Silver-Modified Biochar: Investigating NO2 Adsorption and Reduction Efficiency at Different Temperatures
Abstract
1. Introduction
2. Results and Discussion
2.1. TGA and Proximate Analyses
2.2. Ultimate Analysis and Mineral Composition
2.3. Discussion of Ag-BCC Synthesis Protocol
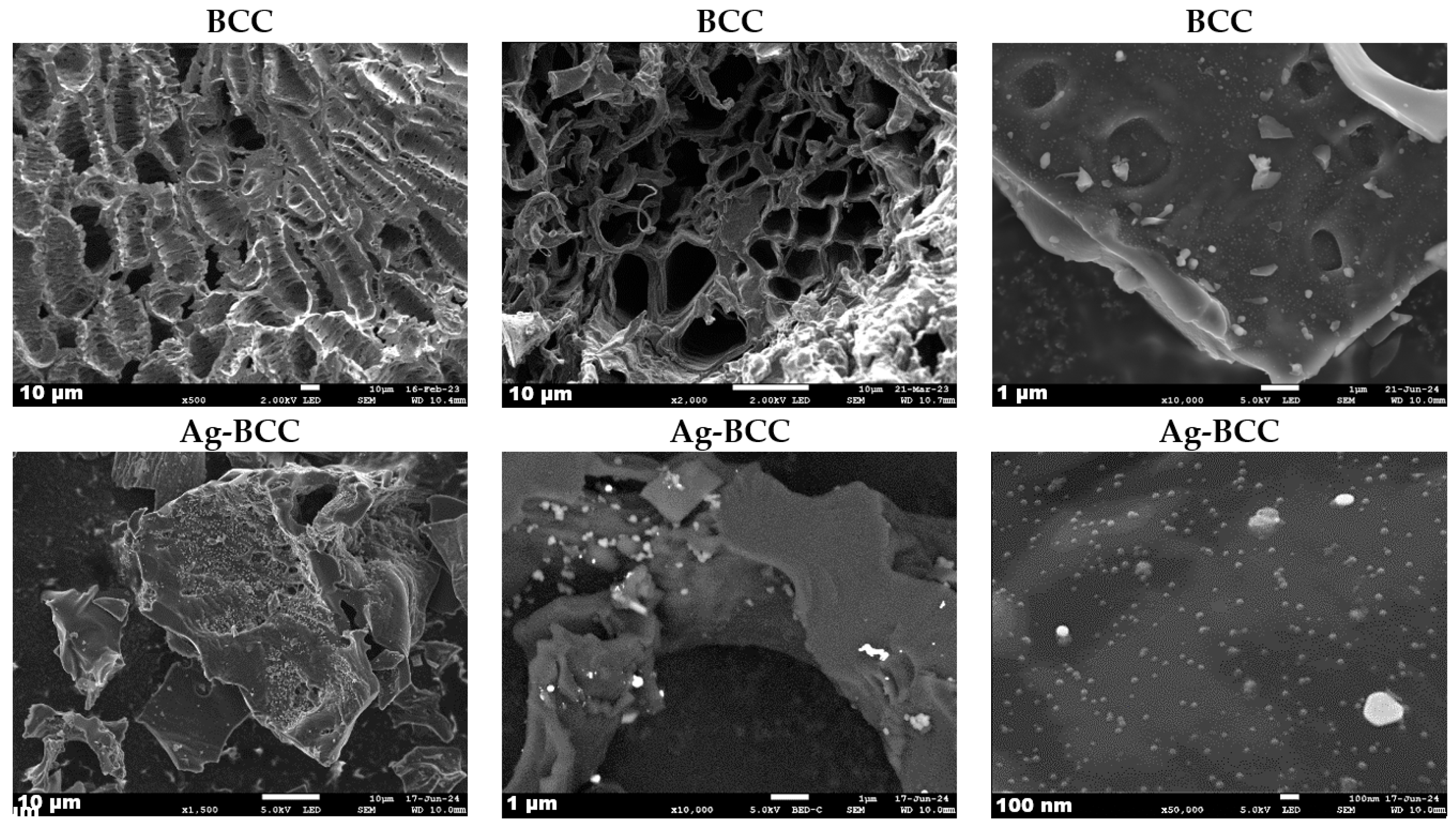
2.4. Textural Properties
2.5. Structural Properties
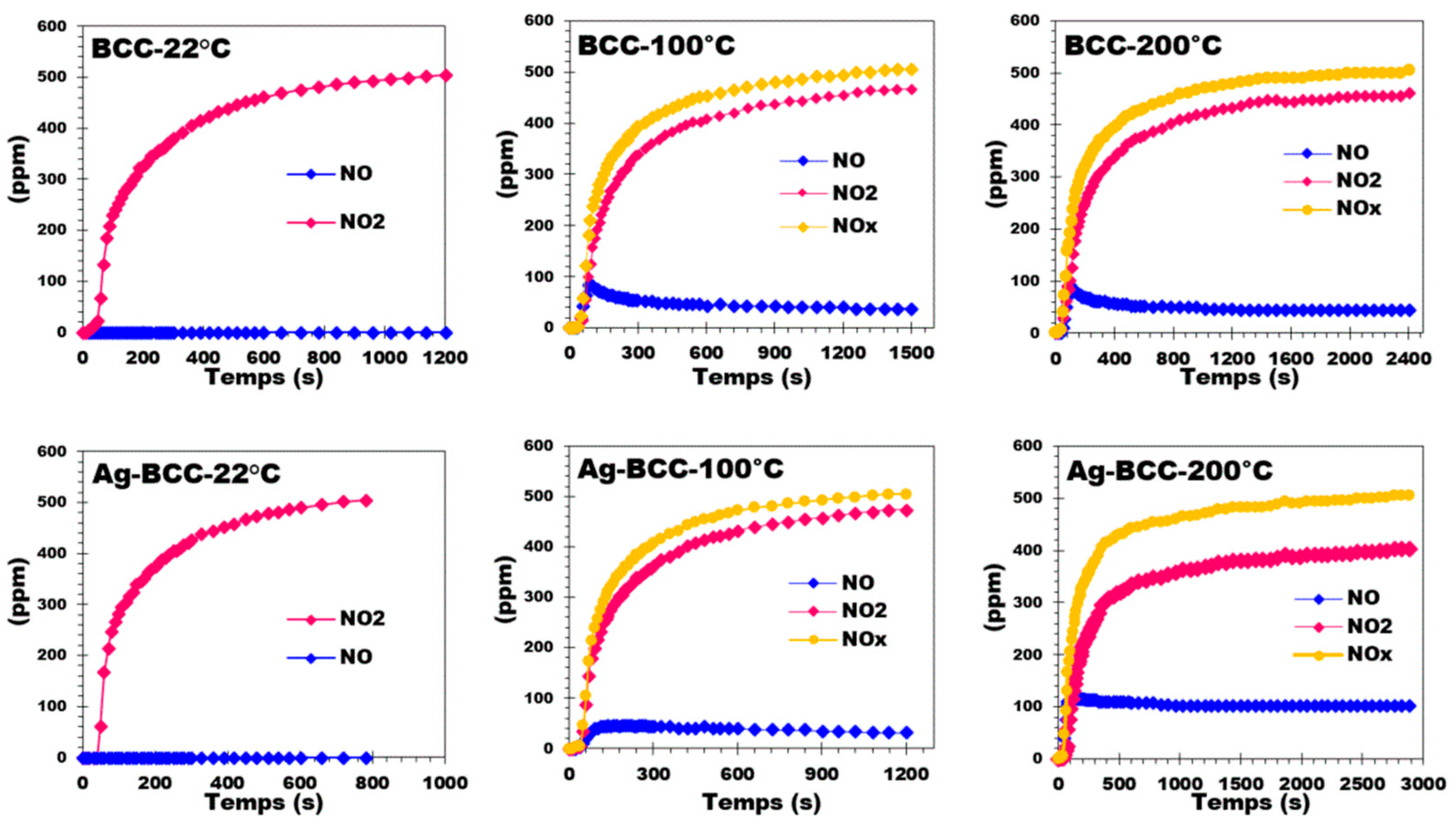
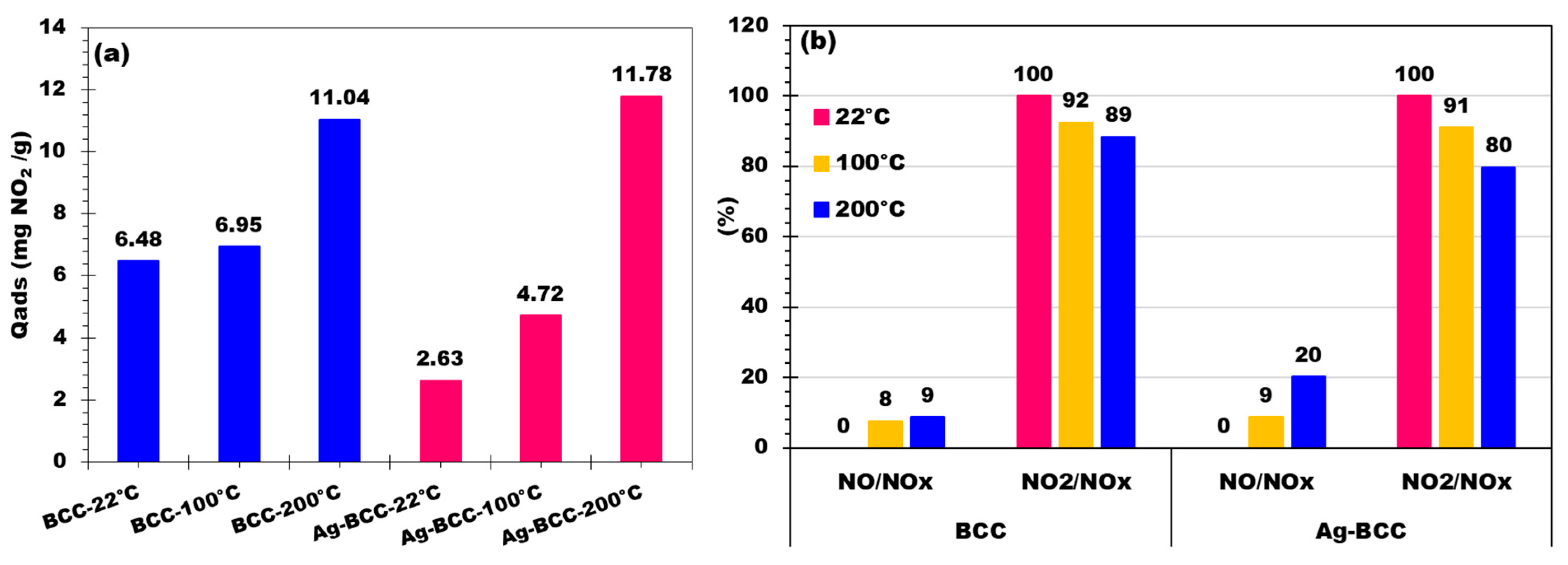
2.6. NO2 Adsorption and Distribution of NO and NO2
2.7. Modeling the Breakthrough Curves
2.8. Proposed Possible Mechanism for NO2 Adsorption on Ag-BCC
- i.
- Metallic silver acts as a reducing agent, converting NO2 into NO gas while forming silver (I) oxide (Ag2O). This step is consistent with silver’s redox capabilities and its ability to form stable oxides. The reduction of NO2 leads to the formation of NO, which is detected as part of the NOx concentration in the experimental results.
- ii.
- Silver oxide (Ag2O) reacts further with NO2, resulting in the formation of silver nitrite (Ag[NO2]) while regenerating metallic silver. This highlights the dynamic oxidation states of silver during the reaction process. Again, NO is produced and contributes to the observed NOx concentration in the measurements.
- iii.
- Silver nitrite (Ag[NO2]) decomposes into silver nitrate (Ag[NO3]), regenerates metallic silver, and releases NO gas. The NO produced in this step is part of the NOx measured in the experiments.
3. Materials and Methods
3.1. Preparation of Biochar
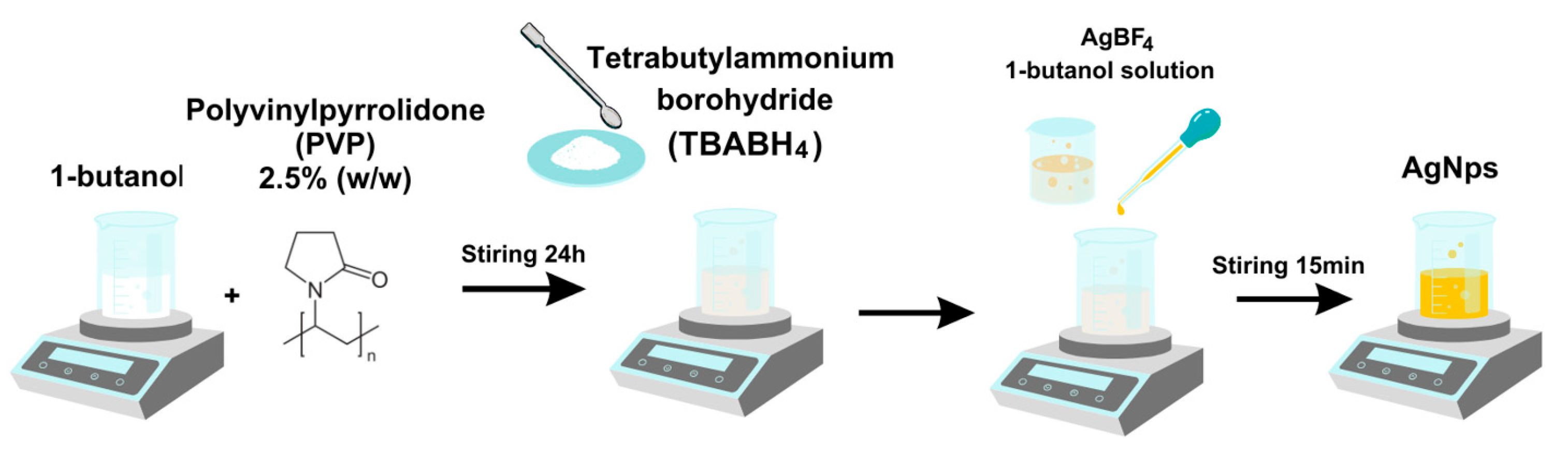
3.2. Synthesis of Silver Nanoparticle Dispersion
3.3. Impregnation of BCC with Silver Nanoparticles
3.4. Characterization Methods
3.5. Adsorption Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.M.; Bandosz, T.J. Ce(III) Doped Zr-Based MOFs as Excellent NO2 Adsorbents at Ambient Conditions. ACS Appl. Mater. Interfaces 2013, 5, 10565–10573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T.; Gu, Q.; Shang, J. Adsorption Removal of NO2 Under Low-Temperature and Low-Concentration Conditions: A Review of Adsorbents and Adsorption Mechanisms. Adv. Mater. 2025, 37, 2401623. [Google Scholar] [CrossRef] [PubMed]
- Bello, E.; Margarit, V.J.; Gallego, E.M.; Schuetze, F.; Hengst, C.; Corma, A.; Moliner, M. Deactivation and regeneration studies on Pd-containing medium pore zeolites as passive NOx adsorbers (PNAs) in cold-start applications. Microporous Mesoporous Mater. 2020, 302, 110222. [Google Scholar] [CrossRef]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 2019, 3, 108–118. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Thogersen, J.R.; Corma, A. Efficient One-Pot Preparation of Cu-SSZ-13 Materials using Cooperative OSDAs for their Catalytic Application in the SCR of NOx. ChemCatChem 2013, 5, 3316–3323. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NO by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Shang, S.; Yang, C.; Tian, Y.; Tao, Z.; Smith, M.; Zhang, H.; Zhang, L.; Li, L.; Gu, Q.; Zhou, H.-C.; et al. Designing multivariate porphyrin-based metal-organic frameworks with Ni/Co dual-metal atom sites for cooperative NO2 capture and NO retention. Sep. Purif. Technol. 2023, 320, 124080. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Levasseur, B.; Ebrahim, A.M.; Bandosz, T.J. Interactions of NO2 with Amine-Functionalized SBA-15: Effects of Synthesis Route. Langmuir 2012, 28, 5703–5714. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, L.; Jankowska, A. Mesoporous silica-based catalysts for selective catalytic reduction of NOx with ammonia—Recent advances. Adv. Inorg. Chem. 2022, 79, 209–245. [Google Scholar]
- Huang, G.; Yang, J.; Lv, C.; Li, D.; Fang, D. Research progress of NH3-SCR over carbon-based catalysts for NOx removal. J. Environ. Chem. Eng. 2023, 11, 110966. [Google Scholar] [CrossRef]
- Shirahama, N.; Moon, S.; Choi, K.-H.; Enjoji, T.; Kawano, S.; Korai, Y.; Tanoura, M.; Mochida, I. Mechanistic study on adsorption and reduction of NO2 over activated carbon fibers. Carbon 2002, 40, 2605–2611. [Google Scholar] [CrossRef]
- Jeguirim, M.; Belhachemi, M.; Limousy, L.; Bennici, S. Adsorption/reduction of nitrogen dioxide on activated carbons: Textural properties versus surface chemistry—A review. Chem. Eng. J. 2018, 347, 493–504. [Google Scholar] [CrossRef]
- Ebrahim, A.M.; Bandosz, T.J. Effect of amine type on acidic toxic gas adsorption at ambient conditions on modified CuBTC. J. Environ. Chem. Eng. 2022, 10, 107261. [Google Scholar] [CrossRef]
- Sun, J.; Gao, W.; Fei, H.; Zhao, G. Efficient and selective electrochemical reduction of nitrate to N2 by relay catalytic effects of Fe-Ni bimetallic sites on MOF-derived structure. Appl. Catal. B Environ. 2022, 301, 120829. [Google Scholar] [CrossRef]
- Khan, S.; Wasfi, A.; Mushtaq, M.; Awwad, F.; Tit, N.M. Transition-Metal Single Atom Catalyst Embedded in C2n for Toxic-Gas Reduction Reaction and Selective Gas-Sensing Application: Atomic-Scale Study. SSRN Electron. J. 2022, 599, 154037. [Google Scholar] [CrossRef]
- Sunil Kumar, M.; Alphin, M.S.; Senthil Kumar, P.; Raja, S. A review on zeolite catalyst for deNOx performance in ammonia–selective catalytic reduction. Fuel 2023, 334, 126828. [Google Scholar] [CrossRef]
- Ko, S.; Gao, F.; Yao, X.; Yi, H.; Tang, X.; Wang, C.; Liu, H.; Luo, N.; Qi, Z. Synthesis of metal–organic frameworks (MOFs) and their application in the selective catalytic reduction of NOx with NH3. New J. Chem. 2022, 46, 15758–15775. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Sun, J.; Xu, R.; Li, P.; Jing, L. Improved Performance of g-C3N4 for Optoelectronic Detection of NO2 Gas by Coupling Metal–Organic Framework Nanosheets with Coordinatively Unsaturated Ni(II) Sites. ACS Appl. Mater. Interfaces 2023, 15, 11961–11969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sheveleva, A.M.; Lee, D.; Chen, Y.; Iuga, D.; Franks, W.T.; Ma, Y.; Li, J.; Li, L.; Cheng, Y.; et al. Modulation of Uptake and Reactivity of Nitrogen Dioxide in Metal-Organic Framework Materials. Angew. Chemie 2023, 135, e202302602. [Google Scholar] [CrossRef]
- Wang, X.; Li, K.; Liang, G.; Zhao, Y.; Su, R.; Luan, Z.; Li, L.; Xi, H. Amino-modified zirconia aerogels for the efficient filtration of NO2: Effects of water on the removal mechanisms. Environ. Sci. Nano 2021, 8, 3722–3734. [Google Scholar] [CrossRef]
- Shang, S.; Wen, C.; Yang, C.; Tian, Y.; Wang, C.; Shang, J. The low-temperature NO2 removal by tailoring metal node in porphyrin-based metal-organic frameworks. Sci. Total Environ. 2021, 801, 149710. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Drané, M.; Zbair, M.; Hajjar-Garreau, S.; Josien, L.; Michelin, L.; Bennici, S.; Limousy, L. Unveiling the Potential of Corn Cob Biochar: Analysis of Microstructure and Composition with Emphasis on Interaction with NO2. Materials 2023, 17, 159. [Google Scholar] [CrossRef]
- Zbair, M.; Drané, M.; Limousy, L. NO2 Adsorption on Biochar Derived from Wood Shaving Litter: Understanding Surface Chemistry and Adsorption Mechanisms. Clean Technol. 2024, 6, 973–993. [Google Scholar] [CrossRef]
- Seredych, M.; Bashkova, S.; Pietrzak, R.; Bandosz, T.J. Interactions of NO2 and NO with Carbonaceous Adsorbents Containing Silver Nanoparticles. Langmuir 2010, 26, 9457–9464. [Google Scholar] [CrossRef]
- Illan-Gomez, M.J.; Linares-Solano, A.; Radovic, L.R.; Salinas-Martinez de Lecea, C. NO Reduction by Activated Carbons. 5. Catalytic Effect of Iron. Energy Fuels 1995, 9, 540–548. [Google Scholar] [CrossRef]
- Carabineiro, S.A.; Brás Fernandes, F.; Ramos, A.M.; Vital, J.; Silva, I.F. Vanadium as a catalyst for NO, N2O and CO2 reaction with activated carbon. Catal. Today 2000, 57, 305–312. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Yang, J. Spatially Nanoconfined Architectures: A Promising Design for Selective Catalytic Reduction of NOx. ChemCatChem 2020, 12, 5599–5610. [Google Scholar] [CrossRef]
- Shariff, A.; Mohamad Aziz, N.S.; Ismail, N.I.; Abdullah, N. Corn Cob as a Potential Feedstock for Slow Pyrolysis of Biomass. J. Phys. Sci. 2016, 27, 123–137. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Zbair, M.; Ainassaari, K.; Drif, A.; Ojala, S.; Bottlinger, M.; Pirilä, M.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Toward new benchmark adsorbents: Preparation and characterization of activated carbon from argan nut shell for bisphenol A removal. Environ. Sci. Pollut. Res. 2018, 25, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Skreiberg, A.; Skreiberg, Ø.; Sandquist, J.; Sørum, L. TGA and macro-TGA characterisation of biomass fuels and fuel mixtures. Fuel 2011, 90, 2182–2197. [Google Scholar] [CrossRef]
- Frikha, K.; Limousy, L.; Arif, M.B.; Thevenin, N.; Ruidavets, L.; Zbair, M.; Bennici, S. Exhausted Grape Marc Derived Biochars: Effect of Pyrolysis Temperature on the Yield and Quality of Biochar for Soil Amendment. Sustainability 2021, 13, 11187. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Mo, Z.; Shi, Q.; Zeng, H.; Lu, Z.; Bi, J.; Zhang, H.; Rinklebe, J.; Lima, E.C.; Rashid, A.; Shahab, A. Efficient removal of Cd(II) from aqueous environment by potassium permanganate-modified eucalyptus biochar. Biomass Convers. Biorefinery 2024, 14, 77–89. [Google Scholar] [CrossRef]
- Bashkova, S.; Bandosz, T.J. Adsorption/Reduction of NO2 on Graphite Oxide/Iron Composites. Ind. Eng. Chem. Res. 2009, 48, 10884–10891. [Google Scholar] [CrossRef]
- Pietrzak, R. Active Carbons Obtained from Bituminous Coal for NO2 Removal under Dry and Wet Conditions at Room Temperature. Energy Fuels 2009, 23, 3617–3624. [Google Scholar] [CrossRef]
- Levasseur, B.; Gonzalez-Lopez, E.; Rossin, J.A.; Bandosz, T.J. Effect of Reduction Treatment on Copper Modified Activated Carbons on NOx Adsorption at Room Temperature. Langmuir 2011, 27, 5354–5365. [Google Scholar] [CrossRef] [PubMed]
- Bashkova, S.; Bandosz, T.J. The effects of urea modification and heat treatment on the process of NO2 removal by wood-based activated carbon. J. Colloid Interface Sci. 2009, 333, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ligero, A.; Calero, M.; Martín-Lara, M.Á.; Blázquez, G.; Solís, R.R.; Pérez, A. Fixed-bed CO2 adsorption onto activated char from the pyrolysis of a non-recyclable plastic mixture from real urban residues. J. CO2 Util. 2023, 73, 102517. [Google Scholar] [CrossRef]
- Chu, K.H. Breakthrough curve analysis by simplistic models of fixed bed adsorption: In defense of the century-old Bohart-Adams model. Chem. Eng. J. 2020, 380, 122513. [Google Scholar] [CrossRef]
- Bohart, G.S.; Adams, E.Q. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Clark, R.M. Evaluating the cost and performance of field-scale granular activated carbon systems. Environ. Sci. Technol. 1987, 21, 573–580. [Google Scholar] [CrossRef]
- Hu, Q.; Xie, Y.; Zhang, Z. Modification of breakthrough models in a continuous-flow fixed-bed column: Mathematical characteristics of breakthrough curves and rate profiles. Sep. Purif. Technol. 2020, 238, 116399. [Google Scholar] [CrossRef]
- Kagawa, S.; Furukawa, H.; Iwamoto, M. Reaction of silver(I) oxide with nitrogen monoxide. J. Chem. Soc. Dalt. Trans. 1979, 74–78. [Google Scholar] [CrossRef]
- Sun, M.; Hanif, A.; Wang, T.; Gu, Q.; Shang, J. Ambient temperature NO2 removal by reversible NO2 adsorption on copper-based metal-organic frameworks (MOFs)-derived nanoporous adsorbents. Sep. Purif. Technol. 2023, 314, 123563. [Google Scholar] [CrossRef]
- Yang, M.; Xia, J.J. Preparation and Characterization of Platinum Nanorods Using Ascorbic Acid as the Reducing Agent. Adv. Mater. Res. 2013, 774–776, 577–580. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- García-Barrasa, J.; López-de-Luzuriaga, J.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Open Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Mills, G.; Hajek, B. Spontaneous formation of silver particles in basic 2-propanol. J. Phys. Chem. 1993, 97, 11542–11550. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Zbair, M.; Dutournié, P.; Bennici, S. Thermochemical sorption heat storage: Investigate the heat released from activated carbon beads used as porous host matrix for MgSO4 salt. J. Energy Storage 2023, 59, 106452. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Zbair, M.; Dutournié, P.; Gervasini, A.; Vaulot, C.; Bennici, S. Toward new low-temperature thermochemical heat storage materials: Investigation of hydration/dehydration behaviors of MgSO4/Hydroxyapatite composite. Sol. Energy Mater. Sol. Cells 2022, 240, 111696. [Google Scholar] [CrossRef]






| Proximate Analysis (wt.%, Wet Basis) | ||||
|---|---|---|---|---|
| Humidity | VM * | FC ** | Ash | |
| CC | 4.10 | 75.69 | 18.81 | 1.40 |
| BCC | 2.03 | 9.90 | 83.05 | 5.02 |
| Element | CC | BCC | Ag-BCC |
|---|---|---|---|
| C | 48.00 | 87.30 | 93 |
| H | 6.04 | 1.82 | - |
| O | 43.00 | 2.64 | 1.66 |
| N | 0.77 | 0.86 | - |
| S | 0.13 | 0.13 | 0.07 |
| Element | CC | BCC | Ag-BCC |
|---|---|---|---|
| Mg | 0.051 | 0.381 | 0.213 |
| Al | 0.051 | 0.101 | 0.106 |
| Si | 0.661 | 1.149 | 0.432 |
| P | 0.108 | 0.367 | 0.139 |
| Na | 0.015 | 0.089 | 0.066 |
| Cl | 0.249 | 1.141 | 0.715 |
| K | 0.826 | 3.804 | 2.527 |
| Ca | 0.057 | 0.111 | 0.063 |
| Fe | 0.032 | 0.084 | 0.039 |
| Zn | 0.003 | 0.011 | 0.019 |
| Br | 0.002 | 0.006 | - |
| Ag | - | - | 0.945 |
| Sample | A | r (1/min) | n | R2 | Reduced Chi-Sqr |
|---|---|---|---|---|---|
| BCC-22 °C | 1.61 × 1014 | 31.442 | 20.87 | 0.983 | 0.001 |
| BCC-100 °C | 2.47 × 1013 | 29.184 | 18.288 | 0.988 | 0.001 |
| BCC-200 °C | 7.55 × 1011 | 25.626 | 17.903 | 0.992 | 0.001 |
| Ag-BCC-22 °C | 1.62 × 1015 | 34.132 | 20.652 | 0.974 | 0.003 |
| Ag-BCC-100 °C | 6.14 × 109 | 21.366 | 14.510 | 0.982 | 0.002 |
| Ag-BCC-200 °C | 4.34 × 109 | 20.405 | 14.466 | 0.989 | 0.001 |
| Sample | N0 (mg/cm3) | KBA (cm3/mg.min) | R2 | Reduced Chi-Sqr |
|---|---|---|---|---|
| BCC-22 °C | 564 | 1.289 | 0.911 | 0.008 |
| BCC-100 °C | 648 | 1.297 | 0.911 | 0.008 |
| BCC-200 °C | 814 | 0.729 | 0.947 | 0.003 |
| Ag-BCC-22 °C | 421 | 1.764 | 0.896 | 0.010 |
| Ag-BCC-100 °C | 535 | 1.352 | 0.907 | 0.009 |
| Ag-BCC-200 °C | 782 | 1.067 | 0.931 | 0.007 |
| Sample | KDR (1/min) | s | R2 | Reduced Chi-Sqr |
|---|---|---|---|---|
| BCC-22 °C | 0.441 | 1.584 | 0.984 | 0.008 |
| BCC-100 °C | 0.367 | 1.706 | 0.989 | 0.001 |
| BCC-200 °C | 0.315 | 1.553 | 0.994 | 0.001 |
| Ag-BCC-22 °C | 0.592 | 1.718 | 0.975 | 0.002 |
| Ag-BCC-100 °C | 0.465 | 1.580 | 0.984 | 0.001 |
| Ag-BCC-200 °C | 0.300 | 1.566 | 0.992 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, F.; Camilo, F.F.; Zbair, M.; Limousy, L.; Brendle, J. Silver-Modified Biochar: Investigating NO2 Adsorption and Reduction Efficiency at Different Temperatures. Catalysts 2025, 15, 392. https://doi.org/10.3390/catal15040392
Tavares F, Camilo FF, Zbair M, Limousy L, Brendle J. Silver-Modified Biochar: Investigating NO2 Adsorption and Reduction Efficiency at Different Temperatures. Catalysts. 2025; 15(4):392. https://doi.org/10.3390/catal15040392
Chicago/Turabian StyleTavares, Flavia, Fernanda F. Camilo, Mohamed Zbair, Lionel Limousy, and Jocelyne Brendle. 2025. "Silver-Modified Biochar: Investigating NO2 Adsorption and Reduction Efficiency at Different Temperatures" Catalysts 15, no. 4: 392. https://doi.org/10.3390/catal15040392
APA StyleTavares, F., Camilo, F. F., Zbair, M., Limousy, L., & Brendle, J. (2025). Silver-Modified Biochar: Investigating NO2 Adsorption and Reduction Efficiency at Different Temperatures. Catalysts, 15(4), 392. https://doi.org/10.3390/catal15040392









