A Characteristic Study of Polylactic Acid/Organic Modified Montmorillonite (PLA/OMMT) Nanocomposite Materials after Hydrolyzing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hot Pressed Film Specimen
2.3. Preparation of Injection Molding Specimen
2.4. Decomposition Test
2.5. Property Analysis
2.5.1. Wide-Angle X-ray Diffraction (WAXD)
2.5.2. Transmission Electron Microscopy (TEM)
2.5.3. Thermogravimetric Analyzer (TGA)
2.5.4. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
3.1. Crystalline of PLA/OMMT Nanocomposites
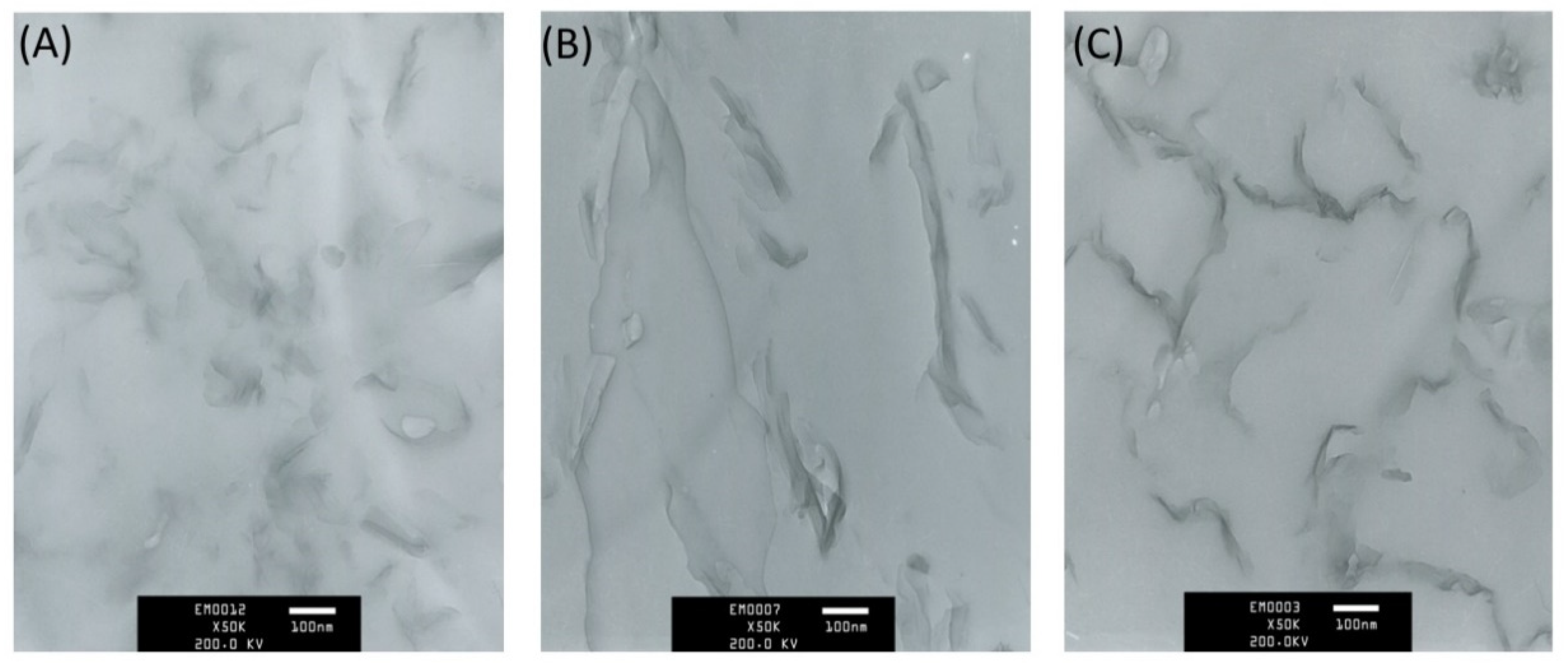
3.2. The Morphology of the PLA/OMMT Nanocomposites
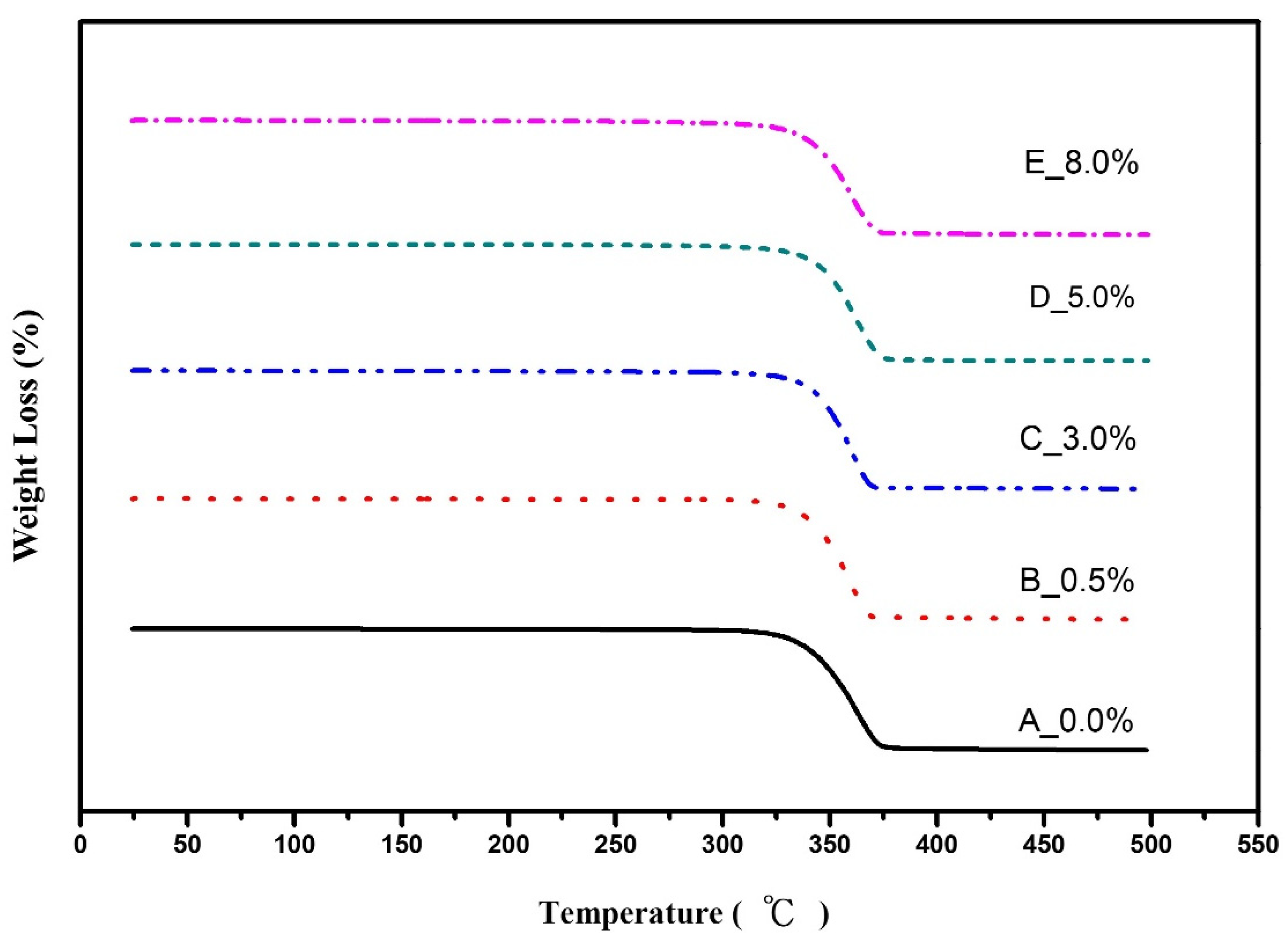
3.3. Heat Resistance
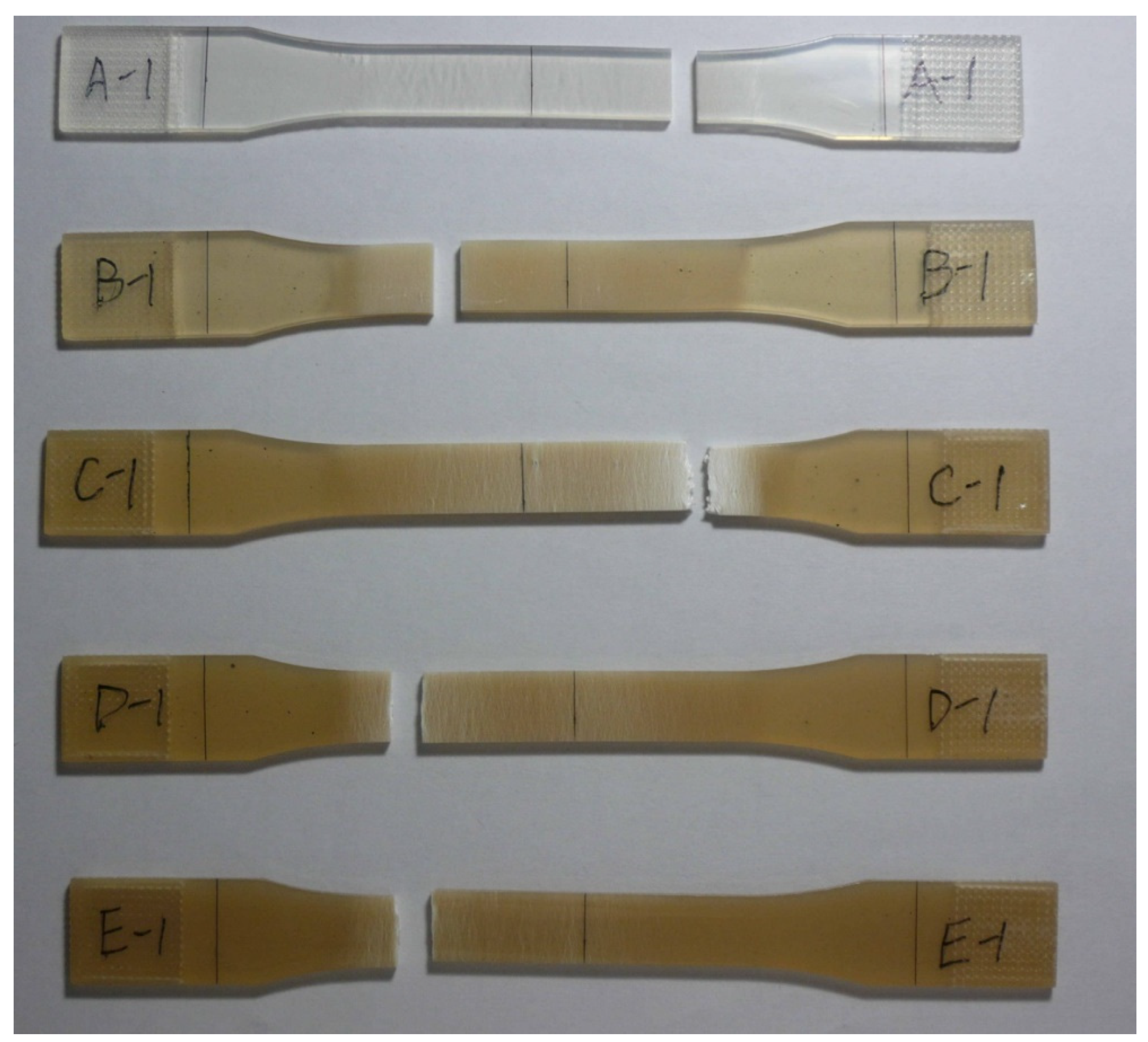
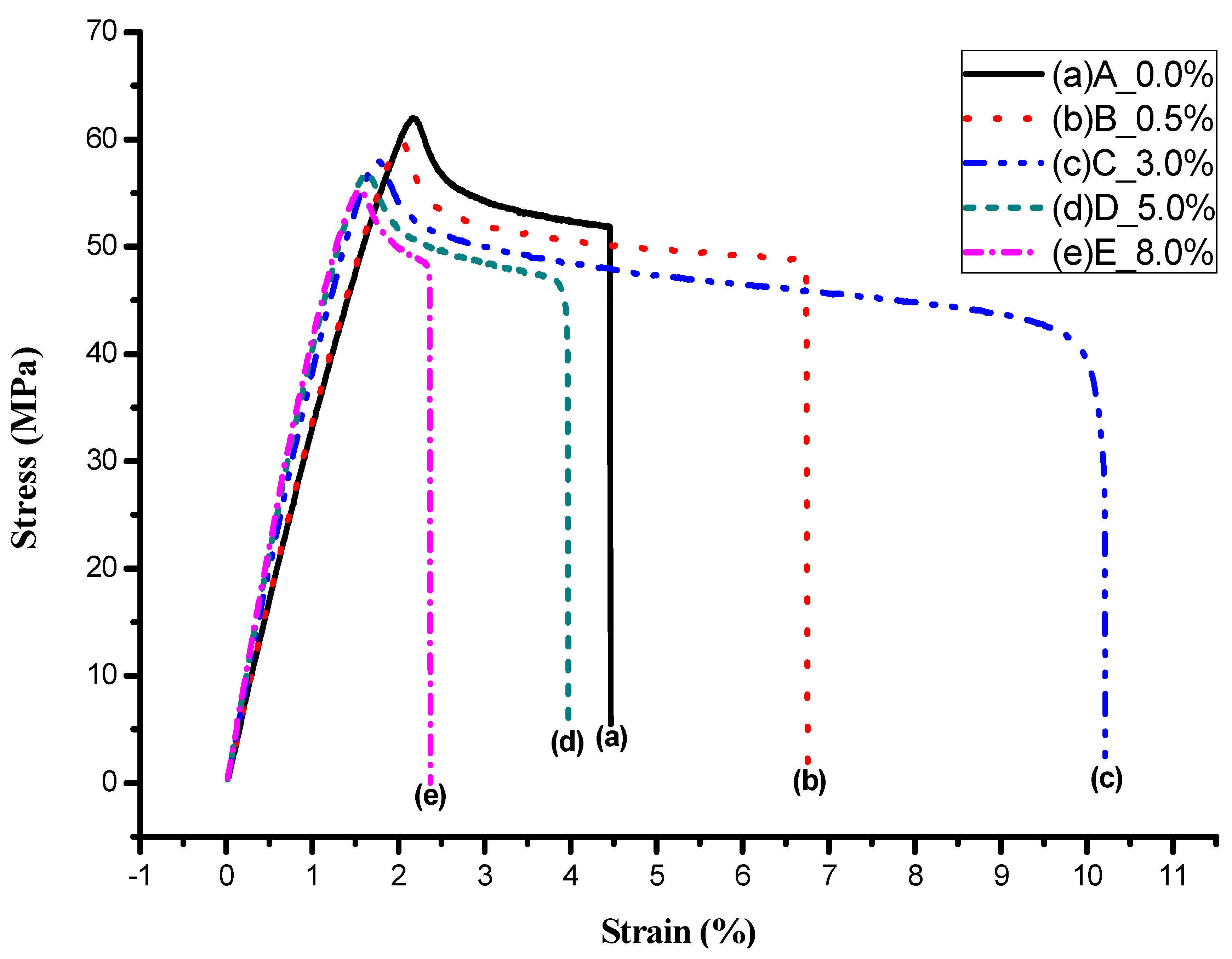
3.4. Tensile Strength
3.5. Hydrolysis
3.5.1. Before Hydrolysis
3.5.2. After Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nurul, M.F.; Jayaraman, K.; Bhattacharyya, D.; Mohamad, M.H.; Saurabh, C.K.; Hussin, M.H.; Abdul Khalil, H.P.S. Green composites made of bamboo fabric and poly (lactic) acid for packaging applications—A review. Materials 2016, 9, 435. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Havstad, M.R. Chapter 5—Biodegradable Plastics. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 97–129. [Google Scholar]
- Weng, Y.X.; Jin, Y.J.; Meng, Q.Y.; Wang, L.; Zhang, M.; Wang, Y.Z. Biodegradation behavior of poly (butylene adipate-co-terephthalate)(PBAT), poly (lactic acid)(PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Dahman, Y. Poly (Lactic Acid): Green and Sustainable Plastics. Ferment. Technol. 2014, 2, e121. [Google Scholar] [CrossRef] [Green Version]
- Darie-Niţă, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpă, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2016, 133, 43223. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.; Asim, M.; Saba, N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2018, 40, 446–463. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Mallegni, N.; Massa, C.A.; Aliotta, L.; Lazzeri, A. Thermal, Mechanical, Viscoelastic and Morphological Properties of Poly (lactic acid) based Biocomposites with Potato Pulp Powder Treated with Waxes. Materials 2019, 12, 990. [Google Scholar] [CrossRef] [Green Version]
- Alemán, C.; Lotz, B.; Puiggali, J. Crystal Structure of the α-Form of Poly(L-lactide). Macromolecules 2001, 34, 4795–4801. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Betancourt, N.G.; Cree, D.E. Mechanical properties of poly (lactic acid) composites reinforced with CaCO3 eggshell based fillers. MRS Adv. 2017, 2, 2545–2550. [Google Scholar] [CrossRef] [Green Version]
- Aliotta, L.; Gigante, V.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A. Evaluation of mechanical and interfacial properties of bio-composites based on poly (lactic acid) with natural cellulose fibers. Int. J. Mol. Sci. 2019, 20, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCutcheon, C.J.; Zhao, B.; Jin, K.; Bates, F.S.; Ellison, C.J. Crazing Mechanism and Physical Aging of Poly(lactide) Toughened with Poly(ethylene oxide)-block-poly(butylene oxide) Diblock Copolymers. Macromolecules 2020, 53, 10163–10178. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Odent, J.; Raquez, J.; Dubois, P. Highly Toughened Polylactide-Based Materials through Melt-Blending Techniques. In Biodegradable Polyesters; Fakirov, S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Berlin, Germany, 2015. [Google Scholar]
- Nyambo, C.; Mohanty, A.K.; Misra, M. Polylactide-based renewable green composites from agricultural residues and their hybrids. Biomacromolecules 2010, 11, 1645–1660. [Google Scholar] [CrossRef]
- Van den Berg, S.A.; Zuilhof, H.; Wennekes, T. Clickable Polylactic Acids by Fast Organocatalytic Ring-Opening Polymerization in Continuous Flow. Macromolecules 2016, 49, 2054–2062. [Google Scholar] [CrossRef]
- Hwang, J.J.; Liu, H.J. Influence of Organophilic Clay on the Morphology, Plasticizer-Maintaining Ability, Dimensional Stability, and Electrochemical Properties of Gel Polyacrylonitrile (PAN) Nanocomposite Electrolytes. Macromolecules 2002, 35, 7314–7319. [Google Scholar] [CrossRef]
- Cui, Y.; Kumar, S.; Rao Kona, B.; van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 2015, 5, 63669–63690. [Google Scholar] [CrossRef]
- Francis, V.; Jain, P.K. Experimental investigations on fused deposition modelling of polymer-layered silicate nanocomposite. Virtual Phys. Prototyp. 2016, 11, 109–121. [Google Scholar] [CrossRef]
- Goh, K.; Heising, J.K.; Yuan, Y.; Karahan, H.E.; Wei, L.; Zhai, S.; Koh, J.X.; Htin, N.M.; Zhang, F.; Wang, R.; et al. Sandwich-Architectured Poly(lactic acid)–Graphene Composite Food Packaging Films. ACS Appl. Mater. Interfaces 2016, 8, 9994–10004. [Google Scholar] [CrossRef] [PubMed]
- Hamed, N.S.; Razavi, R.S.; Loghman-Estarki, M.R.; Ramazani, M. The effect of organoclay on the morphology and mechanical properties of PAI/clay nanocomposites coatings prepared by the ultrasonication assisted process. Ulrason. Sonochem. 2017, 38, 306–316. [Google Scholar]
- Sato, S.; Yamauchi, J.; Takahashi, Y.; Kanehashi, S.; Nagai, K. Effects of Nanofiller-Induced Crystallization on Gas Barrier Properties in Poly(lactic acid)/Montmorillonite Composite Films. Ind. Eng. Chem. Res. 2020, 59, 12590–12599. [Google Scholar] [CrossRef]
- Pluta, M. Morphology and properties of polylactide modified by thermal treatment, filling with layered silicates and plasticization. Polymer 2004, 45, 8239–8251. [Google Scholar] [CrossRef]
- Basu, A.; Nazarkovsky, M.; Ghadi, R.; Khan, W.; Domb, A.J. Poly(lactic acid)-based nanocomposites. Polym. Adv. Technol. 2016, 28, 919–930. [Google Scholar] [CrossRef]
- Chafidz, A.; Tamzysi, C.; Kistriyani, L.; Kusumaningtyas, R.D.; Hartanto, D. Mechanical Properties of PP/clay Nanocomposites Prepared from Masterbatch: Effect of Nanoclay Loadings and Re-Processing. Key Eng. Mater. 2019, 805, 59–64. [Google Scholar] [CrossRef]
- Nagy, D.; Kókai, E. Polymer-based nanocomposites with nanoclay. IOP Conf. Ser. Mater. Sci. Eng. 2018, 448, 012021. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, P.; Lv, P.; Lemstra, P.J.; Cai, X.; Yang, W.; Dong, W.; Chen, M.; Liu, T.; Du, M.; et al. Excellent UV Resistance of Polylactide by Interfacial Stereocomplexation with Double-Shell-Structured TiO2 Nanohybrids. ACS Appl. Mater. Interfaces 2020, 12, 49090–49100. [Google Scholar] [CrossRef]
- De Azevedo Gonçalves Mota, R.C.; de Menezes, L.R.; da Silva, E.O. Poly(lactic acid) polymers containing silver and titanium dioxide nanoparticles to be used as scaffolds for bioengineering. J. Mater. Res. 2021. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and Antibacterial Performance of Novel Polylactic Acid-Graphene Oxide-Silver Nanoparticle Hybrid Nanocomposite Mats Prepared By Electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Li, F.; Zhang, C.; Weng, Y. Improvement of the Gas Barrier Properties of PLA/OMMT Films by Regulating the Interlayer Spacing of OMMT and the Crystallinity of PLA. ACS Omega 2020, 5, 18675–18684. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, W.H.; Zhang, H.; Qu, J.P. Constructing Bone-Mimicking High-Performance Structured Poly(lactic acid) by an Elongational Flow Field and Facile Annealing Process. ACS Appl. Mater. Interfaces 2020, 12, 13411–13420. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.; Botelho, G.; Oliveira, M.; Machado, A.V. Influence of clay organic modifier on the thermal-stability of PLA based nanocomposites. Appl. Clay Sci. 2014, 88–89, 144–150. [Google Scholar] [CrossRef]
- Alves, J.L.; Rosa, P.T.V.; Realinho, V.; Antunes, M.; Velasco, J.I.; Morales, A.R. The effect of Brazilian organic-modified montmorillonites on the thermal stability and fire performance of organoclay-filled PLA nanocomposites. Appl. Clay Sci. 2020, 194, 105697:1–105697:8. [Google Scholar] [CrossRef]
- Huang, S.M.; Hwang, J.J.; Liu, H.J.; Lin, L.H. Crystallization Behavior of Poly(L-lactic acid)/Montmorillonite Nanocomposites. J. Appl. Polym. Sci. 2010, 117, 434–442. [Google Scholar] [CrossRef]





| Sample Label (Clay Ratio) | A (0.0%) | B (0.5%) | C (3.0%) | D (5.0%) | E (8%) |
|---|---|---|---|---|---|
| PLA amount (g) | 40 | 40 | 40 | 40 | 40 |
| OMMT amount (g) | 0 | 0.2 | 1.2 | 2 | 3.2 |
| Sample Label | A | B | C | D | E |
|---|---|---|---|---|---|
| PLA amount (g) | 4000 | 4000 | 4000 | 4000 | 4000 |
| OMMT amount (g) | 0 | 20 | 120 | 200 | 320 |
| Clay ratio (%) | 0.0% | 0.5% | 3.0% | 5.0% | 8.0% |
| Zone 1 temperature (°C) | 160 | 160 | 160 | 160 | 160 |
| Zone 2 temperature (°C) | 163 | 162 | 160 | 155 | 150 |
| Zone 3 temperature (°C) | 183 | 182 | 180 | 175 | 170 |
| Zone 4 temperature (°C) | 188 | 187 | 185 | 180 | 178 |
| Zone 5 temperature (°C) | 192 | 192 | 190 | 185 | 183 |
| Zone 6 temperature (°C) | 184 | 182 | 180 | 175 | 173 |
| Zone 7 temperature (°C) | 177 | 177 | 175 | 170 | 168 |
| Feed chamber (°C) | 173 | 172 | 170 | 165 | 163 |
| Melt pressure (MPa) | 11.36 | 11.36 | 11.36 | 11.36 | 11.36 |
| Feeder speed (rpm) | 51~54 | 51~54 | 51~54 | 51~53 | 50~49 |
| Pelletizing machine speed (rpm) | 150~160 | 150~160 | 150~160 | 150~160 | 150~160 |
| Temperature | Tdeg (°C) | T5 wt% (°C) | |
|---|---|---|---|
| OMMT wt% | |||
| 0.0 | 336.84 | 325.14 | |
| 0.5 | 337.33 | 325.94 | |
| 3.0 | 339.08 | 326.48 | |
| 5.0 | 338.46 | 326.27 | |
| 8.0 | 335.00 | 326.27 | |
| Samples | A | B | C | D | E |
|---|---|---|---|---|---|
| OMMT (wt%) | 0.0 | 0.5 | 3.0 | 5.0 | 8.0 |
| Specimen area A0 (mm2) | 40.96 | 39.94 | 39.69 | 39.91 | 40.32 |
| Elongation (Lf-L0) (mm) | 2.54 | 3.84 | 5.81 | 2.26 | 1.35 |
| Elongation at Break (%) | 4.46 | 6.74 | 10.19 | 3.97 | 2.36 |
| Loading Pmax (N) | 2538.2 | 2389.3 | 2301.9 | 2265.9 | 2226.5 |
| Tensile Strength Pmax/A0 (MPa) | 61.97 | 59.82 | 58.00 | 56.77 | 55.22 |
| Loading Pf (N) | 2123.6 | 1935.0 | 1163.3 | 1624.5 | 1891.9 |
| Pulling Strength Pf/A0 (MPa) | 51.85 | 48.45 | 29.31 | 40.70 | 46.92 |
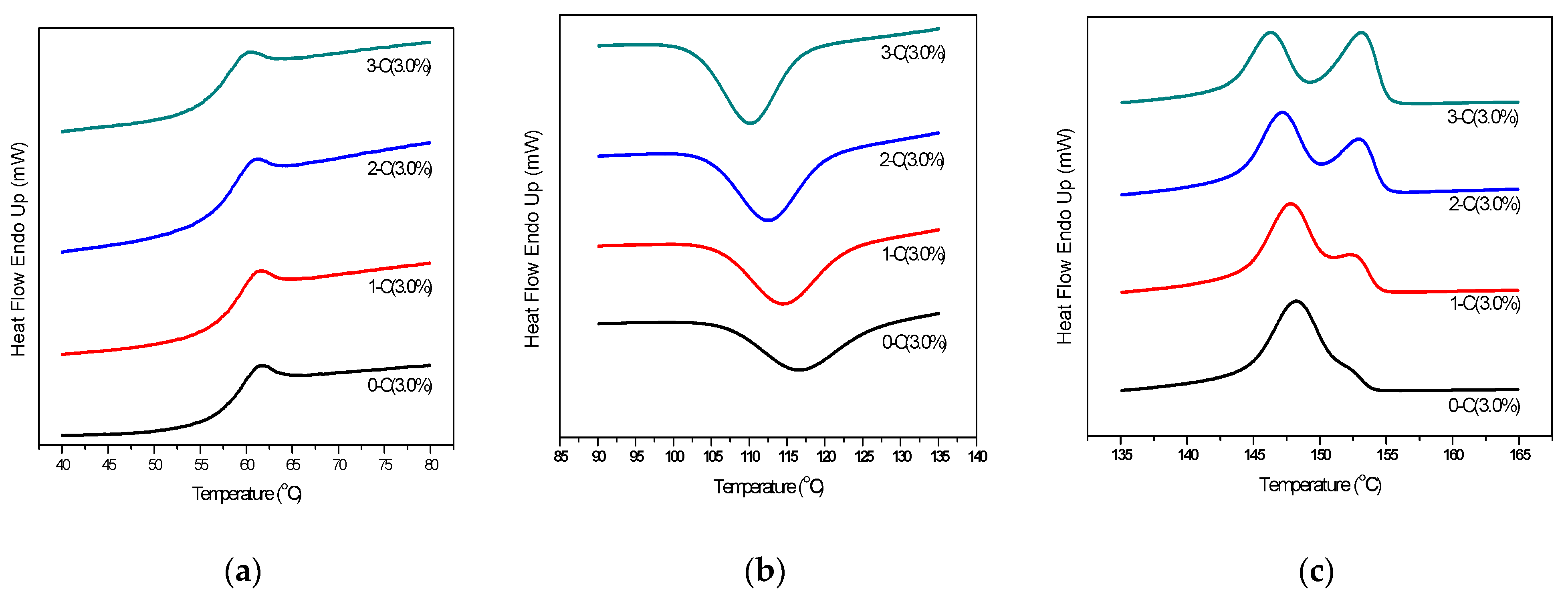
| Hydrolysis Week | OMMT (wt%) | Tg (°C) | Tc (°C) | Tm (°C) | |
|---|---|---|---|---|---|
| Tm1 | Tm2 | ||||
| WEEK 0 | 0.0 | 58.98 | -- | 148.2 | -- |
| 0.5 | 58.82 | 116.3 | 148.0 | -- | |
| 3.0 | 58.93 | 116.4 | 148.2 | -- | |
| 5.0 | 58.83 | 121.3 | 149.5 | -- | |
| 8.0 | 58.76 | 121.4 | 149.5 | -- | |
| WEEK 3 | 0.0 | 58.45 | -- | 148.4 | -- |
| 0.5 | 58.66 | 114.7 | 147.6 | 152.3 | |
| 3.0 | 58.65 | 114.4 | 147.8 | 152.7 | |
| 5.0 | 58.65 | 116.5 | 149.0 | 152.9 | |
| 8.0 | 58.12 | 116.9 | 148.3 | 153.2 | |
| WEEK 6 | 0.0 | 58.96 | -- | 148.5 | -- |
| 0.5 | 58.63 | 112.6 | 147.5 | 152.7 | |
| 3.0 | 58.47 | 113.8 | 147.1 | 153.1 | |
| 5.0 | 58.30 | 115.2 | 147.9 | 153.2 | |
| 8.0 | 57.43 | 116.8 | 147.5 | 152.9 | |
| WEEK 9 | 0.0 | 58.65 | -- | 149.0 | -- |
| 0.5 | 58.60 | 112.7 | 147.1 | 153.1 | |
| 3.0 | 58.50 | 112.7 | 146.3 | 153.2 | |
| 5.0 | 56.56 | 113.0 | 146.6 | 153.2 | |
| 8.0 | 56.99 | 113.7 | 145.9 | 152.7 | |
| OMMT Amount (wt%) | ΔTg (°C) | ΔTc (°C) | ΔTm (°C) | |
|---|---|---|---|---|
| ΔTm1 | * ΔTm2 | |||
| 0.0 | −0.33 | -- | +0.8 | -- |
| 0.5 | −0.22 | −3.6 | −0.9 | +0.8 |
| 3.0 | −0.43 | −3.7 | −1.9 | +0.5 |
| 5.0 | −2.27 | −8.3 | −2.9 | +0.3 |
| 8.0 | −1.77 | −7.7 | −3.6 | +0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.-M.; Hwang, J.-J.; Liu, H.-J.; Zheng, A.-M. A Characteristic Study of Polylactic Acid/Organic Modified Montmorillonite (PLA/OMMT) Nanocomposite Materials after Hydrolyzing. Crystals 2021, 11, 376. https://doi.org/10.3390/cryst11040376
Huang S-M, Hwang J-J, Liu H-J, Zheng A-M. A Characteristic Study of Polylactic Acid/Organic Modified Montmorillonite (PLA/OMMT) Nanocomposite Materials after Hydrolyzing. Crystals. 2021; 11(4):376. https://doi.org/10.3390/cryst11040376
Chicago/Turabian StyleHuang, Su-Mei, Jiunn-Jer Hwang, Hsin-Jiant Liu, and An-Miao Zheng. 2021. "A Characteristic Study of Polylactic Acid/Organic Modified Montmorillonite (PLA/OMMT) Nanocomposite Materials after Hydrolyzing" Crystals 11, no. 4: 376. https://doi.org/10.3390/cryst11040376
APA StyleHuang, S.-M., Hwang, J.-J., Liu, H.-J., & Zheng, A.-M. (2021). A Characteristic Study of Polylactic Acid/Organic Modified Montmorillonite (PLA/OMMT) Nanocomposite Materials after Hydrolyzing. Crystals, 11(4), 376. https://doi.org/10.3390/cryst11040376






