Enhanced Adsorption Ability of CoS-Doped CuS for Promoting Electrochemical Oxidation of HMF
Abstract
1. Introduction
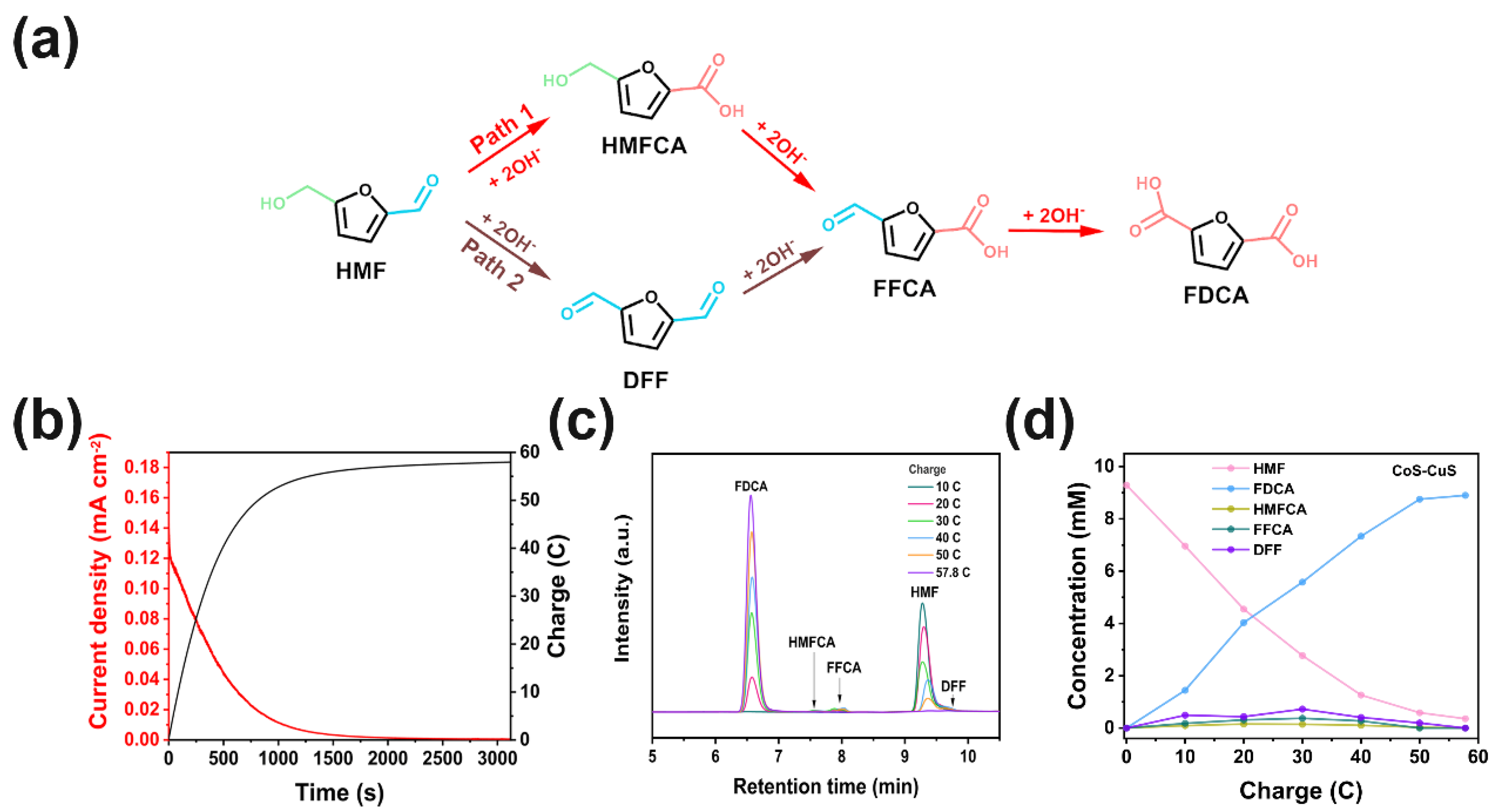
2. Results
3. Materials and Methods
3.1. Materials and Characterizations
3.2. Synthesis of CoS–CuS and Control Catalysts
3.3. In Situ FT-IR Testing
3.4. Electrochemical Measurement
3.5. HPLC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Balmaceda, M.M. Differentiation, materiality, and power: Towards a political economy of fossil fuels. Energy Res. Soc. Sci. 2018, 39, 130–140. [Google Scholar] [CrossRef]
- Cheng, Y.; Jabeen, S.; Lei, S.; Liu, N.; Liu, Y.; Liu, Y.; Li, Y.; Wu, X.; Tong, Z.; Yu, J. N-doped carbon dots—Modulated interfacial charge transfer and surface structure in FeNbO4 photocatalysts for enhanced CO2 conversion selectivity to CH4. Chem. Eng. J. 2024, 498, 155576. [Google Scholar] [CrossRef]
- Salman, M.; Long, X.; Dauda, L.; Mensah, C.N. The impact of institutional quality on economic growth and carbon emissions: Evidence from Indonesia, South Korea, and Thailand. J. Clean. Prod. 2019, 241, 118331. [Google Scholar] [CrossRef]
- Brosemer, K.; Schelly, C.; Gagnon, V.; Arola, K.L.; Pearce, J.M.; Bessette, D.; Olabisi, L.S. The energy crises revealed by COVID: Intersections of Indigeneity, inequity, and health. Energy Res. Soc. Sci. 2020, 68, 101661. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, J.; Tian, G.; Gong, Y.; Guo, B.; Cheng, F. Regulations on the corporate social irresponsibility in the supply chain under the multiparty game: Taking China’s organic food supply chain as an example. J. Clean. Prod. 2021, 317, 128459. [Google Scholar] [CrossRef]
- Fu, M.; Gu, L.; Zhen, Z.; Sun, M.; Tian, L. Optimal carbon tax income distribution and health welfare spillover effect based on health factors. Appl. Energy 2020, 276, 115475. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, W.; Rauf, A.; Ozturk, I. Transitioning from conventional energy to clean renewable energy in G7 countries: A signed network approach. Energy 2024, 307, 132655. [Google Scholar] [CrossRef]
- Jiao, H.; Al-Tohamy, R.; Li, F.; Schagerl, M.; Sun, J.; Ali, S.S. Harnessing wastewater—Based microalgae for biohydrogen production. Process Saf. Environ. Prot. 2024, 190, 372–385. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1901–1929. [Google Scholar] [CrossRef]
- Zhuang, C.; Li, W.; Chang, Y.; Li, S.; Zhang, Y.; Li, Y.; Gao, J.; Chen, G.; Kang, Z. Coordination environment dominated catalytic selectivity of photocatalytic hydrogen and oxygen reduction over switchable gallium and nitrogen active sites. J. Mater. Chem. A 2024, 12, 5711–5718. [Google Scholar] [CrossRef]
- Suzuki, K.; Tsuji, N.; Shirai, Y.; Hassan, M.A.; Osaki, M. Evaluation of biomass energy potential towards achieving sustainability in biomass energy utilization in Sabah, Malaysia. Biomass Bioenergy 2017, 97, 149–154. [Google Scholar] [CrossRef]
- Irfan, M.; Zhao, Z.; Panjwani, M.K.; Mangi, F.H.; Li, H.; Jan, A.; Ahmad, M.; Rehman, A. Assessing the energy dynamics of Pakistan: Prospects of biomass energy. Energy Rep. 2020, 6, 80–93. [Google Scholar] [CrossRef]
- Wu, X.; Tong, Z.; Liu, Y.; Li, Y.; Cheng, Y.; Yu, J.; Cao, P.; Zhuang, C.; Shi, Q.; Liu, N. Modification of the CuO electronic structure for enhanced selective electrochemical CO2 reduction to ethylene. Nano Res. 2024, 17, 7194–7202. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R.; He, Z.; Kandasamy, S. A review on low temperature combustion engines: Performance, combustion and emission characteristics. Renew. Sustain. Energy Rev. 2019, 116, 109404. [Google Scholar] [CrossRef]
- Zhu, J.; Cheng, F.; Wang, F.; Wen, S.; Liu, X. Selective Oxidation of 5-hydroxymethylfurfural to 2,5-Diformylfuran Over a Vanadium Manganese Oxide Catalyst. Catal. Lett. 2022, 152, 2280–2287. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Yang, Q.; Apaliya, M.T. Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 2018, 78, 180–187. [Google Scholar] [CrossRef]
- Trapasso, G.; Chícharo, B.; Gherardi, T.; Redolfi-Bristol, D.; Aricò, F. Iron(III) Sulfate—Mediated Synthesis of 2,5-Furandicarboxylic Acid Dimethyl Ester from Galactaric Acid. Catalysts 2023, 13, 1114. [Google Scholar] [CrossRef]
- Thiensuwan, N.; Sankaranarayanan, S.; Yokoi, T.; Ngamcharussrivichai, C. Exfoliated Layered Metal Oxide—Supported Ruthenium Catalysts for Base—Free Oxidation of 5-hydroxymethylfurfural into a Renewable Bioplastic Precursor. ACS Sustain. Chem. Eng. 2023, 11, 11424–11436. [Google Scholar] [CrossRef]
- Ahmed, S.; Cardinaels, R.; Abu-Jdayil, B.; Munam, A.; Iqbal, M.Z. Toughening Brittle Poly(ethylene Furanoate) with Linear Low—Density Polyethylene via Interface Modulation Using Reactive Compatibilizers. ACS Omega 2025, 10, 5756–5769. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Dai, Y.; Zhang, Y.; Jiang, M.; Zhou, G. High performance biobased poly(ethylene 2,5-furandicarboxylate) nanocomposites for food and cosmetics packaging materials: PMDA chain extended and TiO2 NPs functionalized. Arab. J. Chem. 2023, 16, 105228. [Google Scholar] [CrossRef]
- Kwaw, E.; Yongkun, M.; William, T.; Tibiru, A.M.; Sackle, S.A.; Meng, W.; Xiao, L. Effect of pulsed light treatment on the phytochemical, volatile, and sensorial attributes of lactic-acid-fermented mulberry juice. Int. J. Food Prop. 2018, 21, 213–228. [Google Scholar] [CrossRef]
- Cui, H.; Dai, Y.; Lin, L. Enhancing antibacterial efficacy of nisin in pork by poly-γ-glutamic acid/poly-l-lysine nanoparticles encapsulation. J. Food Saf. 2018, 38, e12475. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.; Zhang, Z.; Lv, B.; Li, J.; Liu, Z.; Zhu, W.; Tao, F.; Lv, G.; Yang, Y. High efficient catalytic oxidation of 5-hydroxymethylfurfural into 2,5-Furandicarboxylic acid under benign conditions with nitrogen–doped graphene encapsulated Cu nanoparticles. J. Energy Chem. 2020, 50, 96–105. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, M.; Xian, J.; Sun, Y.; Li, G. Recent advances in the electrocatalytic synthesis of 2,5-furandicarboxylic acid from 5-(hydroxymethyl)furfural. J. Mater. Chem. A 2021, 9, 20164–20183. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Cheng, F.; Zhou, S.; Xu, Q.; Yin, D.; Liu, X. V–doped MoO3 nanorods for highly selective oxidation of 5-hydroxymethylfurfural to bio-monomer 2,5-furandicarboxylic acid. Renew. Energy 2024, 226, 120409. [Google Scholar] [CrossRef]
- Wu, T.; Fan, X.; Wang, C.; Wu, L.; Bai, Y.; Jia, G. The first principles study of the dual-atom catalyst based on g-C3N5 for efficient nitrogen fixation. Appl. Surf. Sci. 2025, 682, 161648. [Google Scholar] [CrossRef]
- Davidson, M.G.; Elgie, S.; Parsons, S.; Young, T.J. Production of HMF, FDCA and their derived products: A review of life cycle assessment (LCA) and techno-economic analysis (TEA) studies. Green Chem. 2021, 23, 3154. [Google Scholar] [CrossRef]
- Massaro, M.C.; Monteverde, A.H.A. Techno-Economic Analysis of FDCA Production through Electrocatalytic Processes. J. Electrochem. Soc. 2022, 169, 054515. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Choi, M.; Kim, H.; Jeong, K.; Nam, K.T. Scaling Up Biomass Electrorefining: A 100-Liter Continuous-Flow Reactor for FDCA Production with >95% Carbon Efficiency. Joule 2023, 7, 1842–1857. [Google Scholar]
- Wei, L.; Dong, Z.; Chen, R.; Wu, Q.; Li, J. Review of carbon–based nanocomposites as electrocatalyst for H2O2 production from oxygen. Ionics 2022, 28, 4045–4063. [Google Scholar] [CrossRef]
- Lang, Z.; Wang, X.; Jabeen, S.; Cheng, Y.; Liu, N.; Liu, Z.; Gan, T.; Zhuang, Z.; Li, H.; Wang, D. Destabilization of Single–Atom Catalysts: Characterization, Mechanisms, and Regeneration Strategies. Adv. Mater 2025, 37, 2418942. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Niu, J.; Yang, C.; Qin, J.; Zhang, X.; Liu, R. The OER/ORR activities of copper oxyhydroxide series electrocatalysts. Mol. Catal. 2023, 537, 112942. [Google Scholar] [CrossRef]
- Li, J.; Qiu, R.; Zhang, S.; Peng, L.; Dong, Y.; Jiang, Y.; Li, Y.; Fang, N.; Yu, J.; Dong, J.-C. Synergistically Enhanced Co-Adsorption of Reactant and Hydroxyl on Platinum-Modified Copper Oxide for High–Performance HMF Oxidation. Adv. Mater. 2025, 37, 2417684. [Google Scholar] [CrossRef]
- Yu, H.; Li, F.; Zhang, Y.; Wang, C.; Liu, S.; Zhou, W.; Li, H.; Sun, Y. Boosting the Electrocatalytic Oxidation of Biomass-Derived Aldehydes on Cu-Co Oxide/Hydroxide Hierarchical Nanostructures. ACS Catal. 2021, 11, 5069–5078. [Google Scholar]
- Liu, S.; Dou, S.; Meng, J.; Liu, Y.; Liu, Y.; Yu, H. Efficient biobased carboxylic acids synthesis by synergistic electrocatalysis of multi–active sites on bimetallic Cu–Co oxide/oxyhydroxide. Appl. Catal. B Environ. 2023, 331, 122709. [Google Scholar] [CrossRef]
- Liao, S.; Shi, S.; Hu, J.; Yao, W.; Liu, S.; Wang, W.; Xiao, W.; Zhao, D.; Wang, S.; Chen, C. Enhanced electrooxidation of 5-hydroxymethylfurfural over a ZIF–67@β–Ni(OH)2/NF heterostructure catalyst: Synergistic effects and mechanistic insights. J. Colloid Interface Sci. 2025, 688, 806–817. [Google Scholar] [CrossRef]
- Gong, C.; Meng, X.; Jin, C.; Yang, M.; Liu, J.; Sheng, K.; Pu, Y.; Ragauskas, A.; Ji, G.; Zhang, X. Green synthesis of cellulose formate and its efficient conversion into 5-hydroxymethylfurfural. Ind. Crops Prod. 2023, 192, 115985. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhao, T.; Li, F.; Zhang, M.; Li, J.; Zou, Y.; Wang, W.; Cobbina, S.J.; Wu, X. Adsorption properties of macroporous adsorbent resins for separation of anthocyanins from mulberry. Food Chem. 2016, 194, 712–722. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Bai, H.; Li, H.; Zhao, H.; Wang, F.; Fan, W.; Shi, W. Adsorption–Activation Bifunctional Center of Al/Co–Base Catalyst for Boosting 5-hydroxymethylfurfural Oxidation. Adv. Energy Mater 2024, 14, 2402789. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, X.; Tao, S.; Wu, J.; Wang, H.; Wei, X.; Wang, T.; Zhou, B.; Lu, Y.; Frauenheim, T. Heterogeneous–Interface–Enhanced Adsorption of Organic and Hydroxyl for Biomass Electrooxidation. Adv. Mater 2022, 34, 2204089. [Google Scholar] [CrossRef]
- Yao, Y.; He, J.; Yang, X.; Peng, L.; Zhu, X.; Li, K.; Qu, M. Superhydrophilic/underwater superaerophobic self–supporting CuS/Cu foam electrode for efficient oxygen evolution reaction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 127934. [Google Scholar] [CrossRef]
- Li, M.C.; Qian, Y.T.; Du, J.M.; Wu, H.R.; Zhang, L.Y.; Li, G.; Li, K.D.; Wang, W.M.; Kang, D.J. CuS Nanosheets Decorated with CoS2 Nanoparticles as an Efficient Electrocatalyst for Enhanced Hydrogen Evolution at All pH Values. ACS Sustain. Chem. Eng. 2019, 7, 14016–14022. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave Chemistry for Inorganic Nanomaterials Synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Rao, B.N.; Satyanarayana, N. Review—Development of Inorganic Nanostructures by Microwave Synthesis Technique. ECS J. Solid State Sci. Technol. 2021, 10, 103003. [Google Scholar]
- Komarneni, S.; Li, Q.; Roy, R. Microwave–hydrothermal synthesis of ceramic powders. Mater. Res. Bull. 1992, 27, 1393–1405. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Kubokawa, M.; Tsuji, T. Microwave–Assisted Synthesis of Metallic Nanostructures in Solution. Chem. Eur. J. 2005, 11, 3417–3424. [Google Scholar] [CrossRef]
- Raghavendra, K.V.G.; Rao, K.M.; Kumar, N.T.U. Hydrothermal synthesis of CuS/CoS nano composite as an efficient electrode for the supercapattery applications. J. Energy Storage 2021, 41, 102749. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Liu, N.; Li, Y.; Cheng, Y.; Cao, P.; Liu, Y.; Yuan, X.; Zhang, X.; Li, H. Modification strategies on nickel–based electrocatalysts for energy–efficient anodic reactions. Nano Res. 2025, 18, 94907014. [Google Scholar] [CrossRef]
- Gao, H.; Fang, M.; Zhang, Z.; Han, Y.; Wang, D.; Wang, Y.; Xia, H.; Zhu, X.; Miao, S.; Kang, X. Electronic coupling of iron–cobalt in Prussian blue towards improved peroxydisulfate activation. J. Colloid Interface Sci. 2025, 678, 1087–1098. [Google Scholar] [CrossRef]
- Peng, C.; Luo, G.; Zhang, J.; Chen, M.; Wang, Z.; Sham, T.-K.; Zhang, L.; Li, Y.; Zheng, G. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol. Nat. Commun. 2021, 12, 1580. [Google Scholar] [CrossRef]
- Qi, R.; Chen, F.; Zhong, Z.; Jia, Y.; Yang, Y.; Yun, Z.; Ye, Q. Multi–morphology CuS catalyst for selective electrocatalytic of CO2 conversion to formate. J. Alloys Compd. 2024, 1008, 176713. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Ravi, G.; Hong, S.I.; Velauthapillai, D.; Thambidurai, M.; Dang, C.; Al-Mohaimeed, A.M.; Al-onazi, W.A. CuS@β–SnS nanocomposite electrocatalysts for efficient electrochemical water oxidation. Int. J. Hydrogen Energy 2021, 46, 3387–3400. [Google Scholar] [CrossRef]
- Zhuang, C.; Chang, Y.; Li, W.; Li, S.; Xu, P.; Zhang, H.; Zhang, Y.; Zhang, C.; Gao, J.; Chen, G. Light–Induced Variation of Lithium Coordination Environment in g–C3N4 Nanosheet for Highly Efficient Oxygen Reduction Reactions. ACS Nano 2024, 18, 5206–5217. [Google Scholar] [CrossRef]
- Kundu, J.; Khilari, S.; Bhunia, K. Ni-Doped CuS as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. Catal. Sci. Technol. 2019, 9, 406–417. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, T.; Dong, C.-L.; Huang, Y.-C.; Li, Y.; Chen, J.; Zou, Y.; Wang, S. Tuning the Selective Adsorption Site of Biomass on Co3O4 by Ir Single Atoms for Electrosynthesis. Adv. Mater. 2021, 33, 2007056. [Google Scholar] [CrossRef]
- Zhou, B.; Dong, C.-L.; Huang, Y.-C.; Zhang, N.; Wu, Y.; Lu, Y.; Yue, X.; Xiao, Z.; Zou, Y.; Wang, S. Activity origin and alkalinity effect of electrocatalytic biomass oxidation on nickel nitride. J. Energy Chem. 2021, 61, 179–185. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, Y.; Sun, M.; Huang, Q.; Sun, K.; Ma, J.; Li, J.; Tan, H.; Li, M.; Pan, Y. Cooperative Rh-O5/Ni(Fe) Site for Efficient Biomass Upgrading Coupled with H2 Production. J. Am. Chem. Soc. 2023, 145, 17577–17587. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Fan, C.; Liu, N.; Yin, J.; Li, Y.; Cheng, Y.; Yuan, X.; Zhang, X.; Liu, Y. A nanoflower-on-nanowire heterogeneous electrocatalyst for enhanced interfacial water activation in nitrate reduction reaction. Nano Res. 2025, 18, 94907135. [Google Scholar] [CrossRef]
- Barwe, S.; Weidner, J.; Cychy, S.; Morales, D.M.; Dieckhöfer, S.; Hiltrop, D.; Masa, J.; Muhler, M.; Schuhmann, W. Electrocatalytic Oxidation of 5-(Hydroxymethyl)furfural Using High-Surface-Area Nickel Boride. Angew. Chem. Int. Ed. 2018, 57, 11460–11464. [Google Scholar] [CrossRef]
- Poerwoprajitno, A.R.; Gloag, L.; Watt, J.; Cychy, S.; Cheong, S.; Kumar, P.V.; Benedetti, T.M.; Deng, C.; Wu, K.-H.; Marjo, C.E. Faceted Branched Nickel Nanoparticles with Tunable Branch Length for High-Activity Electrocatalytic Oxidation of Biomass.Angew. Chem. Int. Ed. 2020, 59, 15487–15491. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, P.; Liu, Y.; Yang, R.; Li, Y.; Cheng, Y.; Yu, J.; Zhang, X.; Phiri, P.; Yuan, X.; Yang, Y.; et al. Enhanced Adsorption Ability of CoS-Doped CuS for Promoting Electrochemical Oxidation of HMF. Catalysts 2025, 15, 422. https://doi.org/10.3390/catal15050422
Cao P, Liu Y, Yang R, Li Y, Cheng Y, Yu J, Zhang X, Phiri P, Yuan X, Yang Y, et al. Enhanced Adsorption Ability of CoS-Doped CuS for Promoting Electrochemical Oxidation of HMF. Catalysts. 2025; 15(5):422. https://doi.org/10.3390/catal15050422
Chicago/Turabian StyleCao, Peng, Yunliang Liu, Ruihua Yang, Yaxi Li, Yuanyuan Cheng, Jingwen Yu, Xinyue Zhang, Peter Phiri, Xinya Yuan, Yi Yang, and et al. 2025. "Enhanced Adsorption Ability of CoS-Doped CuS for Promoting Electrochemical Oxidation of HMF" Catalysts 15, no. 5: 422. https://doi.org/10.3390/catal15050422
APA StyleCao, P., Liu, Y., Yang, R., Li, Y., Cheng, Y., Yu, J., Zhang, X., Phiri, P., Yuan, X., Yang, Y., Liu, N., Liu, Y., & Li, H. (2025). Enhanced Adsorption Ability of CoS-Doped CuS for Promoting Electrochemical Oxidation of HMF. Catalysts, 15(5), 422. https://doi.org/10.3390/catal15050422






