Effect of Sm3+ Substitutions on the Lithium Ionic Conduction and Relaxation Dynamics of Li5+2xLa3Nb2−xSmxO12 Ceramics
Abstract
:1. Introduction
2. Materials and Methods
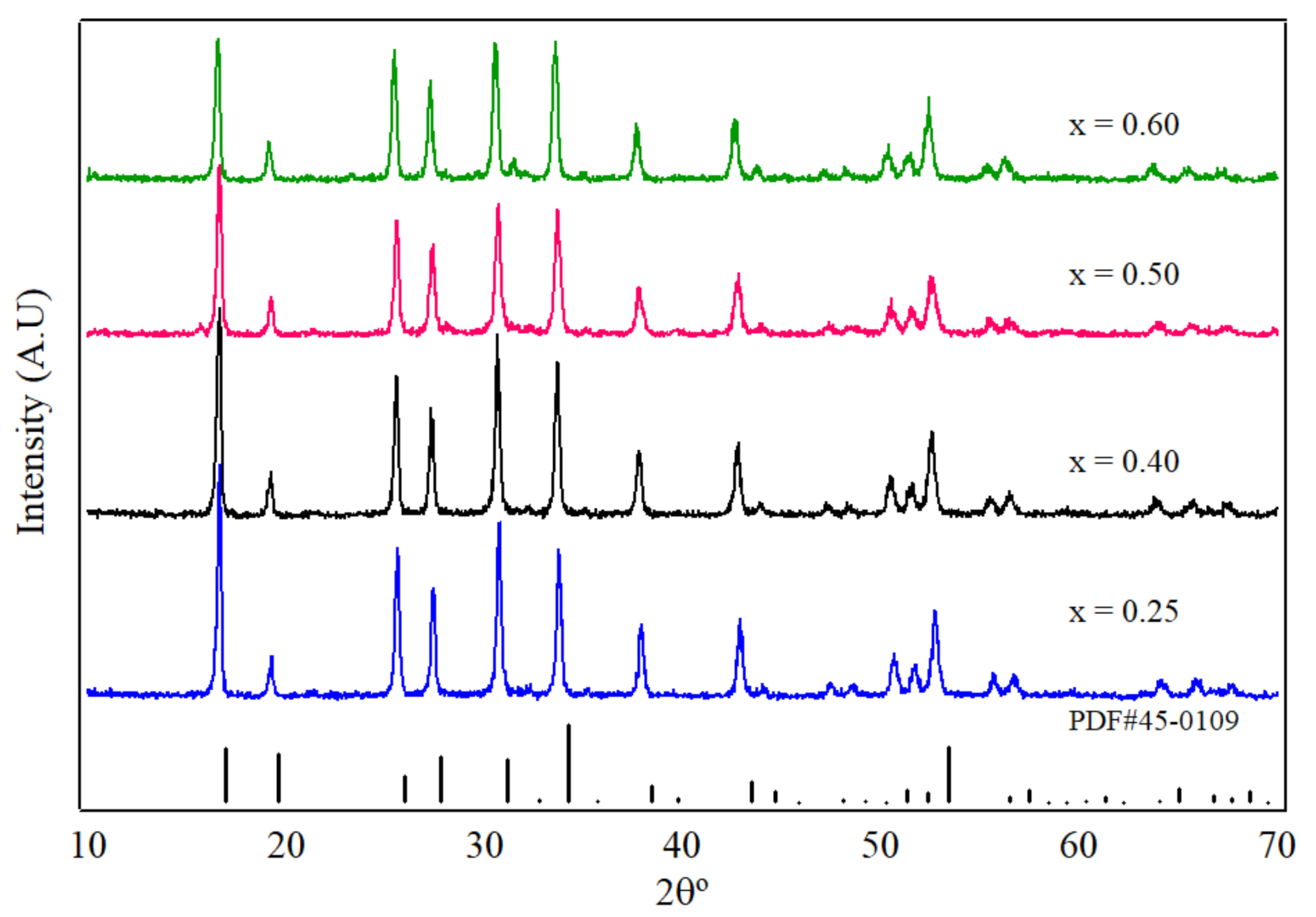
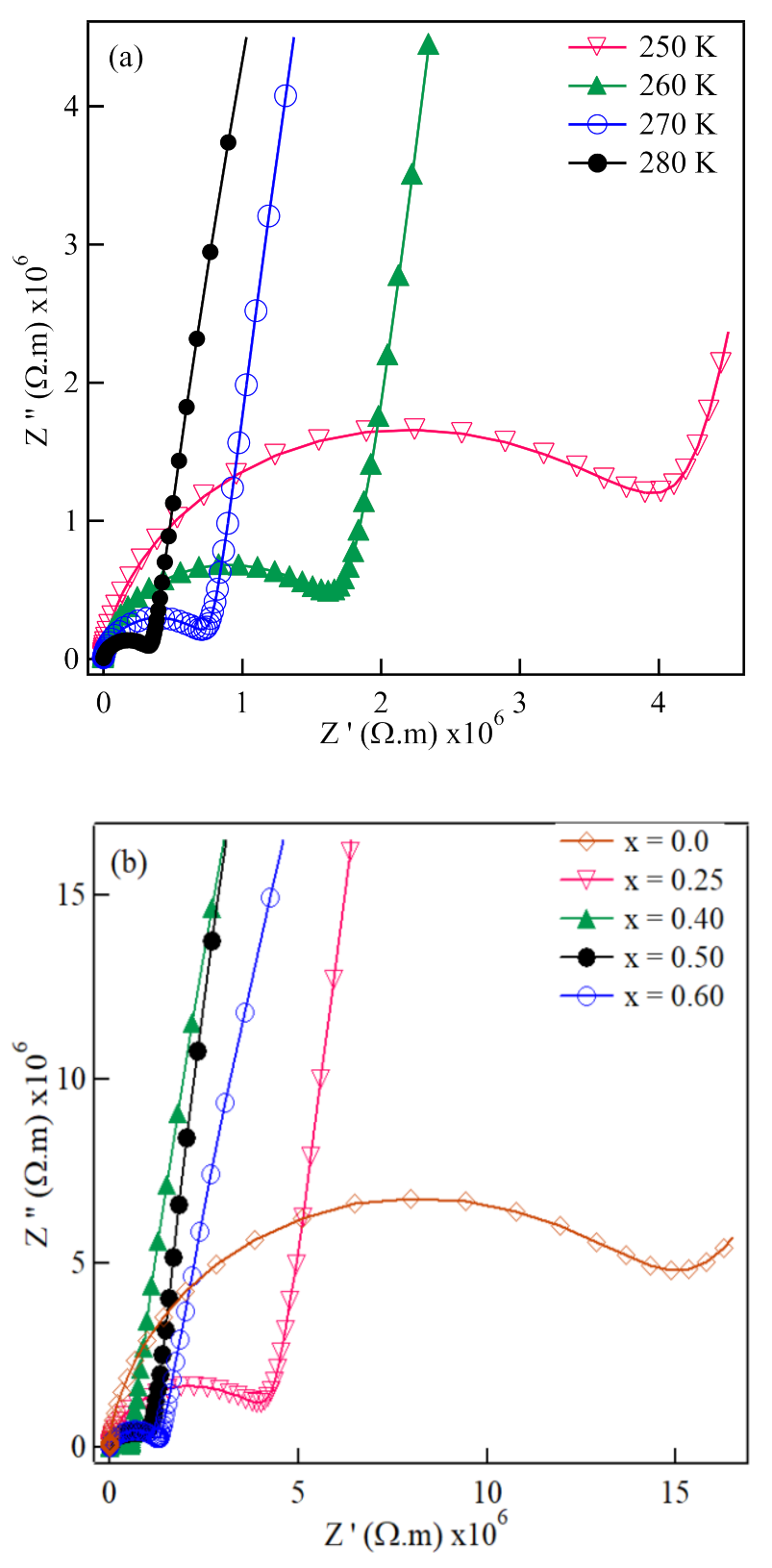
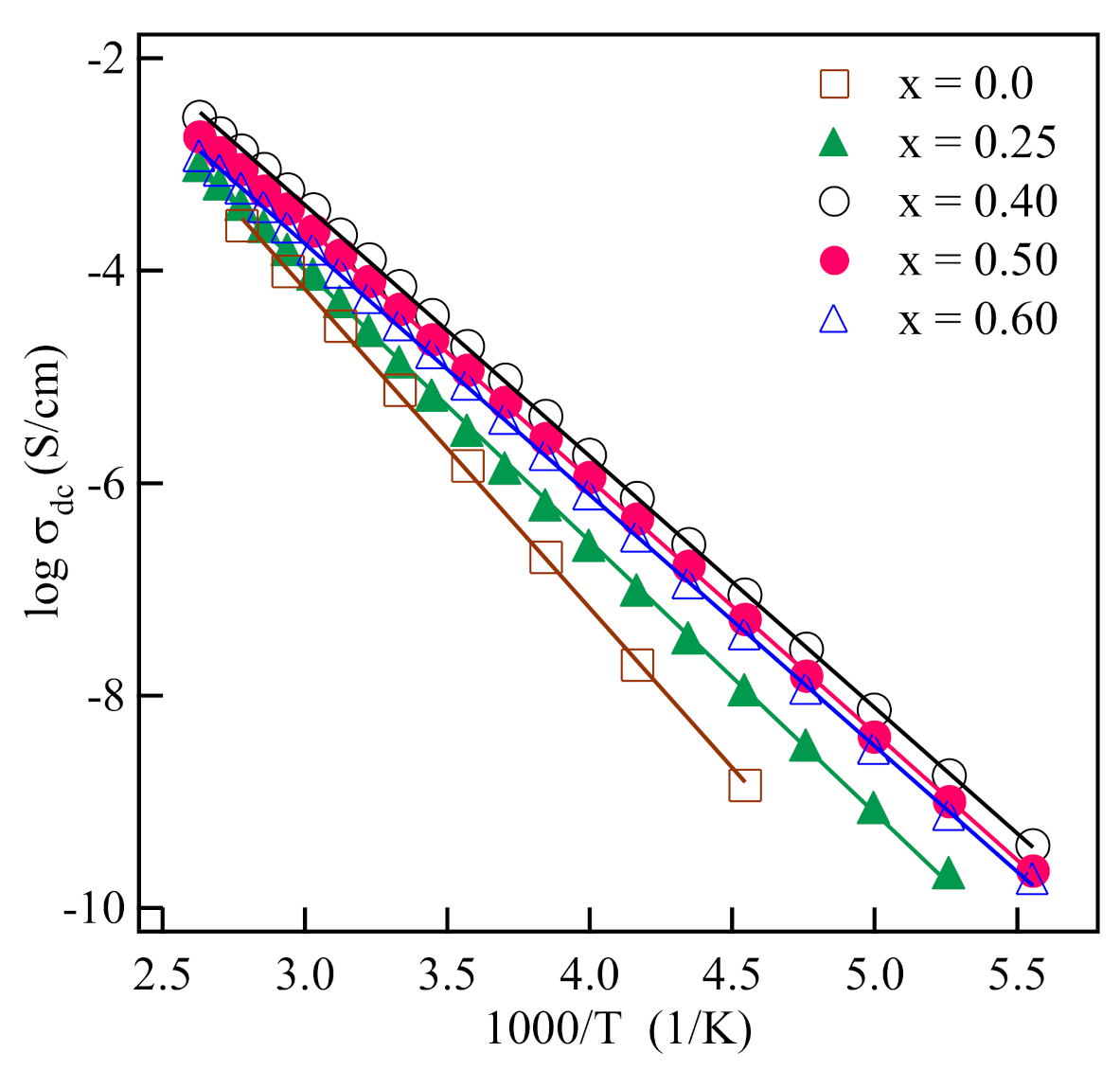
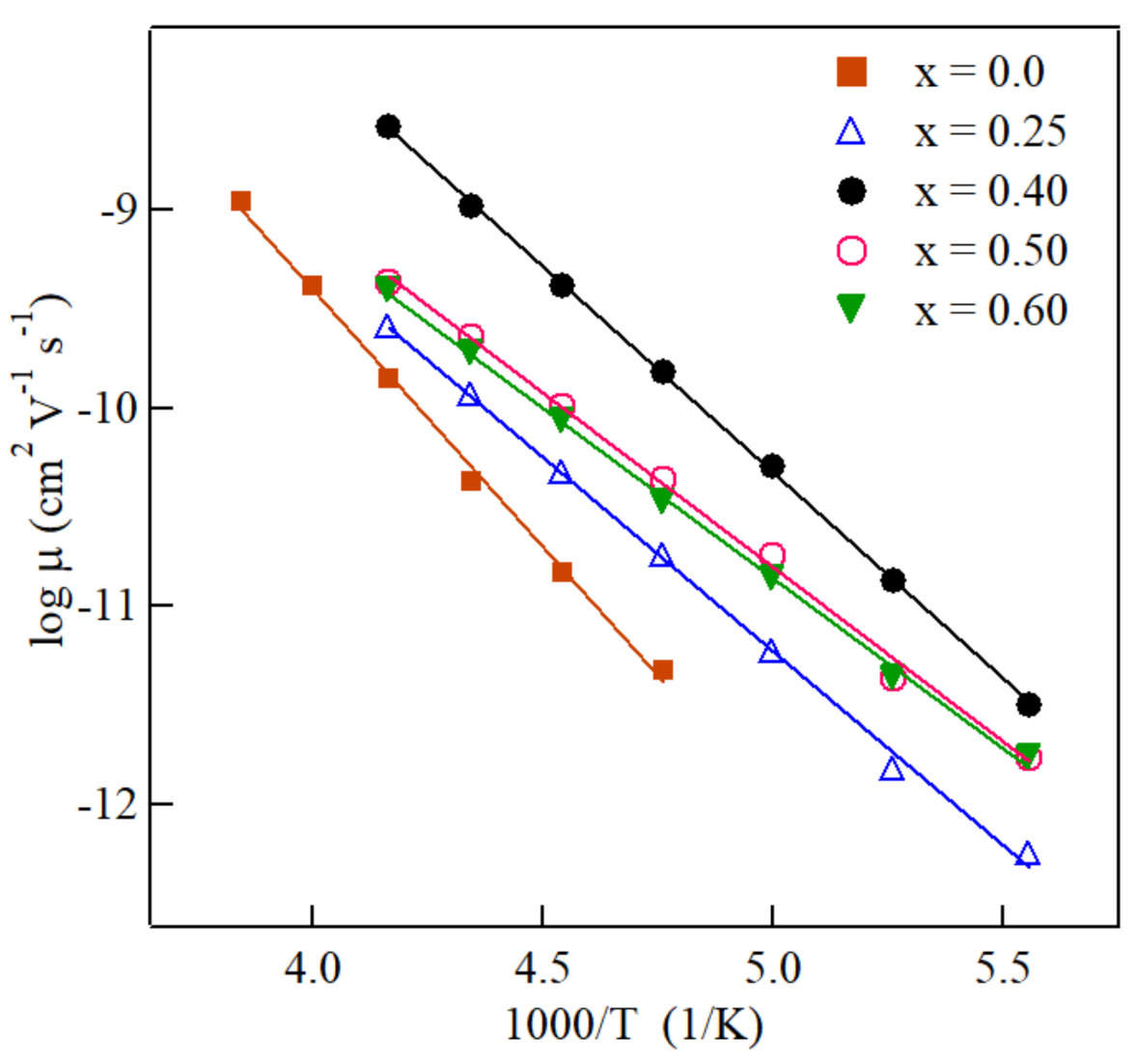
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.H.; Murchison, A.J.; Ferreira, J.A.; Singh, P.; Goodenough, J.B. Glass amorphous alkali-ion solid electrolytes and their performance in symmetrical cells. Energy Environ. Sci. 2016, 9, 948–954. [Google Scholar] [CrossRef]
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef]
- Kim, K.H.; Torgeson, D.R.; Borsa, F.; Martin, S.W. Two separate Li+ ionic motions observed by 7Li and 11B NMR in xLi2S + (1 − x)B2S3 glassy fast ionic conductors. Solid State Ion. 1996, 90, 29–38. [Google Scholar] [CrossRef]
- Fu, J. Fast Li+ ion conducting glass-ceramics in the system Li2O–Al2O3–GeO2–P2O5. Solid State Ion. 1997, 104, 191–194. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Q.; Tsai, C.-L.; Tietz, F.; Guillon, O. A Novel Sol-Gel Method for Large-Scale Production of Nanopowders: Preparation of Li1.5Al0.5Ti1.5(PO4)3 as an Example. J. Am. Ceram. Soc. 2016, 99, 410–414. [Google Scholar] [CrossRef]
- Waetzig, K.; Heubner, C.; Kusnezof, M. Reduced Sintering Temperatures of Li+ Conductive Li1.3Al0.3Ti1.7(PO4)3 Ceramics. Crystals 2020, 10, 408. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Li6ALa2Ta2O12 (A = Sr, Ba): Novel garnet-like oxides for fast lithium ion conduction. Adv. Funct. Mater. 2005, 15, 107–112. [Google Scholar] [CrossRef]
- Thangadurai, V.; Kaack, H.; Weppner, W. Novel fast lithium ion conduction in garnet-type Li5La3M2O12 (M = Nb, Ta). J. Am. Ceram. Soc. 2003, 86, 437–440. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Investigations on electrical conductivity and chemical compatibility between fast lithium ion conducting garnet-like Li6BaLa2Ta2O12 and lithium battery cathodes. J. Power Sources 2005, 142, 339–344. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Effect of lithium ion content on the lithium ion conductivity of the garnet-like structure Li5+xBaLa2Ta2O11.5+0.5x (x = 0–2). Appl. Phys. A 2008, 91, 615–620. [Google Scholar] [CrossRef]
- Murugan, R.; Thangaduraiand, V.; Weppner, W. Lithium ion conductivity of Li5+xBaxLa3−xTa2O12 (x = 0–2) with garnet-related structure in dependence of the barium content. Ionics 2007, 13, 195–203. [Google Scholar] [CrossRef]
- Murugan, R.; Thangaduraiand, V.; Weppner, W. Lattice parameter and sintering temperature dependence of bulk and grain-boundary conduction of garnet-like solid Li-electrolytes. J. Electrochem. Soc. 2008, 155, A90–A101. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnettype Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Takahashi, Y.; Hayakawa, H.; Akimoto, J. Synthesis and crystallographic studies of garnet-related lithium-ion conductors Li6CaLa2Ta2O12 and Li6BaLa2Ta2O12. Solid State Ionics 2009, 180, 602–606. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, Q.; Guo, Y.; Li, Z.; Qiang, Y. The ionic conductivity of Li6BaLa2M2O12 with coexisting Nb and Ta on the M sites. Ionics 2013, 19, 697–700. [Google Scholar] [CrossRef]
- Kokal, I.; Ramanujachary, K.V.; Notten, P.H.L.; Hintzen, H.T. Sol-gel synthesis and lithium ion conduction properties of garnet-type Li6BaLa2Ta2O12. Mater. Res. Bull. 2012, 47, 1932–1935. [Google Scholar] [CrossRef]
- Baral, A.K.; Narayanan, S.; Ramezanipour, F.; Thangadurai, V. Evaluation of fundamental transport properties of Li-excess garnet-type Li5+2xLa3Ta2−xYxO12 (x = 0.25, 0.5 and 0.75) electrolytes using ac impedance and dielectric spectroscopy. Phys. Chem. Chem. Phys. 2014, 16, 11356–11365. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Wang, X.P.; Wang, W.G.; Fang, Q.F. Sol-gel synthesis and electrical properties Li5La3Ta2O12 lithium ionic conductors. Solid State Ion. 2010, 181, 33–36. [Google Scholar] [CrossRef]
- Campanella, D.; Belanger, D.; Paolella, A. Beyond garnets, phosphates and phosphosulfides solid electrolytes: New ceramic perspectives for all solid lithium metal batteries. J. Power Sources 2021, 482, 228949. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Thompson, T.; Sakamoto, J.; Huq, A.; Wolfenstine, J.; Allen, J.L.; Bernstein, N.; Stewart, D.A.; Johannes, M.D. Structure and stoichiometry in supervalent doped Li7La3Zr2O12. Chem. Mater. 2015, 27, 3658–3665. [Google Scholar] [CrossRef]
- Allen, J.L.; Wolfenstine, J.; Rangasamy, E.; Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 2012, 206, 315–319. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, A.D.; Mo, Y.; et al. Garnet-type solid-state electrolytes: Materials, interfaces and batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Al-Quaimi, M. Origin of the enhanced Li+ ionic conductivity in Gd+3 substituted Li5+2xLa3Nb2−xGdxO12 lithium conducting garnets. Phys. Chem. Chem. Phys. 2015, 17, 16007–16014. [Google Scholar] [CrossRef]
- Nemori, H.; Matsuda, Y.; Matsui, M.; Yamamoto, O.; Takeda, Y.; Imanishi, N. Relationship between lithium content and ionic conductivity in the Li5+2xLa3Nb2−xScxO12 system. Solid State Ion. 2014, 266, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Stockham, M.P.; Dong, B.; Ding, Y.; Lib, Y.; Slater, P.R. Evaluation of the effect of site substitution of Pr doping in the lithium garnet system Li5La3Nb2O12. Dalton Trans. 2020, 49, 10349. [Google Scholar] [CrossRef]
- Pinzaru, D.; Thangadurai, V. Synthesis, structure and Li ion conductivity of garnet-like Li5+2xLa3Nb2−xSmxO12 (0≤ x ≤ 0.7). J. Electrochem. Soc. 2014, 161, A2060. [Google Scholar] [CrossRef] [Green Version]
- Almond, D.P.; Ducan, G.K.; West, A.R. The determination of hopping rates and carrier concentrations in ionic conductors by a new analysis of ac conductivity. Solid State Ion. 1983, 8, 159–164. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Yamada, K. Hopping rates and concentration of mobile fluoride ions in Pb1−xSnxF2 solid solutions. J. Chem. Phys. 2007, 127, 124507. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Rao, R.P. Ion transport and phase transition in Li7−xLa3(Zr2−xMx)O12 (M = Ta5+, Nb5+, x = 0, 0.25). J. Mater. Chem. 2012, 22, 1426–1434. [Google Scholar] [CrossRef]
- Cussen, E.J. The structure of lithium garnets: Cation disorder and clustering in a new family of fast Li+ conductors. Chem. Commun. 2006, 4, 412–413. [Google Scholar] [CrossRef] [Green Version]
- O’Challaghan, M.P.; Cussen, E.J. Lithium dimer formation in the Li-conducting garnets Li5+xBaxLa3−xTa2O12 (0 < x ≤ 1.6). Chem. Commun. 2007, 2048–2050. [Google Scholar]
- Ahmad, M.M. Estimation of the concentration and mobility of mobile Li+ in the cubic garnet-type Li7La3Zr2O12. RSC Adv. 2015, 5, 25824–25829. [Google Scholar] [CrossRef]
- Macedo, P.B.; Moynihan, C.T.; Bose, R. The role of ionic diffusion in polarization in vitreous ionic conductors. Phys. Chem. Glasses 1972, 13, 171–179. [Google Scholar]

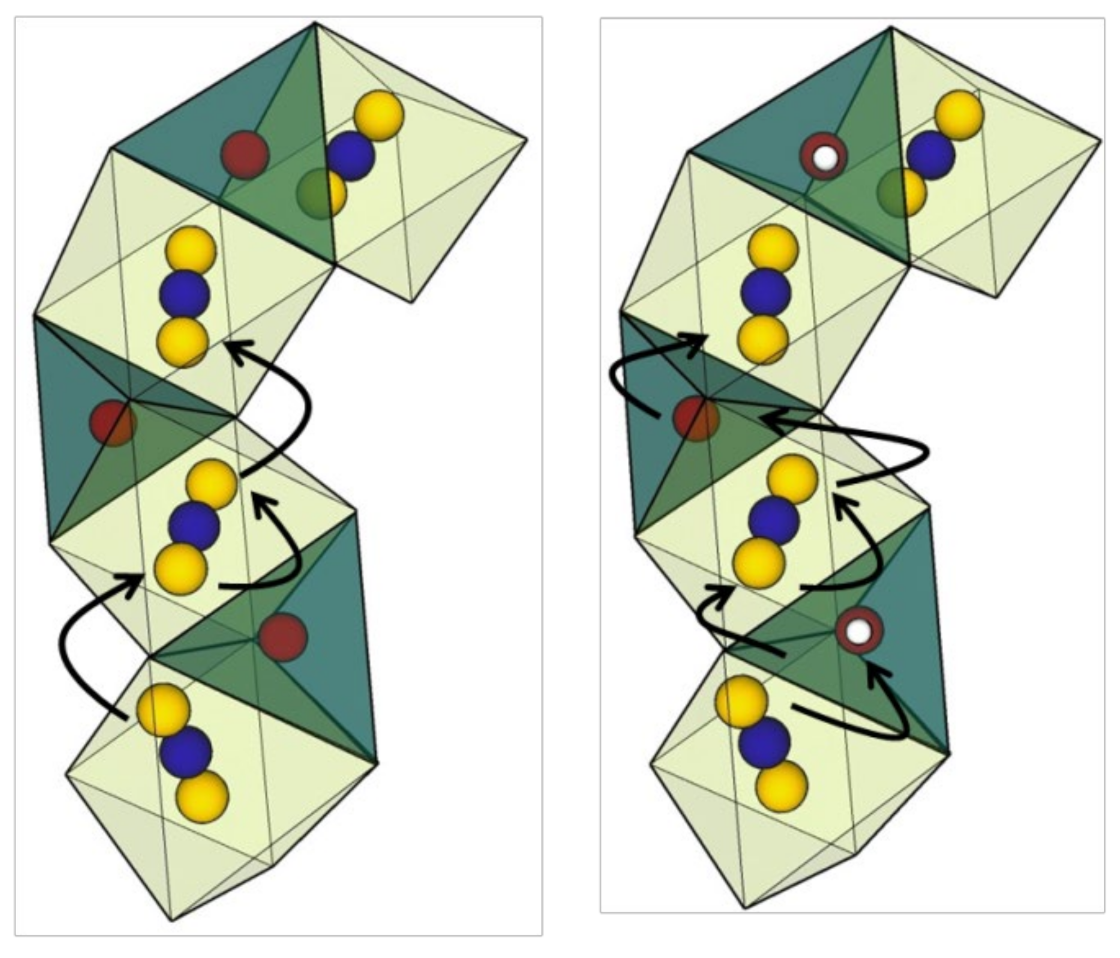
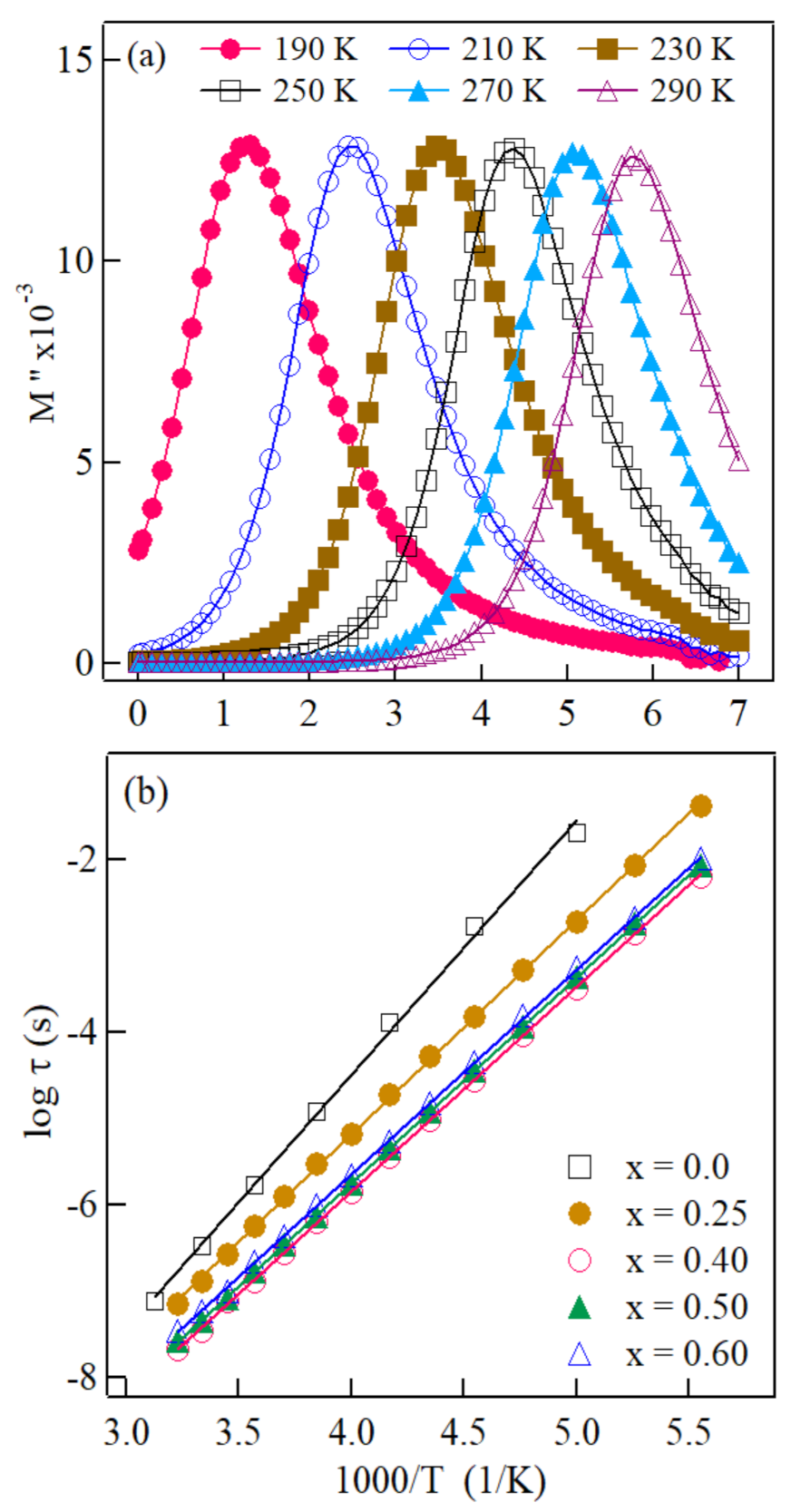
| X | ΔEσdc(eV) | ΔEμ(eV) | ||
|---|---|---|---|---|
| 0.00 | 7.49 × 10−6 | 0.60 | 0.52 | 0.58 |
| 0.25 | 1.35 × 10−5 | 0.51 | 0.39 | 0.49 |
| 0.40 | 7.04 × 10−5 | 0.47 | 0.40 | 0.47 |
| 0.50 | 4.31 × 10−5 | 0.47 | 0.35 | 0.47 |
| 0.60 | 3.04 × 10−5 | 0.47 | 0.34 | 0.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.M.; Kotb, H.M.; Alshoaibi, A.; Alouane, M.H.H.; Aljaafari, A.; Khater, H.A. Effect of Sm3+ Substitutions on the Lithium Ionic Conduction and Relaxation Dynamics of Li5+2xLa3Nb2−xSmxO12 Ceramics. Crystals 2021, 11, 95. https://doi.org/10.3390/cryst11020095
Ahmad MM, Kotb HM, Alshoaibi A, Alouane MHH, Aljaafari A, Khater HA. Effect of Sm3+ Substitutions on the Lithium Ionic Conduction and Relaxation Dynamics of Li5+2xLa3Nb2−xSmxO12 Ceramics. Crystals. 2021; 11(2):95. https://doi.org/10.3390/cryst11020095
Chicago/Turabian StyleAhmad, Mohamad M., H. Mahfoz Kotb, Adil Alshoaibi, M. H. Hadj Alouane, Abdullah Aljaafari, and Hassan A. Khater. 2021. "Effect of Sm3+ Substitutions on the Lithium Ionic Conduction and Relaxation Dynamics of Li5+2xLa3Nb2−xSmxO12 Ceramics" Crystals 11, no. 2: 95. https://doi.org/10.3390/cryst11020095








