (λ ≥ 400 nm), pH = 7, [H2O2] = 100 μL, [Catalyst] = 20 mg | 100%, 25 min | [100] |
| Fe2O3@C-coupled Bi2MoO6 | TC (25 mg L−1) | 500 W Xe lamp with a 420 nm UV cut-off filter, pH = 3, [H2O2] = 1 mmol, [Catalyst] = 40 mg | 93.2%, 50 min | [107] |
| black-BiOCl/F2O3 | TC (10 mg L−1) | 300 W Xe lamp, (λ < 400 nm), [H2O2] = 75 μL, [Catalyst] = 50 mg | 92%, 25 min | [93] |
| α-Fe2O3/BiOI | MO (10 mg L−1) | Xenon lamp, (λ < 420 nm), pH = 6.5, [H2O2] = 10 mM, [Catalyst] = 0.05 g | 99.5%, 15 min | [108] |
| (H2PO4−, Ni2+)–α-Fe2O3/Bi2S3 | p-NP (0.2 mM) | Visible light, pH = 3, [H2O2] = 1.3 mM, [Catalyst] = 0.04 g | 91.3%, 30 min | [109] |
| Fe3O4@rGO@CdS/Bi2S3 | MB (25 ppm) | solar light, pH = 7, [H2O2] = 0.0536 M, [Catalyst] = 10 mg | 99%, 70 min | [110] |
| Fe3O4@La-BiFeO3 | NOR (20 mg L−1) | 500 W Xe lamp, (400 nm < λ < 780 nm) pH = 5, [H2O2] = 20 mM, [Catalyst] = 0.7 g L−1 | 96.6%, 60 min | [111] |
| Catalysts | Pollutants | Condition | Removal Efficiency | Reference |
|---|---|---|---|---|
| Fe2O3/g-C3N4 | Amaranth (0.03 mM) | 300 W Xe lamp with a 420 nm UV cut-off filter, pH = 3, [H2O2] = 1 mL, [Catalyst] = 50 mg | 97.6%, 10 min | [130] |
| Fe2O3/g-C3N4 | TC (20 mg L−1) | 300 W Xe lamp, pH = 4, [H2O2] = 50 mM, [Catalyst] = 50 mg | 96.4%, 180 min | [131] |
| Fe2O3/g-C3N4 | TC (5 mg L−1) | 500 W Xe lamp with a 420 nm UV cut-off filter, pH = 5.5, [H2O2] = 50 mM, [Catalyst] = 20 mg | 90.7%, 120 min | [132] |
| α-Fe2O3@g-C3N4@NH2-MIL-101(Fe) | TC (10 mg L−1) | 300 W Xe lamp, pH = 5, [H2O2] = 20 mM, [Catalyst] = 10 mg | 98.33%, 120 min | [122] |
| α-Fe2O3@g-C3N4 | TC (40 mg L−1) | 100 W LED lamp (λ = 420 nm), pH = 5.5, [H2O2] = 10 mM, [Catalyst] = 0.05 g | 92%, 60 min | [133] |
| α-Fe2O3/g-C3N4/SiO2 | RhB (10 ppm) | 100 W LED lamp (λ = 420 nm), pH = 3, [H2O2] = 7 × 10−4 M, [Catalyst] = 0.06 g | 97%, 120 min | [134] |
| Fe2O3/S doped g-C3N4 | Norfloxacin (5 mg L−1) | Visible light, pH = 4, [H2O2] = 6 g L−1, [Catalyst] = 50 mg | 100%, 25 min | [135] |
| α-Fe2O3/g-C3N4 | Phenol (50 mg L−1) | 350 W Xe lamp, pH = 2.5, [H2O2] = 45 mM, [Catalyst] = 10 mg | 90%, 70 min | [136] |
| α-Fe2O3/g-C3N4 | Acetaminophen (ACT) (20 mg L−1) | 35 W Xe lamp with a 420 nm UV cut-off filter, pH = 5, [H2O2] = 5 mM, [Catalyst] = 0.1 g L−1 | 100%, 25 min | [137] |
| TiO2/α-Fe2O3/Fe-g-C3N4 | LEV (15 mg L−1) | Xe lamp, pH = 2, [H2O2] = 8 mM, [Catalyst] = 0.5 g L−1 | 93.8%, 90 min | [138] |
| Fe2O3–Fe–CN | TC (20 mg L−1) | 300 W Xe lamp with a 400 nm UV cut-off filter, pH = 7, [H2O2] = 300 μL, [Catalyst] = 0.02 g | 99.88%, 40 min | [139] |
| Fe2O3/g-C3N4 | MO (20 mg L−1) | 1000 W tungsten/halogen lamp (Philips) with a 420 nm UV cut-off filter, pH = 3, [H2O2] = 50 μL, [Catalyst] = 50 mg | 90%, 90 min | [140] |
| Fe2O3/Boron-doped g-C3N4 | Amoxicillin | Sunlight, pH = 4, [H2O2] = 30 μL, [Catalyst] = 0.02 g | 93%, 30 min | [141] |
| g-C3N4/Fe2O3 | TC (20 mg L−1) | 300 W Xe lamp, [H2O2] = 50 mM, [Catalyst] =20 mg | 87%, 60 min | [142] |
| γ-Fe2O3/g-C3N4 | Oxytetracycline (OTC) (20 mg L−1) | 300 W Xe lamp with a 420 nm UV cut-off filter, pH = 4.89, [H2O2] = 150 μL, [Catalyst] = 0.1 g | 85.7%, 60 min | [143] |
| α-Fe2O3/NCDs/g-C3N4 | Indole (50 mg L−1) | 350 W Xe lamp, pH = 5, [H2O2] = 90 mM, [Catalyst] = 0.15 g L−1 | 85%, 150 min | [144] |
| MLD/CN/Fe3O4 | TC (20 mg L−1) | 300 W Xe lamp with a 420 nm UV cut-off filter, pH = neutral, [H2O2] = 80 mM, [Catalyst] = 0.5 g L−1 | 95.8%, 80 min | [145] |
| g-C3N4/Fe3O4@MIL-100(Fe) | Ciprofloxacin (CIP) (200 mg L−1) | Visible light with a 420 nm UV cut-off filter, pH = 3, [H2O2] = 2.64 g L−1, [Catalyst] = 0.67 g L−1 | 94.7%, 120 min | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, C.; Wang, L.; Hou, C. Recent Advances in Iron Oxide-Based Heterojunction Photo-Fenton Catalysts for the Elimination of Organic Pollutants. Catalysts 2025, 15, 391. https://doi.org/10.3390/catal15040391
Wu Y, Wang C, Wang L, Hou C. Recent Advances in Iron Oxide-Based Heterojunction Photo-Fenton Catalysts for the Elimination of Organic Pollutants. Catalysts. 2025; 15(4):391. https://doi.org/10.3390/catal15040391
Chicago/Turabian StyleWu, Yiqian, Cong Wang, Lan Wang, and Chen Hou. 2025. "Recent Advances in Iron Oxide-Based Heterojunction Photo-Fenton Catalysts for the Elimination of Organic Pollutants" Catalysts 15, no. 4: 391. https://doi.org/10.3390/catal15040391
APA StyleWu, Y., Wang, C., Wang, L., & Hou, C. (2025). Recent Advances in Iron Oxide-Based Heterojunction Photo-Fenton Catalysts for the Elimination of Organic Pollutants. Catalysts, 15(4), 391. https://doi.org/10.3390/catal15040391









