Abstract
The increase in CO2 emissions has been identified as a core driving factor in the intensification of the greenhouse effect. In order to achieve the dual-carbon vision, research on CO2 capture and its catalytic conversion is receiving growing attention. Due to the high chemical stability of CO2 itself, traditional separation technologies find it difficult to capture it onto catalysts. Currently, using hydrocarbons as carriers for catalytic reactions is the most common and efficient method. In recent years, metal-organic frameworks (MOFs) have shown their irreplaceable importance in CO2 capture and catalytic conversion due to their unique adjustable and controllable pore structures and multiple active sites. This study integrates various classification criteria of MOFs, proposes a cooperative mechanism between metal doping and functional groups, and also reveals the CO2 capture and catalytic conversion processes. In addition, we have conducted an in-depth discussion on the future development of continuous-flow microreactor technology and provided performance and property relationship diagrams for multiple MOF series, offering valuable reference material for future research in related fields.
1. Introduction
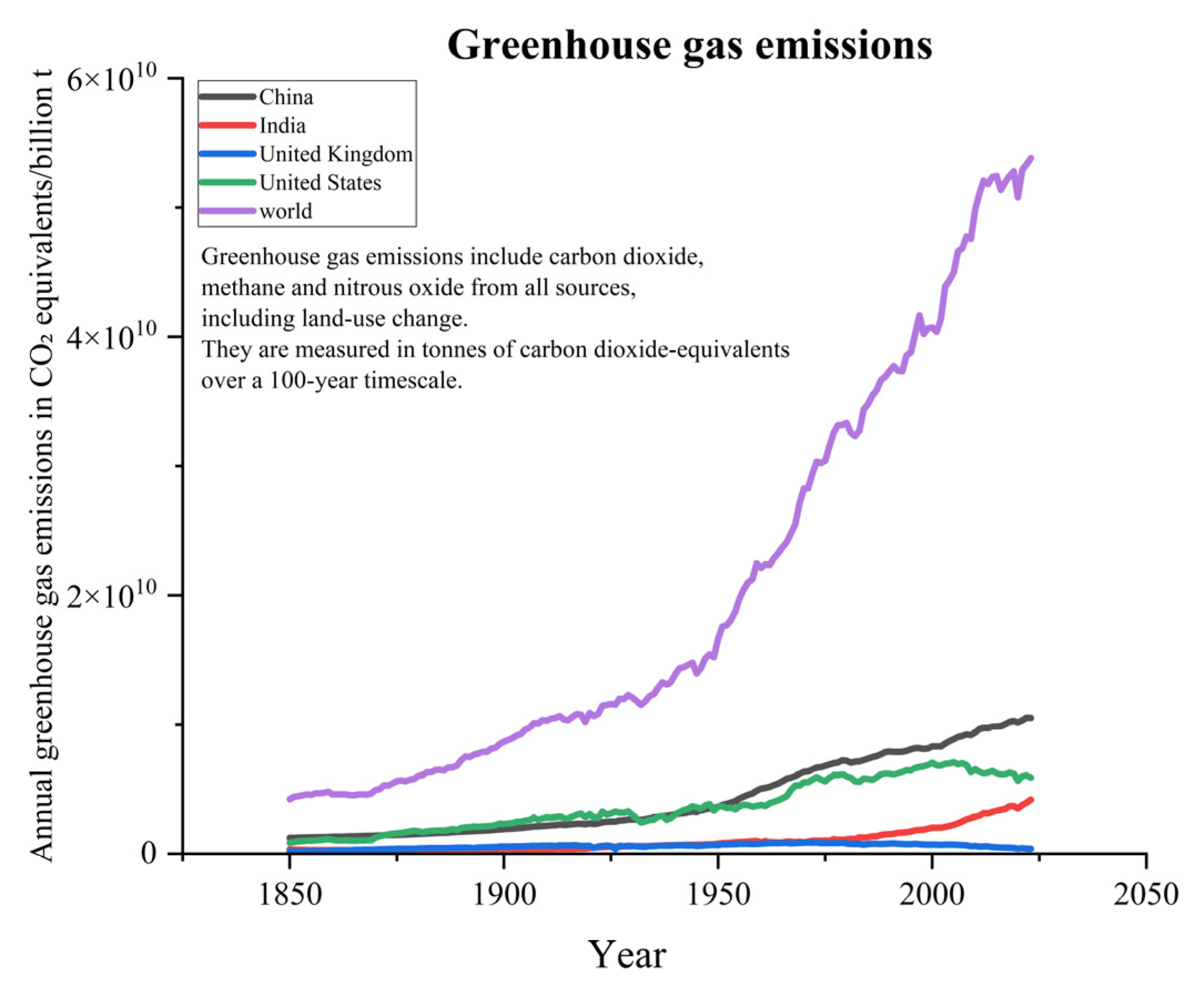
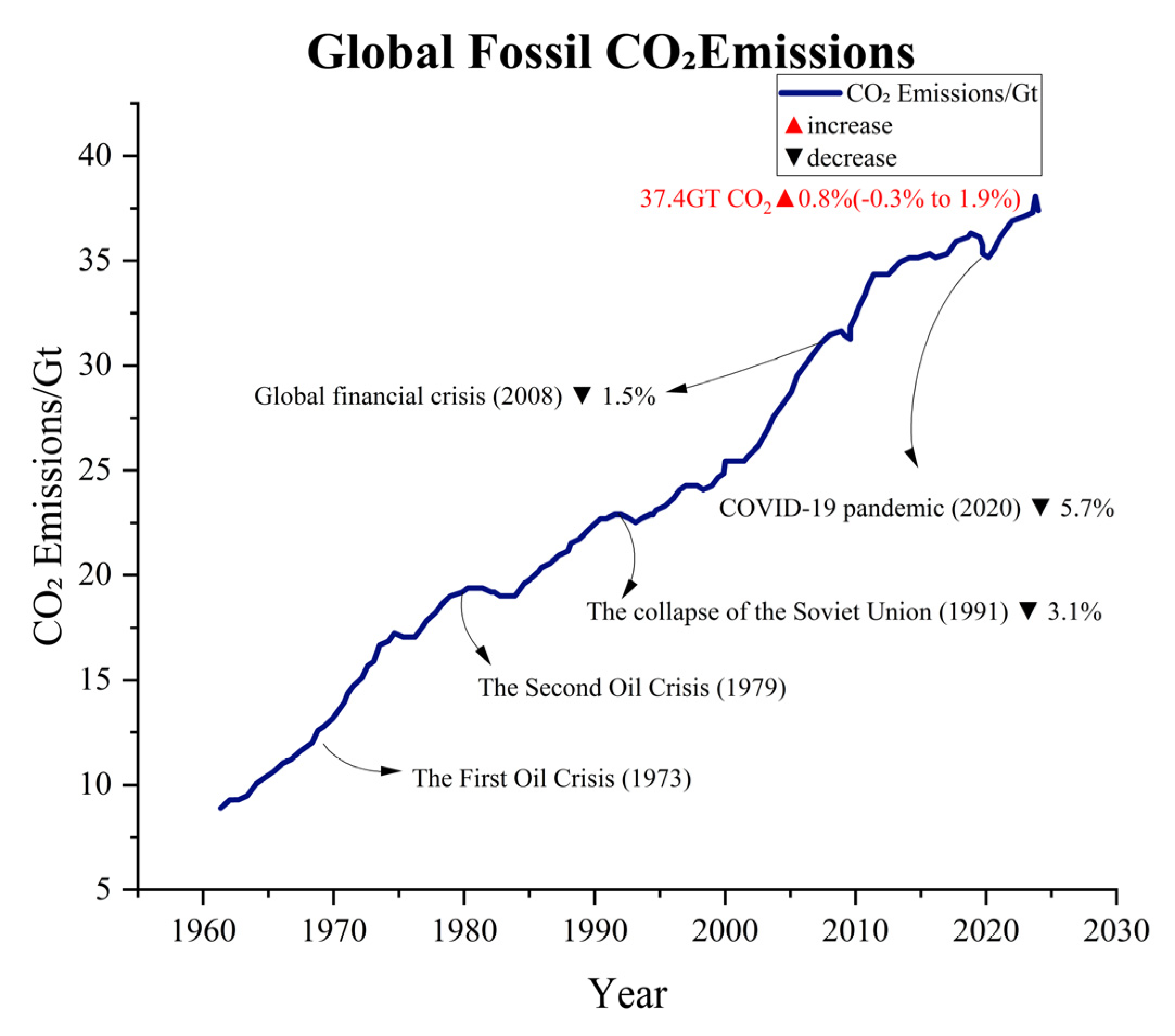
The global population is growing exponentially, while modern industries are also developing rapidly. As a result, the reliance on fossil fuel combustion is quite high. With the annual average consumption of fossil fuels continuously rising, this has led to consistently high levels of greenhouse gas emissions, such as CO2 and methane (see Figure 1) [1,2]. According to data from the Global Carbon Project (GCP), since the 1960s, global fossil fuel CO2 emissions have steadily increased, with projections suggesting that by 2024, emissions will reach 37.4 billion tons (see Figure 2) [3,4]. Although emissions have experienced significant fluctuations during certain historical phases, such as the oil crisis of the 1970s, the global economic downturn in the early 2000s, and the large-scale COVID-19 pandemic, this trend has remained persistent. The large-scale emission of greenhouse gases has contributed to the intensification of global warming, which has further triggered an increase in extreme weather events. It is worth mentioning that global warming has led to a series of negative impacts, including but not limited to glacier melt, droughts, floods, tsunamis, increased pests and diseases, declining soil quality, as well as changes in crop growth cycles and planting regions [4,5]. It is important to emphasize that although methane has 25 times the greenhouse effect of CO2 per kilogram, its overall emission in the atmosphere is relatively small due to its primary sources being livestock and landfills. Additionally, its presence in the atmosphere is relatively short, around 10–12 years, while CO2 can remain in the atmosphere for centuries. Therefore, CO2 is considered the leading cause of the greenhouse effect. According to the data released by the International Energy Agency (IEA), there has been a significant increase in the concentration of CO2 in the atmosphere. The concentration of CO2 has risen from approximately 280 parts per million (ppm) before the industrial revolution to over 420 ppm currently, which is a 52% increase compared to pre-industrial levels. In order to better address the various issues caused by CO2 emissions, there is an urgent need to develop efficient CO2 capture and catalytic conversion technologies.
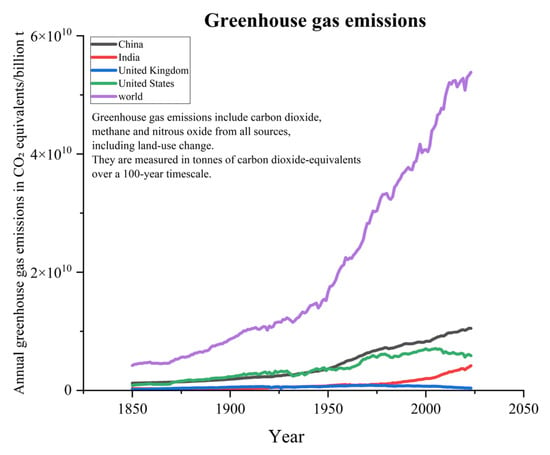
Figure 1.
Global greenhouse gas emissions graph [2].
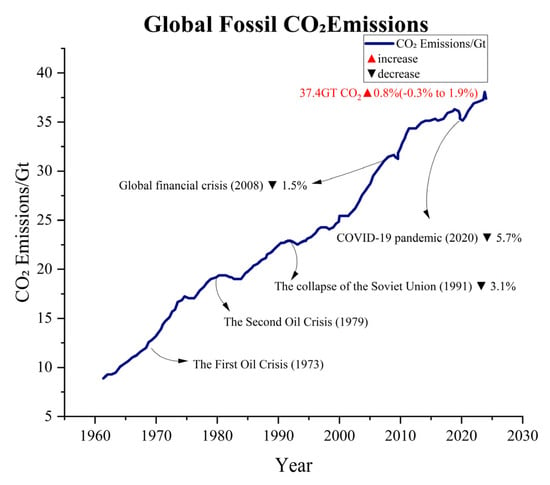
Figure 2.
Global fossil CO2 emissions (GCP data) [3].
In recent years, MOFs have garnered significant attention in the field of CO2 capture and catalytic conversion, due to their distinctive structures and exceptional properties [6]. The specific surface area of certain MOFs can reach thousands of square meters per gram, providing a substantial surface area for the adsorption and storage of various substances. In the field of gas storage, these materials exhibit particularly promising adsorption capabilities for CO2 and other gases, a property crucial in addressing the pressing issue of global warming. Concurrently, metal-organic framework (MOF) materials have demonstrated promise in the capture and conversion of CO2 at moderate temperatures (typically 523–773 K), highlighting their potential for practical applications. The structural parameters of MOFs, such as their pore size and shape, can be meticulously tailored through the judicious selection of metal ions and organic ligands. This allows the design and synthesis of MOFs with customized pore structures, ensuring their effectiveness in adsorbing and separating substances of various molecular sizes and shapes. The presence of abundant functional groups on the surfaces of MOFs has led to their remarkable performance in catalysis and sensing. In the context of catalysis, MOFs are used as catalysts or catalyst supports, thereby enhancing selectivity and efficiency in chemical reactions. For instance, MOFs can provide numerous adsorption sites, facilitating the adsorption of CO2. Their adaptable pore configuration can be customized through the strategic design of organic ligands to achieve selective adsorption of CO2. These materials can achieve high efficiency in capturing CO2 and converting it into valuable products. In addition, some MOFs have potential catalytic activity sites, with their metal ions and organic ligands serving as the active centers for the catalytic conversion of CO2 into methanol, formic acid, carbonate, and other valuable products.
2. Metal-Organic Framework Materials (MOFs)
MOFs are self-assembled from metal ions or metal clusters and organic ligands through ligand bonding, forming crystalline materials with a regular pore structure, a highly ordered porous structure, and an ultra-high specific surface area. The unique structure of MOFs gives them the advantages of both inorganic and organic materials, and they exhibit many excellent properties. The unique structure of MOFs enables them to combine the advantages of both inorganic and organic materials. They have many excellent properties and great potential for application in various fields.
2.1. Classification of MOFs
MOFs have a variety of structural characteristics, which has led to the emergence of multiple classification methods. By adopting various classification strategies to study MOFs, we can systematically explore the interrelationship between their structure and function, thereby providing solid theoretical support for efficient material design. This paper classifies MOFs from multiple perspectives.
2.1.1. Categorized by Ligand Type
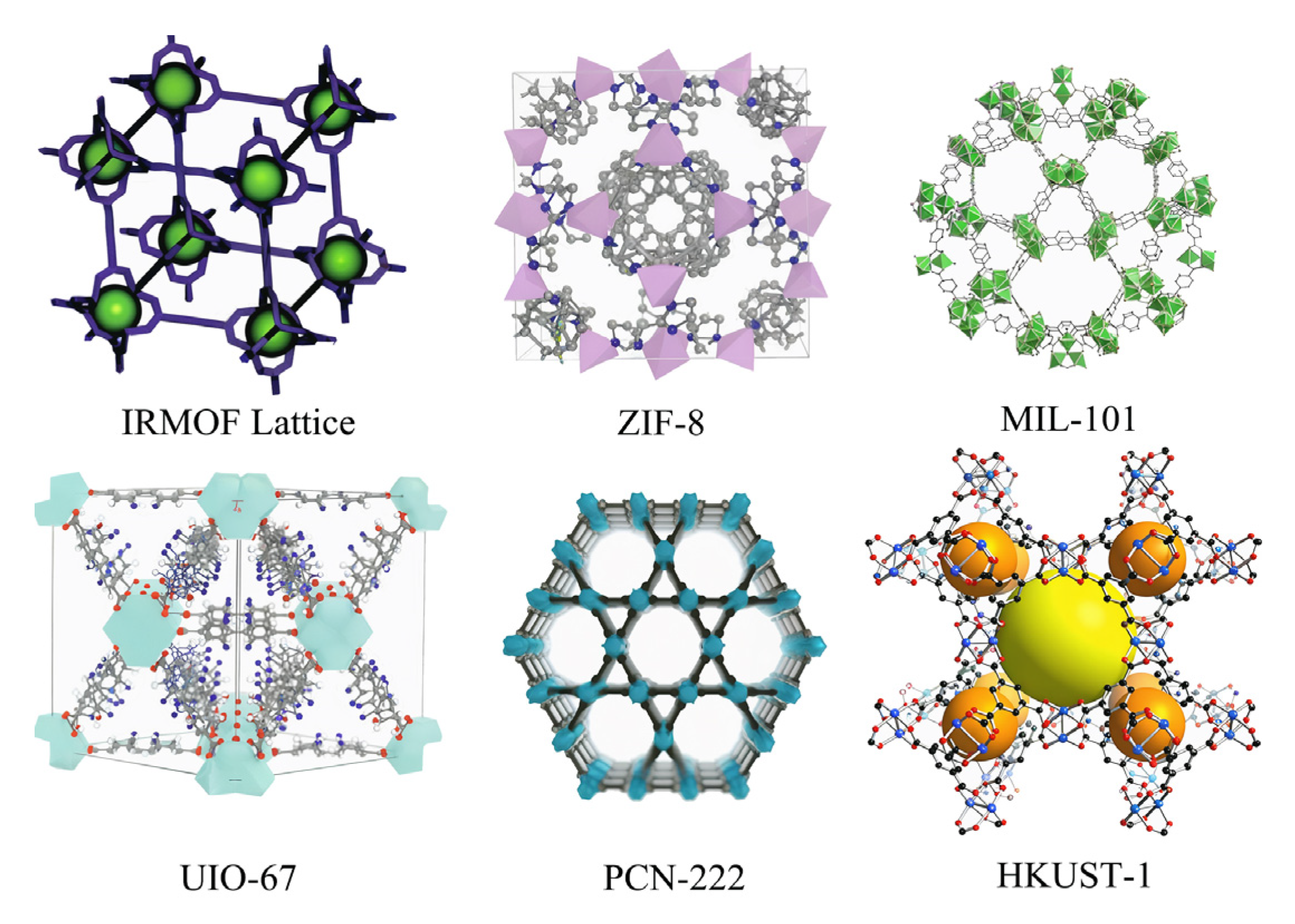
A popular series of MOFs, classified according to the ligand situation, encompasses approximately six categories, which are further divided into six series: IRMOFs structure series, ZIF structure series, MIL structure series, UIO structure series, HKUST series, and PCN structure series. The molecular structure diagrams of these materials are shown in Figure 3.
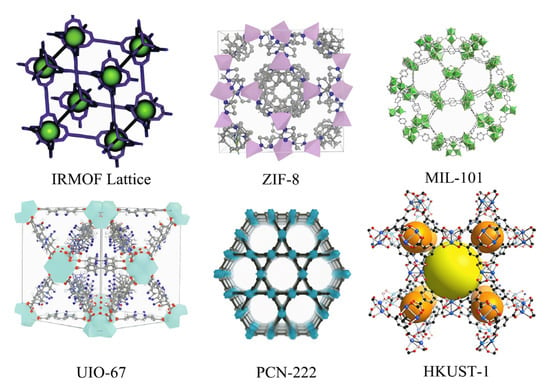
Figure 3.
Molecular structure diagrams of the six major MOF materials [7,8].
Among the popular MOFs mentioned above, the IRMOF series is a microporous crystal structure constructed in the form of an octahedron using inorganic groups and aromatic carboxylic acid ligands. The structural unit (Zn4O) is formed through self-assembly with organic ligands and is most studied for its large specific surface area, regular pore structure, and large pore volume.
The ZIF series is characterized by a zeolite-like structure, representing a class of compounds formed with a nitrogen-containing heterocyclic ring as a ligand through the binding reaction of Zn or Co with an imidazole ligand. In comparison to other types, the coordination of nitrogen-containing heterocyclic ligands with metal ions confers enhanced flexibility and adjustability. Additionally, these compounds exhibit distinctive properties, such as high porosity and favorable chemical stability.
The MIL series, also known as Levashield skeleton materials, is composed of carboxylates and trivalent cations. The existing MILs include MIL-53, MIL-100, and MIL-101. These materials possess a large specific surface area, a flexible skeleton, and a high degree of stability. The pore size of MIL-101(Cr) can reach up to about 3.4 nm, and its specific surface area is as high as 4100 m2·g−1, providing typical examples of its macromolecular catalytic applications in specific contexts. The activity and stability of MIL-101(Cr) in specific catalytic reactions provide typical examples of its macromolecular catalytic applications. The “breathing effect” of the MIL-53 series (with metal centers such as Al, Cr, etc.) and its property of reversible structural transformation under different solvents, temperatures, or pressure environments are discussed. The high thermal stability of MIL series materials (partial temperature resistance > 573 K) [7].
The UIO structure series, developed at the University of Oslo, is based on zirconium (Zr) clusters and is characterized by its ultra-high chemical stability, with excellent resistance to strong acids and radiation.
The HKUST series, developed by a team from the Hong Kong University of Science and Technology, has found widespread application in catalysis and sensing technologies. These materials are distinguished by their open metal sites and high catalytic activity. Representative materials include HKUST-1 and HKUST-101, which have been utilized for methane storage or electrocatalytic CO2 reduction. The synthesis and crystal structure of HKUST-1, which is hypothesized to possess open Cu2+ sites, have been shown to interact with guest molecules (e.g., CO2, CH4, etc.) to enhance gas adsorption [8,9]. HKUST-1 (or its isostructural material MOF-199) was derivatized as a Cu-based electrocatalyst for the electrochemical reduction of CO2, and the relationship between the structure of the material, the preparation conditions, and the catalytic activity was explored [10].
PCN (Porous Coordination Network) is a class of high-performance MOFs developed by Professor Hong-Cai Zhou’s team at Texas A&M University. The fundamental design objective was to construct framework structures with high porosity. Strong stability and functional tunability can be achieved by precisely regulating the combination of metal nodes and organic ligands. PCN is a series of materials that have attracted much attention in the fields of gas adsorption, catalysis, and drug delivery due to their diverse topologies and excellent performance. A variety of nanoscale MOFs (including some from the PCN series) have been utilized in bioimaging and radiotherapy applications, demonstrating their good biocompatibility and high drug/contrast agent loading capacity [11].
The common MOF materials, along with their corresponding characteristics and limitations, are shown in Table 1.

Table 1.
Characteristics of common MOFs.
There are several additional series being developed as the research progresses. For example, the Bio-MOF series, particularly Bio-MOF-1, which is zinc-centered, exhibited relatively low toxicity in cellular viability assays. For instance, cell viability was maintained above 80–90% in certain studies, with the tested concentrations ranging from 50–100 mg·L−1. The pores of Bio-MOF-1 are capable of interacting with biomolecules, such as nucleotides. This interaction makes Bio-MOF-1 a potential drug carrier material. Some MOFs containing nucleotide ligands (including Bio-MOF-1) are biocompatible and maintain high cell viability in assays measuring cell toxicity at concentrations up to several hundred micrograms per milliliter. In vitro tests have shown low toxicity to mammalian cells (e.g., HeLa, MCF-7, etc.) [12,13,14]. Furthermore, Bio-MOF doped with silver ions or silver nanoparticles has been shown to achieve a 99% kill rate for both Gram-negative and Gram-positive bacteria within 24 h. The antibacterial effect is attributed to the material’s ability to act gradually. The antimicrobial effect is attributed to the slow release of metal ions (or silver nanoparticles) from the material and the porous structure of the MOF itself [15].
2.1.2. Classified According to the Cambridge Structural Database (CSD)
The University of Cambridge, in conjunction with information management techniques such as databases, has developed algorithms based on several key chemical and physical characteristics. These characteristics break down the overall family of MOFs into several subgroups for the purpose of classification. This classification is based on characteristics such as surface chemistry, metal cluster families, and crystallographic properties. Thus, researchers can determine how MOFs interact with specific molecules or assess their physicochemical stability. Researchers can understand and query various aspects, such as metal clusters, network and pore dimensions, surface chemistry (i.e., functional groups), and chirality [16].
2.1.3. Classification Based on Substructure Units (SBUs)
MOFs can be categorized based on the secondary structural units (SBUs), with this categorization method emphasizing the nature of the nodes and connectors, thereby enabling MOFs to develop unique applications in numerous fields. Given that the structure of metal-organic frameworks consists of metal ion clusters and organic connectors, the SBUs are related to metal-containing nodes, and the nature of the nodes and connectors can determine the network structure, topology, physical, mechanical, and morphological characteristics of MOFs and many other properties. Following the classification of MOFs into designated systems, the manipulation of nodes and organic connectors can yield a substantial number of MOFs with distinctive properties, which possess unique applications in numerous fields [17].
2.1.4. Classification by Function
Metal-organic frameworks (MOFs) can be divided into five categories: adsorption and separation, sensing, catalysis, supercapacitors, and luminescence. The applications of MOFs are very broad, including catalysis, gas adsorption and separation, membrane separation, energy storage devices, and sensor applications.
The classification of metal-organic framework materials is a complex and extensive task that requires various classification methods. Researchers and practitioners are advised to adopt classifications that meet their specific needs to ensure a comprehensive understanding and effective use of MOFs.
2.2. Synthesis of Metal-Organic Skeleton Material MOFs
There are several general methods for synthesizing MOFs:
- (1)
- Solvothermal/Hydrothermal Synthesis
Hydrothermal synthesis is the most traditional method of MOFs preparation. It generally involves the use of organic solvents to dissolve the metal precursor and the organic linker. The mixture is then placed into the lining of polytetrafluoroethylene (PTFE). The autoclave is subsequently used to heat the mixture, thereby obtaining MOF crystals. However, the use of organic heating requires the employment of toxic solvents, such as methanol, ethanol, and acetonitrile, among others. These solvents are typically used above their respective boiling points, a practice that can cause environmental concerns due to the release of volatiles. Furthermore, the extended reaction times inherent to this process can lead to significant energy consumption and reduced yields [18].
- (2)
- Microwave-Assisted Synthesis
The employment of microwave-assisted synthesis, which involves the utilization of microwave heating within an oscillatory field, the amalgamation of substrates (metal salts and organic ligands), and suitable solvents after permanent dipole moment coupling, results in molecular rotation. This, in turn, leads to rapid heating, which accelerates the kinetics of the chemical reaction, reduces the nucleation time, and decreases the crystal growth time. The crystals obtained by this method are small in size, making them well-suited for the synthesis of nanoscale MOFs [18].
- (3)
- Mechanochemical Synthesis
The direct reaction of the metal salt with the ligand is driven by mechanical forces, such as ball milling, and no (or only trace) solvent is required. The products prepared by this method are suitable for reactions involving insoluble ligands. Furthermore, the products may be amorphous or of low crystallinity, requiring further processing.
- (4)
- Diffusion Method
The rate of crystal growth is governed by the slow diffusion of reactants (e.g., in layered solvent systems). Typically, this process requires prolonged standing of the reaction solution, often taking several days.
- (5)
- Ultrasound-Assisted Synthesis
Ultrasonication is a synthesis method in which the energy source is ultrasound. This method utilizes the cavitation effect of ultrasound to promote the mixing of reactants and the nucleation of crystals, resulting in a significant increase in the nucleation rate [18].
- (6)
- Electrochemical Synthesis
Electrochemical methods utilize a metal electrode as a metal source, which dissolves and combines with a ligand under the influence of an electric field to produce MOFs. In general, electrochemical synthesis enables the continuous dissolution of the anode, thereby driving the reaction of metal ions with a polyacid, which can result in the formation of highly crystalline powdered MOFs. The electrochemical method also has clear advantages, such as high efficiency, low-temperature requirements, and the absence of the need for metal salts. It is imperative to acknowledge that the addition of organic solvents is frequently employed to facilitate the dissolution of organic ligands [18].
- (7)
- Specialized Methods
The advent of research has given rise to a plethora of novel methodologies for the synthesis of MOFs, resulting in a wide array of outcomes.
A statistical summary of the advantages and disadvantages of several major methods for preparing MOFs is provided in Table 2.

Table 2.
Comparison of advantages and disadvantages of several major synthesis methods for MOFs.
2.3. General Modification Strategies for Metal-Organic Skeleton Materials MOFs
The three existing modification strategies are as follows: the introduction of metal sites, the introduction of functional groups, and the preparation of composites with carbon nanomaterials. These three modification methods overlap with the aforementioned strategies. In addition, the modification of MOFs can be achieved by altering the reaction conditions during hydrothermal synthesis.
The incorporation of metal sites into metal-organic skeleton materials has been demonstrated to engender structural and property diversity, thereby enhancing the selective absorption of CO2. A notable example is the work of Bai Juan, who modified Zr-MOF through the introduction of metal doping and alterations to organic ligands. Subsequent to Ce doping, significant alterations were observed in the crystallinity, crystal morphology, particle size, pore volume, oxygen vacancy content, and acid-base properties of the material. The DMC yield of the resulting Zr-Ce-MOF was determined to be 5.02 mmol at 433 K and 4.6 MPa, indicating its optimal weak basicity and the presence of medium-strength acid sites. Furthermore, experiments involving the alteration of the structure of organic ligands revealed that Zr-1C=C-2C yielded 2.78 mmol DMC at 423 K and 2.6 MPa, while Zr-BDC-Br yielded 5.82 mmol DMC at 423 K and 4.6 MPa, thereby enhancing the material’s properties [19]. Lillerud’s team successfully synthesized a Zr-MOF (metal-organic framework) with the acronym UiO-66, which possesses an ultra-high specific surface area and excellent thermal stability properties. They achieved this feat by linking hexanuclear Zr clusters with a simple ligand, 1,4-benzene dicarboxylate (BDC), to obtain a robust three-dimensional porous structure [20]. Mutyala et al. synthesized MOFs (UiO-66) and TETA/UiO-66 with Zr as the metal center by adding triethylenetetramine (TETA) to the synthesis process. The results of comparative tests revealed that the modified materials produced much higher CO2 uptake and reduced uptake of other substances, thereby improving the separation parameter [21]. Pazoki revealed that the incorporation of Li+ into Cu-BDC diminishes CH4 adsorption at lower pressures, while the converse is observed at higher pressures, a finding that holds considerable promise for industrial CO2/CH4 adsorption separations [22]. In his study, Wu Dong examined the interaction of metal Li-modified chem-4Li MOF (doped with metal Li in IRMOF-1) with CO2. His research encompassed a systematic investigation into the CO2 trapping and separation efficacy of this modified material for various industrial systems, including CO2/H2, CO2/N2, CO2/O2, CO2/CO, and CO2/C2H4. Additionally, he conducted a detailed analysis of the alterations in the pore structure and properties induced by the introduction of the metal Li modification. Furthermore, he analyzed the reasons and mechanisms for the strong interaction between the material and CO2, as well as the changes in the adsorption sites of adsorbed guest molecules in different pore structures within the material and the resulting changes in the pore structure of the adsorbed guest molecules. The study also examined the reasons and mechanisms behind the strong interaction between the material and CO2, as well as the changes in adsorption sites of adsorbed guest molecules in different pore structures within the material and the resulting microphase separation effect. The findings of this study demonstrate that metal Li modification is an effective method to enhance the separation performance of the materials [23]. Asghar et al. [24] synthesized MOFs (Cu-BDC) with Cu as the metal-centered site and prepared hexamethylenetetramine (HMTA)-loaded Cu-BDC ⊃ HMTA through ligation. The results demonstrated that the adsorption capacity of the modified MOFs was significantly higher than that of the pre-modified MOFs [24]. Wu Dong studied Cu-BTC with a pore-cage-pore structure (also known as [Cu3(TMA)2-(H2O)3]). The material is prepared by the reaction of copper nitrate solution with mesitylene triformate under certain conditions and has a three-dimensional intersecting central pore of 9.5 Å. Typically, the ligand-unsaturated sites on the metal are occupied by water molecules, which are delocalized upon heating, leaving the ligand-unsaturated sites (bare). It has been shown that high porosity and good chemical stability are also reflected in Cu-BTC, making it potentially useful in gas adsorption and separation [23].
The incorporation of functional groups has been demonstrated to enhance the CO2 uptake capacity of MOFs. For instance, Yurduen et al. synthesized a MOF (MIL-88B) with Fe(III) as the metal center by following the method of functional groups [25]. Similarly, Mahdipoor et al. synthesized MOFs (MIL-101(Fe)-NH2) using amino substituent-modified terephthalic acid as the ligand [26]. The resulting materials exhibited superior CO2 adsorption and separation properties. In a similar study, Mahdipoor et al. prepared MOF-5, ZIF-8, and other materials, which exhibited superior performance compared to conventional adsorbent materials due to the precise control of the Fe/BDC ratio during the reaction [26].
A comparison of the aforementioned approaches reveals the distinct advantages of composites prepared with carbon nanomaterials. These composites offer a superior degree of selection and manipulation while preserving the structural and morphological integrity of the raw materials. Extant research has demonstrated that the integration of nanofiber membranes and the synthesis of MOF nanoparticles can yield adsorptive high-performance materials with the capacity to separate CO2 from mixed gas streams [27].
Modifying the reaction conditions in hydrothermal synthesis is a strategy to alter the structure and properties of MOFs. This modification is achieved by adjusting reaction conditions, including the type and ratio of solvents, reaction temperature and time, concentration of metal ions, and the amount of ligands. These conditions are based on the preparation of MOFs through hydrothermal synthesis. For instance, the choice of solvent affects solubility and polarity, which in turn influence the solubility and reactivity of metal ions and organic ligands. This, in turn, impacts the crystallization process and the structure of MOFs.
3. CO2 Capture and Catalytic Conversion by MOFs
Considering the aforementioned strategies for structural adjustments of MOFs, metal doping, and functionalization improvements, these methods have considerable potential for optimization in the adsorption performance and the mechanism of CO2 capture.
3.1. CO2 Capture by MOFs
Substantial research has demonstrated that MOFs exhibit remarkable properties, including a low regeneration temperature, high porosity and adjustability, good chemical stability, and selective adsorption. This suggests that MOFs possess considerable potential in the field of CO2 capture. This potential extends beyond the scope of single adsorption and encompasses its potential for development, manufacturing, and industrialization. For instance, Yaghi’s team developed MOF-303 (chemical formula Al(OH)(PZDC)), which can adsorb water efficiently at a relative humidity as low as 16% and release the water by mild heating at only 287 K, with a regeneration enthalpy as low as 5 kJ·mol−1. The synthesis on a kilogram scale is enabled by multi-metal coordination and green water-based synthesis methods. Notably, the regeneration energy consumption of this material is 60% lower than that of traditional silica gel [28]. As an illustration, Fe3O4@UiO-66-SO4 is a material that, through sulfate interfacial modification, reduces CO2 desorption energy consumption by 44.7% and the regeneration temperature to less than 353 K (whereas conventional materials usually require 393 K). The core–shell structure of Fe3O4 (the core) and UiO-66-SO4 (the shell) combines the advantages of magnetic separation with low regeneration energy consumption, making it suitable for industrial flue gas treatment [29]. Lian Shen’s research utilized the facile modification properties of metal nodes in high porosity MOFs to prepare enhanced catalytic materials using the post-modification method and investigate their applications in oxygen and hydrogen peroxide reduction [30]. Wu Dong’s research demonstrated that the UiO-66 (Zr) series materials exhibit elevated levels of thermal and chemical stability. This enhanced stability allows the materials to maintain their structural integrity during processes such as adsorption, separation, and other applications. Consequently, this ensures the maintenance of their properties and provides a reliable basis for their utilization in practical industrial settings [23]. In the study of noble gas adsorption, Chen Hao used a Monte Carlo method based on high-throughput screening to investigate the effects of various factors in MOFs on the adsorption and separation performance of Kr/Xe. Four types of functional groups were computationally screened along with three types of backbone structures. The findings of the study indicated that the functional groups had a more significant influence on the adsorption and separation properties than the skeletons and metal centers. The interpenetrating structures demonstrated superior performance in single-component adsorption, while the non-interpenetrating structures exhibited enhanced selectivity for Xe in the binary hybrid system [31]. Three primary factors have been identified as influential in the process of CO2 capture by MOFs: the presence of organic functional groups, the composition of the metal, and the conditions under which adsorption occurs [19,26]. The present study demonstrates that enhancing the adsorption capacity of MOFs for CO2 can be achieved by altering the organic ligands, incorporating metals as dopants, reducing the regeneration temperature, and modifying the external conditions.
Alteration of organic ligands: In general, the solvothermal method is employed to enhance the material’s capacity for CO2 adsorption by modifying the carbon chain structure, the number of coordination groups, and the functional groups of the organic ligands. These modifications result in substantial structural alterations in the material’s properties, including crystallinity, crystal morphology, particle size, pore structure, acidity, and alkalinity. Consequently, these alterations lead to an improvement in the material’s adsorption capacity for CO2. For instance, alterations in the carbon chain structure of organic ligands have been observed to result in changes in the crystallinity of the material, the evolution of the crystal morphology from tetrahedral to rectangular, a shift in pore size from microporous to mesoporous, a decrease in thermal stability, and an increase in Lewis acid sites. These changes in properties have been shown to lead to an increase in the adsorption capacity of the material for CO2. Microporous MOFs (pore size < 2 nm) possess a high specific surface area and structural selectivity for small molecules; however, they are often poorly suited due to difficulties in mass transfer and the limited encapsulation of large functional guest molecules [32]. To overcome these limitations, mesoporous MOFs (2–50 nm) have emerged as a subject of intense research interest. Synthesized MOFs frequently contain guest molecules, such as solvents, unreacted linkers, clusters, and modifiers. These guest molecules generally require removal through an activation process. However, mesoporous MOFs are prone to collapse during this process. Consequently, research into the design and control of porosity, including methods to better maintain porosity for the intended application, is a perpetual central theme [33]. In a seminal study, Wu et al. pioneered a novel method for synthesizing a highly ordered macroporous MIL-125 material via a solvent evaporation-induced self-assembly route [34]. This innovative approach yielded a macroporous MIL-125 material with a remarkable specific surface area of up to 1083 m2·g−1. Subsequent investigations revealed that this material exhibited remarkable stability in immobilizing CO2, demonstrating good substrate tolerance and excellent yields in UV-irradiated CO2 carbonylation coupling reactions [35].
Doped metals: Metal elements, as the earliest studied electrocatalysts for CO2 reduction, hold an important position in the field of electrocatalysis. The CO2 adsorption properties of MOFs centered on different metals containing modified metals are shown in Table 3. During the 1980s and 1990s, Hori et al. published a series of research results on the use of various metals for electrocatalytic CO2 reduction [36,37,38], which still have a profound impact on today’s scientific research. A taxonomy of metals based on their reduction products has been proposed, classifying metals into four categories. The first category includes Pt, Ni, Fe, Co, and other metals with low hydrogen overpotential and a strong carbon monoxide adsorption capacity. These metals can poison the CO2 reduction site, thereby converting hydrogen into formate. The second category includes Sn, Pb, Bi, In, etc., due to the difficulty in adsorbing CO intermediates. These metals can be easily protonated after detachment, thus generating formic acid or formate. The third group includes Au, Ag, Zn, and others. These metals can adsorb CO2 intermediates and break the C-O bond, with the generated carbon monoxide easily detaching from the surface of the electrode to become the main product. Copper is the only metal element capable of generating C1–C3 hydrocarbon products due to its ability to react with them, making hydrogen the main product. The selective adsorption capacity of copper for carbon monoxide, along with its ability to hydrogenate adsorbed carbon monoxide, leads to the generation of C1–C3 hydrocarbon products. The subsequent formation of C-C bonds enables the production of C2 and higher hydrocarbon products. The performance of the catalytic conversion of CO2 is significantly influenced by doping with different metal elements during the preparation of MOFs. It has been demonstrated that Zr/Ce-based MOFs with the UiO-66 structure can be synthesized by doping Ce metal into the MOFs. This material has the potential to catalyze the conversion of CO2 to DMC. It has been observed that doping with Ce metal leads to substantial alterations in the structural properties of the MOFs, including an enhancement of the crystallinity, evolution of the crystal morphology from tetrahedral to spherical, reduction in particle size, increase in pore volume, increase in oxygen vacancy content, and changes in acid-base properties, among others. These property changes lead to enhanced adsorption of CO2 by the materials [19]. The enhancement of defects and acidic sites in bimetallic MOFs contributes to their superior performance. This is attributable to the elevated number of metal defect sites and Lewis acid content present in bimetallic MOFs compared to monometallic ones. These properties are advantageous for CO2 adsorption and separation, resulting in enhanced CO2 adsorption efficiency [27].
A reduction in the regeneration temperature has been demonstrated as a viable strategy for achieving simultaneous energy savings and enhanced service life of MOFs. This approach is supported by existing studies, which have shown that modifying existing MOFs can result in a decrease in the regeneration temperature. For instance, a modified MOF developed at the University of California, Berkeley, exhibits a regeneration temperature of 323 K, while the regeneration temperature of an amine solution (currently employed for CO2 absorption) ranges from 353 to 383 K. The regeneration temperature of the modified MOF is considerably lower than that of the amine solution. The lower regeneration temperature, combined with the advantage that the modified MOFs do not require heating of the aqueous solution used by conventional amine absorbents, results in reduced energy consumption for the new material when utilized as a CO2 trap. Yaghi’s team developed MOF-303 (chemical formula: Al(OH)(PZDC)), which exhibits remarkable efficacy in adsorbing water at relative humidity as low as 16%. This material requires only mild heating at 287 K to release the water, and its regeneration enthalpy is as low as 5 kJ·mol−1. The material’s synthesis is scalable to a kilogram through multi-metal coordination and green water-based synthesis, and its regeneration energy consumption is 60% lower than that of traditional silica gel [28].
Alterations in external conditions: Modifications to the external conditions during the reaction have been demonstrated to enhance the efficacy of adsorption. For instance, researchers at Oregon State University observed that copper-based MOFs exhibited more than a twofold increase in CO2 adsorption efficiency when initially exposed to ammonia, requiring less energy for regeneration and demonstrating enhanced stability compared to amine-based adsorbents [27]. Beyond this approach to enhancing performance, it has been determined that MOFs have the capacity to further improve their adsorption performance through activation. For instance, Yang et al. [39] augmented the CO2 adsorption of prepared MIL-100 (Cr) from 3.52 to 7.18 mmol·g−1 by calibrating the vacuum activation temperature. In the case of UiO-66, Ahmadijokani et al. demonstrated that varying activation solvents and vacuums yielded different product yields and CO2 adsorption, underscoring the pivotal role of the vacuum activation temperature in product formation [40]. Furthermore, the layering of this substance with acid treatment, followed by the synthesis of plagioclase zeolite with the aid of PEG or PVP and a layered composite, has been shown to result in the construction of a disordered layered UiO-66 heterostructure on plagioclase zeolite. This has been demonstrated to exhibit excellent adsorption selectivity for CO2 and CH4 [41]. It is important to note that trapping efficiency can be influenced by several external factors. For instance, Yan et al. were able to enhance CO2 adsorption from 7.23 mmol·g−1 to 10.67 mmol·g−1 at 273 K and 10000 Pa by soaking and allowing HKUST-1 in ethanol containing a small amount of NaCl and NH4Cl at room temperature several times [42]. In addition to the aforementioned factors, it has been demonstrated that the incorporation of nanofibrous membranes and the synthesis of MOF nanoparticles yields materials with high performance in adsorption, capable of separating CO2 from mixed gas streams [43].

Table 3.
CO2 Adsorption Properties of MOFs Centered on Different Metals Containing Modified Metals Table.
Table 3.
CO2 Adsorption Properties of MOFs Centered on Different Metals Containing Modified Metals Table.
| MOF | Metal Center | Specific Surface Area/m2·g−1 | CO2 Adsorption Capacity/mmol·g−1 | Pressure/MPa | Selective CO2/N2 | Reference |
|---|---|---|---|---|---|---|
| MIL-53 | Al | 634 | 10.4 | 30 | 50 | [44,45] |
| MIL-53 | Mn0.27Al0.73 | 1748 | 11.8 | 30 | 83 | [46] |
| MIL-53 | Mn0.5Al0.5 | 1817 | 13.3 | 30 | 95 | [46] |
| MIL-53 | Mn0.6Al0.4 | 1576 | 12.8 | 30 | 45 | [46] |
| MIL-100 | Fe | 1811 | 2.6 | 1 | 49.6 | [47] |
| MIL-100 | Al, Fe | 1993 | 3.3 | 1 | 76.5 | [46] |
| MIL-120 | Al | 3084 | 4.8 | 10 | - | [46] |
| MOF-74 | Mg | 1640 | 8.0 | 1 | 233 | [48,49,50] |
| MOF-74 | Zn0.14Mg0.86 | 1277 | 0.5 | 1 | - | [51] |
| MOF-74 | Zn0.48Mg0.52 | 794 | 2.1 | 1 | - | [51] |
| MOF-74 | Zn0.75Mg0.25 | 668 | 3.2 | 1 | - | [51] |
| MOF-74 | Ni | 1274 | 5.2 | 1.1 | 11.3 | [41,47] |
| MOF-74 | Pd, Ni | 1115 | 12.2 | 32 | 14.6 | [52] |
| MOF-74 | Co | 1404 | 3.7 | 1.1 | 9.7 | [52] |
| MOF-74 | Pd, Co | 1088 | 11.4 | 32 | 12.4 | [52] |
| MOF-5 | Zn | 1858 | 0.5 | 1 | - | [53] |
| MOF-11 | Zn | 2096 | 14.7 | 35 | - | [54] |
| MOF-210 | Zn | 6240 | - | 50 | - | [55] |
| UiO-66 | Zr | 1455 | 4.3 | 20 | - | [56] |
| HKUST-1 | Cu | 1440 | 3.4 | 1 | - | [57] |
| HKUST-1 | Li, Cu | 1000 | 2.6 | 2 | - | [58] |
| Cu3(BTC)2 | Cu | 1781 | 10.7 | 35 | - | [54] |
| [Ni6(OH)4(COO)8(H2O)6] | Ni | 468 | 1.4 | 1 | - | [58] |
| [Ni4.1Co1.9(OH)4(BTB)8/3(H2O)6] | Ni4.1Co1.9 | 491 | 1.7 | 1 | - | [58] |
| [Ni3.1Co2.9(OH)4(BTB)8/3(H2O)6] | Ni3.1Co2.9 | 526 | 1.9 | 1 | - | [58] |
| [Ni2.8Co3.2(OH)4(BTB)8/3(H2O)6] | Ni2.8Co3.2 | 819 | 2.3 | 1 | - | [58] |
3.2. Catalytic Conversion of CO2 by MOFs
The catalytic conversion of CO2 by MOFs will vary depending on the material itself, and in the reduction process, MOFs contain the following main catalysts.
- (1)
- Photocatalysis
MOFs have the capacity to function as a photocatalyst in the conversion of CO2. MOFs are hybrid materials formed by the coordination of metal ions or clusters of ions with organic molecules. To a certain extent, MOFs can be regarded as both organic molecules linked by inorganic nanoparticles and nanoparticles isolated by organic molecules. There are several reasons for using MOFs in the photocatalytic reduction of CO2 [59,60,61]: from a structural point of view, MOFs are characterized by their porous structure and large specific surface area. This feature not only indicates that they have more active sites, but also that they can adsorb more CO2. Meanwhile, the special structure can increase the local CO2 concentration, which is conducive to the reaction. Furthermore, the integration of isomeric organic ligands with chromophoric groups or metal ions that possess an isomeric structure during the synthesis of MOFs has been demonstrated to enhance the light absorption efficiency of MOFs, broaden the absorption spectra, or improve catalytic performance. With respect to the ligand type, the organic ligands of MOFs typically contain functional groups such as N-H2, -OH, -CH=CH2, and others. These functional groups not only provide a substantial number of bonding sites but also introduce new ligands when they are unsaturated or coordinated with solvent molecules. The introduction of molecules with -OH, -NH2, -COOH, etc., into MOFs through coordination enables dye functionalization, and their combination with other inorganic materials to form new hybrid materials can enhance catalytic performance. In terms of plasticity, the morphology and size of MOFs can be controlled by manipulating them during synthesis, for instance, by adding surfactants, using solvents, and generating ultrathin nanosheets, thereby exposing more catalytic sites and enhancing catalytic activity.
According to existing literature, MOFs play at least one role in catalyzing and absorbing light in the photocatalytic CO2 reduction reaction. Accordingly, MOFs involved in catalytic reactions can be categorized into three types: MOF photocatalysts, MOF catalysts, and MOF photosensitizers. For instance, Wang et al. [62] synthesized ZIF-9 through a series of controls involving the use of Co2+, benzimidazole, tris-bipyridine ruthenium as a photosensitizer, and triethanolamine as a sacrificial agent. The photocatalytic reduction of CO2 was subsequently carried out in a mixture of acetonitrile and water, and a total of about 350 μmol of hydrogen and carbon monoxide was produced from 0.8 μmol of ZIF-9 after a 2.5-h reaction (hydrogen: carbon monoxide = 3:4), with a conversion number of 450. Subsequent X-ray diffraction analysis revealed that the crystalline structure of ZIF-9 remained unaltered, indicating that the MOFs did not function as reactants and were capable of maintaining their stability during the catalytic process. It is noteworthy that the authors’ experiments demonstrated that the MOFs functioned as a catalyst in the catalytic reaction exclusively as the catalytic component, with the light-absorbing component typically comprising a metal complex (e.g., tris(pyridine)ruthenium/iridium) or a semiconductor (e.g., metal-semiconductor, organic-semiconductor) or semiconductors (e.g., metal semiconductors, organic semiconductors). In this system, metal complexes or semiconductors may exhibit minimal catalytic activity, and the activity of the reaction system is significantly enhanced by the incorporation of the MOFs. Consequently, this type of MOF is frequently referred to as a cocatalyst in the literature [63].
Two broad strategies have been developed to enhance photocatalytic efficiency. The first strategy involves the use of a functionalized ligand, such as a porphyrin ligand. PCN-222 has been shown to achieve visible-light-driven CO2 reduction (CO generation at rates of up to 120 μmol/g·h) via a porphyrin ligand [64]. Metal doping has also been demonstrated to enhance photocatalytic efficiency to 0.8% by loading Co2+ onto Ti-MOF-NH2 [65]. These enhancement strategies generally begin with charge transfer and photocatalytic processes. There are three charge transfer pathways: LMCT (ligand-metal charge transfer), MLCT (metal–ligand charge transfer), and LLCT (ligand-ligand charge transfer). The electron transfer process is closely associated with chemical bonding, as illustrated in Figure 4. A variety of materials and systems exhibit electron transfer and redox processes under photoexcitation, including TTPA materials, MIL-125-NH2 materials, the TCH-related photocatalytic reaction process, the BDC-NH2 removal process, porphyrin-[Ni] cluster-related photocatalytic reactions, and the photocatalytic degradation of CBZ/IBP under visible light. Refer to Figure 5 for a visual representation of each of these elements.
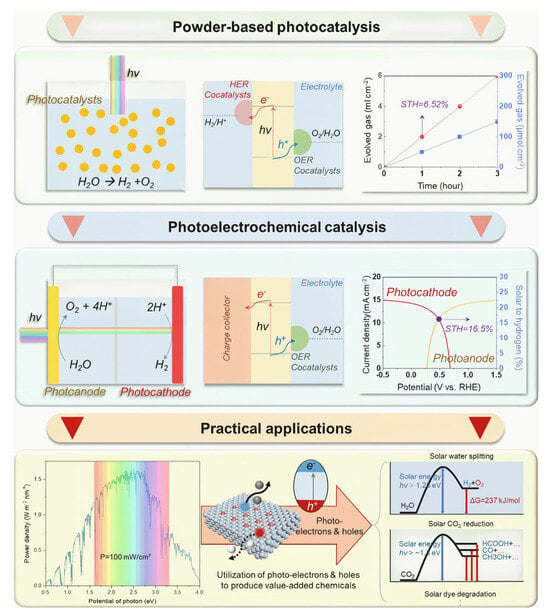
Figure 4.
The represented systems and application mechanisms of photocatalysis Ref: [66].
- (2)
- Electrocatalytic
Electrocatalysis is defined as the catalytic action that accelerates the charge transfer reaction between the electrode and electrolyte interface. MOFs have emerged as a subject of intense research interest in the field of CO2 trapping and electrocatalytic reduction due to their advantageous properties, including tunable pore structures, high specific surface areas, and well-defined active sites. However, the inherent limitations of pristine MOFs, including conductivity, stability, and selectivity, impose significant constraints on their practical applications. To address these challenges, modification strategies such as metal doping, ligand functionalization, and composite hybridization have been employed to enhance the performance of MOFs. The selection of suitable electrode materials is paramount for optimizing battery performance, thereby accelerating the reaction rate. During the discharge process, the selected electrode materials function as catalysts, modulating the reaction rate or electrode orientation without undergoing substantial changes themselves. In the domain of electrocatalysis, MOFs have demonstrated efficacy in the production of hydrogen and oxygen, the reduction of oxygen, and the reduction of CO2, among other applications. Xu et al. modified hybrid nanostructures with CeOx nanoparticles on ZIF-67-derived hollow CoS surfaces through in situ generation [67]. It was found that proper control of the amount of CeOx on the CoS surface could achieve precise tuning of the Co2+/Co3+ ratio, which could induce defects and thus enhance the oxygen release reaction (OER) activity. Wang et al. synthesized a series of FeNi bimetallic MIL-101 materials by the hydrothermal method and used them for OER directly [68]. These metal-organic frameworks (MOFs) contain a substantial number of catalytically active MO6 octahedra and bare hydrophilic carboxyl groups, which enhance OER activity. MOFs possess a greater number of active sites and a larger surface area, making them highly useful in electrocatalysis. The combination of MOFs with other materials can improve their stability and thus enhance their electrocatalytic activity, providing a direction for the development of electrocatalysis. A plethora of strategies have been proposed for enhancing electrocatalytic modification, including metal-centered modulation, mono- and bimetallic doping, and ligand-functionalized amine and polar group modification. A domestic study has been undertaken, focusing on the following areas: Xiao’s team enhanced CO2 adsorption up to 3.8 mmol·g−1 (1 bar) by amine-functionalizing MOF-177 (TEPA-modified) [69], and the data significantly showed that it was superior to conventional physical adsorption materials. Hydrogen bonding modulation: The team from Sun Yat-sen University introduced NH2 groups into Co porphyrin MOFs and stabilized the Co-CO2 intermediates through weak hydrogen bonding, with 99.4% FE and 2.7 s−1 TOF, which is leading performance in the international arena. A comprehensive analysis reveals that domestic and international studies have distinct foci within the broader context of MOF-based electrocatalysis. Foreign countries are at the forefront of developing mechanisms and designing highly selective catalysts, while domestic efforts prioritize cost-effective modifications and industrial adaptation.
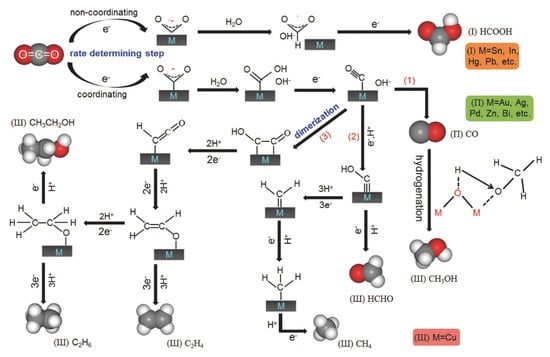
Figure 5.
Selectivity of different metal catalysts for electrochemical CO2 reduction products Ref: [70].
Figure 5.
Selectivity of different metal catalysts for electrochemical CO2 reduction products Ref: [70].
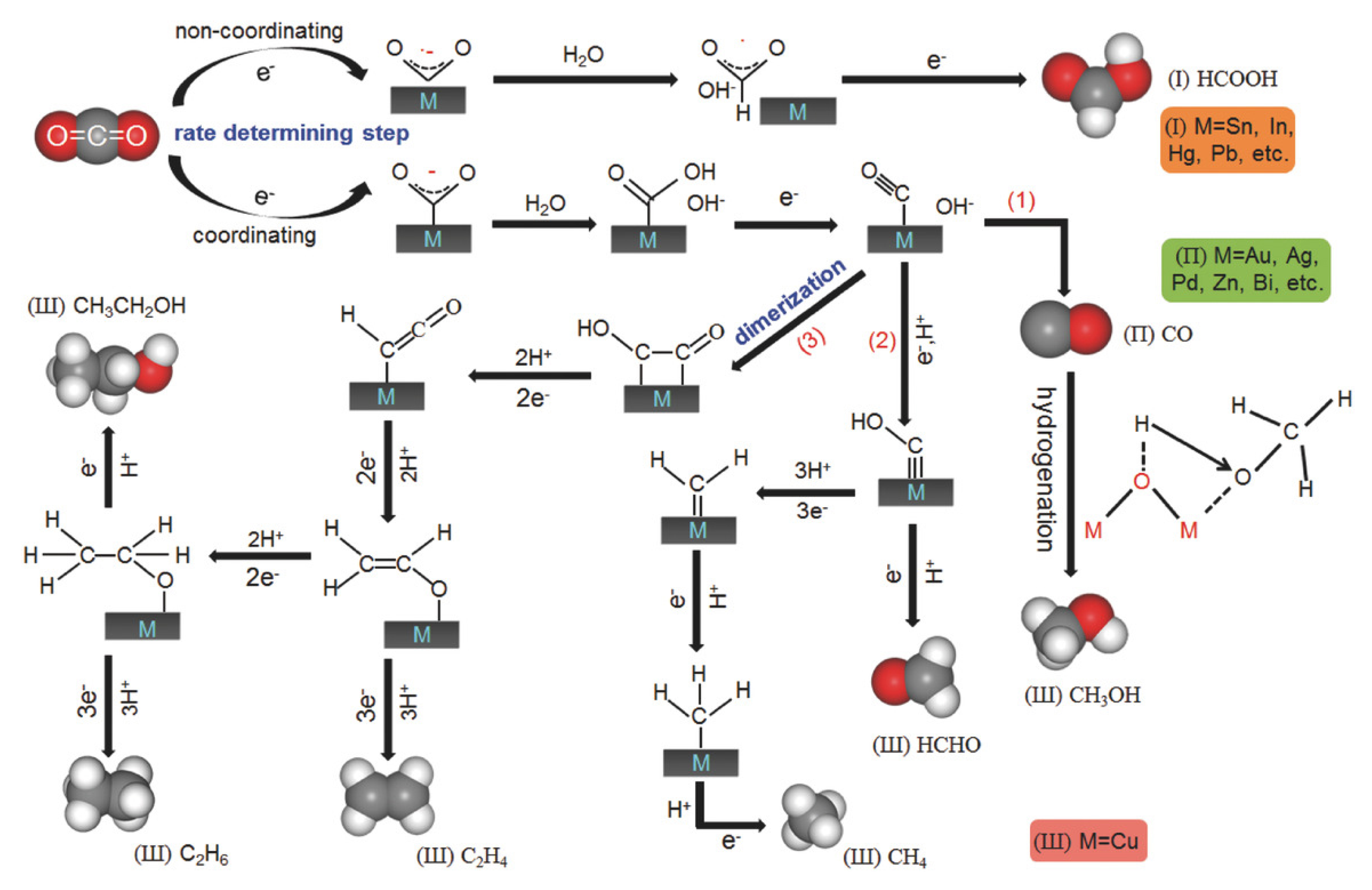
- (3)
- Thermal Catalysis
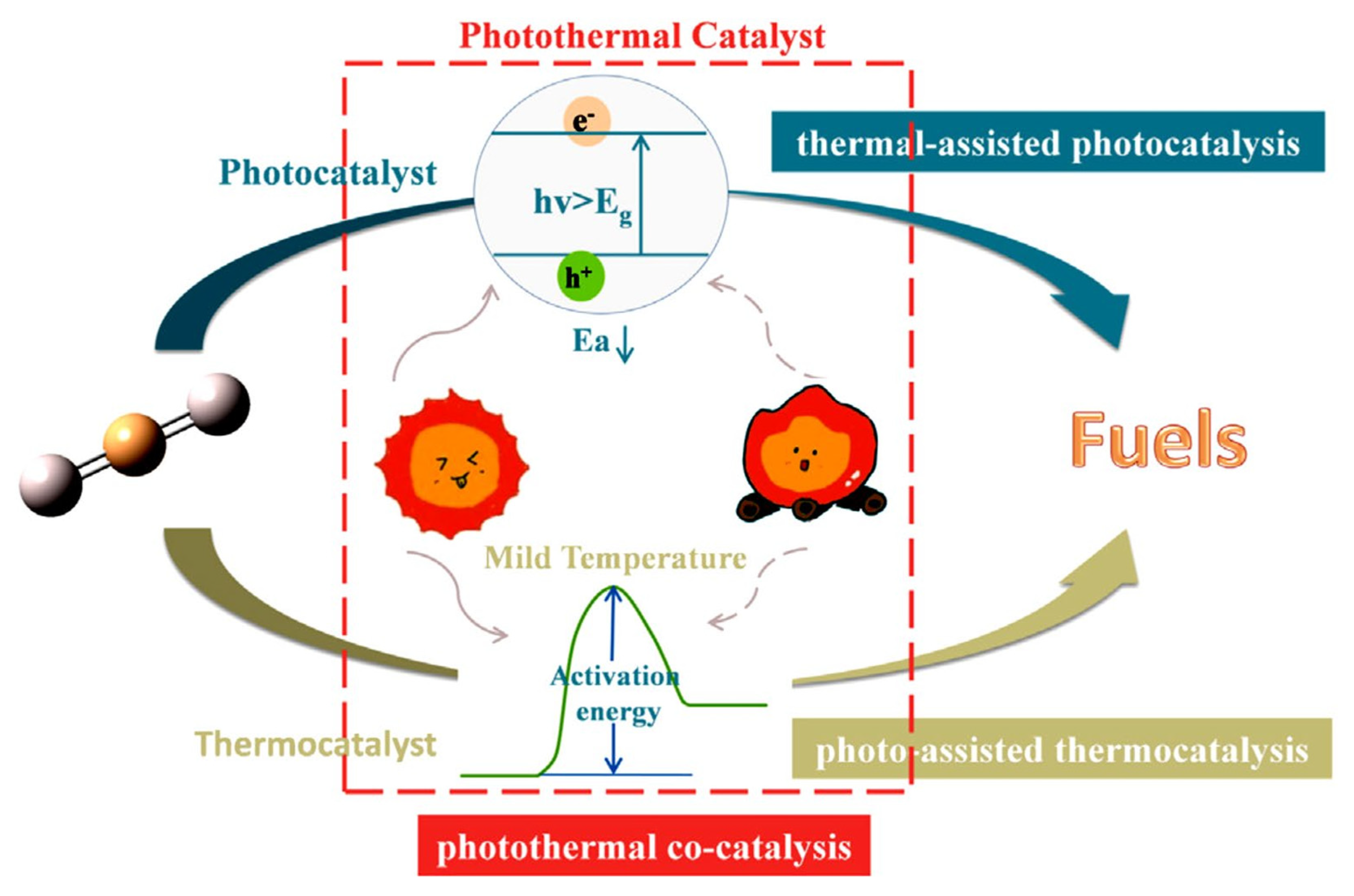
The thermal catalytic reduction of CO2 is the process of converting CO2 into high-value-added chemicals, such as methane, methanol, and low-carbon olefins, through the action of catalysts under high-temperature conditions (as shown in Figure 6). MOFs with tunable metal nodes and organic ligands are promising catalysts for a variety of applications, including CO2 cycloaddition reactions, hydrogenation of unsaturated hydrocarbons, and epoxidation of olefins. The modification of MOFs has been shown to significantly enhance catalytic activity and selectivity. This enhancement results from the unique pore structure, tunable active sites, and the metal–ligand synergistic effect of the modified materials. The modification strategies for thermal catalysis can be broadly categorized into three distinct classes: metal doping and alloying, structural modulation and functionalization, and composite hybridization and pyrolytic derivatization. The core mechanisms include active site modulation, where metal nodes (e.g., Zr, Fe, Cu, etc.) in MOFs can act as Lewis acid sites to promote CO2 adsorption and activation. Electronic structure optimization: Metal doping or alloying (e.g., bimetallic Ag/Cu-MOF) has been demonstrated to regulate the electron density and accelerate the formation and conversion of intermediates (e.g., -COOH, -HCOO). Pore domain-limiting effect: The microporous structure of MOFs has been shown to stabilize reaction intermediates through the spatial domain-limiting effect and prolong their residence time, thus improving product selectivity. The use of organic ligands containing sulfoxide groups, amide, amino, or pyridine groups is a viable approach for synthesizing functionalized MOFs. The advantage of these groups is that they can undergo further modification to enhance efficiency and achieve targeted modifications. For instance, Zou et al. successfully assembled Zn2+ with tricarboxylic acid ligands to form new MOFs [71]. Subsequent to this, single-crystal to single-crystal metal cation exchange was performed with Cu2+ and Co2+. Of particular note, a catalytic study of the cycloaddition reaction of CO2 with epoxides to generate related carbonates showed that the MOFs of Zn2+ had the highest catalytic activity. Aniruddha et al. prepared a highly crystalline Hf (IV) MOF, and the resulting Hf-UiO-66-N2H3 was found to be useful for the benzaldehyde and malononitrile Knoevenagel condensation reaction, showing effective catalytic activity [32].
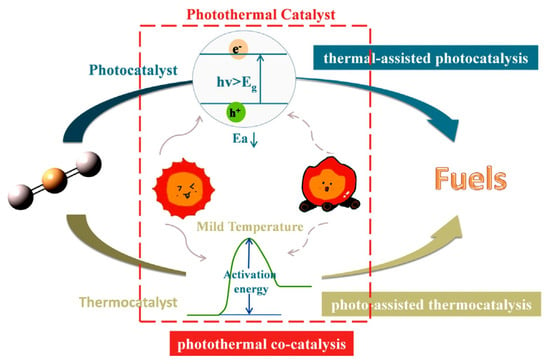
Figure 6.
Three Types of Photothermal Catalyst Reactions Ref: [72].
- (4)
- Multiphase Catalysis
MOFs have been identified as optimal carriers for multiphase catalytic CO2 conversion due to their high specific surface area, tunable pore structure, and well-defined active sites. Figure 7 shows the microstructure of Bi2O2S/PCN-20 obtained by using different detection methods. Through modification strategies such as metal doping, ligand functionalization, and complex hybridization, MOFs can achieve efficient catalytic activity and selectivity. These modified frameworks can be applied in classical multiphase catalytic reactions, including the CO2 cycloaddition reaction, CO2-assisted alkane dehydrogenation, and electrocatalytic CO2 reduction. Enhanced strategies for multiphase catalysis encompass metal node modulation and bimetallic synergistic effects. Bimetallic MOFs (e.g., BiZnMOF) markedly reduce the binding energy of reaction intermediates by optimizing the electronic structure of BiZn bimetallic centers and exhibit a 92% Faraday efficiency in the electrocatalytic reduction of CO2 to formate (HCOO-) (−0.9 V vs. RHE) [73]. Metal cluster active site: trinuclear metal clusters in Fe2M-TATAB (M=Cu, Co, Zn, etc.). MOFs provide open Lewis acid sites to catalyze the cycloaddition reaction of CO2 with epoxides >95% conversion [74]. Ligand functionalization, porphyrin ligand with a photosensitive moiety: PCN-222 (Zr-based porphyrin MOF) has been shown to drive the reduction of CO2 to CO in the visible spectrum with a quantum efficiency of 0.8%, a process that occurs through the light-trapping ability of the porphyrin ligand [75].
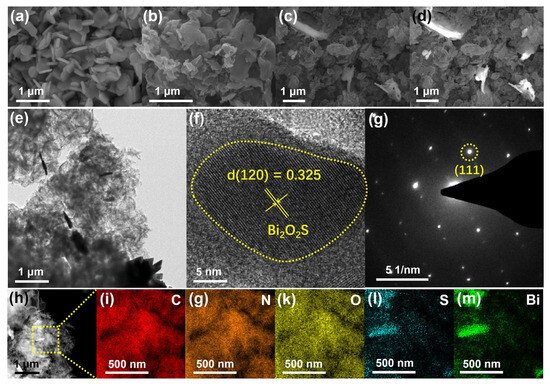
Figure 7.
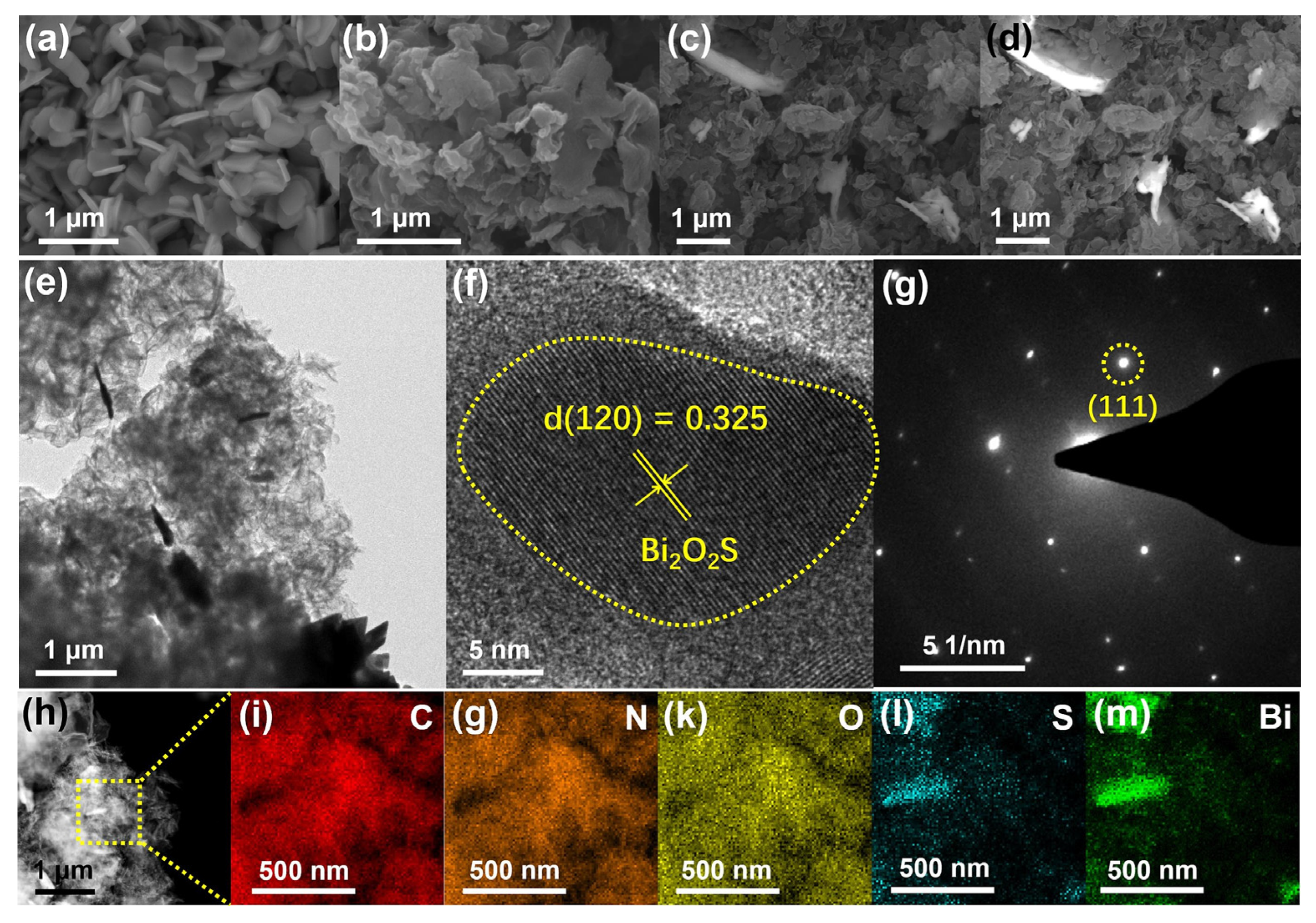
SEM images of Bi2O2S (a) and PCN (b); SEM (c) and corresponding EBSD (d) images of Bi2O2S/PCN-20; TEM (e), HRTEM (f), SAED (g), HADDF (h) and corresponding elemental mapping images (i–m) of Bi2O2S/PCN-20 Ref: [76].
The comparison of several CO2 catalytic conversion technologies are shown in Table 4. After comparative analysis, it is believed that electrocatalysis may be more suitable in distributed environments (such as exhaust gas treatment for electric vehicles). It can achieve low-carbon conversion by using off-peak electricity from the grid, but the issue of high energy consumption still needs to be addressed. Photocatalysis is particularly suitable for remote areas with abundant sunlight, such as desert photovoltaic power stations equipped with carbon capture devices, which aligns with the ‘solar energy + CO2 conversion’ green energy strategy. However, its large-scale application still requires further research and breakthroughs. In large-scale industrial applications, such as CO2 recovery at coal-to-gas plants, thermocatalysis appears more suitable and relies on advanced high-temperature treatment technologies for efficient conversion. Although heterogeneous catalysis holds an irreplaceable position as a core industrial technology, achieving its sustainability still requires further breakthroughs in material innovation and process optimization. The development of non-precious metal catalysts (such as Fe/Co bimetallic MOFs) and enhancing their activity through improved synergistic effects could become a new trend in future research.

Table 4.
Comparison of several CO2 catalytic conversion technologies.
4. Challenges and Future Perspectives
In recent years, MOFs have demonstrated significant advancements in the field of CO2 capture and catalytic conversion. However, to further enhance the efficacy of these materials, it is imperative to overcome the current technical limitations through material innovation and exploration of mechanisms.
4.1. Technological Problems
- (1)
- Stability
The harsh preservation conditions and stability of some MOF materials require enhancement because their structures are vulnerable to damage in harsh environments, including humidity, elevated temperatures, and strong acids and bases. This can compromise their performance and service life. For instance, some MOF materials based on carboxylic acid ligands are susceptible to hydrolysis in water, resulting in structural collapse. Chae et al. reported that MOF-5 was rapidly deactivated in the presence of water. Although its high specific surface area was favorable for gas adsorption, the intrusion of water led to the fracture of the framework structure, which seriously limited its reliability in practical applications [77].
- (2)
- Cost
The industrialization of MOFs applications is hindered by the necessity of precious metal modification or complex synthesis steps in some MOFs. The use of precious metals in the modification process or the employment of complex synthesis steps in some MOFs results in high preparation costs and complex production processes, thereby hindering industrialization. Consequently, recent studies have focused on the development of cost-effective, readily scalable modification strategies for synthesis. Wang et al. demonstrated that MOFs derived from noble metal modification exhibited substantial activity enhancement in CO2 reforming catalysis. However, due to the cost of the noble metal itself and the complexity of the modification process, the overall preparation cost remained high, impeding the industrialization and promotion of the scheme [78].
- (3)
- Adaptation to Working Conditions
The CO2 capture and catalytic conversion performance of MOFs in an atmosphere containing impurities (e.g., SOx, NOx, H2O) is affected. Kim et al. demonstrated that the CO2 adsorption capacity of certain MOFs was considerably diminished, and the catalytic activity was significantly suppressed in an environment containing water vapor and other impurities. This resulted in considerable uncertainty regarding their application under practical working conditions [79].
4.2. Frontier Research Directions
- (1)
- Machine Learning Assisted Design
The current state of research on MOF catalytic mechanisms is characterized by two significant limitations. Firstly, the experimental dataset is relatively small, with approximately 200 data points, which restricts the scope of analysis and hinders the development of more sophisticated machine learning methods. Secondly, the predictive accuracy of traditional machine learning methods, such as random forest and decision tree algorithms, ranges from 0.65 to 0.72, which falls short of the precision required for advanced design [80]. To address these challenges, an active learning framework with a migration learning strategy has been proposed. The utilization of an existing inorganic porous material database (e.g., CSD) for pre-training has been demonstrated to effectively mitigate the learning errors of small samples. Concurrently, the active learning framework is constructed, where the key synthesis parameters (metal electronegativity, ligand rigidity index) are screened using Bayesian optimization, thereby enhancing experimental efficiency. The algorithm in question has the capacity to predict the structure of MOFs with a high degree of stability and activity, while concurrently constructing efficient catalytic sites.
- (2)
- Replacement of Precious Metals with Cheaper Metals and Green Synthesis
Ru-based metal-organic frameworks (MOFs) (e.g., Ru-UiO-67) exhibit remarkable catalytic activity (TOF: 250 h−1), yet the metal cost share is substantial at 78% (320/g). The employment of a Fe/Co bimetallic system (FeCo-MOF-74) enables the maintenance of high activity while concomitantly reducing the cost [81]. The development of continuous production technology is imperative. Conventional solvothermal synthesis of ZIF-8 yields 65% (batch mode), whereas the microreactor continuous flow process can enhance the yield to 92%, achieving a 5.6-fold increase in the spatiotemporal yield. This technology has been successfully implemented in a kiloton MOF production line, resulting in a 42% reduction in unit energy consumption [82].
The development of high-performance modified MOF materials is expected to provide new technical means and solutions to solve the global CO2 emission problem, promote the sustainable development of the energy sector, and have broad application prospects in the fields of industrial exhaust gas treatment, energy storage and conversion, and environmental protection. A comprehensive investigation into the structure-property relationship of modified MOFs is essential to elucidate the impact of these materials on the CO2 capture and conversion mechanism. Such an investigation will contribute to the enrichment and development of theoretical knowledge in the fields of materials science and chemical engineering, thereby providing theoretical guidance for the design and development of novel and efficient CO2 capture and conversion materials. In the context of big data, there is a need to prioritize system integration and innovation in constructing a MOF/membrane composite system and an artificial intelligence-assisted platform. Future research will focus on multifunctional integrated design, real environment adaptation, and cross-disciplinary technology fusion to promote the practical application of MOFs in the field of carbon neutralization.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, Y.J.; Han, J.F.; Wang, F.; Liu, X.S.; Liu, Y.J.; Yan, X.; Zhang, G.S.; Ma, J.X.; Wei, T.; Jin, Z.W.; et al. Global warming changes patterns of runoff and sediment flux in Tibetan Yangtze River headwater. J. Hydrol. 2025, 656, 133009. [Google Scholar] [CrossRef]
- Available online: https://ourworldindata.org/grapher/ghg-emissions-by-sector (accessed on 22 April 2025).
- Available online: https://baijiahao.baidu.com/s?id=1816323030870707434&wfr (accessed on 22 April 2025).
- Chagolla-Aranda, M.A.; Simá, E.; Hernández-López, I.; Piña-Ortiz, A.; Ávila-Hernández, A. Dynamic thermal evaluation of a green roof system under warm weather conditions: Case of Tropical and Dry climates. J. Build. Eng. 2025, 103, 112053. [Google Scholar] [CrossRef]
- Joseph, M.; Charlotte, L.O.; Silvia, C.; Luísa, G.C.; Felipe, D.; Lynn, V.D.; Jeff, O.; Tim, N. Key tropical crops at risk from pollinator loss due to climate change and land use. Sci. Adv. 2023, 9, eadh0756. [Google Scholar] [CrossRef]
- Li, W.H.; An, X.F.; Chen, J.J.; Dong, F. Functionally graded porous materials derived from MOFs based on cellulose skeleton support strategy for low energy consumption carbon dioxide separation from flue gas. Chem. Phys. Lett. 2023, 832, 140890. [Google Scholar] [CrossRef]
- Luo, H.H.; Yu, L.Q.; Liu, C.; Chen, N.N.; Xue, K.H.; Liu, W.D.; Zhu, H.F.; Zhang, Y.P. Comprehensive review of synthesis strategies and performance enhancement of metal-organic frameworks and their derivatives for photocatalytic applications. Journal of Energy Chemistry. 2025, 103, 408–439. [Google Scholar] [CrossRef]
- Deyko, G.S.; Glukhov, L.M.; Isaeva, V.I.; Vergun, V.V.; Chernyshev, V.V.; Kapustin, G.I.; Kustov, L.M. Adsorption of methane and ethane on HKUST-1 metal-organic framework and mesoporous silica composites. Mendeleev Commun. 2023, 33, 817–820. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Loredana, V.; Bartolomeo, C.; Sachin, C.; Silvia, B.; Merete, H.N.; Søren, J.; Karl, P.L. Disclosing the complex structure of UiO-66 metal organic framework: A synergic combination of experiment and theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Bahareh, B.; Saeed, D. Highly efficient visible-light photocatalytic nitrogen fixation via single-atom iron catalyst site and synergistic effect of Zr-cluster in zirconium-based porphyrinic metal-organic frameworks (PCN-222). J. Solid State Chem. 2023, 324, 124079. [Google Scholar] [CrossRef]
- Glove, T.; Gregory, W.P.; Bryan, J.S.; David, B.; Omar, Y. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Subham, S.; Sumit, M.; Debajit, S. Luminescent lanthanide metal organic frameworks (LnMOFs): A versatile platform towards organomolecule sensing. Coord. Chem. Rev. 2022, 470, 214707. [Google Scholar] [CrossRef]
- Tu, T.N.; Nguyen, M.V.; Nguyen, H.L.; Brian, Y.; Kyle, E.C.; Selçuk, D. Designing bipyridine-functionalized zirconium metal–organic frameworks as a platform for clean energy and other emerging applications. Coord. Chem. Rev. 2018, 364, 33–50. [Google Scholar] [CrossRef]
- Du, X.Y.; Wu, G.; Dou, X.L.; Ding, Z.Y.; Xie, J. Recent advances of fluorescence MOF-based sensors for the freshness of aquatic products. Microchem. J. 2024, 203, 110901. [Google Scholar] [CrossRef]
- Peyman, Z.M.; Aurelia, L.; Liu, X.W.; Rocio, B.; Wang, S.D. Targeted classification of metal–organic frameworks in the Cambridge structural database (CSD). Chem. Sci. 2020, 11, 8373–8387. [Google Scholar] [CrossRef]
- Niu, Y.F.; Cui, L.T.; Han, J.; Zhao, X.L. Solvent-mediated secondary building units (SBUs) diversification in a series of MnII-based metal-organic frameworks (MOFs). J. Solid State Chem. 2016, 241, 18–25. [Google Scholar] [CrossRef]
- Xie, G.M.; Bai, X.Y.; Yu, F.; Yuang, Q.Y.; Wang, Z.J. Oxygen vacancy engineering in MOF-derived AuCu/ZnO bimetallic catalysts for methanol synthesis via CO2 hydrogenation. Catal. Today 2024, 434, 114702. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Zhang, S.; Gu, Y.H.; Wang, Z.; Wang, J.T.; Li, L.; Wang, Y.J.; Wang, H.; Qian, J.D. Catalytic systems for the direct synthesis of dimethyl carbonate from carbon dioxide and methanol containing dehydrating agent, a review. J. Fuel Chem. Technol. 2023, 51, 1593–1616. [Google Scholar] [CrossRef]
- Yuan, Y.X.; Liao, Q.L.; Zhao, T.X. Synthesis of UiO-66-NH2@PILs core-shell composites for CO2 conversion into cyclic carbonates via synergistic catalysis under solvent- and additive-free conditions. Colloids Surf. A Physicochem. Eng. Asp. 2025, 704, 135492. [Google Scholar] [CrossRef]
- Mutyala, S.; Jonnalagadda, M.; Ibrahim, S.M. Effect of modification of UiO-66 for CO2 adsorption and separation of CO2/CH4. J. Mol. Struct. 2020, 1227, 129506. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, D.; Chen, H.Y.; Qian, Y.; Xi, H.X.; Xia, Q.B. Enhancement effect of lithium-doping functionalization on methanol adsorption in copper-based metal-organic framework. Chem. Eng. Sci. 2015, 123, 1–10. [Google Scholar] [CrossRef]
- Plant, D.F.; Maurin, G.; Deroche, I.; Llewellyn, P.L. Investigation of CO2 adsorption in Faujasite systems: Grand canonical monte carlo and molecular dynamics simulations based on a new derived Na+–CO2 force field. Microporous Mesoporous Mater. 2007, 99, 70–78. [Google Scholar] [CrossRef]
- Aisha, A.; Naseem, I.; Tayyaba, N.; Majid, A.; Timothy, L. Easun Efficient One-Pot Synthesis of a Hexamethylenetetramine-Doped Cu-BDC Metal-Organic Framework with Enhanced CO2 Adsorption. Nanomaterials 2019, 9, 1063. [Google Scholar] [CrossRef]
- Aysu, Y.; Yuda, Y. A controlled synthesis strategy to enhance CO2 adsorption capacity of MIL-88B type MOF crystallites by the crucial role of narrow micropores. Ind. Eng. Chem. Res. 2019, 58, 14058–14072. [Google Scholar] [CrossRef]
- Rasha, A. A review on modified MOFs as CO2 adsorbents using mixed metals and functionalized linkers. Samarra J. Pure Appl. Sci. 2023, 5, 1–18. [Google Scholar] [CrossRef]
- Zhou, J.W.; Liu, M.; Chen, X.; Bai, S.Y.; Sun, J.H. Interfacial growth strategy for synthesizing Mg-MOF-74@clinoptilolites with hierarchical structures for enhancing adsorptive separation performance of CO2/CH4, CH4/N2 and CO2/N2. Surf. Interfaces 2024, 54, 105106. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Nguyenc, H.L.; Hanikel, N.; Li, K.K.-Y.; Zhou, Z.H.; Ma, T.; Yaghi, O.M. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air. Nat. Protoc. 2023, 18, 136–156. [Google Scholar] [CrossRef]
- Liu, L.J.; Zuo, L.Y.; Zhai, X.J.; Xiao, X.P.; Fan, H.T.; Li, B.; Wang, L.Y. A novel hexagonal prism of Zr-based MOF@ZnIn2S4 core−shell nanorod as an efficient photocatalyst for hydrogen evolution. Appl. Catal. B Environ. Energy 2025, 361, 124686. [Google Scholar] [CrossRef]
- Fang, X.X.; Zhu, Y.Z.; Dong, H.T.; Ma, N.; Dai, W. Ability evaluation of thiophenic sulfurs capture with a novel (MOF-818)-on-(Cu-BTC) composite in the presence of moisture. Microporous Mesoporous Mater. 2022, 333, 111756. [Google Scholar] [CrossRef]
- Ivan, V.G.; Vladimir, Y.G. High-throughput screening of Metal−Organic frameworks for helium recovery from natural gas. Microporous Mesoporous Mater. 2024, 368, 113021. [Google Scholar] [CrossRef]
- Aniruddha, D.; Nagaraj, A.; Amarajothi, D.; Shyam, B. A highly catalytically active Hf(IV) metal-organic framework for Knoevenagel condensation. Microporous Mesoporous Mater. 2019, 284, 459–467. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.J.; Liu, X.Y.; Hanna, S.L.; Wang, X.J.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Xing, X.F.; Wang, D.; Zhang, J.Z.; Chu, J.M.; Yu, C.C.; Wei, Z.T.; Hu, M.L.; Zhang, X.; Li, Z.X. Highly ordered hierarchically macroporous MIL-125 with high specific surface area for photocatalytic CO2 fixation. ACS Sustain. Chem. Eng. 2019, 8, 148–153. [Google Scholar] [CrossRef]
- Dipendu, S.; Deng, S.G. Hydrogen adsorption on metal-organic framework MOF-177. Tsinghua Sci. Technol. 2010, 15, 363–376. [Google Scholar] [CrossRef]
- Yoshio, H.; Katsuhei, K.; Akira, M.; Shin, S. Production of methane and ethylene in electrochemical reduction of carbon dioxide at copper electrode in aqueous hydrogencarbonate solution. Chem. Lett. 1986, 15, 897–898. [Google Scholar] [CrossRef]
- Yoshio, H.; Hidetoshi, W.; Toshio, T.; Osamu, K. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Yoshio, H.; Akira, M.; Ryutaro, T. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2309–2326. [Google Scholar] [CrossRef]
- Yang, J.F.; Bai, H.H.; Zhang, F.F.; Liu, J.Q.; Winarta, J.; Wang, Y.; Mu, B. Effects of activation temperature and densification on adsorption performance of MOF MIL-100(Cr). J. Chem. Eng. Data 2019, 64, 5814–5823. [Google Scholar] [CrossRef]
- Farhad, A.; Salman, A.; Hossein, M.; Mashallah, R.; Tejraj, M.A.; Mohammad, A. Impact of scale, activation solvents, and aged conditions on gas adsorption properties of UiO-66. J. Environ. Manag. 2020, 274, 111155. [Google Scholar] [CrossRef]
- Jia, B.Y.; Sun, J.H.; Bing, L.J.; Bai, S.Y. Nonionic surfactant-assisted strategy towards disorderly layered UiO-66-on-clinoptilolites heterostructure as efficient adsorbent for selective adsorption of CO2 and CH4. Mater. Lett. 2022, 328, 133147. [Google Scholar] [CrossRef]
- Yan, X.L.; Komarneni, S.; Zhang, Z.Q.; Yan, Z.F. Extremely enhanced CO2 uptake by HKUST-1 metal–organic framework via a simple chemical treatment. Microporous Mesoporous Mater. 2014, 183, 69–73. [Google Scholar] [CrossRef]
- Allmond, K.; Stone, J.; Harp, S.; Mujibur, K. Synthesis and electrospraying of nanoscale MOF (Metal Organic Framework) for high-performance CO2 adsorption membrane. Nanoscale Res. Lett. 2017, 12, 6. [Google Scholar] [CrossRef]
- Sandrine, B.; Philip, L.L.; Christian, S.; Franck, M.; Thierry, L.; Gérard, F. Different adsorption behaviors of methane and CO2 in the isotypic nanoporous metal terephthalates MIL-53 and MIL-47. J. Am. Chem. Soc. 2005, 127, 13519–13521. [Google Scholar] [CrossRef]
- Saptasree, B.; Debabrata, S.; Christos, D.M.; Karam, B.I.; Xie, H.M.; Wang, X.L.; Michael, L.B.; Nathaniel, M.B.; Vinayak, P.D.; Timur, I.; et al. Suitability of a diamine functionalized metal–organic framework for direct air capture. Chem. Sci. 2023, 14, 9380–9388. [Google Scholar] [CrossRef]
- Hussein, R.A.; Zana, H.R.; Liu, L.H.; Wang, S.B.; Liu, S.M. Striking CO2 capture and CO2/N2 separation by Mn/Al bimetallic MIL-53. Polyhedron 2021, 193, 114898. [Google Scholar] [CrossRef]
- Van, N.L.; Van, C.N.; Huu, T.N.; Hoai, D.T.; Thach, N.T.; Woo-Sik, K. Facile synthesis of bimetallic MIL-100(Fe, Al) for enhancing CO2 adsorption performance. Microporous Mesoporous Mater. 2023, 360, 112716. [Google Scholar] [CrossRef]
- Garzón-Tovar, L.; Carné-Sánchez, A.; Carbonell, C.; Imaz, I.; Maspoch, D. Optimised room temperature, water-based synthesis of CPO-27-M metal–organic frameworks with high space-time yields. J. Mater. Chem. A 2015, 3, 20819–20826. [Google Scholar] [CrossRef]
- Zhuang, X.L.; Zhang, S.T.; Tang, Y.J.; Yu, F.; Li, Z.M.; Pang, H. Recent progress of MOF/MXene-based composites: Synthesis, functionality and application. Coord. Chem. Rev. 2023, 490, 215208. [Google Scholar] [CrossRef]
- Yang, D.A.; Cho, H.Y.; Kim, J.; Yang, S.T.; Ahn, W.S. CO2 capture and conversion using Mg-MOF-74 prepared by a sonochemical method. Energy Environ. Sci. 2012, 5, 6465–6473. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Liang, L.; Zhang, X.; Xu, P.; Sun, J.M. Facile one-pot synthesis of Zn/Mg-MOF-74 with unsaturated coordination metal centers for efficient CO2 adsorption and conversion to cyclic carbonates. ACS Appl. Mater. Interfaces 2021, 13, 61334–61345. [Google Scholar] [CrossRef]
- Abhijit, K.A.; Lin, K.S. Improving CO2 adsorption capacities and CO2/N2 separation efficiencies of MOF-74(Ni, Co) by doping palladium-containing activated carbon. Chem. Eng. J. 2016, 284, 1348–1360. [Google Scholar] [CrossRef]
- Deng, H.X.; Christian, J.D.; Hiroyasu, F.; Ricardo, B.F.; John, T.; Carolyn, B.K.; Wang, B.; Omar, M.Y. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.M.; Omar, M.Y. Metal−organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Hiroyasu, F.; Nakeun, K.; Yong, B.G.; Naoki, A.; Sang, B.C.; Eunwoo, C. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Farrando-Pérez, J.; Martinez-Navarrete, G.; Gandara-Loe, J.; Reljic, S.; Garcia-Ripoll, A.; Fernandez, E.; Silvestre-Albero, J. Controlling the adsorption and release of ocular drugs in metal–organic frameworks: Effect of polar functional groups. Inorg. Chem. 2022, 61, 18861–18872. [Google Scholar] [CrossRef]
- Zhou, L.L.; Niu, Z.D.; Jin, X.; Tang, L.H.; Zhu, L.P. Effect of lithium doping on the structures and CO2 adsorption properties of metal-organic frameworks HKUST-1. Chem. Sel. 2018, 3, 12865–12870. [Google Scholar] [CrossRef]
- Cui, P.; Li, J.J.; Dong, J.; Zhao, B. Modulating CO2 adsorption in metal–organic frameworks via metal-Ion doping. Inorg. Chem. 2018, 57, 6135–6141. [Google Scholar] [CrossRef] [PubMed]
- Christian, S.D.; Liu, Y.Z.; Kyle, E.C.; Omar, M.Y. The role of reticular chemistry in the design of CO2 reduction catalysts. Nat. Mater. 2018, 17, 301–307. [Google Scholar] [CrossRef]
- Fu, Y.G.; Sun, D.G.; Chen, Y.J.; Huang, R.K.; Ding, Z.X.; Fu, X.Z.; Li, Z.H. An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Nai, J.W.; Zhang, J.T.; Xiong, W. Construction of single-crystalline prussian blue analog hollow nanostructures with tailorable topologies. Chem 2018, 4, 1967–1982. [Google Scholar] [CrossRef]
- Wang, S.B.; Yao, W.S.; Lin, J.L.; Ding, Z.X.; Wang, X.C. Cobalt imidazolate metal–organic frameworks photosplit CO2 under mild reaction conditions. Angew. Chem. Int. Ed. 2014, 53, 1034–1038. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Li, S.M.; Huang, N.Y.; Li, X.R. Recent advances in metal–organic framework-derived materials for electrocatalytic and photocatalytic CO2 reduction. Coord. Chem. Rev. 2024, 510, 215858. [Google Scholar] [CrossRef]
- Wang, P.; Ba, X.H.; Zhang, X.W.; Gao, H.Y.; Han, M.Y.; Zhao, Z.Y.; Chen, X.; Wang, L.M.; Diao, X.M.; Wang, G. Direct Z-scheme heterojunction of PCN-222/CsPbBr3 for boosting photocatalytic CO2 reduction to HCOOH. Chem. Eng. J. 2023, 457, 141248. [Google Scholar] [CrossRef]
- Fu, Y.H.; Yang, H.; Du, R.F.; Tu, G.M.; Xu, C.H.; Zhang, F.M.; Fan, M.H.; Zhu, W.D. Enhanced photocatalytic CO2 reduction over Co-doped NH2-MIL-125(Ti) under visible light. RSC Adv. 2017, 7, 42819–42825. [Google Scholar] [CrossRef]
- Lin, H.; Ma, M.; Qi, H.; Wang, X.; Xing, Z.; Alowasheeir, A.; Tang, H.P.; Jun, C.J.; Yamauchi, Y.; Liu, S.D. 3D-Printed photocatalysts for revolutionizing catalytic conversion of solar to chemical energy. Prog. Mater. Sci. 2025, 151, 101427. [Google Scholar] [CrossRef]
- Xu, H.J.; Cao, J.; Shan, C.F.; Wang, B.K.; Xi, P.X.; Liu, W.S.; Tang, Y. MOF-derived hollow CoS decorated with CeOx nanoparticles for boosting oxygen evolution reaction electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 8654–8658. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, C.C.; Li, D.D.; Guo, W.J.; Zhong, D.Z.; Zhao, Q. FeNi-based bimetallic MIL-101 directly applicable as an efficient electrocatalyst for oxygen evolution reaction. Microporous Mesoporous Mater. 2019, 286, 92–97. [Google Scholar] [CrossRef]
- Gaikwad, S.; Kim, Y.; Gaikwad, R.; Han, S. Enhanced CO2 capture capacity of amine-functionalized MOF-177 metal organic framework. J. Environ. Chem. Eng. 2021, 9, 105523. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hu, Y.; Ma, L.B.; Zhu, G.Y.; Wang, Y.R.; Xue, X.L.; Chen, R.P.; Yang, S.Y.; Jin, Z. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Zou, R.Y.; Li, P.Z.; Zeng, Y.F.; Liu, J.; Zhao, R.; Duan, H.; Luo, Z.; Wang, J.G.; Zou, R.Q.; Zhao, Y.L. Bimetallic metal-organic frameworks: Probing the lewis acid site for CO2 conversion. Small 2016, 12, 2334–2343. [Google Scholar] [CrossRef]
- Sun, M.Y.; Zhao, B.H.; Chen, F.P.; Liu, C.B.; Lu, S.Y.; Yu, Y.F.; Zhang, B. Thermally-assisted photocatalytic CO2 reduction to fuels. Chem. Eng. J. 2021, 408, 127280. [Google Scholar] [CrossRef]
- Rui, Y.; Huang, Q.; Sha, X.L.; Gao, B.B.; Peng, J. Regulation of bimetallic coordination centers in MOF catalyst for electrochemical CO2 reduction to formate. Int. J. Mol. Sci. 2023, 24, 18. [Google Scholar] [CrossRef]
- Naoki, O.; Sayaka, U. Metal–oxo-cluster-based crystals as solid catalysts. Chem Catal. 2023, 37, 100607. [Google Scholar] [CrossRef]
- Di, Z.; Qi, Y.; Yu, X.; Hu, F. The progress of metal-organic framework for boosting CO2 conversion. Catalysts 2022, 12, 1582. [Google Scholar] [CrossRef]
- Xu, N.; Li, J.M.; Wang, Y.J.; Akram, M.Y.; Hu, B.; Dong, H.J. Adjustment of charge transfer behavior for layered photocatalysts through fabricating face-to-face 2D/2D S-scheme heterojunction toward efficient carbon dioxide reduction. Sep. Purif. Technol. 2025, 354, 129518. [Google Scholar] [CrossRef]
- Chae, H.K.; Diana, Y.S.; Kim, J.; Go, Y.B.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Q.; Xu, H.T.; Su, Y.Q.; Xu, Z.L.; Wang, K.F.; Wang, W.Z. Noble metal (Pt, Au@Pd) nanoparticles supported on metal organic framework (MOF-74) nanoshuttles as high-selectivity CO2 conversion catalysts. J. Catal. 2019, 370, 70–78. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P.; Li, G.; Webley, P.A. Effect of flue gas impurities on CO2 capture performance from flue gas at coal-fired power stations by vacuum swing adsorption. Energy Procedia 2009, 1, 1115–1122. [Google Scholar] [CrossRef]
- Noureddine, J.; Ali, B.A.; Imed, R.F. Machine learning for MOF catalyst design: Current status and future perspectives. Artif. Intell. Rev. 2023, 56, 3853–3876. [Google Scholar] [CrossRef]
- Anantharaj, S. Ru-tweaking of non-precious materials: The tale of a strategy that ensures both cost and energy efficiency in electrocatalytic water splitting. J. Mater. Chem. A 2021, 9, 6710–6731. [Google Scholar] [CrossRef]
- Chen, Y.T.; Tang, S.K. Solvothermal synthesis of porous hydrangea-like zeolitic imidazole framework-8 (ZIF-8) crystals. J. Solid State Chem. 2019, 276, 68–74. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).