Fe or Ni Catalytic Hydrothermal Depolymerization with Ethanol for Efficient Anaerobic Digestion of Corn Stover
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solid Fraction Transformation Analysis
2.1.1. Chemical Composition Changes
2.1.2. Structural Modification
FTIR
XRD
Surface Lignin Area and Hydrophobicity
2.1.3. Accessibility Improvement
2.2. Liquid Fraction Characteristics
2.2.1. RS and pH
2.2.2. VFAs
2.2.3. TPC
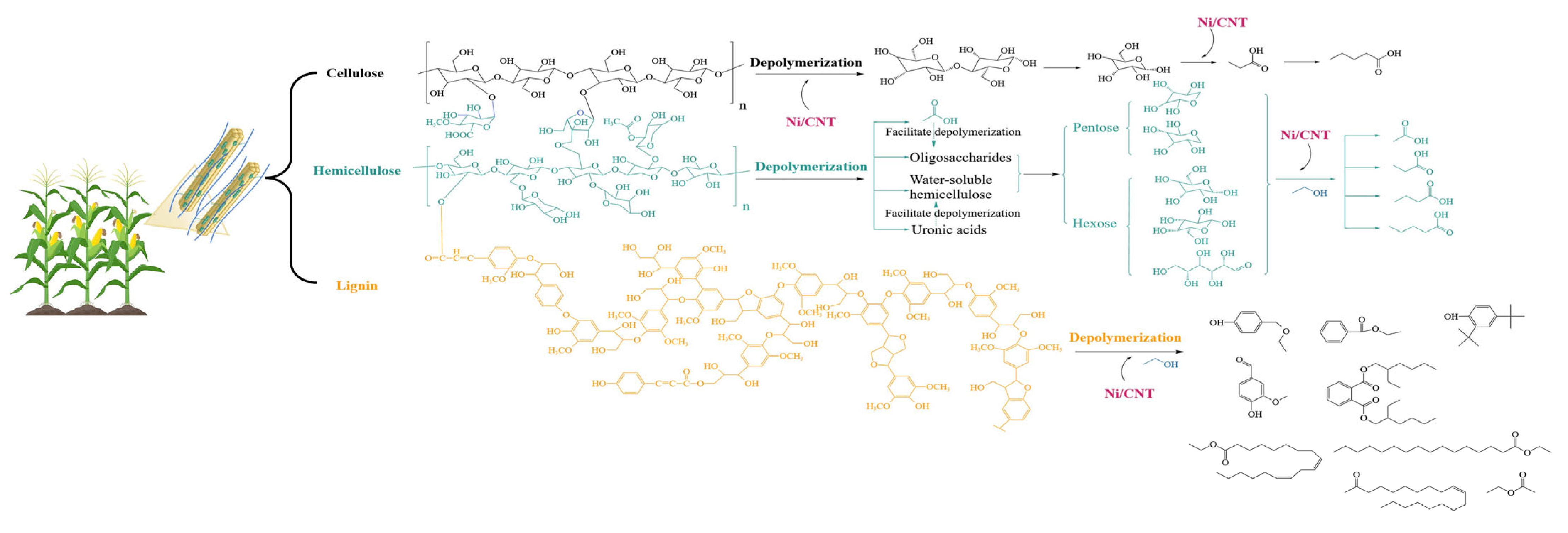
2.3. Possible Depolymerization Conversion Pathways
2.4. AD Performances
2.4.1. Daily Biogas Production
2.4.2. Biomethane Yield
2.4.3. Substrate Conversion
3. Materials and Methods
3.1. Materials
3.2. DC Methods
3.3. Batch AD Experiment
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | corn stover |
| AD | anaerobic digestion |
| DC | depolymerization with catalyst |
| BY | biomethane yield |
| CrI | crystallinity index |
| RS | reducing sugar |
| VFAs | volatile fatty acids |
| TPC | total phenolic content |
| DC-E | depolymerization with catalyst-ethanol |
| HP | hydrothermal depolymerization |
| TS | total solids |
| VS | volatile solids |
| TC | Total carbon |
| TN | total nitrogen |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Xiang, C.; Tian, D.; Hu, J.; Huang, M.; Shen, F.; Zhang, Y.; Yang, G.; Zeng, Y.; Deng, S. Why can hydrothermally pretreating lignocellulose in low severities improve anaerobic digestion performances? Sci. Total Environ. 2021, 752, 141929. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Zhang, L.; Zuo, X.; Li, X.; Yuan, H. Substance bioconversion, hydrolases activity, and metagenomic analysis to unravel the enhanced biomethanation of corn stover with urea-hydrothermal pretreatment. J. Environ. Manag. 2023, 333, 117466. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, C.; Yuan, H.; Li, X. Enhanced hydrothermal depolymerization with Fe/Ni Loaded C catalysts for improving anaerobic digestion performance of corn stover. Energies 2025, 18, 192–204. [Google Scholar] [CrossRef]
- Du, J.; Qian, Y.; Xi, Y.; Lü, X. Hydrothermal and alkaline thermal pretreatment at mild temperature in solid state for physicochemical properties and biogas production from anaerobic digestion of rice straw. Renew. Energ. 2019, 139, 261–267. [Google Scholar] [CrossRef]
- Jindal, M.; Uniyal, P.; Thallada, B. Reductive catalytic fractionation as a novel pretreatment/lignin-first approach for lignocellulosic biomass valorization: A review. Bioresour. Technol. 2023, 385, 129396. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-T.; Li, Z.-K.; Yan, H.-L.; Lei, Z.-P.; Yan, J.-C.; Ren, S.-B.; Wang, Z.-C.; Kang, S.-G.; Shui, H.-F. Catalytic hydrogenolysis of lignin and model compounds over highly dispersed Ni-Ru/Al2O3 without additional H2. Fuel 2022, 326, 125027. [Google Scholar] [CrossRef]
- Klein, I.; Saha, B.; Abu-Omar, M.M. Lignin depolymerization over Ni/C catalyst in methanol, a continuation: Effect of substrate and catalyst loading. Catal. Sci. Technol. 2015, 5, 3242–3245. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Zou, W.; Lv, Y.; Liu, Y.; Chen, L. Selective hydrodeoxygenation of lignin and its derivatives without initial reaction pressure using MOF-derived carbon-supported nickel composites. ACS Sustain. Chem. Eng. 2022, 10, 5430–5440. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, Y.; Yang, S.; Zhuang, K.; Wang, J. Staged biorefinery of Moso bamboo by integrating polysaccharide hydrolysis and lignin reductive catalytic fractionation (RCF) for the sequential production of sugars and aromatics. Ind. Crops Prod. 2021, 164, 113358. [Google Scholar] [CrossRef]
- Huang, X.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Catalytic depolymerization of lignin in supercritical ethanol. ChemSusChem 2014, 7, 2276–2288. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Yan, M.; Ye, J.; Wang, K.; Jiang, J. Green production of lignocellulose nanofibrils by FeCl3-catalyzed ethanol treatment. Int. J. Biol. Macromol. 2023, 224, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Li, H.; Xiao, L.-P.; Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, Z.; Wu, Z.; Wang, P.; Xiao, F.-S. Basic carrier promoted Pt-catalyzed hydrogenolysis of alkaline lignin. Catal. Today 2021, 365, 193–198. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bosch, S.; Vangeel, T.; Ennaert, T.; Koelewijn, S.-F.; Van den Bossche, G.; Courtin, C.M.; Schutyser, W.; Sels, B.F. Synergetic effects of alcohol/water mixing on the catalytic reductive fractionation of poplar wood. ACS Sustain. Chem. Eng. 2016, 4, 6894–6904. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, W.; Li, L.; Huang, M.; Ma, C.; He, Y.-C. Enhancing enzymatic hydrolysis of waste sunflower straw by clean hydrothermal pretreatment. Bioresour. Technol. 2023, 383, 129236. [Google Scholar] [CrossRef]
- Guan, R.; Li, X.; Wachemo, A.C.; Yuan, H.; Liu, Y.; Zou, D.; Zuo, X.; Gu, J. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 637–638, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.; Yuan, H.; Yan, B.; Yang, G.; Lu, Y.; Li, J.; Zuo, X. Enhancement of biomethane production and decomposition of physicochemical structure of corn straw by combined freezing-thawing and potassium hydroxide pretreatment. Energy 2023, 268, 126633. [Google Scholar] [CrossRef]
- Wu, X.; Fan, Z.; Mwansa, S.; Huang, C.; Yong, Q. Use of hydrogen peroxide to prime the autohydrolysis and enzymatic hydrolysis efficiency of wheat straw pulp residues. Fuel 2023, 346, 128283. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, X.; Resende, F.L.P.; Zhou, J.; Wang, M.; Dichiara, A.B. Hydropyrolysis of residual Camellia sinensis and Its cellulose and lignin fractions over nickel nanoparticles confined inside carbon nanotube microreactors at atmospheric pressure. ACS Sustain. Chem. Eng. 2021, 9, 10827–10836. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, N.; Chen, N.; Liu, T.; Feng, C. Study on the remediation of groundwater nitrate pollution by pretreated wheat straw and woodchips. Environ. Res. 2024, 263, 120226. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Zuo, X.; Yan, B.; Li, X.; Yuan, H. Depolymerization of corn stover by urea-hydrothermal pretreatment for efficient biomethane production and microbial community analysis of anaerobic digestion. J. Clean. Prod. 2022, 380, 134978. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, H.; den Boer, E.; Wu, W.; Wang, Q.; Gao, M.; Vo, D.-V.N.; Guo, M.; Xia, C. Effect of microwave/hydrothermal combined ionic liquid pretreatment on straw: Rumen anaerobic fermentation and enzyme hydrolysis. Environ. Res. 2022, 205, 112453. [Google Scholar] [CrossRef]
- Qu, H.; Hu, W.; Li, X.; Xu, R.; Han, X.; Li, J.; Lu, Y.; Ye, Y.; Wang, C.; Wang, Z.; et al. Efficient hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Ni-C3N4 catalysts with ultra-low Ni loading. Chin. J. Catal. 2024, 60, 253–261. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, C.; Shen, B.; Ling, Z.; Huang, C.; Li, X.; Lai, C.; Yong, Q. Comparative study on enzymatic digestibility of acid-pretreated poplar and larch based on a comprehensive analysis of the lignin-derived recalcitrance. Bioresour. Technol. 2021, 319, 124225. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, C.; Lin, W.; Bian, B.; Lai, C.; Ling, Z.; Yong, Q. A structure-activity understanding of the interaction between lignin and various cellulase domains. Bioresour. Technol. 2022, 351, 127042. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, W.; Ma, C.; He, Y.-C. Efficient co-production of reducing sugars and xylooligosaccharides via clean hydrothermal pretreatment of rape straw. Bioresour. Technol. 2023, 388, 129727. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, D.; Tang, W.; Ma, C.; He, Y.-C. Improved enzymatic saccharification of bulrush via an efficient combination pretreatment. Bioresour. Technol. 2023, 385, 129369. [Google Scholar] [CrossRef]
- Ding, C.; Hu, X.; Sun, W.; Hailili, R.; Liao, F.; Shu, C.; Huang, J.; Huang, L.; Wang, N. Interface of Ni-MgCr2O4 spinel promotes the autothermal reforming of acetic acid through accelerated oxidation of carbon-containing intermediate species. ACS Catal. 2023, 13, 4560–4574. [Google Scholar] [CrossRef]
- Xu, C.; Wu, B.; Zhao, P.; Wang, Y.; Yang, H.; Mi, Y.; Zhou, Y.; Ma, T.; Zhang, S.; Wu, L.; et al. Biological saccharification coupled with anaerobic digestion using corn straw for sustainable methane production. Bioresour. Technol. 2023, 367, 128277. [Google Scholar] [CrossRef]
- Li, D.; Wang, Q.; Li, J.; Li, Z.; Yuan, Y.; Yan, Z.; Mei, Z.; Liu, X. Mesophilic-hydrothermal-thermophilic (M-H-T) digestion of green corn straw. Bioresour. Technol. 2016, 202, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, X.; Xu, S.; Chen, M.; Yu, Q.; Xie, J. Combined hydrothermal pretreatment of agricultural and forestry wastes to enhance anaerobic digestion for methane production. Chem. Eng. J. 2024, 486, 150313. [Google Scholar] [CrossRef]
- Ratha, S.K.; Renuka, N.; Abunama, T.; Rawat, I.; Bux, F. Hydrothermal liquefaction of algal feedstocks: The effect of biomass characteristics and extraction solvents. Renew. Sust. Energ. Rev. 2022, 156, 111973. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.-W.; Rao, J.; Tian, R.; Sun, S.-N.; Peng, F. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Cao, W.; Sun, C.S.; Li, X.; Qiu, J.; Liu, R. Methane production enhancement from products of alkaline hydrogen peroxide pretreated sweet sorghum bagasse. RSC Adv. 2017, 7, 16173. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Gu, S.; Luo, K.H. State-of-the-art catalytic hydrogenolysis of lignin for the production of aromatic chemicals. Catal. Sci. Technol. 2018, 8, 6275–6296. [Google Scholar] [CrossRef]
- Ding, C.; Zhong, W.; Cao, Y.; Ma, T.; Ye, H.; Fang, Z.; Feng, Y.; Zhao, S.; Yang, J.; Li, Y.; et al. Fe-MOF-based catalysts for oxygen evolution reaction: Microenvironment regulated by organic ligands, metals and carbonization synergistically. Chem. Eng. Sci. 2025, 302, 120888. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Qiu, X.; Xu, C. Hydrothermal treatment of lignocellulosic biomass towards low-carbon development: Production of high-value-added bioproducts. Energy Chem. 2024, 6, 100133. [Google Scholar] [CrossRef]
- Cherian, T.; Eranhottu, S.; Mandal, K.K.; Cherian, B.; Kurien, S. Carbon nanomaterials based catalytic conversion of biomass: An overview. Next Res. 2025, 2, 100268. [Google Scholar] [CrossRef]
- Raikwar, D.; Majumdar, S.; Shee, D. Effects of solvents in the depolymerization of lignin into value-added products: A review. Biomass Convers. Biorefin. 2023, 13, 11383–11416. [Google Scholar] [CrossRef]
- Jia, P.; Wang, J.; Zhang, W. Catalytic hydrothermal liquefaction of lignin over carbon nanotube supported metal catalysts for production of monomeric phenols. J. Energy Inst. 2021, 94, 1–10. [Google Scholar] [CrossRef]
- Idrus, A.; Dwiatmoko, A.A.; Maryati, Y. Lignin depolymerization using nickel-based catalysts: A mini review. Inorg. Chem. Commun. 2025, 174, 113901. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, Y.; Chen, S.; Ding, M.; Yao, J. Carbon nitride nanotube-based materials for energy and environmental applications: A review of recent progresses. J. Mater. Chem. A 2020, 8, 25626–25648. [Google Scholar] [CrossRef]
- Ayala-Mercado, I.D.; Weber, B.; Durán-García, M.D. Use of hydrothermal pretreatment to enhance biogas production from pelagic. Bioenerg. Res. 2022, 15, 1639–1648. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew. Energ. 2016, 87, 592–598. [Google Scholar] [CrossRef]
- Li, X.; Wu, M.; Xue, Y. Nickel-loaded shrimp shell biochar enhances batch anaerobic digestion of food waste. Bioresour. Technol. 2022, 352, 127092. [Google Scholar] [CrossRef]
- Akansu, H.; Arbag, H.; Tasdemir, H.M.; Yasyerli, S.; Yasyerli, N.; Dogu, G. Nickel-based alumina supported catalysts for dry reforming of biogas in the absence and the presence of H2S: Effect of manganese incorporation. Catal. Today 2022, 397, 37–49. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; van Dillen, A.J.; de Jongh, P.E.; de Jong, K.P. Application of ordered mesoporous materials as model supports to study catalyst preparation by impregnation and drying. In Scientific Bases for the Preparation of Heterogeneous Catalysts; Elsevier: Amsterdam, The Netherlands, 2006; pp. 95–102. [Google Scholar]
- Scarsella, M.; de Caprariis, B.; Damizia, M.; De Filippis, P. Heterogeneous catalysts for hydrothermal liquefaction of lignocellulosic biomass: A review. Biomass Bioenergy 2020, 140, 105662. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Yuan, H.; Li, X.; Zuo, X. Two-Step pretreatment of hydrothermal with ammonia for cow bedding: Pretreatment characteristics, anaerobic digestion performance and kinetic analysis. Waste Biomass Valorization 2021, 12, 5675–5687. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.; Yang, R.; Liu, C.; Hu, X.; Luo, S.; Gong, E.; Ye, J. The relationship between reducing sugars and phenolic retention of brown rice after enzymatic extrusion. J. Cereal Sci. 2017, 74, 244–249. [Google Scholar] [CrossRef]
- Puangbanlang, C.; Sirivibulkovit, K.; Nacapricha, D.; Sameenoi, Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta 2019, 198, 542–549. [Google Scholar] [CrossRef] [PubMed]

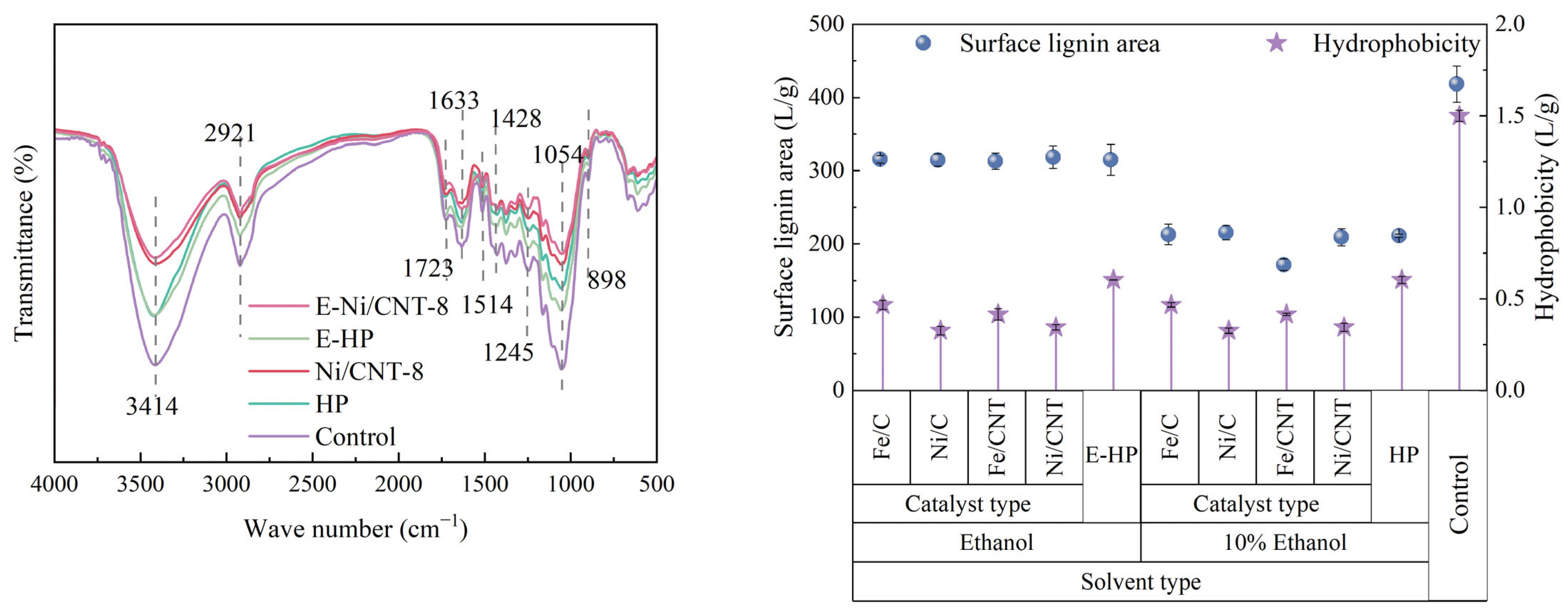



| E-Ni/CNT | E-HP | Ni/CNT | HP | Control | |
|---|---|---|---|---|---|
| CrI | 36.3 | 34.8 | 32.6 | 36.0 | 38.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yuan, H.; Li, X. Fe or Ni Catalytic Hydrothermal Depolymerization with Ethanol for Efficient Anaerobic Digestion of Corn Stover. Catalysts 2025, 15, 429. https://doi.org/10.3390/catal15050429
Wang X, Yuan H, Li X. Fe or Ni Catalytic Hydrothermal Depolymerization with Ethanol for Efficient Anaerobic Digestion of Corn Stover. Catalysts. 2025; 15(5):429. https://doi.org/10.3390/catal15050429
Chicago/Turabian StyleWang, Xitong, Hairong Yuan, and Xiujin Li. 2025. "Fe or Ni Catalytic Hydrothermal Depolymerization with Ethanol for Efficient Anaerobic Digestion of Corn Stover" Catalysts 15, no. 5: 429. https://doi.org/10.3390/catal15050429
APA StyleWang, X., Yuan, H., & Li, X. (2025). Fe or Ni Catalytic Hydrothermal Depolymerization with Ethanol for Efficient Anaerobic Digestion of Corn Stover. Catalysts, 15(5), 429. https://doi.org/10.3390/catal15050429






