Progress in Catalytic Oxidation of Noble Metal-Based Carbon Monoxide: Oxidation Mechanism, Sulfur Resistance, and Modification
Abstract
1. Introduction
2. Noble Metal Catalysts for Catalytic Oxidation of CO
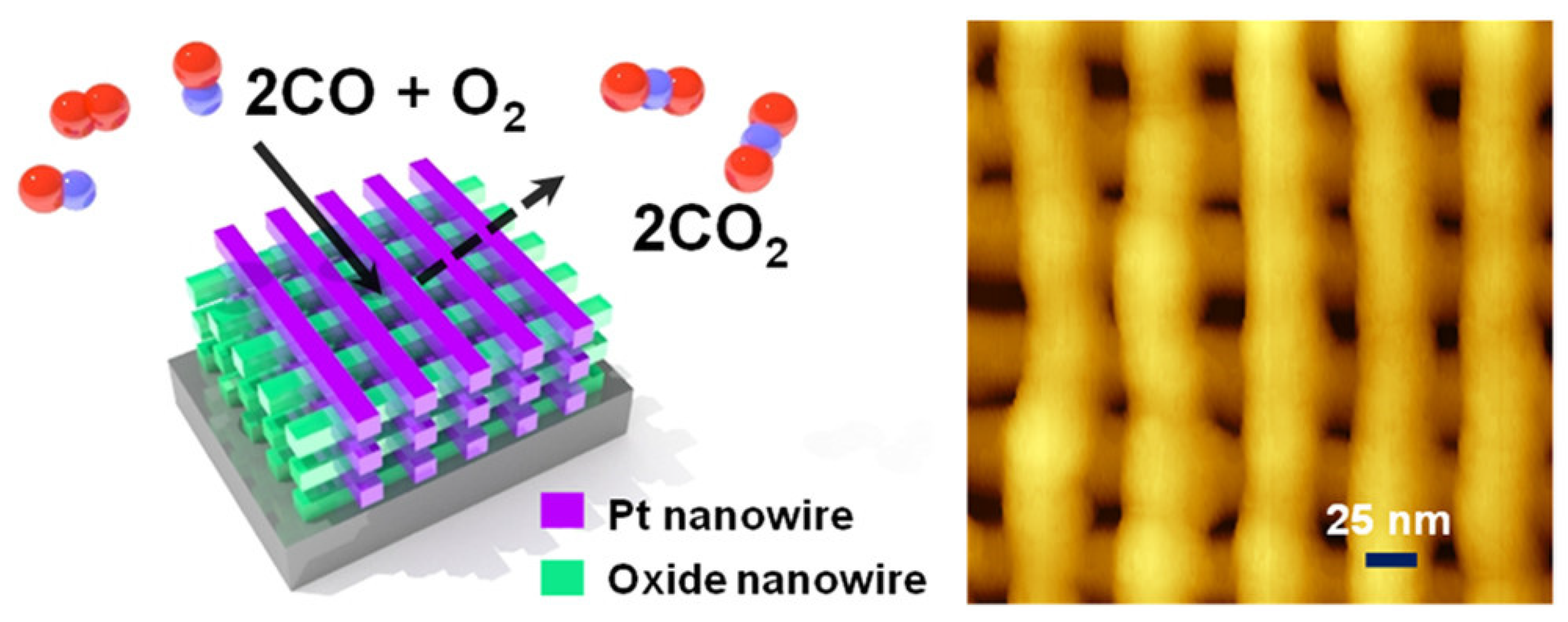
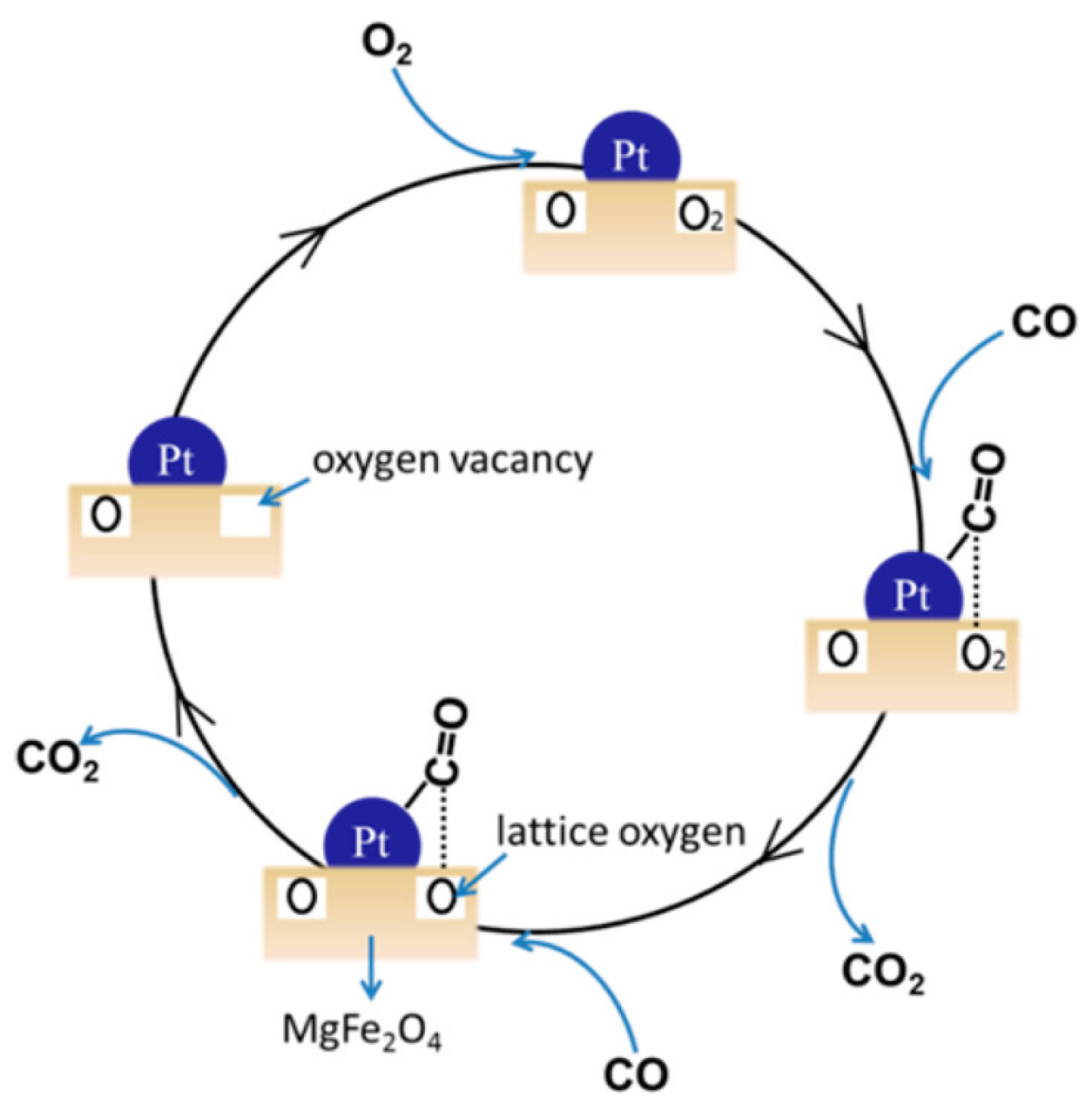
2.1. Pt-Based Catalysts for Catalytic Oxidation of CO
2.2. Pd-Based Catalysts for Catalytic Oxidation of CO
2.3. Pt-Pd Based Catalysts for Catalytic Oxidation of CO
2.4. Au-Based Catalysts for Catalytic Oxidation of CO
2.5. Other Noble Metal Catalysts
2.5.1. Rh-Based Noble Metal Catalysts
2.5.2. Ag-Based Noble Metal Catalysts
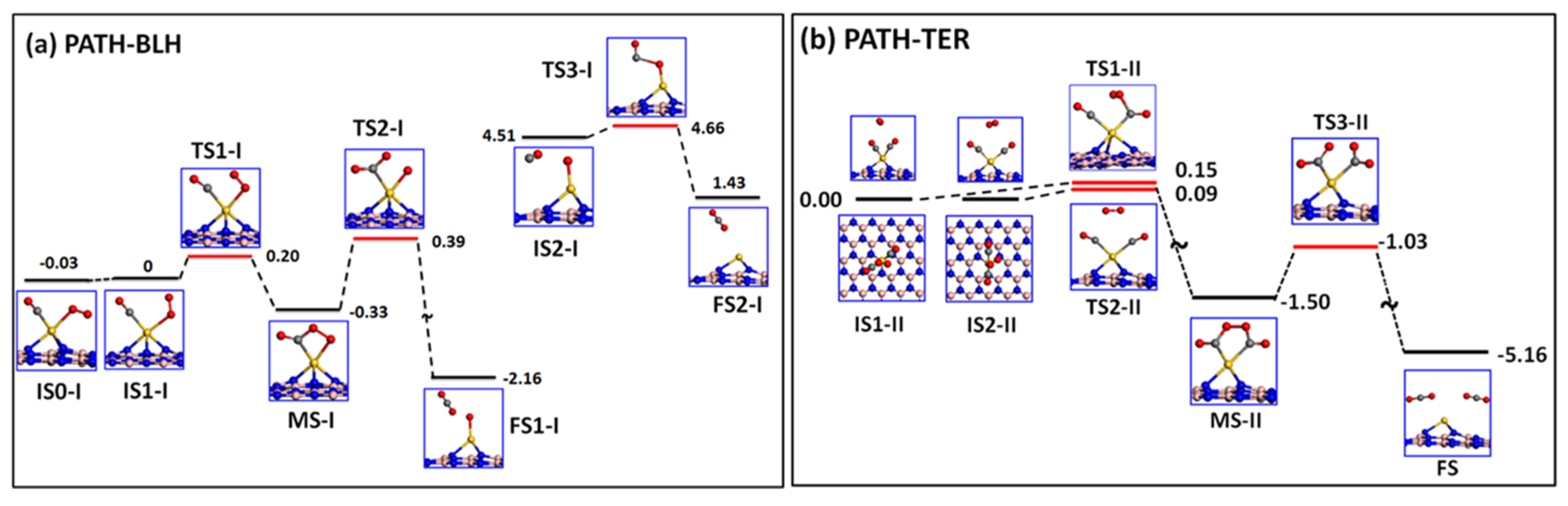
3. Mechanism of Catalytic Oxidation of CO
4. Mechanism of Sulfur Poisoning Deactivation of Catalysts
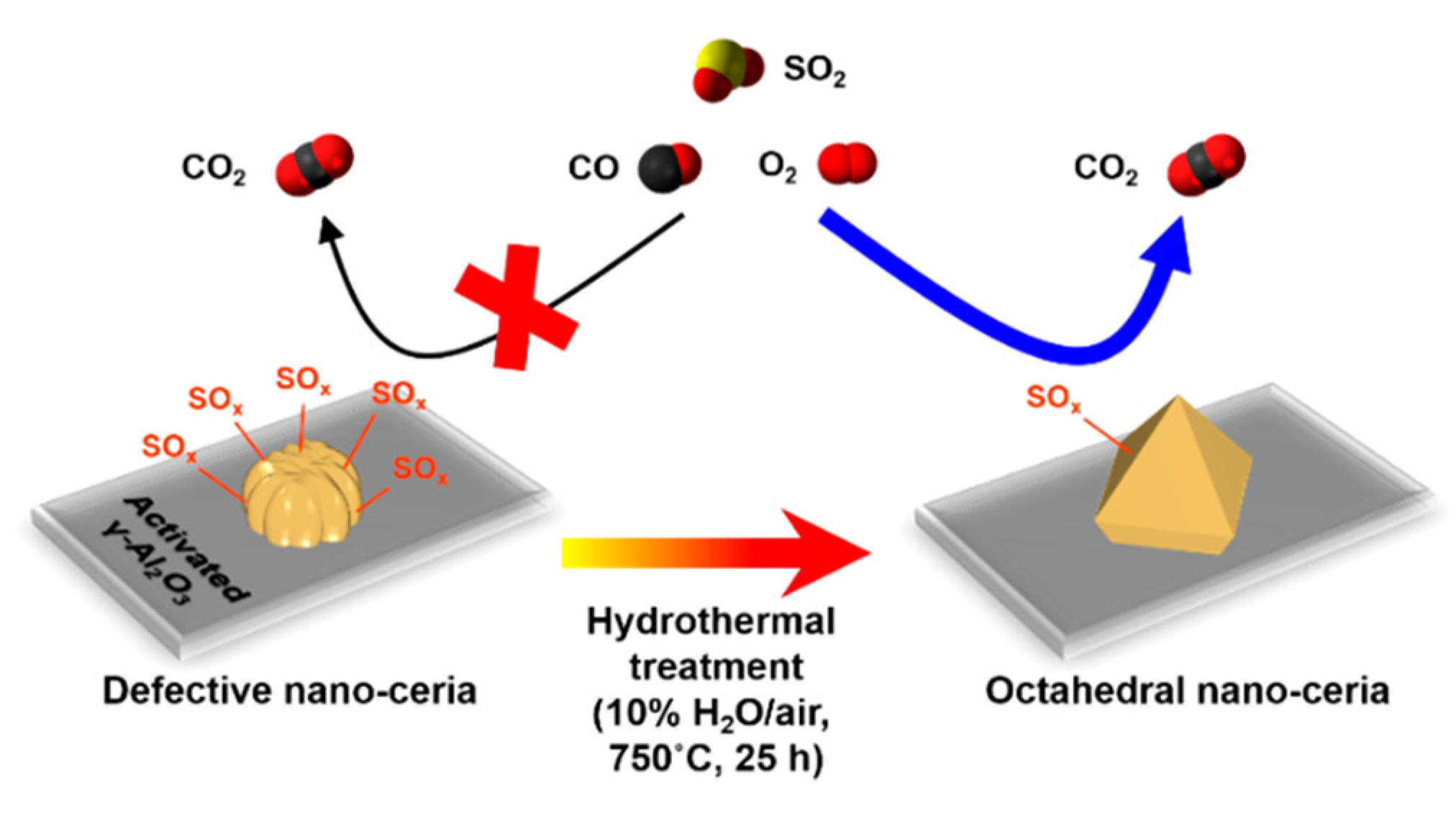
5. Strategies for Sulfur Resistance in Catalytic Oxidation of CO Catalysts
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bickerstaff, K.; Walker, G. Public understandings of air pollution: The “localisation” of environmental risk. Glob. Environ. Change-Human Policy Dimens. 2001, 11, 133–145. [Google Scholar] [CrossRef]
- Kumar, G.; Sampath, S.; Jeena, V.; Anjali, R. Carbon Monoxide Pollution Levels at Environmentally Different Sites. J. Ind. Geophys. Union 2008, 12, 31–40. [Google Scholar]
- Dey, S.; Dhal, G.C. Deactivation and regeneration of hopcalite catalyst for carbon monoxide oxidation: A review. Mater. Today Chem. 2019, 14, 100180. [Google Scholar] [CrossRef]
- Blumenthal, I. Carbon monoxide poisoning. J. R. Soc. Med. 2001, 94, 270–272. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, P. A Review on CO Oxidation over Copper Chromite Catalyst. Catal. Rev. 2012, 54, 224–279. [Google Scholar] [CrossRef]
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B-Environ. 2016, 197, 105–115. [Google Scholar] [CrossRef]
- Si, R.; Liu, J.; Yang, K.; Chen, X.; Dai, W.; Fu, X. Temperature-programed surface reaction study of CO oxidation over Au/TiO2 at low temperature: An insight into nature of the reaction process. J. Catal. 2014, 311, 71–79. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Liu, C. Perspective on CO oxidation over Pd-based catalysts. Catal. Sci. Technol. 2014, 5, 69–81. [Google Scholar] [CrossRef]
- Satsuma, A.; Osaki, K.; Yanagihara, M.; Ohyama, J.; Shimizu, K. Activity controlling factors for low-temperature oxidation of CO over supported Pd catalysts. Appl. Catal. B-Environ. 2013, 132–133, 511–518. [Google Scholar] [CrossRef]
- Gholap, R.V.; Chaudhari, R.V. Absorption of carbon monoxide with reversible reaction in cuprous chloride solutions. Ind. Eng. Chem. Res. 1988, 27, 2105–2110. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Tian, M. Catalytic oxidation of CO on noble metal-based catalysts. Environ. Sci. Pollut. Res. 2021, 28, 24847–24871. [Google Scholar] [CrossRef] [PubMed]
- Boronin, A.I.; Slavinskaya, E.M.; Figueroba, A.; Stadnichenko, A.I.; Kardash, T.Y.; Stonkus, O.A.; Fedorova, E.A.; Muravev, V.V.; Svetlichnyi, V.A.; Bruix, A.; et al. CO oxidation activity of Pt/CeO2 catalysts below 0 °C: Platinum loading effects. Appl. Catal. B-Environ. 2021, 286, 119931. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Hong, S.C. Effect of Pt Particle Size and Valence State on the Performance of Pt/TiO2 Catalysts for CO Oxidation at Room Temperature. J. Phys. Chem. C 2016, 120, 17996–18004. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, P.; Hu, J.; Tu, Y.; Gong, Z.; Cui, Y.; Zheng, Y.; Chen, M.; Zhang, W.; Ma, C.; et al. Electron penetration triggering interface activity of Pt-graphene for CO oxidation at room temperature. Nat. Commun. 2021, 12, 5814. [Google Scholar] [CrossRef]
- Liu, L.; Qiao, B.; He, Y.; Zhou, F.; Yang, B.; Deng, Y. Catalytic co-oxidation of CO and H2 over FeOx-supported Pd catalyst at low temperatures. J. Catal. 2012, 294, 29–36. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Chen, M.; Weng, W.; Wan, H. Size and support effects for CO oxidation on supported Pd catalysts. Sci. China Chem. 2010, 53, 2047–2056. [Google Scholar] [CrossRef]
- Liang, Q.; Li, J.; He, H.; Yue, T.; Tong, L. Effects of SO2 and H2O on low-temperature NO conversion over F-V2O5-WO3/TiO2 catalysts. J. Environ. Sci. 2020, 90, 253–261. [Google Scholar] [CrossRef]
- Guan, H.; Lin, J.; Qiao, B.; Yang, X.; Li, L.; Miao, S.; Liu, J.; Wang, A.; Wang, X.; Zhang, T. Catalytically Active Rh Sub-Nanoclusters on TiO2 for CO Oxidation at Cryogenic Temperatures. Angew. Chem. Int. Ed. 2016, 55, 2820–2824. [Google Scholar] [CrossRef]
- Knell, A.; Barnickel, P.; Baiker, A.; Wokaun, A. CO oxidation over Au/ZrO2 catalysts: Activity, deactivation behavior, and reaction mechanism. J. Catal. 1992, 137, 306–321. [Google Scholar] [CrossRef]
- Jain, D.; Madras, G. Mechanistic Insights and Kinetics of CO Oxidation over Pristine and Noble Metal Modified Fe2O3 Using Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Ind. Eng. Chem. Res. 2017, 56, 2008–2024. [Google Scholar] [CrossRef]
- Yeste, M.P.; Vidal, H.; García-Cabeza, A.L.; Hernández-Garrido, J.C.; Guerra, F.M.; Cifredo, G.A.; González-Leal, J.M.; Gatica, J.M. Low temperature prepared copper-iron mixed oxides for the selective CO oxidation in the presence of hydrogen. Appl. Catal. A-Gen. 2018, 552, 58–69. [Google Scholar] [CrossRef]
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B-Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Bunluesin, T.; Gorte, R.J.; Graham, G.W. Studies of the water-gas-shift reaction on ceria-supported Pt, Pd, and Rh: Implications for oxygen-storage properties. Appl. Catal. B Environ. 1998, 15, 107–114. [Google Scholar] [CrossRef]
- Kung, H.H.; Kung, M.C.; Costello, C.K. Supported Au catalysts for low temperature CO oxidation. J. Catal. 2003, 216, 425–432. [Google Scholar] [CrossRef]
- Feng, C.; Liu, X.; Zhu, T.; Hu, Y.; Tian, M. Catalytic oxidation of CO over Pt/TiO2 with low Pt loading: The effect of H2O and SO2. Appl. Catal. A Gen. 2021, 622, 118218. [Google Scholar] [CrossRef]
- Yuan, C.; Yao, N.; Wang, X.; Wang, J.; Lv, D.; Li, X. The SiO2 supported bimetallic Ni–Ru particles: A good sulfur-tolerant catalyst for methanation reaction. Chem. Eng. J. 2015, 260, 1–10. [Google Scholar] [CrossRef]
- Dawody, J.; Skoglundh, M.; Olsson, L.; Fridell, E. Sulfur deactivation of Pt/SiO2, Pt/BaO/Al2O3, and BaO/Al2O3 NOx storage catalysts: Influence of SO2 exposure conditions. J. Catal. 2005, 234, 206–218. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kamiuchi, N.; Muroyama, H.; Matsui, T.; Eguchi, K. Effect of reduction treatment on CO oxidation over Pt/SnO2 catalyst. Catal. Today 2011, 164, 169–175. [Google Scholar] [CrossRef]
- Taira, K.; Einaga, H. The Effect of SO2 and H2O on the Interaction Between Pt and TiO2(P-25) During Catalytic CO Oxidation. Catal. Lett. 2019, 149, 965–973. [Google Scholar] [CrossRef]
- Farhan, S.M.; Wang, P.; Yin, J.; Yi, J.; Chen, Z. Optimizing Pt/Pd Ratios for Enhanced Low-Temperature Catalytic Oxidation of CO and C3H6 on Al2O3 Support. Catal. Lett. 2024, 154, 4678–4691. [Google Scholar] [CrossRef]
- Šmit, G.; Zrnčević, S.; Lázár, K. Adsorption and low-temperature oxidation of CO over iron oxides. J. Mol. Catal. A-Chem. 2006, 252, 103–106. [Google Scholar] [CrossRef]
- Cruz, A.R.M.; Ramon, A.P.; Gomes, J.F.; Assaf, J.M. CO oxidation and CO-PROX reactions over Au catalysts supported on different metal oxides: A comparative study. Braz. J. Chem. Eng. 2020, 37, 667–677. [Google Scholar] [CrossRef]
- Huang, H.Y.; Long, R.Q.; Yang, R.T. A highly sulfur resistant Pt-Rh/TiO2/Al2O3 storage catalyst for NOx reduction under lean-rich cycles. Appl. Catal. B-Environ. 2001, 33, 127–136. [Google Scholar] [CrossRef]
- Freund, H.-J.; Meijer, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef]
- Donnelly, R.G.; Modell, M.; Baddour, R.F. Modeling chemisorption kinetics: Carbon monoxide on polycrystalline platinum. J. Catal. 1978, 52, 239–260. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Elementary Steps in the Catalytic Oxidation of Carbon Monoxide on Platinum Metals. Adv. Catal. 1979, 28, 1–78. [Google Scholar] [CrossRef]
- Slavinskaya, E.; Stonkus, O.; Kibis, L.; Lashina, E.; Zadesenets, A.; Kardash, T.; Korenev, S.; Podyacheva, O.; Boronin, A. New Perspectives of Pt-CeO2 System: Stabilization on MWCNTs for Boosting Activity and Water-Resistance in CO Oxidation at Ambient Temperatures. ChemCatChem 2024, 16, e202400116. [Google Scholar] [CrossRef]
- Ohyama, J.; Sato, M.; Tsushida, M.; Awaya, K.; Machida, M.; Uruga, T.; Higashi, K. Dynamics of CeO2-supported Pt nanoparticles in CO oxidation reaction revealed by millisecond time-resolved HERFD-XANES spectroscopy. Catal. Sci. Technol. 2025, 15, 2544–2550. [Google Scholar] [CrossRef]
- Song, W.; Deng, Y.; Lv, Z.; Su, M.; Dong, L.L.; Zheng, H.; Wang, D.; Yuan, S.; Ouyang, L. Effect of cobalt on CeO2 nanorod supported Pt catalyst: Structure, performance, kinetics and reaction mechanism in CO oxidation. Chem. Eng. J. 2024, 296, 120212. [Google Scholar] [CrossRef]
- Cai, J.; Yu, Z.; Li, J. Effect of Preparation Methods on the Performance of Pt/TiO2 Catalysts for the Catalytic Oxidation of Carbon Monoxide in Simulated Sintering Flue Gas. Catalysts 2021, 11, 804. [Google Scholar] [CrossRef]
- Hong, X.; Sun, Y. Effect of Preparation Methods on the Performance of Pt/CeO2 Catalysts for the Catalytic Oxidation of Carbon Monoxide. Catal. Lett. 2016, 146, 2001–2008. [Google Scholar] [CrossRef]
- Liu, J.; Ding, T.; Zhang, H.; Li, G.; Cai, J.; Zhao, D.; Tian, Y.; Xian, H.; Bai, X.; Li, X. Engineering surface defects and metal–support interactions on Pt/TiO2(B) nanobelts to boost the catalytic oxidation of CO. Catal. Sci. Technol. 2018, 8, 4934–4944. [Google Scholar] [CrossRef]
- Liu, S.P.; Zhao, M.; Sun, G.E.; Gao, W.; Jiang, Q. Different effects of water molecules on CO oxidation with different reaction mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 8341–8348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Wu, S.; Yang, X.; Jia, M.; Zhang, W.; Liu, G. Room Temperature CO Oxidation over Pt/MgFe2O4: A Stable Inverse Spinel Oxide Support for Preparing Highly Efficient Pt Catalyst. ACS Appl. Mater. Interfaces 2016, 8, 26683–26689. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Kim, J.; Chan, X.; Kim, T.J.; Kim, D.H. Influence of the Defect Concentration of Ceria on the Pt Dispersion and the CO Oxidation Activity of Pt/CeO2. J. Phys. Chem. C 2018, 122, 4972–4983. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, C.-J. Enhanced Activity for CO Oxidation over WO3 Nanolamella Supported Pt Catalyst. ACS Appl. Mater. Interfaces 2014, 6, 12860–12867. [Google Scholar] [CrossRef]
- Lou, Y.; Ma, J.; Cao, X.; Wang, L.; Dai, Q.; Zhao, Z.; Cai, Y.; Zhan, W.; Guo, Y.; Hu, P.; et al. Promoting Effects of In2O3 on Co3O4 for CO Oxidation: Tuning O2 Activation and CO Adsorption Strength Simultaneously. ACS Catal. 2014, 4, 4143–4152. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, J. CO Oxidation on Metal Oxide Supported Single Pt atoms: The Role of the Support. Ind. Eng. Chem. Res. 2017, 56, 6916–6925. [Google Scholar] [CrossRef]
- An, K.; Alayoglu, S.; Musselwhite, N.; Plamthottam, S.; Melaet, G.; Lindeman, A.E.; Somorjai, G.A. Enhanced CO Oxidation Rates at the Interface of Mesoporous Oxides and Pt Nanoparticles. J. Am. Chem. Soc. 2013, 135, 16689–16696. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Gao, E.; Zhu, J.; Yao, S.; Li, J. Revealing the role of oxygen vacancies on α-MnO2 of different morphologies in CO oxidation using operando DRIFTS-MS. Appl. Surf. Sci. 2023, 618, 156643. [Google Scholar] [CrossRef]
- Song, H.C.; Lee, G.R.; Jeon, K.; Lee, H.; Lee, S.W.; Jung, Y.S.; Park, J.Y. Engineering Nanoscale Interfaces of Metal/Oxide Nanowires to Control Catalytic Activity. ACS Nano 2020, 14, 8335–8342. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhao, S.; Yang, J.; Huang, W.-H.; Chen, C.-L.; Chen, T.; Zhao, Y.; Chen, G.; Qiu, Y.; Gu, L. Hydrogen Spillover Induced PtCo/CoOx Interfaces with Enhanced Catalytic Activity for CO Oxidation at Low Temperatures in Humid Conditions. Small 2024, 20, e2309181. [Google Scholar] [CrossRef] [PubMed]
- Kochubey, D.I.; Pavlova, S.N.; Novgorodov, B.N.; Kryukova, G.N.; Sadykov, V.A. The Influence of Support on the Low-Temperature Activity of Pd in the Reaction of CO Oxidation: 1. The Structure of Supported Pd. J. Catal. 1996, 161, 500–506. [Google Scholar] [CrossRef]
- Pavlova, S.N.; Sadykov, V.A.; Bulgakov, N.N.; Bredikhin, M.N. The Influence of Support on the Low-Temperature Activity of Pd in the Reaction of CO Oxidation: 3. Kinetics and Mechanism of the Reaction. J. Catal. 1996, 161, 517–523. [Google Scholar] [CrossRef]
- Li, A.; Wang, P.; Yi, J.; Farhan, S.M.; Zhang, L.; Zhao, L.; Lei, L. Influence of M-Doped (M = Ba, Zr, La, and Ce) for Enhanced CO and C3H6 Catalytic Oxidation over Pd/Al2O3 Catalysts. J. Phys. Chem. C 2024, 128, 14638–14648. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Banerjee, S.; Choudhary, V.R. Catalysts for combustion of methane and lower alkanes. Appl. Catal. A-Gen. 2002, 234, 1–23. [Google Scholar] [CrossRef]
- Méthivier, C.; Béguin, B.; Brun, M.; Massardier, J.; Bertolini, J.C. Pd/SiC Catalysts: Characterization and Catalytic Activity for the Methane Total Oxidation. J. Catal. 1998, 173, 374–382. [Google Scholar] [CrossRef]
- Zhu, G.; Han, J.; Zemlyanov, D.Y.; Ribeiro, F.H. The Turnover Rate for the Catalytic Combustion of Methane over Palladium Is Not Sensitive to the Structure of the Catalyst. J. Am. Chem. Soc. 2004, 126, 9896–9897. [Google Scholar] [CrossRef]
- Sheintuch, M.; Schmidt, J.; Lecthman, Y.; Yahav, G. Modelling catalyst—Support interactions in carbon monoxide oxidation catalysed by Pd/SnO2. Appl. Catal. 1989, 49, 55–65. [Google Scholar] [CrossRef]
- Schryer, D.R.; Upchurch, B.T.; Van Norman, J.D.; Brown, K.G.; Schryer, J. Effects of pretreatment conditions on a Pt/SnO2 catalyst for the oxidation of CO in CO2 lasers. J. Catal. 1990, 122, 193–197. [Google Scholar] [CrossRef]
- Daneshvar, K.; Krishna Dadi, R.; Luss, D.; Balakotaiah, V.; Kang, S.B.; Kalamaras, C.M.; Epling, W.S. Experimental and modeling study of CO and hydrocarbons light-off on various Pt-Pd/γ-Al2O3 diesel oxidation catalysts. Chem. Eng. J. 2017, 323, 347–360. [Google Scholar] [CrossRef]
- Kang, S.B.; Hazlett, M.; Balakotaiah, V.; Kalamaras, C.; Epling, W. Effect of Pt:Pd ratio on CO and hydrocarbon oxidation. Applied Catalysis B. Environ. 2018, 223, 67–75. [Google Scholar] [CrossRef]
- Haruta, M.; Yamada, N.; Kobayashi, T.; Iijima, S. Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J. Catal. 1989, 115, 301–309. [Google Scholar] [CrossRef]
- Qin, X.; Chen, M.; Chen, X.; Zhang, J.; Wang, X.; Fang, J.; He, H.; Zhang, C. Regulating the surface Au sites of Au/TiO2 catalyst for achieving co-oxidation of HCHO and CO at room temperature. Appl. Catal. B-Environ. Energy 2023, 330, 122663. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Zhang, B.; Si, R.; Han, B.; Hong, F.; Niu, Y.; Sun, L.; Li, L.; Qiao, B.; et al. Boosting the catalysis of gold by O2 activation at Au-SiO2 interface. Nat. Commun. 2020, 11, 558. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Du, F.; Zhao, Y.; Gao, P.; Chen, H.; Jiao, Z.; Li, X.; Zhao, T.; Sun, Y. Enhanced Interactions between Gold and MnO2 Nanowires for Water Oxidation: A Comparison of Different Chemical and Physical Preparation Methods. ACS Sustain. Chem. Eng. 2015, 3, 2049–2057. [Google Scholar] [CrossRef]
- Wolf, A.; Schüth, F. A systematic study of the synthesis conditions for the preparation of highly active gold catalysts. Appl. Catal. A Gen. 2002, 226, 1–13. [Google Scholar] [CrossRef]
- Gąsior, M.; Grzybowska, B.; Samson, K.; Ruszel, M.; Haber, J. Oxidation of CO and C3 hydrocarbons on gold dispersed on oxide supports. Catal. Today 2004, 91–92, 131–135. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Tsubota, S.; Nakamura, T.; Haruta, M. The influence of the preparation methods on the catalytic activity of platinum and gold supported on TiO2 for CO oxidation. Catal. Lett. 1997, 44, 83–87. [Google Scholar] [CrossRef]
- Chen, M.S.; Goodman, D.W. Structure–activity relationships in supported Au catalysts. Catal. Today 2006, 111, 22–33. [Google Scholar] [CrossRef]
- Shimizu, T.; Ota, M.; Sato, Y.; Inomata, H.; Nakagawa, Y.; Nanba, T. Preparation of Rh/CeO2 Using Supercritical CO2 and Its Catalytic Application for Automotive Exhaust. J. Jpn. Pet. Inst. 2013, 56, 312–316. [Google Scholar] [CrossRef][Green Version]
- Rafaj, Z.; Krutel, J.; Nehasil, V. Oxygen Exchange between Catalyst and Active Support during CO Oxidation on Rh/CeO2(111) and Rh/CeO2(110): Isotope Labeled 18O Study. J. Phys. Chem. C 2021, 125, 15959–15966. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Wang, Y.; Liu, N.; Zuo, Y.; Cui, L. Study of catalytic activity at the Ag/Al-SBA-15 catalysts for CO oxidation and selective CO oxidation. Chem. Eng. J. 2016, 283, 1097–1107. [Google Scholar] [CrossRef]
- Mytareva, A.I.; Kanaev, S.A.; Bokarev, D.A.; Kazakov, A.V.; Baeva, G.N.; Stakheev, A.Y. Alumina-Supported Silver Catalyst for O3-Assisted Catalytic Abatement of CO: Effect of Ag Loading. Top. Catal. 2023, 66, 1064–1070. [Google Scholar] [CrossRef]
- Widmann, D.; Behm, R.J. Activation of Molecular Oxygen and the Nature of the Active Oxygen Species for CO Oxidation on Oxide Supported Au Catalysts. Acc. Chem. Res. 2014, 47, 740–749. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A-Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Huang, R.; Kim, K.; Kim, H.J.; Jang, M.G.; Han, J.W. Size-Controlled Pd Nanoparticles Loaded on Co3O4 Nanoparticles by Calcination for Enhanced CO Oxidation. ACS Appl. Nano Mater. 2020, 3, 486–495. [Google Scholar] [CrossRef]
- Abdel Aal, S. CO catalytic oxidation on Pt-doped single wall boron nitride nanotube: First-principles investigations. Surf. Sci. 2016, 644, 1–12. [Google Scholar] [CrossRef]
- Najafi, M. Study of oxidation of carbon monoxide on the surface of sn-doped carbon nanotube. Chin. J. Struct. Chem. 2019, 38, 524–532. [Google Scholar] [CrossRef]
- Mao, K.; Li, L.; Zhang, W.; Pei, Y.; Zeng, X.C.; Wu, X.; Yang, J. A Theoretical Study of Single-Atom Catalysis of CO Oxidation Using Au Embedded 2D h-BN Monolayer: A CO-Promoted O2 Activation. Sci. Rep. 2014, 4, 5441. [Google Scholar] [CrossRef]
- Wang, C.; Gu, X.-K.; Yan, H.; Lin, Y.; Li, J.; Liu, D.; Li, W.-X.; Lu, J. Water-Mediated Mars–Van Krevelen Mechanism for CO Oxidation on Ceria-Supported Single-Atom Pt1 Catalyst. ACS Catal. 2017, 7, 887–891. [Google Scholar] [CrossRef]
- Ordóñez, S.; Paredes, J.R.; Díez, F.V. Sulphur poisoning of transition metal oxides used as catalysts for methane combustion. Appl. Catal. A-Gen. 2008, 341, 174–180. [Google Scholar] [CrossRef]
- Ordóñez, S.; Hurtado, P.; Sastre, H.; Díez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulphur dioxide: Development of a deactivation model. Appl. Catal. A-Gen. 2004, 259, 41–48. [Google Scholar] [CrossRef]
- Carlo, G.D.; Melaet, G.; Kruse, N.; Liotta, L.F.; Pantaleo, G.; Venezia, A.M. Combined sulfating and non-sulfating support to prevent water and sulfur poisoning of Pd catalysts for methane combustion. Chem. Commun. 2010, 46, 6317–6319. [Google Scholar] [CrossRef]
- Zi, X.; Liu, L.; Xue, B.; Dai, H.; He, H. The durability of alumina supported Pd catalysts for the combustion of methane in the presence of SO2. Catal. Today 2011, 175, 223–230. [Google Scholar] [CrossRef]
- Somorjai, G.A. On the mechanism of sulfur poisoning of platinum catalysts. J. Catal. 1972, 27, 453–456. [Google Scholar] [CrossRef][Green Version]
- Oudar, J. Sulfur adsorption and poisoning of metallic catalysts. Catal. Rev. Sci. Eng. 1980, 22, 171–195. [Google Scholar] [CrossRef]
- Gracia, F.J.; Guerrero, S.; Wolf, E.E.; Miller, J.T.; Kropf, A.J. Kinetics, operando FTIR, and controlled atmosphere EXAFS study of the effect of sulfur on Pt-supported catalysts during CO oxidation. J. Catal. 2005, 233, 372–387. [Google Scholar] [CrossRef]
- Xue, E.; Seshan, K.; Ross, J.R.H. Roles of supports, Pt loading and Pt dispersion in the oxidation of NO to NO2 and of SO2 to SO3. Appl. Catal. B-Environ. 1996, 11, 65–79. [Google Scholar] [CrossRef]
- Taira, K.; Nakao, K.; Suzuki, K.; Einaga, H. SOx Tolerant Pt/TiO2 Catalysts for CO Oxidation and the Effect of TiO2 Supports on Catalytic Activity. Environ. Sci. Technol. 2016, 50, 9773–9780. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K.; Lambert, R.M.; Hubbard, C.P.; Hurley, R.G.; McCabe, R.W.; Gandhi, H.S. The Origin of SO2 Promotion of Propane Oxidation over Pt/Al2O3 Catalysts. J. Catal. 1999, 184, 491–498. [Google Scholar] [CrossRef]
- Ren, S.; Liang, W.; Fang, H.; Zhu, Y. Performance and poisoning analysis of organic sulfur resistance of Pd-Ce catalyst in catalytic oxidation of VOCs. J. Environ. Chem. Eng. 2021, 9, 106640. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Liang, L.; He, H.; Dai, H.; Song, L. Effect of SO2 and H2O in flue gas on the catalytic oxidation of CO by Pt/TiO2. Ind. Catal. 2024, 32, 24–31, (In Chinese with English abstract). [Google Scholar]
- Smirnov, M.Y.; Kalinkin, A.V.; Pashis, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I. Interaction of SO2 with Pt Model Supported Catalysts Studied by XPS. J. Phys. Chem. C 2014, 118, 22120–22135. [Google Scholar] [CrossRef]
- Honkanen, M.; Wang, J.; Kärkkäinen, M.; Huuhtanen, M.; Jiang, H.; Kallinen, K.; Keiski, R.L.; Akola, J.; Vippola, M. Regeneration of sulfur-poisoned Pd-based catalyst for natural gas oxidation. J. Catal. 2018, 358, 253–265. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Szabo, V.; Trong On, D.; Kaliaguine, S. Mesoporous silica supported LaCoO3 perovskites as catalysts for methane oxidation. Micropor. Mesopor. Mat. 2002, 54, 51–61. [Google Scholar] [CrossRef]
- On, D.T.; Nguyen, S.V.; Kaliaguine, S. New SO2 resistant mesoporous La–Co–Zr mixed oxide catalysts for hydrocarbon oxidation. Phys. Chem. Chem. Phys. 2003, 5, 2724–2729. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kreft, S.; Georgi, G.; Fulda, G.; Pohl, M.-M.; Seeburg, D.; Berger-Karin, C.; Kondratenko, E.V.; Wohlrab, S. Improved catalytic methane combustion of Pd/CeO2 catalysts via porous glass integration. Appl. Catal. B-Environ. 2015, 179, 313–320. [Google Scholar] [CrossRef]
- Kim, B.-S.; Bae, J.; Jeong, H.; Choe, C.; Lee, H. Surface Restructuring of Supported Nano-Ceria for Improving Sulfur Resistance. ACS Catal. 2021, 11, 7154–7159. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ablikim, W.; Wang, J.; Chang, H.; Ma, L.; Xu, J.; Ge, M.; Arandiyan, H. CeO2–WO3 Mixed Oxides for the Selective Catalytic Reduction of NOx by NH3 Over a Wide Temperature Range. Catal. Lett. 2011, 141, 1859–1864. [Google Scholar] [CrossRef]
- Liu, F.; Shan, W.; Lian, Z.; Liu, J.; He, H. The smart surface modification of Fe2O3 by WOx for significantly promoting the selective catalytic reduction of NOx with NH3. Appl. Catal. B-Environ. 2018, 230, 165–176. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, W.; An, D.; Tang, C.; Zou, W.; Ge, C.; Tong, Q.; Gao, F.; Sun, J.; Dong, L. Activity enhancement of WO3 modified FeTiOx catalysts for the selective catalytic reduction of NOx by NH3. Catal. Today 2021, 375, 614–622. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X.; Zhu, T.; Feng, C.; Hu, Y.; Tian, M. New insights into the influence mechanism of H2O and SO2 on Pt–W/Ti catalysts for CO oxidation. Catal. Sci. Technol. 2022, 12, 1574–1585. [Google Scholar] [CrossRef]
- Park, K.; Ye, B.; Lee, M.; Lee, G.; Jeong, B.; Kim, D.; Jung, J.; Im, H.; Lee, H.; Kim, H.-D. Sulfur-resistance properties of WS2-added Pt/TiO2 catalysts for selective catalytic oxidation. Catal. Today 2023, 411–412, 113955. [Google Scholar] [CrossRef]
- Yu, C.; Yang, C.; Wang, R.; Dai, G.; Chen, H.; Huang, Z.; Zhao, H.; Zhou, Z.; Wu, X.; Jing, G. Mechanistic insight into the catalytic CO oxidation and SO2 resistance over Mo-decorated Pt/TiO2 catalyst: The essential role of Mo. Chem. Eng. J. 2024, 486, 150319. [Google Scholar] [CrossRef]
- Ma, L.; Yuan, S.; Jiang, T.; Zhu, X.; Lu, C.; Li, X. Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane. Catalysts 2019, 9, 410. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Kim, K.-J.; Hong, G.-R.; Ahn, S.-Y.; Kim, B.-J.; Shim, J.-O.; Roh, H.-S. Highly sulfur tolerant and regenerable Pt/CeO2 catalyst for waste to energy. Renew. Energy 2021, 178, 334–343. [Google Scholar] [CrossRef]
- Silva, L.P.C.; Freitas, M.M.; Santos, R.M.; Perez, G.; Terra, L.E.; Coutinho, A.C.S.L.S.; Passos, F.B. The effect of metal type on the sulfur tolerance of catalysts supported on niobia for sour water-gas shift reaction. J. Hydro. Energy 2018, 43, 3190–3202. [Google Scholar] [CrossRef]




| Catalyst | Support | GHSV (h−1)/Flow Rate (mL/min) | Reactant | Reaction Temperature (°C) | CO Conversion Rate (%) | Reference |
|---|---|---|---|---|---|---|
| Pd | CeO2 | 480,000 h−1 | 0.45% CO + 10% O2 | 150 | 100 | [9] |
| TiO2 | 100 | 25 | ||||
| Al2O3 | 150 | 80 | ||||
| Pd | FeOx | 15,000 h−1 | 1% CO + 5% O2 | 100 | 98 | [15] |
| Pt | TiO2 | 60,000 h−1 | 1% CO + 1% H2O + 50 ppm SO2 | 220 | 10 | [25] |
| 1% CO without H2O and SO2 | 280 | 100 | ||||
| Pt | CeO2 | 240,000 h−1 | 0.2% CO + 1% O2 | 100 | 92 | [12] |
| Pt | SnO2 | 32,000 h−1 | 5.0% CO + 15% O2 | 103 | 20 | [28] |
| Al2O3 | 147 | 20 | ||||
| Pt | TiO2 | 6,000,000 h−1 | 1% CO + 10% O2 + 20% H2O + 40 ppm SO2 + 40 ppm NO | 500 | 54 | [29] |
| Pt-Pd | Al2O3 | 120,000 h−1 | 1000 ppm C3H6 + 500 ppm NO + 0.15% CO + 10% O2 + 10% H2O | 160 | 80 | [30] |
| Rh | TiO2 | 720,000 h−1 | 1% CO + 5% O2 | −50 | 100 | [18] |
| Au | FeOx | 60,000 h−1 | 20% O2 + 2% CO | 240 | 50 | [31] |
| Au | CeO2 | / | 2:1:22 CO/O2/N2 | 150 | 87.5 | [32] |
| MnO2 | / | 100 | 75 | |||
| Pt-Rh | Al2O3 | 30,000 | 1.01% NO + 1.01% C3H6 + 1.03% CO + 0.99% SO2 | 250 | 90 | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Wang, S.; Yue, T. Progress in Catalytic Oxidation of Noble Metal-Based Carbon Monoxide: Oxidation Mechanism, Sulfur Resistance, and Modification. Catalysts 2025, 15, 415. https://doi.org/10.3390/catal15050415
Tong Y, Wang S, Yue T. Progress in Catalytic Oxidation of Noble Metal-Based Carbon Monoxide: Oxidation Mechanism, Sulfur Resistance, and Modification. Catalysts. 2025; 15(5):415. https://doi.org/10.3390/catal15050415
Chicago/Turabian StyleTong, Yali, Shuo Wang, and Tao Yue. 2025. "Progress in Catalytic Oxidation of Noble Metal-Based Carbon Monoxide: Oxidation Mechanism, Sulfur Resistance, and Modification" Catalysts 15, no. 5: 415. https://doi.org/10.3390/catal15050415
APA StyleTong, Y., Wang, S., & Yue, T. (2025). Progress in Catalytic Oxidation of Noble Metal-Based Carbon Monoxide: Oxidation Mechanism, Sulfur Resistance, and Modification. Catalysts, 15(5), 415. https://doi.org/10.3390/catal15050415









