Bacterial Inactivation and Organic Pollutant Degradation in Slaughterhouse Wastewater Using Ag2O/Ba/TiO2 Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. SWW Sampling
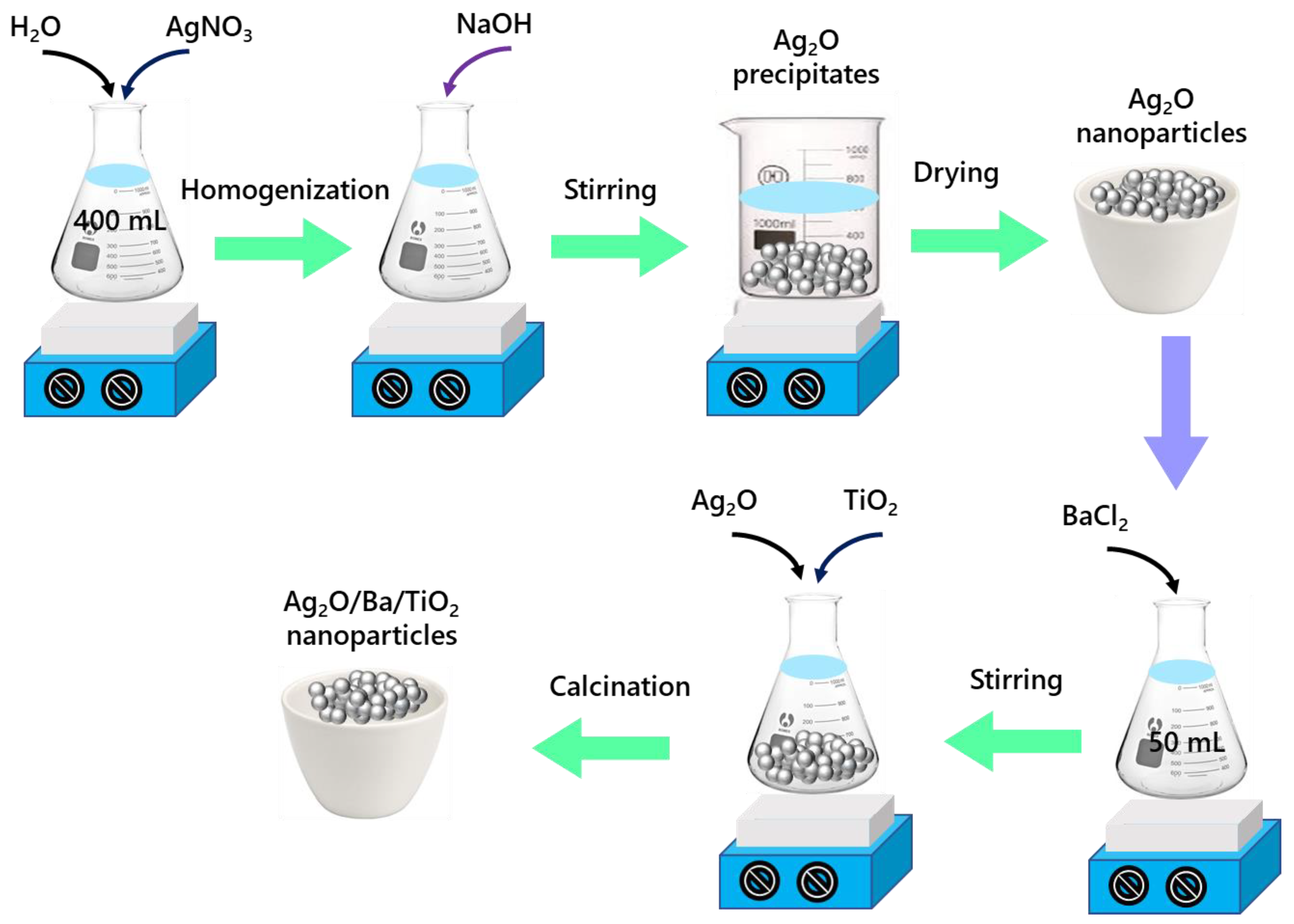
2.3. Synthesis of Ag2O Nanoparticles
2.4. Synthesis of Ag2O/Ba/TiO2 Nanocomposite
2.5. Characterization of Nanomaterials
2.6. Analytical Procedures
2.6.1. COD Determination
2.6.2. Solids Determination
2.7. Colony Forming Unit Tests for Screening
2.8. Antibacterial Activity Testing
Zone of Inhibition Assessment
2.9. Photocatalytic Experiments
2.9.1. Wastewater Degradation
2.9.2. Bacterial Inactivation
2.10. Statistical Analysis
3. Results and Discussion
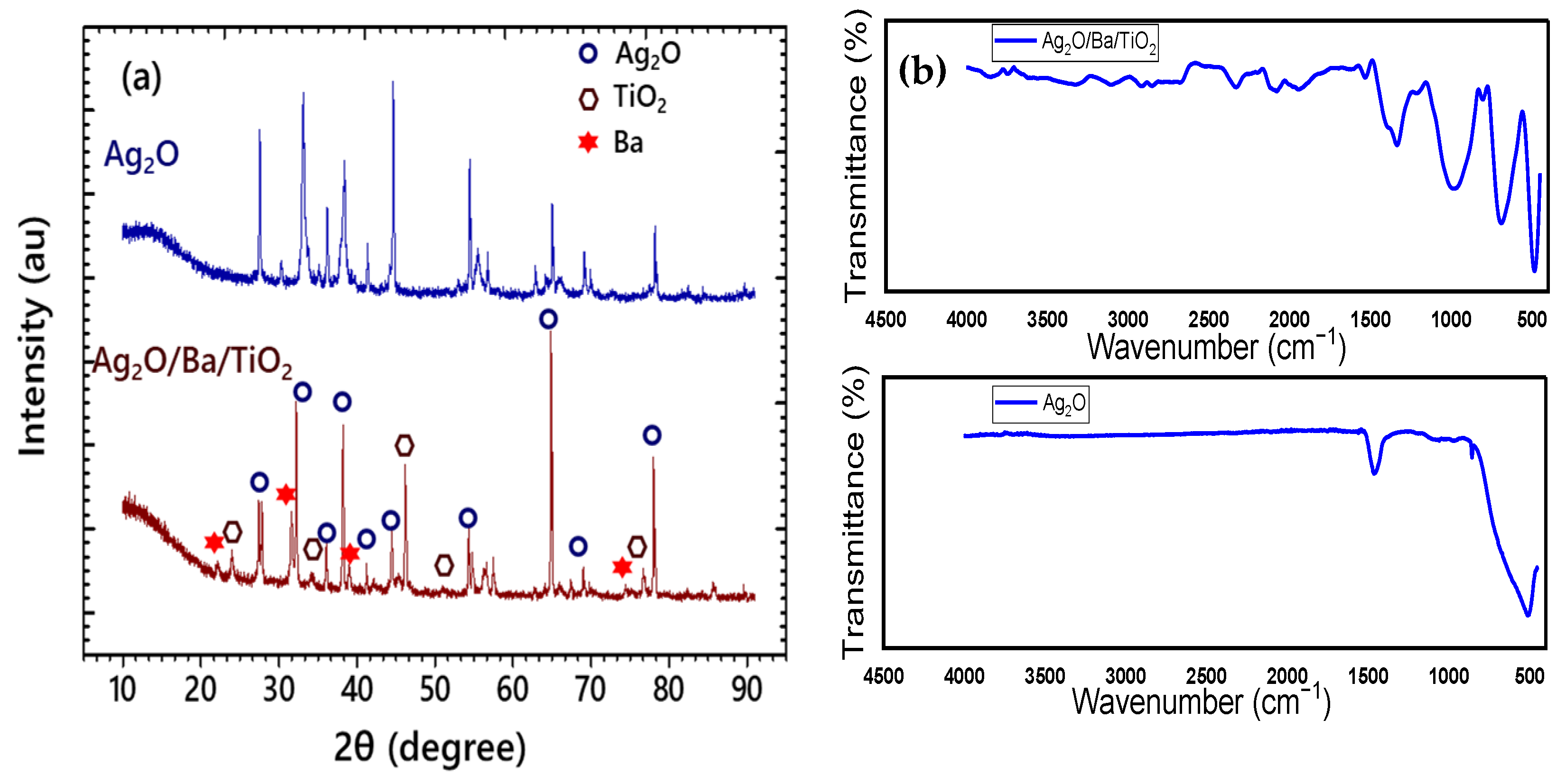
3.1. Structural and Spectroscopic Characteristics of Ag2O/Ba/TiO2 Nanocomposite
3.2. Morphology and Optical Properties of Ag2O/Ba/TiO2 Nanocomposite
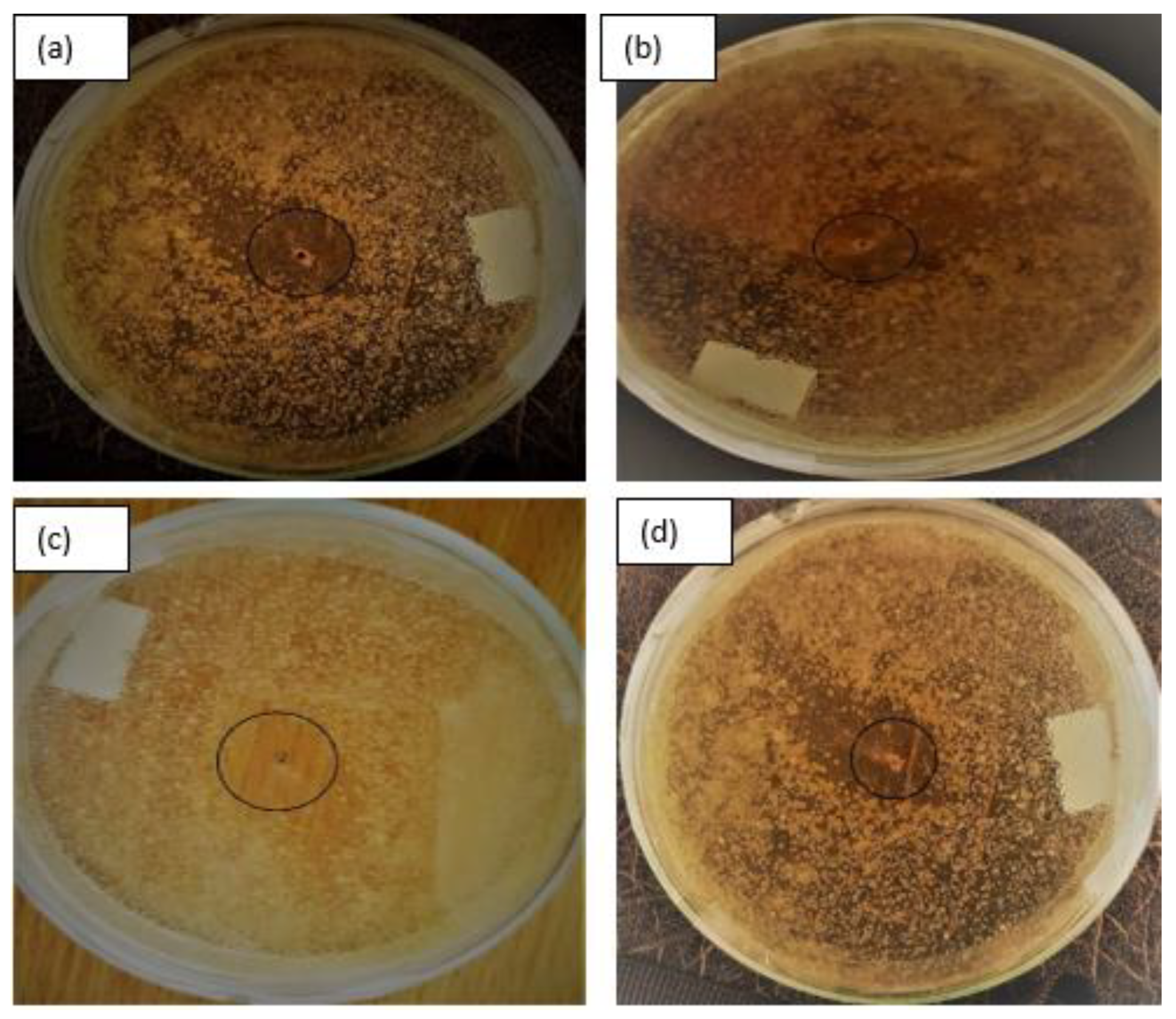
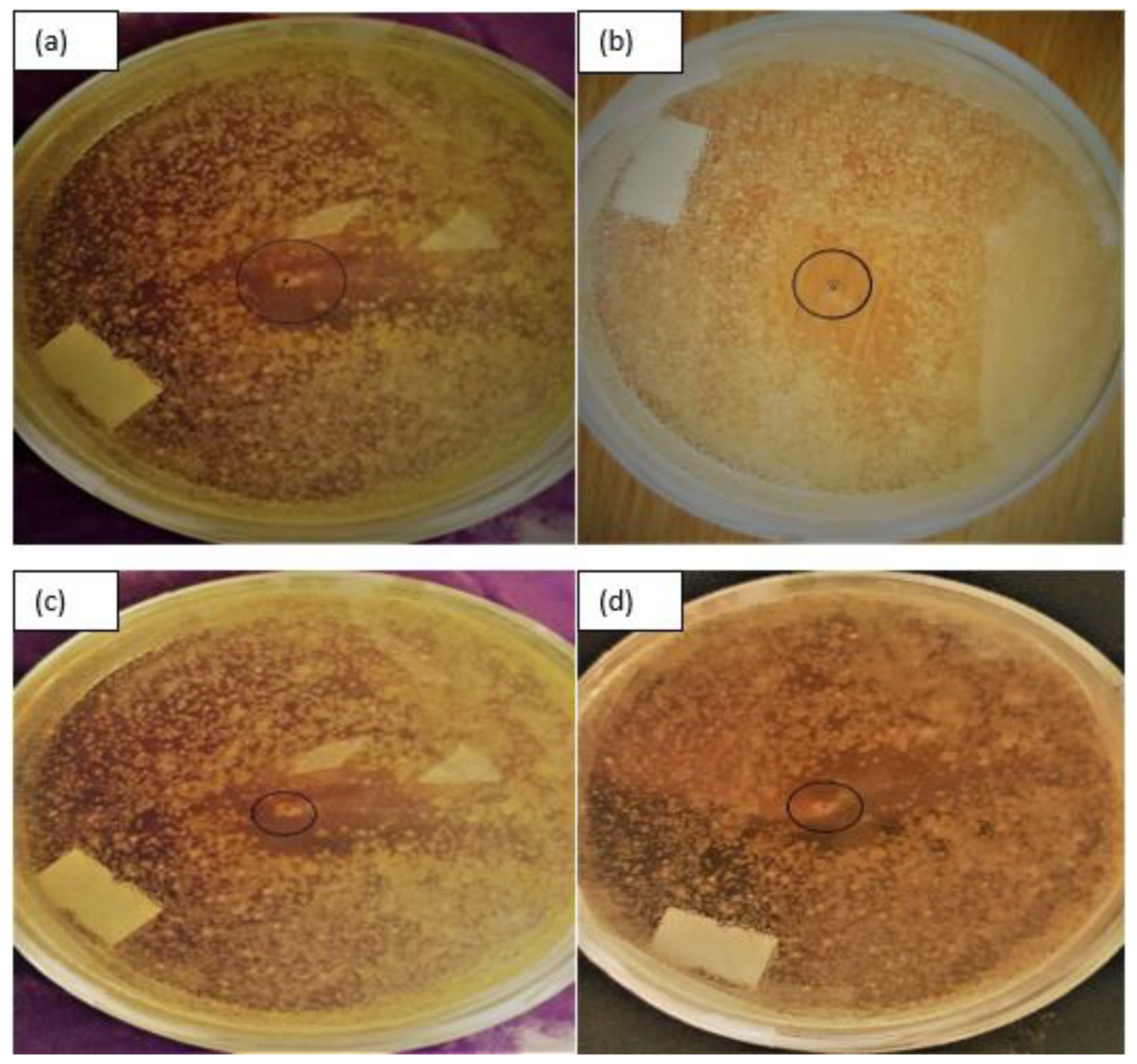
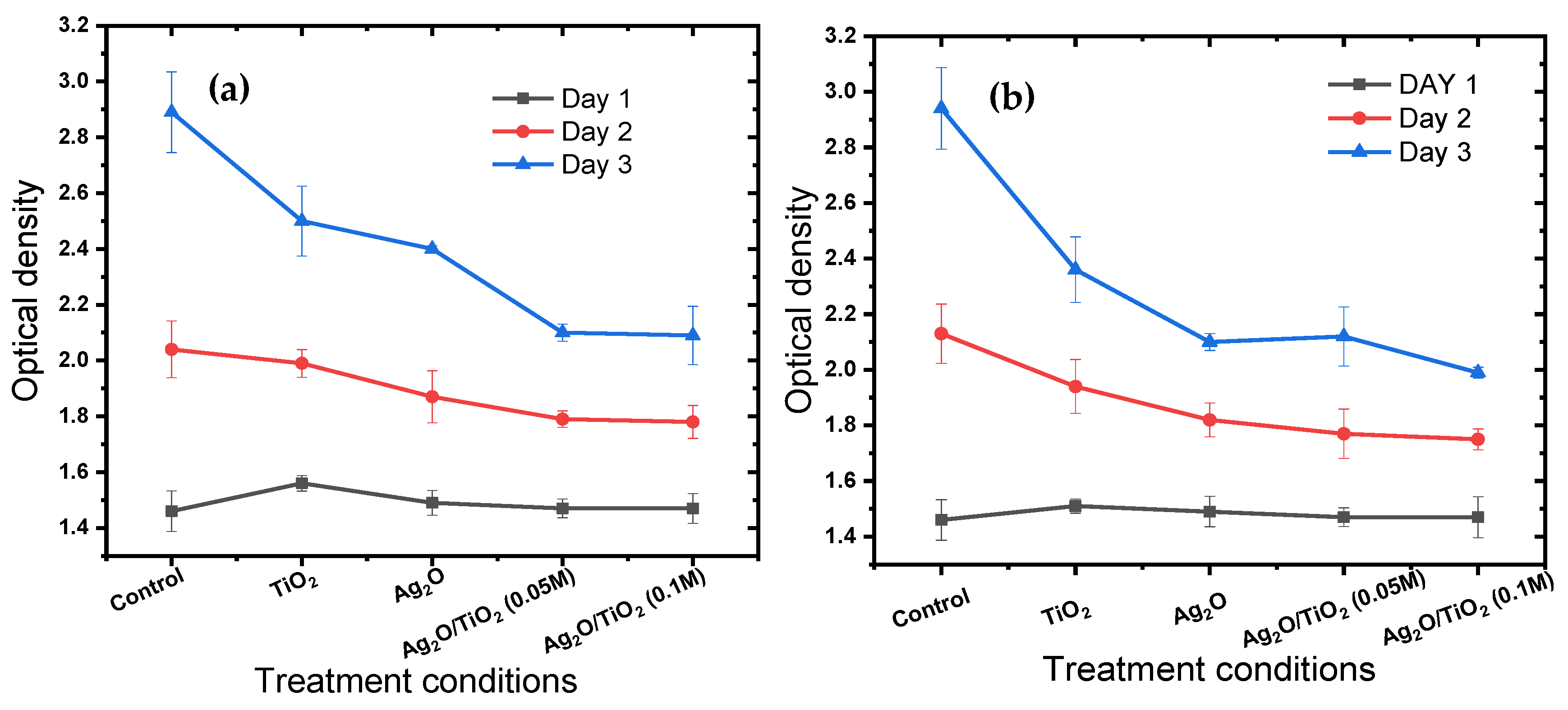
3.3. Antibacterial Activity of Ag2O/Ba/TiO2 Nanocomposite
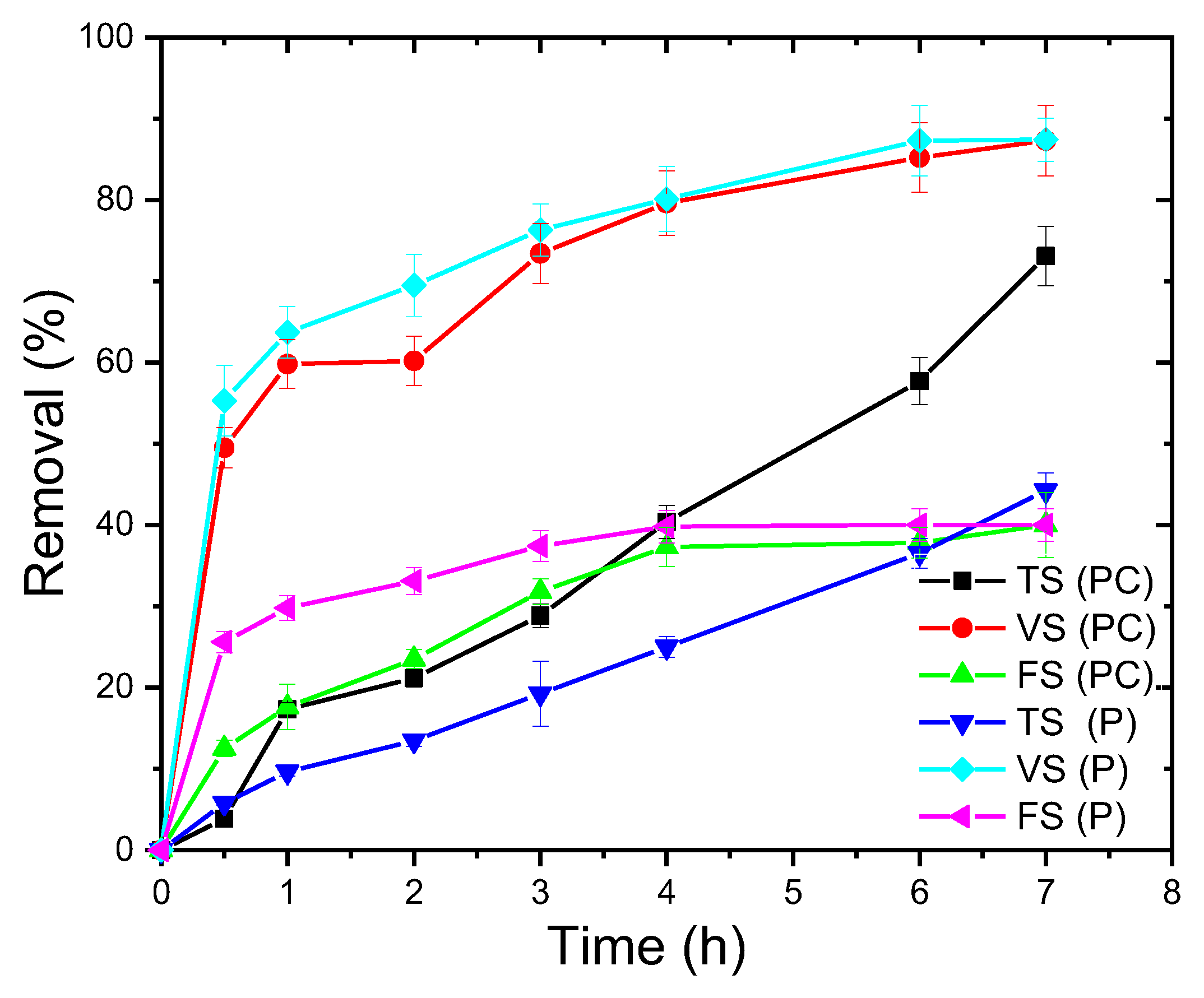
3.4. Organics Removal from SSW Using Ag2O/Ba/TiO2 Nanocomposite
3.5. Kinetic Modeling
3.6. Upscaling and Environmental Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Yuan, R.; Wang, Q.; Jin, S. Consumers’ preferences for the attributes of plant-based meat in China: A best-worst scaling approach. Future Foods 2024, 9, 100384. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Coreño, O.; García, V.; Salazar-González, R. Enhancing industrial swine slaughterhouse wastewater treatment: Optimization of electrocoagulation technique and operating mode. J. Environ. Manag. 2024, 349, 119556. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Basheer, F.; Sengar, A.; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef]
- Zanol, M.B.; Lima, J.P.P.; Assemany, P.; Aguiar, A. Assessment of characteristics and treatment processes of wastewater from slaughterhouses in the state of Minas Gerais, Brazil. J. Environ. Manag. 2024, 358, 120862. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Lecompte, C.; Mehrvar, M. Slaughterhouse wastewater: Treatment, management and resource recovery. In Physico-Chemical Wastewater Treatment and Resource Recovery; IntechOpen: London, UK, 2017; pp. 153–174. [Google Scholar]
- Almandoz, M.C.; Pagliero, C.L.; Ochoa, N.A.; Marchese, J. Composite ceramic membranes from natural aluminosilicates for microfiltration applications. Ceram. Int. 2015, 41, 5621–5633. [Google Scholar] [CrossRef]
- Barrera, M.; Mehrvar, M.; Gilbride, K.A.; McCarthy, L.H.; Laursen, A.E.; Bostan, V.; Pushchak, R. Photolytic treatment of organic constituents and bacterial pathogens in secondary effluent of synthetic slaughterhouse wastewater. Chem. Eng. Res. Des. 2012, 90, 335–1350. [Google Scholar] [CrossRef]
- Sau, A.; Ghosh, S.; Kandar, B.; Ghanta, K.C.; Baltrėnaitė-Gedienė, E.; Dutta, S. Enhanced slaughterhouse wastewater treatment: A comparative approach with phycoremediation and adsorption. J. Indian Chem. Soc. 2024, 101, 101499. [Google Scholar] [CrossRef]
- Del Nery, V.; De Nardi, I.R.; Damianovic, M.H.; Pozzi, E.; Amorim, A.K.; Zaiat, M. Long-term operating performance of a poultry slaughterhouse wastewater treatment plant. Resour. Conserv. Recycl. 2007, 50, 102–114. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Carbajo, J.; García-Muñoz, P. Intensification of Photo-Assisted Advanced Oxidation Processes for Water Treatment: A Critical Review. Catalysts 2023, 13, 401. [Google Scholar] [CrossRef]
- Luiz, D.B.; Genena, A.K.; José, H.J.; Moreira, R.F.; Schröder, H.F. Tertiary treatment of slaughterhouse effluent: Degradation kinetics applying UV radiation or H2O2/UV. Water Sci. Technol. 2009, 60, 1869–1874. [Google Scholar] [CrossRef]
- Hanafiah, M.M.; Nawaz, R.; Ikram, A.; Ishaq, Y.; Alothman, A.A.; Mohammad, S.; Anjum, M.; Arshad, U.; Baki, Z.A. TiO2-mediated visible-driven photocatalytic degradation of phenolic compounds: Predictive modeling and optimization via machine learning techniques. Chem. Eng. Commun. 2025, 1–20. [Google Scholar] [CrossRef]
- Nawaz, R.; Haider, S.; Anjum, M.; Haneef, T.; Oad, V.K.; Aqif, M.; Haider, A.; Khan, R. Photodegrading hazardous pollutants using black TiO2 materials with different morphology and estimation of energy requirement. Mater. Chem. Phys. 2023, 309, 128401. [Google Scholar] [CrossRef]
- Nawaz, R.; Haider, S.; Anjum, M.; Oad, V.K.; Haider, A.; Khan, R.; Aqif, M.; Hanif, T.; Khan, N. Optimized photodegradation of palm oil agroindustry waste effluent using multivalent manganese–modified black titanium dioxide. Environ. Sci. Pollut. Res. 2023, 30, 77850–77874. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, R.; Hanafiah, M.M.; Haider, S.; Anjum, M.; Ali, M.; Khan, R.; Khurshid, S.; Ullah, S.; Aqif, M.; Haider, A.; et al. Development of energy efficient flower-shaped defective TiO2 materials for wastewater remediation of agro-industries and oil refineries. Process Saf. Environ. Prot. 2024, 188, 105–121. [Google Scholar] [CrossRef]
- Nawaz, R.; Arshad, U.; Hanafiah, M.M.; Haider, S.; Anjum, M.; Baki, Z.A.; Khan, R.; Sakawi, Z.; Aqif, M.; Haider, A.; et al. Visible-driven industrial wastewater remediation using black titania: Optimization, energy consumption, treatment, and material preparation costs estimation. Chem. Eng. Sci. 2025, 306, 121257. [Google Scholar] [CrossRef]
- Alafif, Z.O.; Anjum, M.; Ansari, M.O.; Kumar, R.; Rashid, J.; Madkour, M.; Barakat, M.A. Synthesis and characterization of S-doped-rGO/ZnS nanocomposite for the photocatalytic degradation of 2-chlorophenol and disinfection of real dairy wastewater. J. Photochem. Photobiol. A Chem. 2019, 377, 190–197. [Google Scholar] [CrossRef]
- He, N.; Cao, S.; Gu, J.; Uddin, A.; Zhang, C.; Yu, Y.; Chen, H.; Jiang, F. Well-designed oxidized Sb/g-C3N4 2D/2D nanosheets heterojunction with enhanced visible-light photocatalytic disinfection activity. J. Colloid Interface Sci. 2022, 606, 284–1298. [Google Scholar] [CrossRef]
- Anjum, M.; Qadeer, S.; Waqas, M.; Khalid, A.; Miandad, R.; Barakat, M.A.E.F. Antimicrobial Nanomaterials for Water Disinfection. In Nanomaterials for Environmental Applications; CRC Press: Boca Raton, FL, USA, 2022; pp. 231–260. [Google Scholar]
- Chairungsri, W.; Subkomkaew, A.; Kijjanapanich, P.; Chimupala, Y. Direct dye wastewater photocatalysis using immobilized titanium dioxide on fixed substrate. Chemosphere 2022, 286, 131762. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Khanna, A.; Shetty, K.V. Solar light-driven photocatalytic degradation of Anthraquinone dye-contaminated water by engineered Ag@TiO2 core–shell nanoparticles. Desalination Water Treat. 2015, 54, 744–757. [Google Scholar] [CrossRef]
- Moradi, R.; Hamidvand, M.; Ganjali, A. Using of TiO2/Ag2O Nanocomposite in Degradation of Acid Red 18 Dye in Photoreactor by Taguchi Experimental Design. Russ. J. Phys. Chem. A 2019, 93, 1133–1142. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Hernández-Morales, V.A.; Rodríguez-Varela, M.; Guerrero-Araque, D.; Ramirez-Ortega, D.; Castillón, F.; Acevedo-Peña, P.; Zanella, R. Ag2O/TiO2 nanostructures for the photocatalytic mineralization of the highly recalcitrant pollutant iopromide in pure and tap water. Catal. Today 2020, 341, 71–81. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and antimicrobial properties of Ag2O/TiO2 heterojunction. ChemEngineering 2019, 3, 3. [Google Scholar] [CrossRef]
- Wang, X.; Wu, H.F.; Kuang, Q.; Huang, R.B.; Xie, Z.X.; Zheng, L.S. Shape-dependent antibacterial activities of Ag2O polyhedral particles. Langmuir 2010, 26, 2774–2778. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, H.; Yang, R.; Yu, X.; Marfavi, Z.; Lv, Q.; Zhang, G.; Sun, K.; Yuan, C.; Tao, K. Ba2+-doping introduced piezoelectricity and efficient Ultrasound-Triggered bactericidal activity of brookite TiO2 nanorods. J. Colloid Interface Sci. 2024, 670, 742–750. [Google Scholar] [CrossRef]
- Mohapatra, S.; Singh, J.; Satpati, B. Facile synthesis, structural, optical and photocatalytic properties of mesoporous Ag2O/TiO2 nanoheterojunctions. J. Phys. Chem. Solids 2020, 138, 109305. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, S.B.; Jamal, A.; Faisal, M.; Asiri, A.M. Fabrication of highly sensitive acetone sensor based on sonochemically prepared as-grown Ag2O nanostructures. Chem. Eng. J. 2012, 192, 122–128. [Google Scholar] [CrossRef]
- Sivalingam, G.; Nagaveni, K.; Hegde, M.S.; Madras, G. Photocatalytic degradation of various dyes by combustion synthesized nano anatase TiO2. Appl. Catal. B Environ. 2003, 45, 23–38. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Iqbal, M.; Nadeem, M.A.; Hussain, I.; Yousaf, S.; Mustafa, G.; Zafar, M.I.; Nadeem, M.A.; et al. Photocatalytic degradation of textile dyes on Cu2O-CuO/TiO2 anatase powders. J. Environ. Chem. Eng. 2016, 4, 2138–2146. [Google Scholar] [CrossRef]
- Padmini, M.; Balaganapathi, T.; Thilakan, P. Rutile-TiO2: Post heat treatment and its influence on the photocatalytic degradation of MB dye. Ceram. Int. 2022, 48, 16685–16694. [Google Scholar] [CrossRef]
- Jalali, E.; Maghsoudi, S.; Noroozian, E. A novel method for biosynthesis of different polymorphs of TiO2 nanoparticles as a protector for Bacillus thuringiensis from Ultra Violet. Sci. Rep. 2020, 10, 426. [Google Scholar] [CrossRef]
- Chen, J.W.; Pal, A.; Chen, B.H.; Kumar, G.; Chatterjee, S.; Peringeth, K.; Huang, M.H. Shape-Tunable BaTiO3 Crystals Presenting Facet-Dependent Optical and Piezoelectric Properties. Small 2023, 19, 2205920. [Google Scholar] [CrossRef]
- Mugundan, S.; Praveen, P.; Sridhar, S.; Prabu, S.; Mary, K.L.; Ubaidullah, M.; Shaikh, S.F.; Kanagesan, S. Sol-gel synthesized barium doped TiO2 nanoparticles for solar photocatalytic application. Inorg. Chem. Commun. 2022, 139, 109340. [Google Scholar] [CrossRef]
- Wong, K.A.; Lam, S.M.; Sin, J.C. Wet chemically synthesized ZnO structures for photodegradation of pre-treated palm oil mill effluent and antibacterial activity. Ceram. Int. 2019, 45, 1868–1880. [Google Scholar] [CrossRef]
- Shukla, M.; Kumari, S.; Shukla, S.; Shukla, R.K. Potent antibacterial activity of nano CdO synthesized via microemulsion scheme. J. Mater. Environ. Sci. 2012, 3, 678–685. [Google Scholar]
- Rajeshkumar, S.; Kannan, C.; Annadurai, G. Green synthesis of silver nanoparticles using marine brown algae Turbinaria conoides and its antibacterial activity. Int. J. Pharma Bio Sci. 2012, 3, 502–510. [Google Scholar]
- Sánchez, M.; Fernández, M.I.; Ruiz, I.; Canle, M.; Soto, M. Combining Constructed Wetlands and UV Photolysis for the Advanced Removal of Organic Matter, Nitrogen, and Emerging Pollutants from Wastewater. Environments 2023, 10, 35. [Google Scholar] [CrossRef]
- George, C.; Beeldens, A.; Barmpas, F.; Doussin, J.F.; Manganelli, G.; Herrmann, H.; Kleffmann, J.; Mellouki, A. Impact of photocatalytic remediation of pollutants on urban air quality. Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Vargas, J.B.; Akizuki, S.; Kodera, T.; Ida, J.; Cuevas-Rodríguez, G. Cumulative effects of titanium dioxide nanoparticles in UASB process during wastewater treatment. J. Environ. Manag. 2021, 277, 111428. [Google Scholar] [CrossRef]
- Padmavathy, K.S.; Madhu, G.; Haseena, P.V. A study on effects of pH, adsorbent dosage, time, initial concentration and adsorption isotherm study for the removal of hexavalent chromium (Cr (VI)) from wastewater by magnetite nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Ng, K.H.; Lee, C.H.; Khan, M.R.; Cheng, C.K. Photocatalytic degradation of recalcitrant POME waste by using silver doped titania: Photokinetics and scavenging studies. Chem. Eng. J. 2016, 286, 282–290. [Google Scholar] [CrossRef]
- Wint, T.H.M.; Smith, M.F.; Chanlek, N.; Chen, F.; Oo, T.Z.; Songsiriritthigul, P. Physical origin of diminishing photocatalytic efficiency for recycled TiO2 nanotubes and Ag-loaded TiO2 nanotubes in organic aqueous solution. Catalysts 2020, 10, 737. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, X.; Li, Y.; Li, X.; Yang, L.; Ji, L.; He, Y. Preparation of a novel chelating resin containing amidoxime–guanidine group and its recovery properties for silver ions in aqueous solution. Chem. Eng. J. 2012, 209, 394–400. [Google Scholar] [CrossRef]
- Syed, S. Silver recovery aqueous techniques from diverse sources: Hydrometallurgy in recycling. Waste Manag. 2016, 50, 234–256. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Mullani, S.B.; Koli, V.B.; Patil, S.M.; Kasabe, P.J.; Dandge, P.B.; Pawar, S.A.; Delekar, S.D. Ag nanoparticles connected to the surface of TiO2 electrostatically for antibacterial photoinactivation studies. Photochem. Photobiol. 2018, 94, 1249–1262. [Google Scholar] [CrossRef]
- Liu, B.; Mu, L.; Han, B.; Zhang, J.; Shi, H. Fabrication of TiO2/Ag2O heterostructure with enhanced photocatalytic and antibacterial activities under visible light irradiation. Appl. Surf. Sci. 2017, 396, 1596–1603. [Google Scholar] [CrossRef]
- Jia, J.; Giannakis, S.; Li, D.; Yan, B.; Lin, T. Efficient and sustainable photocatalytic inactivation of E. coli by an innovative immobilized Ag/TiO2 photocatalyst with peroxymonosulfate (PMS) under visible light. Sci. Total Environ. 2023, 901, 166376. [Google Scholar] [CrossRef]
- Yang, G.; Yin, H.; Liu, W.; Yang, Y.; Zou, Q.; Luo, L.; Li, H.; Huo, Y.; Li, H. Synergistic Ag/TiO2-N photocatalytic system and its enhanced antibacterial activity towards Acinetobacter baumannii. Appl. Catal. B Environ. 2018, 224, 175–182. [Google Scholar] [CrossRef]
- Zhang, F.; Lee, M.H.; Huang, Y.; Keller, A.A.; Majumdar, S.; Cervantes-Avilés, P.; Tang, X.; Yin, S. Effective water disinfection using magnetic barium phosphate nanoflakes loaded with Ag nanoparticles. J. Clean. Prod. 2019, 218, 173–182. [Google Scholar] [CrossRef]
- Gou, J.; Ma, Q.; Deng, X.; Cui, Y.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q. Fabrication of Ag2O/TiO2-Zeolite composite and its enhanced solar light photocatalytic performance and mechanism for degradation of norfloxacin. Chem. Eng. J. 2017, 308, 818–826. [Google Scholar] [CrossRef]
- Deekshitha; Shetty, V. Solar light active biogenic titanium dioxide embedded silver oxide (AgO/Ag2O@TiO2) nanocomposite structures for dye degradation by photocatalysis. Mater. Sci. Semicond. Process. 2021, 132, 105923. [Google Scholar] [CrossRef]
- Son, H.S.; Im, J.K.; Zoh, K.D. A Fenton-like degradation mechanism for 1, 4-dioxane using zero-valent iron (Fe0) and UV light. Water Res. 2009, 43, 1457–1463. [Google Scholar] [CrossRef]
- Laipan, M.; Zhu, R.; Zhu, J.; He, H. Visible light assisted Fenton-like degradation of Orange II on Ni3Fe/Fe3O4 magnetic catalyst prepared from spent FeNi layered double hydroxide. J. Mol. Catal. A Chem. 2016, 415, 9–16. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.; Wang, R.; Chou, S.; Dong, Z. A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes. J. Hazard. Mater. 2016, 301, 462–470. [Google Scholar] [CrossRef]
- Tekin, G.; Ersöz, G.; Atalay, S. Degradation of benzoic acid by advanced oxidation processes in the presence of Fe or Fe-TiO2 loaded activated carbon derived from walnut shells: A comparative study. J. Environ. Chem. Eng. 2018, 6, 1745–1759. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Klauck, C.R.; Giacobbo, A.; de Oliveira, E.D.L.; da Silva, L.B.; Rodrigues, M.A.S. Evaluation of acute toxicity, cytotoxicity and genotoxicity of landfill leachate treated by biological lagoon and advanced oxidation processes. J. Environ. Chem. Eng. 2017, 5, 6188–6193. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Ebrahim, S.E. Recent advances in nano-semiconductors photocatalysis for degrading organic contaminants and microbial disinfection in wastewater: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100666. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Sanabria, J.; Machuca, F.; Dierolf, C.F.; Pulgarin, C.; Orellana, G. A Comparison of Solar Photocatalytic Inactivation of Waterborne E. coli Using Tris (2,2′-bipyridine) ruthenium (II), Rose Bengal, and TiO2. J. Sol. Energy Eng. 2005, 129, 135–140. [Google Scholar] [CrossRef]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Sacco, O.; Guida, M.; Rizzo, L. Comparison between heterogeneous and homogeneous solar driven advanced oxidation processes for urban wastewater treatment: Pharmaceuticals removal and toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- Herrera Melián, J.A.; Araña, J.; Ortega, J.A.; Martín Muñoz, F.; Tello Rendón, E.; Pérez Peña, J. Comparative study of phenolics degradation between biological and photocatalytic systems. J. Sol. Energy Eng. 2008, 130, 041003. [Google Scholar] [CrossRef]
- Dunlop, P.S.; McMurray, T.A.; Hamilton, J.W.; Byrne, J.A. Photocatalytic inactivation of Clostridium perfringens spores on TiO2 electrodes. J. Photochem. Photobiol. A Chem. 2008, 196, 113–119. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Invironmental Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why five decades of massive research on heterogeneous photocatalysis, especially on TiO2, has not yet driven to water disinfection and detoxification applications? Critical review of drawbacks and challenges. Chem. Eng. J. 2023, 477, 146875. [Google Scholar] [CrossRef]
- Ng, M.; Dalhatou, S.; Wilson, J.; Kamdem, B.P.; Temitope, M.B.; Paumo, H.K.; Djelal, H.; Assadi, A.A.; Nguyen-Tri, P.; Kane, A. Characterization of slaughterhouse wastewater and development of treatment techniques: A review. Processes 2022, 10, 1300. [Google Scholar] [CrossRef]
- Belsky, A.J.; Matzke, A.; Uselman, S. Survey of livestock influences on stream and riparian ecosystems in the western United States. J. Soil Water Conserv. 1999, 54, 419–431. [Google Scholar]
- Cao, W.; Mehrvar, M. Slaughterhouse wastewater treatment by combined anaerobic baffled reactor and UV/H2O2 processes. Chem. Eng. Res. Des. 2011, 89, 1136–1143. [Google Scholar] [CrossRef]


| Photocatalyst | Photocatalyst Concentration (mg/mL) | Bacterial Species/Organic Pollutant | Light Intensity | Organic Removal | Bacterial Inactivation (%) | Reference |
|---|---|---|---|---|---|---|
| Ag/TiO2 NCs | 1 | Escherichia coli | 5 mWcm−2 | - | 100 | [47] |
| TiO2/TiO2 | 1 0.4 | E. coli (ATCC 15597) Methylene blue | 10 mW/cm2 300 W *** | 100 | 100 | [48] |
| Ag/SCA/TiO2NTAs | 0.14 | E. coli | 93.5 W/m2 | - | 2.99 × 102 * | [49] |
| TiO2: Ba | 0.4 1 | E. coli Rhodamine B | - 450 W *** | 100 | 100 | [27] |
| Ag/TiO2-N | 3 | Acinetobacter baumannii | 300 W *** | - | 100 | [50] |
| Ag NPs on FBP | 1 | E. coli | - | - | 100 | [51] |
| Ag2O/TiO2-Zeolite | 0.5 | Norfloxacin | 6.7 mW·cm−2 | 98.7 | - | [52] |
| TiO2 embedded AgO/Ag2O | 1 | Reactive Blue 220 | 125W *** | 100 | - | [53] |
| Ba-TiO2 | 0.2 | Methylene blue | Solar light | 75 | - | [35] |
| Ag2O/Ba/TiO2 | 1 | E. coli COD | Solar light | 98.7 | 4 to 9 ** nm | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, H.; Elahi, I.; Saleem, S.; Nawaz, R.; Ullah, S.; Qadeer, S.; Kabeer, B.; Anjum, M.; Liu, Y.; Shahab, A.; et al. Bacterial Inactivation and Organic Pollutant Degradation in Slaughterhouse Wastewater Using Ag2O/Ba/TiO2 Nanocomposite. Catalysts 2025, 15, 411. https://doi.org/10.3390/catal15050411
Ullah H, Elahi I, Saleem S, Nawaz R, Ullah S, Qadeer S, Kabeer B, Anjum M, Liu Y, Shahab A, et al. Bacterial Inactivation and Organic Pollutant Degradation in Slaughterhouse Wastewater Using Ag2O/Ba/TiO2 Nanocomposite. Catalysts. 2025; 15(5):411. https://doi.org/10.3390/catal15050411
Chicago/Turabian StyleUllah, Habib, Izhar Elahi, Sahar Saleem, Rab Nawaz, Shafi Ullah, Samia Qadeer, Bilal Kabeer, Muzammil Anjum, Yi Liu, Asfandyar Shahab, and et al. 2025. "Bacterial Inactivation and Organic Pollutant Degradation in Slaughterhouse Wastewater Using Ag2O/Ba/TiO2 Nanocomposite" Catalysts 15, no. 5: 411. https://doi.org/10.3390/catal15050411
APA StyleUllah, H., Elahi, I., Saleem, S., Nawaz, R., Ullah, S., Qadeer, S., Kabeer, B., Anjum, M., Liu, Y., Shahab, A., Idris, A. M., & Rao, Z. (2025). Bacterial Inactivation and Organic Pollutant Degradation in Slaughterhouse Wastewater Using Ag2O/Ba/TiO2 Nanocomposite. Catalysts, 15(5), 411. https://doi.org/10.3390/catal15050411










