Fine Fabrication and Optical Waveguide Characteristics of Hexagonal tris(8-hydroxyquinoline)aluminum(Ⅲ) (Alq3) Crystal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Measurement
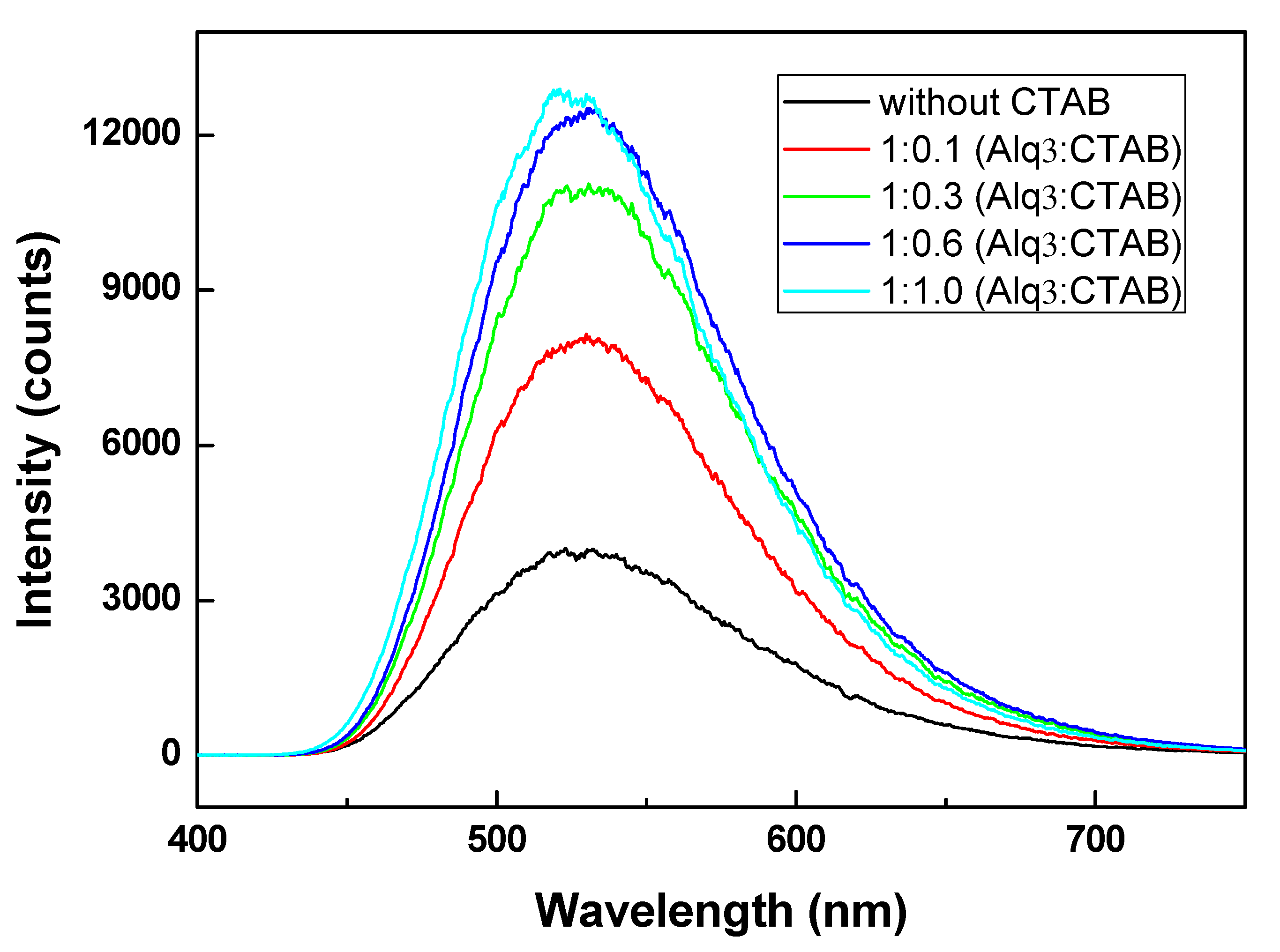
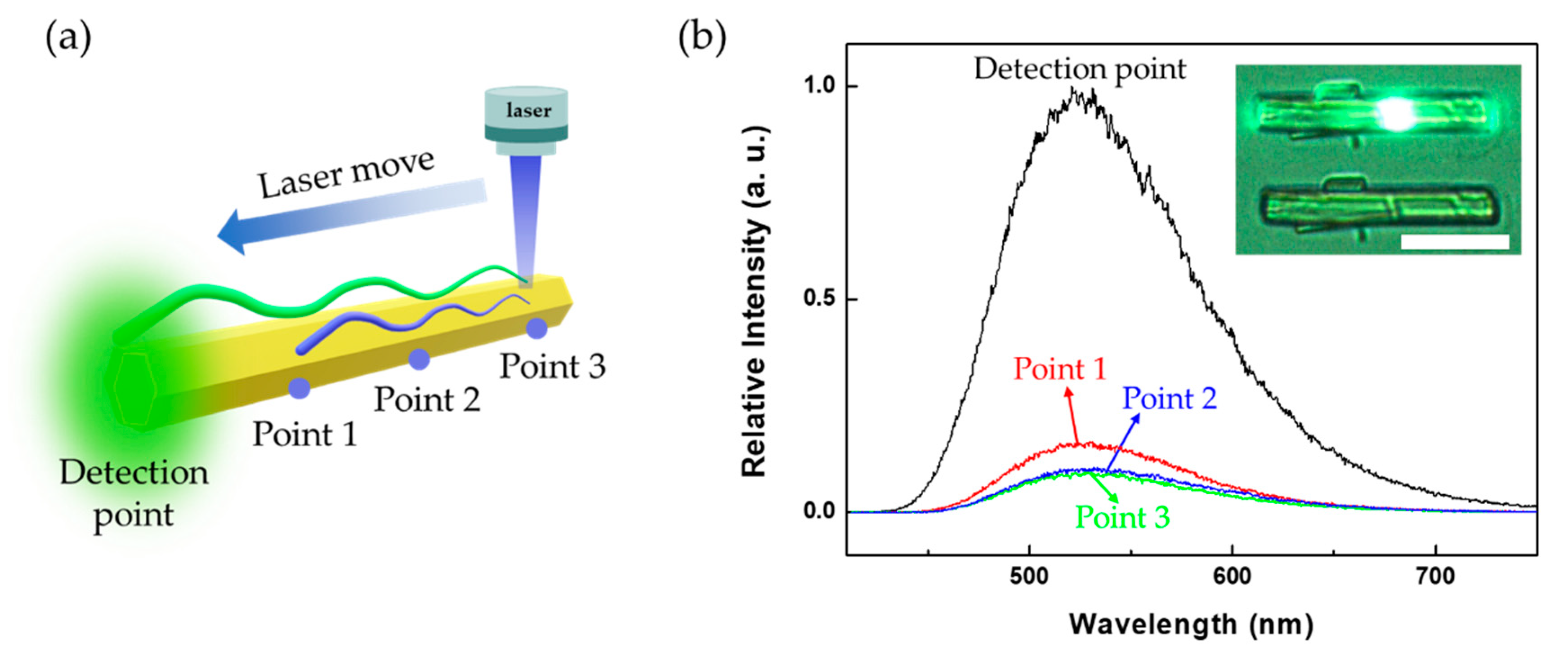
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, Y.S.; Fu, H.; Peng, A.; Ma, Y.; Xiao, D.; Yao, J. Low-dimensional Nanomaterials Based on Small Organic Molecules: Preparation and Optoelectronic Properties. Adv. Mater. 2008, 20, 2859–2876. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Fu, H.B.; Hu, F.Q.; Peng, A.D.; Yang, W.S.; Yao, J.N. Tunable Emission from Binary Organic One-dimensional Nanomaterials: An Alternative Approach to White-light Emission. Adv. Mater. 2008, 20, 79–83. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, M.S.; Joo, J. Hybrid Nanostructures using Π-Conjugated Polymers and Nanoscale Metals: Synthesis, Characteristics, and Optoelectronic Applications. Chem. Soc. Rev. 2010, 39, 2439–2452. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting Polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef]
- Jo, S.G.; Park, D.H.; Kim, B.; Seo, S.; Lee, S.J.; Kim, J.; Kim, J.; Joo, J. Dual-Mode Waveguiding of Raman and Luminescence Signals in a Crystalline Organic Microplate. J. Mater. Chem. C 2014, 2, 6077–6083. [Google Scholar] [CrossRef]
- Yan, R.; Gargas, D.; Yang, P. Nanowire Photonics. Nat. Photon. 2009, 3, 569–576. [Google Scholar] [CrossRef]
- Cui, Q.H.; Zhao, Y.S.; Yao, J. Photonic Applications of One-Dimensional Organic Single-Crystalline Nanostructures: Optical Waveguides and Optically Pumped Lasers. J. Mater. Chem. 2012, 22, 4136–4140. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Y.S.; Yao, J. Optical Waveguides at Micro/Nanoscale Based on Functional Small Organic Molecules. Phys. Chem. Phys. 2011, 13, 9060–9073. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. [Google Scholar] [CrossRef] [PubMed]
- Briseno, A.L.; Holcombe, T.W.; Boukai, A.I.; Garnett, E.C.; Shelton, S.W.; Fréchet, J.J.; Yang, P. Oligo-and Polythiophene/ZnO Hybrid Nanowire Solar Cells. Nano Lett. 2010, 10, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.H.; Kim, B.; Jun, S.; Lee, J.; Park, D.H.; Lee, K.; Kim, J.; Kim, J.; Joo, J. Remote Biosensing with Polychromatic Optical Waveguide using Blue Light-Emitting Organic Nanowires Hybridized with Quantum Dots. Adv. Funct. Mater. 2014, 24, 3684–3691. [Google Scholar] [CrossRef]
- Jo, S.G.; Kim, B.G.; Kim, J.; Kim, J.; Joo, J. Waveguiding Characteristics of Surface Enhanced Raman Scattering Signals Along Crystalline Organic Semiconducting Microrod. Opt. Express 2017, 25, 6215–6226. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, K.; Jung, J.S.; Jo, S.G.; Kim, H.; Lee, H.S.; Kim, J.; Joo, J. Luminescence, Charge Mobility, and Optical Waveguiding of Two-Dimensional Organic Rubrene Nanosheets: Comparison with One-Dimensional Nanorods. Org. Electron. 2012, 13, 2047–2055. [Google Scholar] [CrossRef]
- Briseno, A.L.; Mannsfeld, S.C.; Lu, X.; Xiong, Y.; Jenekhe, S.A.; Bao, Z.; Xia, Y. Fabrication of Field-Effect Transistors from Hexathiapentacene Single-Crystal Nanowires. Nano Lett. 2007, 7, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liao, Q.; Shi, Q.; Fu, H.; Ma, J.; Yao, J. Rubrene Micro-Crystals from Solution Routes: Their Crystallography, Morphology and Optical Properties. J. Mater. Chem. 2010, 20, 159–166. [Google Scholar] [CrossRef]
- Garreau, A.; Duvail, J. Recent Advances in Optically Active Polymer-Based Nanowires and Nanotubes. Adv. Opt. Mater. 2014, 2, 1122–1140. [Google Scholar] [CrossRef]
- Lee, T.; Lin, M.S. Sublimation Point Depression of Tris (8-Hydroxyquinoline) Aluminum (III)(Alq3) by Crystal Engineering. Cryst. Growth Des. 2007, 7, 1803–1810. [Google Scholar] [CrossRef]
- Xie, W.; Fan, J.; Song, H.; Jiang, F.; Yuan, H.; Wei, Z.; Ji, Z.; Pang, Z.; Han, S. Controllable Synthesis of Rice-Shape Alq3 Nanoparticles with Single Crystal Structure. Phys. E Low Dimens. Syst. Nanostruct. 2016, 84, 519–523. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.H.; Choi, J.; Lee, H.; Kim, S.; Park, J.W.; Park, D.H. Growth and Brilliant Photo-Emission of Crystalline Hexagonal Column of Alq3 Microwires. Materials 2018, 11, 472. [Google Scholar] [CrossRef]
- Fukushima, T.; Kaji, H. Green-and Blue-Emitting Tris (8-Hydroxyquinoline) Aluminum (III)(Alq3) Crystalline Polymorphs: Preparation and Application to Organic Light-Emitting Diodes. Org. Electron. 2012, 13, 2985–2990. [Google Scholar] [CrossRef]
- Tamura, H.; Hamada, I.; Shang, H.; Oniwa, K.; Akhtaruzzaman, M.; Jin, T.; Asao, N.; Yamamoto, Y.; Kanagasekaran, T.; Shimotani, H. Theoretical Analysis on the Optoelectronic Properties of Single Crystals of Thiophene-Furan-Phenylene Co-Oligomers: Efficient Photoluminescence due to Molecular Bending. J. Phys. Chem. C 2013, 117, 8072–8078. [Google Scholar] [CrossRef]
- Devi, S.R.; Kalaiyarasi, S.; Zahid, I.M.; Kumar, R.M. Studies on the Growth Aspects, Structural, Thermal, Dielectric and Third Order Nonlinear Optical Properties of Solution Grown 4-Methylpyridinium P-Nitrophenolate Single Crystal. J. Cryst. Growth 2016, 454, 139–146. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, H.; Zhang, Y.; Gao, H.; Su, Z.; Wang, Y. Fac-Alq3 and Mer-Alq3 Nano/Microcrystals with Different Emission and Charge-Transporting Properties. Adv. Mater. 2010, 22, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Cölle, M.; Brütting, W. Thermal, Structural and Photophysical Properties of the Organic Semiconductor Alq3. Phys. Status Solidi A 2004, 201, 1095–1115. [Google Scholar] [CrossRef]
- Rajeswaran, M.; Blanton, T.N. Single-Crystal Structure Determination of a New Polymorph (Ε-Alq3) of the Electroluminescence OLED (Organic Light-Emitting Diode) Material, Tris (8-Hydroxyquinoline) Aluminum (Alq3). J. Chem. Cryst. 2005, 35, 71–76. [Google Scholar] [CrossRef]
- Cölle, M.; Dinnebier, R.E.; Brütting, W. The Structure of the Blue Luminescent Δ-Phase of Tris (8-Hydroxyquinoline) Aluminium (Iii)(Alq3). Chem. Commun. 2002, 2908–2909. [Google Scholar] [CrossRef]
- Cui, C.; Park, D.H.; Kim, J.; Joo, J.; Ahn, D.J. Oligonucleotide Assisted Light-Emitting Alq3 Microrods: Energy Transfer Effect with Fluorescent Dyes. Chem. Commun. 2013, 49, 5360–5362. [Google Scholar] [CrossRef]
- Brinkmann, M.; Gadret, G.; Muccini, M.; Taliani, C.; Masciocchi, N.; Sironi, A. Correlation between Molecular Packing and Optical Properties in Different Crystalline Polymorphs and Amorphous Thin Films of Mer-Tris (8-Hydroxyquinoline) Aluminum (III). J. Am. Chem. Soc. 2000, 122, 5147–5157. [Google Scholar] [CrossRef]
- Hu, J.; Ji, H.; Cao, A.; Huang, Z.; Zhang, Y.; Wan, L.; Xia, A.; Yu, D.; Meng, X.; Lee, S. Facile Solution Synthesis of Hexagonal Alq3 Nanorods and their Field Emission Properties. Chem. Commun. 2007, 3083–3085. [Google Scholar] [CrossRef]
- Xu, G.; Tang, Y.; Tsang, C.; Zapien, J.; Lee, C.; Wong, N. Facile Solution Synthesis without Surfactant Assistant for Ultra Long Alq3 Sub-Microwires and their Enhanced Field Emission and Waveguide Properties. J. Mater. Chem. 2010, 20, 3006–3010. [Google Scholar] [CrossRef]
- Goswami, M.; Nayak, P.K.; Periasamy, N.; Madhu, P.K. Characterisation of Different Polymorphs of Tris (8-Hydroxyquinolinato) Aluminium (III) using Solid-State NMR and DFT Calculations. Chem. Cent. J. 2009, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Chen, D.; Li, D.; Yuan, Y.; Xia, D.; Zhang, Z.; Zhang, H.; Wang, Y. A Green Emissive Amorphous Fac-Alq3 Solid Generated by Grinding Crystalline Blue Fac-Alq3 Powder. Chem. Commun. 2011, 47, 4135–4137. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, M.S.; Kim, J.; Joo, J. Fine Control of Photoluminescence and Optical Waveguiding Characteristics of Organic Rubrene Nanorods using Focused Electron-Beam Irradiation. J. Phys. Chem. C 2014, 118, 30179–30186. [Google Scholar] [CrossRef]
- Cho, E.; Choi, J.; Jo, S.; Park, D.; Hong, Y.K.; Kim, D.; Lee, T.S. A Single-Benzene-Based Fluorophore: Optical Waveguiding in the Crystal Form. ChemPlusChem 2019, 84, 1130–1134. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, S.; Choi, J.; Yoo, S.H.; Oh, S.; Kim, D.H.; Park, D.H. Fine Fabrication and Optical Waveguide Characteristics of Hexagonal tris(8-hydroxyquinoline)aluminum(Ⅲ) (Alq3) Crystal. Crystals 2020, 10, 260. https://doi.org/10.3390/cryst10040260
Park J, Kim S, Choi J, Yoo SH, Oh S, Kim DH, Park DH. Fine Fabrication and Optical Waveguide Characteristics of Hexagonal tris(8-hydroxyquinoline)aluminum(Ⅲ) (Alq3) Crystal. Crystals. 2020; 10(4):260. https://doi.org/10.3390/cryst10040260
Chicago/Turabian StylePark, Jungwoon, Seokho Kim, Jinho Choi, Sung Ho Yoo, Seongjae Oh, Do Hyoung Kim, and Dong Hyuk Park. 2020. "Fine Fabrication and Optical Waveguide Characteristics of Hexagonal tris(8-hydroxyquinoline)aluminum(Ⅲ) (Alq3) Crystal" Crystals 10, no. 4: 260. https://doi.org/10.3390/cryst10040260
APA StylePark, J., Kim, S., Choi, J., Yoo, S. H., Oh, S., Kim, D. H., & Park, D. H. (2020). Fine Fabrication and Optical Waveguide Characteristics of Hexagonal tris(8-hydroxyquinoline)aluminum(Ⅲ) (Alq3) Crystal. Crystals, 10(4), 260. https://doi.org/10.3390/cryst10040260



