The Study of Regioselective Acylation of Geniposide by Using Whole-Cell Biocatalysts in Organic Solvents
Abstract
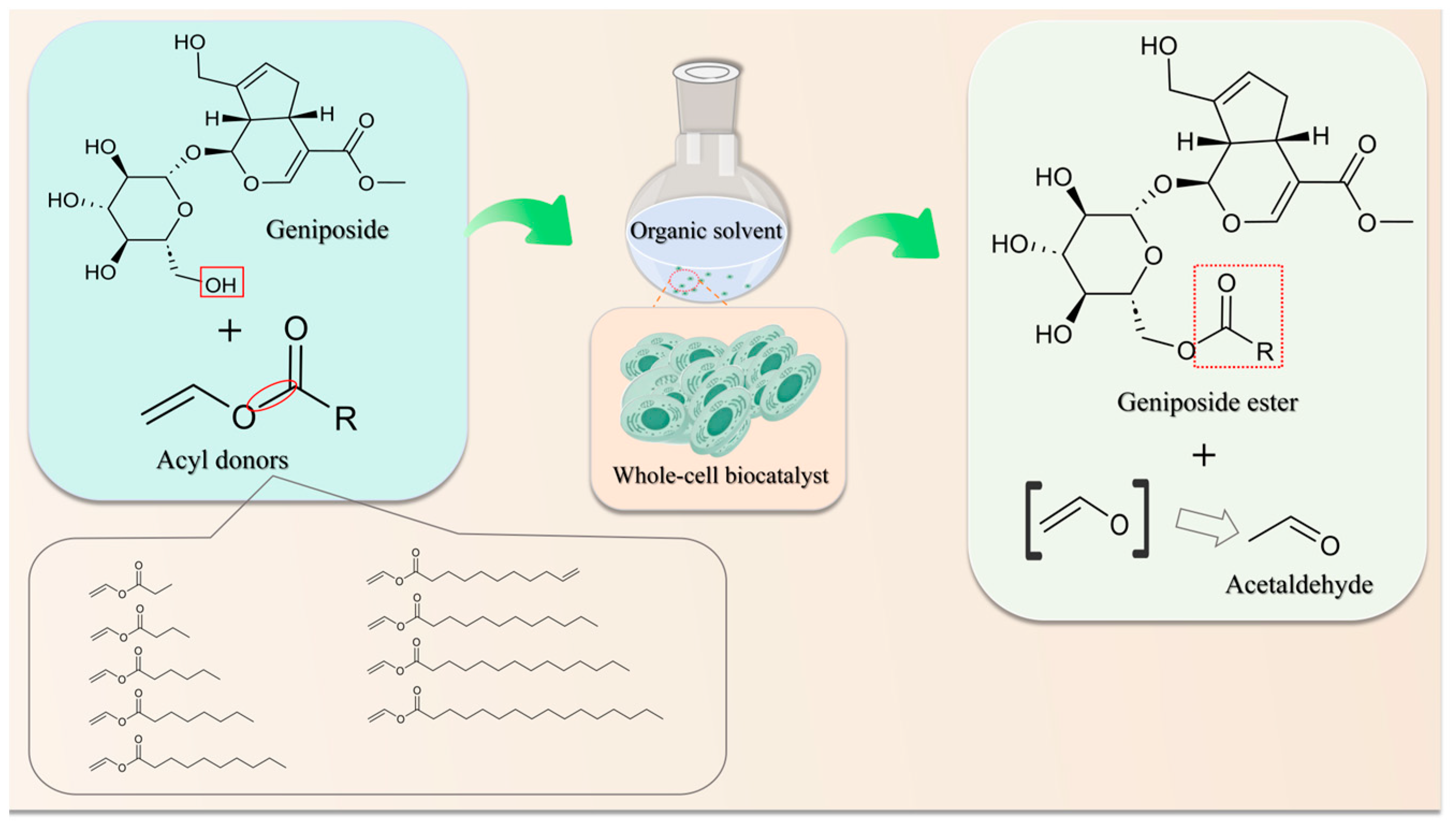
1. Introduction
2. Results and Discussion
2.1. Catalytic Behaviors of Various Microbial Whole Cells in the Acylation of Geniposide
2.2. Effects of Organic Solvents on Decanoylation of Geniposide Catalyzed by A. oryzae Whole Cell
2.3. Influence of Several Crucial Parameters on the Decanoylation of Geniposide
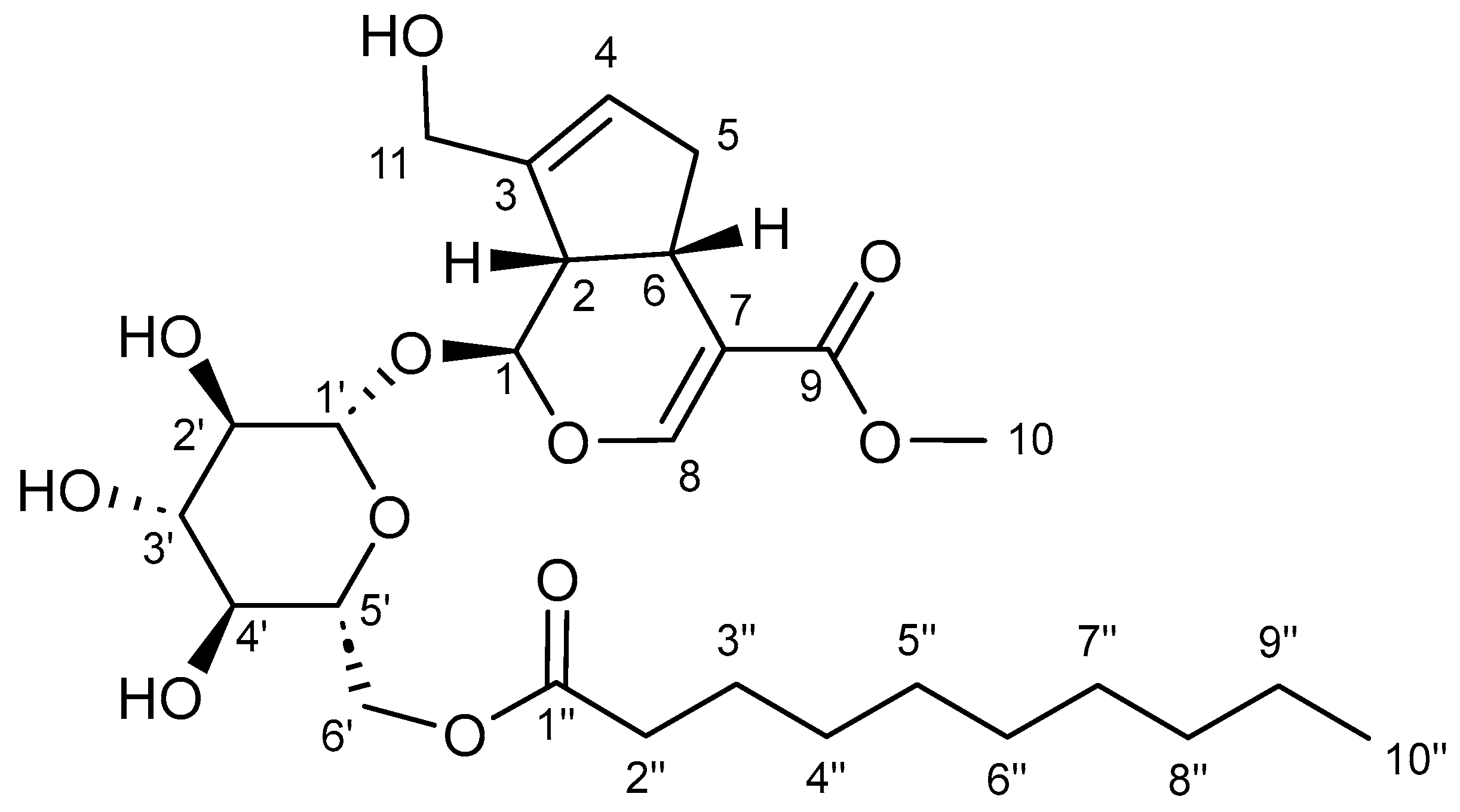
2.4. Product Structure Characterization
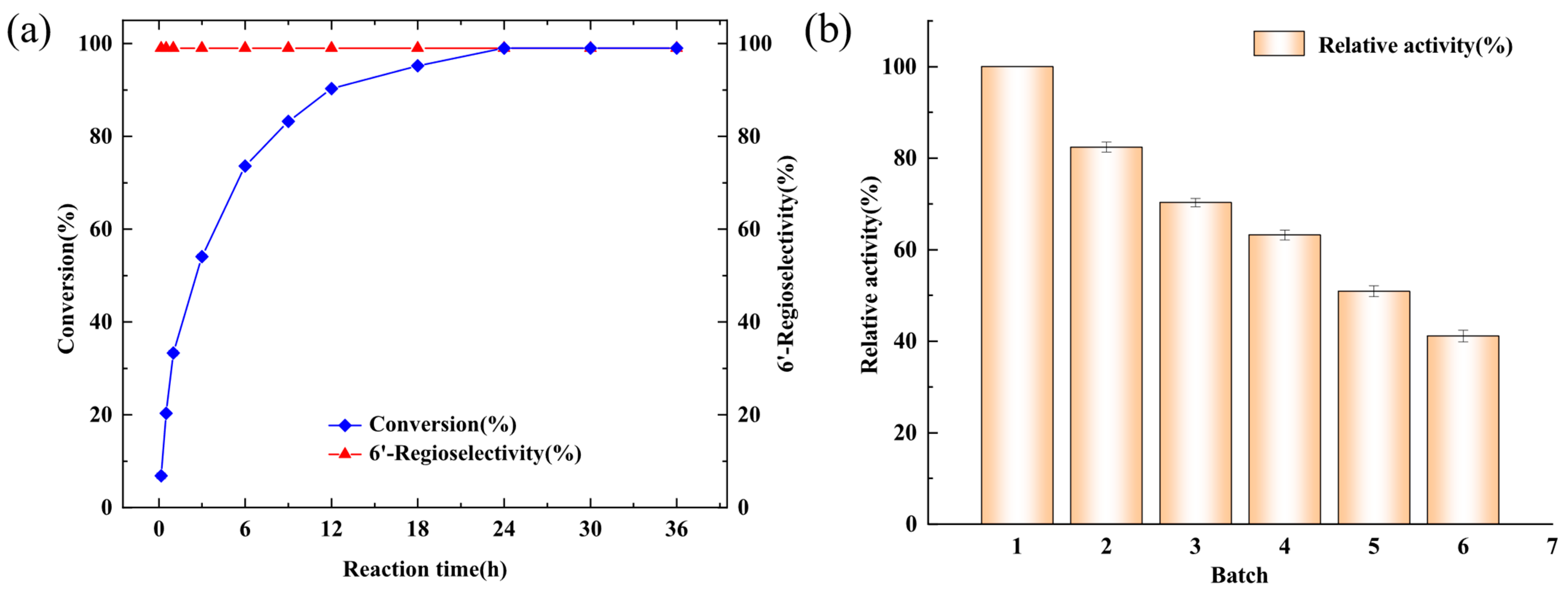
2.5. Reaction Process of Geniposide Decanoylation and Biocatalyst Reusability of Whole Cells
2.6. Regioselective Acylation of Geniposide with Various Aliphatic Acyl Donors
3. Materials and Methods
3.1. Microorganisms and Materials
3.2. Preparation of Whole-Cell Catalysts
3.3. General Procedure for Acylation of Geniposide by Whole Cells
3.4. Biocatalyst Reusability of the Whole-Cell
3.5. HPLC Analysis
3.6. Separation and Structure Identification of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Selka, A.; Abidli, A.; Schiavo, L.; Jeanmart, L.; Hanquet, G.; Lubell, W.D. Recent advances in sustainable total synthesis and chiral pool strategies with emphasis on (−)-sclareol in natural products synthesis. Eur. J. Org. Chem. 2025, 28, e202400983. [Google Scholar] [CrossRef]
- Li, B.T.; Zhao, Y.F.; Zhou, X.M.; Cheng, P.; Yan, X.B.; Zou, T.Y. Geniposide improves depression by promoting the expression of synapse-related proteins through the creb1/six3os1 axis. Gene 2023, 877, 147564. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Taniguchi, M. Geniposide prevents tumor growth by inhibiting colonic interleukin-1beta and monocyte chemoattractant protein-1 via down-regulated expression of cyclooxygenase-2 and thymocyte selection-associated high mobility box proteins tox/tox2 in azoxymethane/dextran sulfate sodium-treated mice. Int. Immunopharmacol. 2023, 118, 110077. [Google Scholar] [CrossRef]
- Li, H.S.; Xi, Y.F.; Xin, X.; Feng, Q.; Hu, Y.Y. Geniposide plus chlorogenic acid reverses non-alcoholic steatohepatitis via regulation of gut microbiota and bile acid signaling in a mouse model in vivo. Front. Pharmacol. 2023, 14, 1148737. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, X.J.; Fan, J.Y.; Zhang, L.; Ji, H.; Xue, J.; Zhang, C.; Chen, R.; Zhao, J.; Chen, J.M.; et al. Geniposide protected against cerebral ischemic injury through the anti-inflammatory effect via the nf-κb signaling pathway. Transl. Neurosci. 2023, 14, 20220273. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, C.; Li, L.J. Geniposide improves bleomycin-induced pulmonary fibrosis by inhibiting nlrp3 inflammasome activation and modulating metabolism. J. Funct. Foods 2023, 104, 105503. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.Q.; Geng, Z.; Bao, L.; Gao, S.R.; Sun, J.; Liu, X.; Yang, X.W.; Zhao, R.H.; Li, S.R.; et al. Geniposide attenuates influenza virus-induced pneumonia by regulating inflammatory cytokines production. Evidences to elucidate the followed pathway. Phytomedicine 2024, 135, 156018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.P.; Li, S.M.; Wang, S.Y.; Ho, C.T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2016, 25, 43–61. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, R.Q.; Rahman, K.; Cao, Z.X.; Zhang, H.; Peng, C. Diverse pharmacological activities and potential medicinal benefits of geniposide. Evid.-Based Complement. Altern. Med. 2019, 2019, 4925682. [Google Scholar] [CrossRef]
- Cai, L.; Mu, Y.R.; Liu, M.M.; Tang, W.J.; Li, R. Antidepressant-like effects of penta-acetyl geniposide in chronic unpredictable mild stress-induced depression rat model: Involvement of inhibiting neuroinflammation in prefrontal cortex and regulating hypothalamic-pituitaryadrenal axis. Int. Immunopharmacol. 2020, 80, 106182. [Google Scholar] [CrossRef]
- Chen, J.S.; Wang, M.X.; Wang, M.M.; Zhang, Y.K.; Guo, X.; Chen, Y.Y.; Zhang, M.Q.; Sun, J.Y.; Liu, Y.F.; Liu, C. Synthesis and biological evaluation of geniposide derivatives as inhibitors of hyperuricemia, inflammatory and fibrosis. Eur. J. Med. Chem. 2022, 237, 114379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Y.J.; Liu, Y.N.; Disasa, D.; Akira, M.; Xiang, L.; Qi, J.H. A new geniposidic acid derivative exerts antiaging effects through antioxidative stress and autophagy induction. Antioxidants 2021, 10, 987. [Google Scholar] [CrossRef]
- Yang, W.M.; Ni, F.; Yao, Z.; Lu, Y.X.; Sun, Y.; Zhu, B.W.; Yuan, H.; Xiong, Q. Enzymatic synthesis and anti-inflammatory activity evaluation of 6′-o-lauroyl genipin. Chin. J. Bioprocess Eng. 2021, 19, 199–206. [Google Scholar]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.L.; Wu, T.T.; Xu, N.N.; Zhao, X.J.; Wang, Z.Y.; Luo, H.Z.; Bilal, M.; Nie, Z.K.; Song, Y.Y. Improving whole-cell biocatalysis for helicid benzoylation by the addition of ionic liquids. Biochem. Eng. J. 2020, 161, 107695. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, W.W.; Ding, B.; Guo, M.; Liang, M.; Pang, H.; Wei, Y.T.; Huang, R.B.; Du, L.Q. Highly efficient bioconversion of icariin to icaritin by whole-cell catalysis. Microb. Cell Factories 2023, 22, 64. [Google Scholar] [CrossRef]
- Smith, M.L.; Gao, H.; Prabhu, P.; Bugada, L.F.; Roth, C.; Mutukuri, D.; Yee, C.; Lee, L.; Ziff, R.M.; Lee, J.-K.; et al. Elucidating structure-performance relationships in whole-cell cooperative enzyme catalysis. Nat. Catal. 2019, 2, 809–819. [Google Scholar] [CrossRef]
- Xin, X.; Li, X.F.; Xiao, X.L.; Tang, Y.Q.; Zhao, G.L. Facile and efficient acylation of bioflavonoids using whole-cell biocatalysts in organic solvents. ACS Sustain. Chem. Eng. 2017, 5, 10662–10672. [Google Scholar] [CrossRef]
- Xu, H.X.; Li, X.F.; Xin, X.; Yuan, K.; Wu, H.; Zhao, G.L. Whole-cell-catalyzed synthesis of phenolic glycoside esters, and their antioxidant and antimelanogenic properties. Ind. Eng. Chem. Res. 2020, 59, 16591–16602. [Google Scholar] [CrossRef]
- Li, X.F.; Xu, H.X.; Zhao, G.L.; Wu, H.; Yu, Y.G.; Lai, F.R.; Xiao, X.L. Highly efficient synthesis of arbutin esters catalyzed by whole cells of candida parapsilosis. RSC Adv. 2018, 8, 10081–10088. [Google Scholar] [CrossRef]
- Wu, T.T.; Zhao, X.J.; Yang, R.L.; Bilal, M.; Wang, Z.Y.; Luo, H.Z.; Xu, N.N.; Nie, Z.K. Catalytic performance of a robust whole-cell biocatalyst in the regioselective synthesis of helicid esters under optimized processing conditions. Catal. Lett. 2020, 150, 1841–1848. [Google Scholar] [CrossRef]
- Rollig, R.; Plikat, C.; Ansorge-Schumacher, M.B. Efficient and selective carboligation with whole-cell biocatalysts in pickering emulsion. Angew. Chem. Int. Ed. 2019, 58, 12960–12963. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.L.; Wang, Y.; Zhao, X.J.; Tong, Z.; Zhu, Q.L.; He, X.X.; Wang, Z.Y.; Luo, H.Z.; Fang, F. A facile and efficient synthesis approach of salidroside esters by whole-cell biocatalysts in organic solvents. Front. Bioeng. Biotechnol. 2022, 10, 1051117. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Zhang, M.; Li, X.; Xin, X.; Lei, F.; Lai, X.; Zhao, G.; Wu, H. Highly efficient whole-cell biosynthesis and cytotoxicity of esculin esters. J. Biotechnol. 2021, 337, 46–56. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Li, N.; Zong, M.H. A simple procedure for the synthesis of potential 6-azauridine prodrugs by thermomyces lanuginosus lipase. J. Mol. Catal. B Enzym. 2009, 59, 212–219. [Google Scholar] [CrossRef]
- Xu, X. Production of specific-structured triacylglycerols by lipase-catalyzed reactions: A review. Eur. J. Lipid Sci. Technol. 2000, 102, 287–303. [Google Scholar] [CrossRef]

| Strains | V0 (mmol/L·h) | Conversion Rate (%) | 6′-Regioselectivity (%) |
|---|---|---|---|
| Rhizopus oryzae GDM 3.406 | Not Detected | Not Detected | Not Detected |
| Aspergillus oryzae GDM 3.446 | 3.3 ± 0.2 | 86.3 ± 0.6 | >99 |
| Pseudomonas aeruginosa GDM 1.443 | 1.5 ± 0.0 | 56.4 ± 0.6 | >99 |
| Pseudomonas stutzeri GDM 1.446 | Not Detected | Not Detected | Not Detected |
| Pseudomonas fluorescens GDM 1.209 | Not Detected | Not Detected | Not Detected |
| Solvents | V0 (mmol/L/h) | Conversion Rate (%) | 6′-Regioselectivity (%) |
|---|---|---|---|
| Acetone | 1.8 ± 0.8 | 63.4 ± 0.6 | >99 |
| Acetonitrile | 3.3 ± 0.2 | 86.3 ± 0.6 | >99 |
| tert-Butanol | Not Detected | 6.1 ± 0.7 | >99 |
| Tetrahydrofuran | 1.5 ± 0.3 | 13. 3 ± 0.9 | >99 |
| Dimethylformamide (DMF) | Not Detected | Not Detected | Not Detected |
| 2-Methyltetrahydrofuran | 1.7 ± 0.4 | 26.2 ± 1.1 | >99 |
| Dimethyl sulfoxide (DMSO) | Not Detected | Not Detected | Not Detected |
| Carbon Numbers | Geniposide (δC) | Geniposide 6′-O-Decanoate (δC) |
|---|---|---|
| Base moiety | ||
| 1 | 95.71 | 96.29 |
| 2 | 45.85 | 45.58 |
| 3 | 144.05 | 144.33 |
| 4 | 125.42 | 125.59 |
| 5 | 37.94 | 38.16 |
| 6 | 34.40 | 34.69 |
| 7 | 110.89 | 110.95 |
| 8 | 151.50 | 151.47 |
| 9 | 166.84 | 166.78 |
| 10 | 50.94 | 50.92 |
| 11 | 59.28 | 59.32 |
| Sugar moiety | ||
| 1′ | 98.58 | 98.87 |
| 2′ | 73.26 | 73.16 |
| 3′ | 77.20 | 76.38 |
| 4′ | 69.96 | 70.20 |
| 5′ | 76.60 | 73.92 |
| 6′ | 60.95 | 63.27 |
| Acyl moiety | ||
| 1″ | 172.64 | |
| 2″ | 33.49 | |
| 3″ | 22.09 | |
| 4″ | 24.43 | |
| 5″ | 28.47 | |
| 6″ | 28.85 | |
| 7″ | 28.65 | |
| 8″ | 31.28 | |
| 9″ | 13.86 | |
| 10″ | 9.88 | |
| Entry | Acyl Donor | V0 (mmol/L·h) | Time (h) | Conversion (%) | 6′-Regioselectivity (%) |
|---|---|---|---|---|---|
| 1 | Vinyl propionate (C3) | 3.4 ± 0.4 | 24 | 88.4 ± 0.9 | >99 |
| 2 | Vinyl butyrate (C4) | 4.2 ± 0.6 | 24 | 87.8 ± 0.4 | >99 |
| 3 | Vinyl hexanoate (C6) | 5.7 ± 0.2 | 24 | 93.2 ± 0.5 | >99 |
| 4 | Vinyl caprylate (C8) | 5.9 ± 0.3 | 24 | 99 | >99 |
| 5 | Vinyl decanoate (C10) | 6.1 ± 0.3 | 24 | 99 | >99 |
| 6 | Vinyl 10-undecenoate (C11) | 6.7 ± 0.6 | 24 | 99 | >99 |
| 7 | Vinyl laurate (C12) | 6.2 ± 0.7 | 24 | 99 | >99 |
| 8 | Vinyl myristate (C14) | 5.5 ± 0.2 | 24 | 99 | >99 |
| 9 | Vinyl palmitate (C16) | 4.2 ± 0.3 | 24 | 97.3 ± 0.2 | >99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Huang, Z.; Zhao, X.; Luo, H.; Tong, Y.; Li, X.; Zhu, C.; Wang, Y.; Sun, Y. The Study of Regioselective Acylation of Geniposide by Using Whole-Cell Biocatalysts in Organic Solvents. Catalysts 2025, 15, 428. https://doi.org/10.3390/catal15050428
Yang R, Huang Z, Zhao X, Luo H, Tong Y, Li X, Zhu C, Wang Y, Sun Y. The Study of Regioselective Acylation of Geniposide by Using Whole-Cell Biocatalysts in Organic Solvents. Catalysts. 2025; 15(5):428. https://doi.org/10.3390/catal15050428
Chicago/Turabian StyleYang, Rongling, Ziling Huang, Xiangjie Zhao, Hongzhen Luo, Yuli Tong, Xiaoyan Li, Chun Zhu, Yu Wang, and Yang Sun. 2025. "The Study of Regioselective Acylation of Geniposide by Using Whole-Cell Biocatalysts in Organic Solvents" Catalysts 15, no. 5: 428. https://doi.org/10.3390/catal15050428
APA StyleYang, R., Huang, Z., Zhao, X., Luo, H., Tong, Y., Li, X., Zhu, C., Wang, Y., & Sun, Y. (2025). The Study of Regioselective Acylation of Geniposide by Using Whole-Cell Biocatalysts in Organic Solvents. Catalysts, 15(5), 428. https://doi.org/10.3390/catal15050428






