Mid-IR Optical Property of Dy:CaF2-SrF2 Crystal Fabricated by Multicrucible Temperature Gradient Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crystal Growth
2.2. Characterizations
3. Results and Discussion
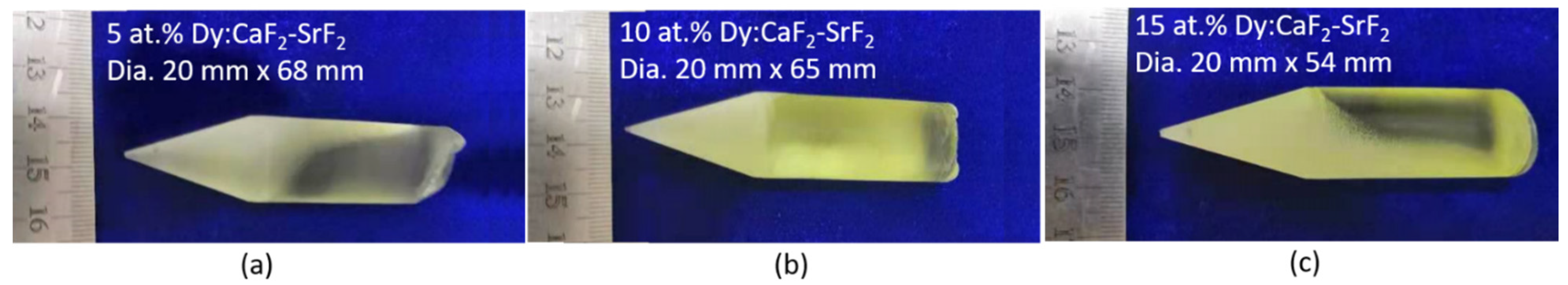
3.1. Crystal Structure and Optical Quality
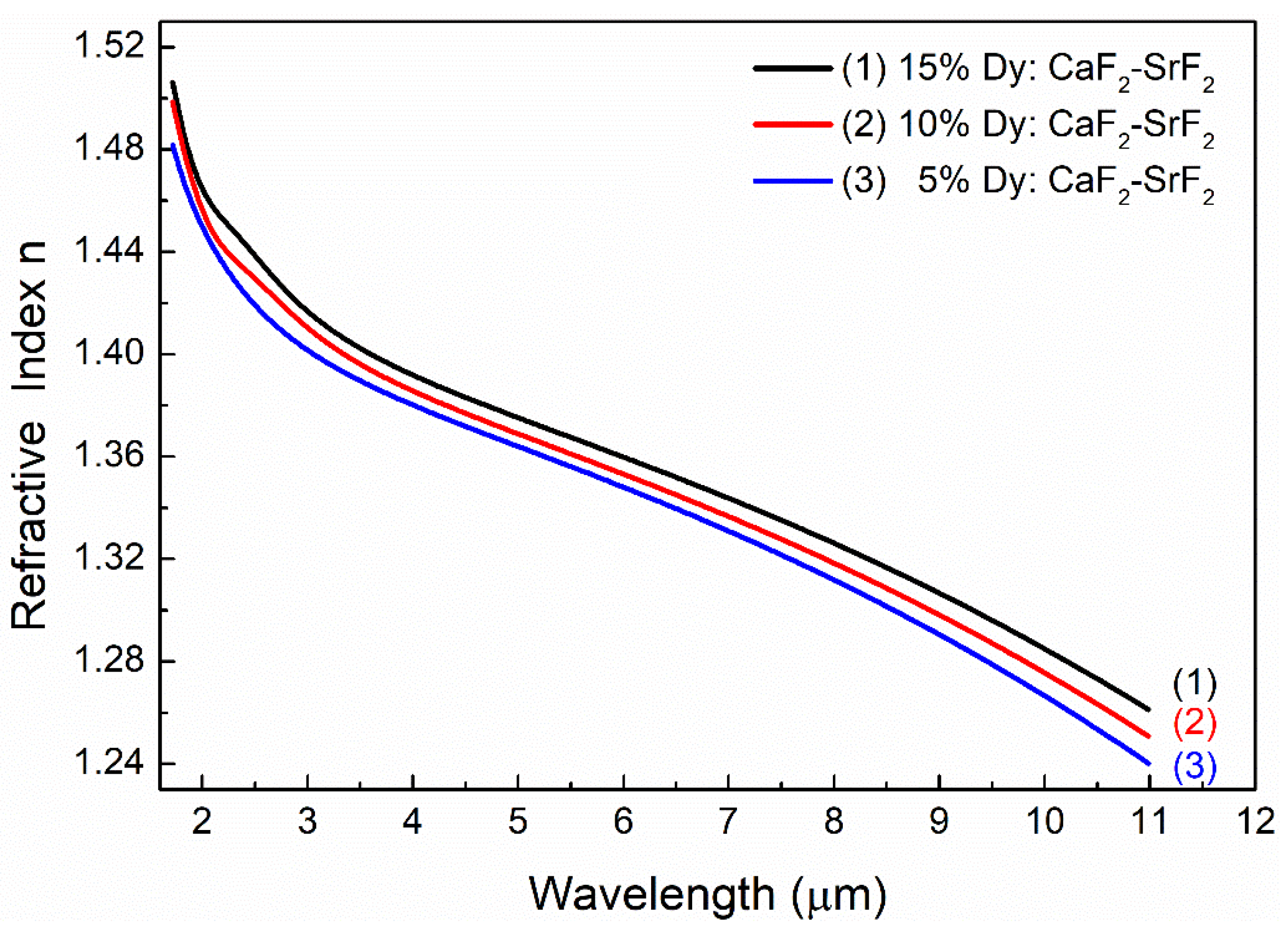
3.2. Refractive Index in the Mid-IR Spectral Range
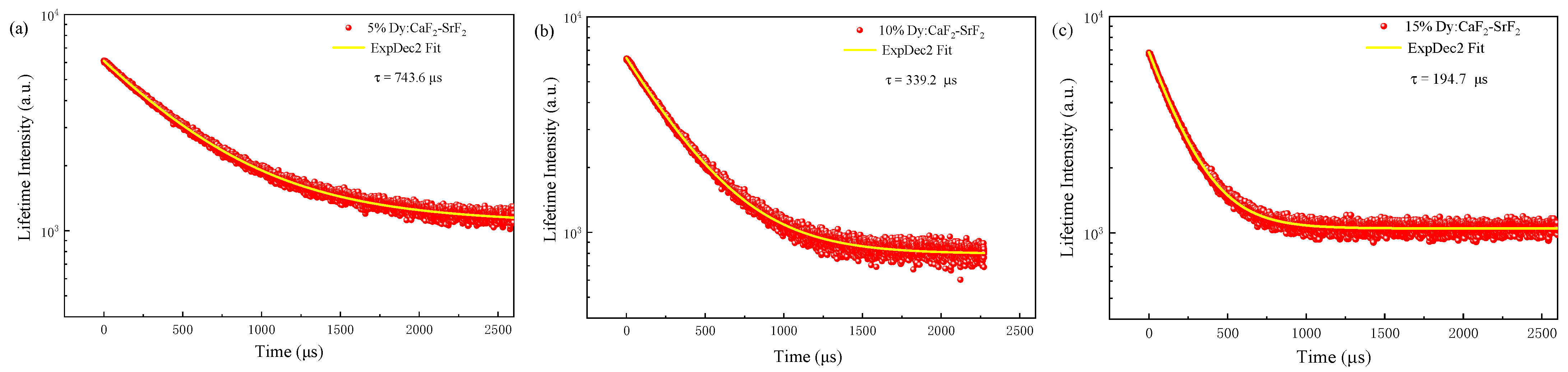
3.3. Absorption and Emission Spectra
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, Y.Y.; Xu, X.D.; Su, L.B.; Xu, J. Research Progress of Mid-infrared Laser Crystals. J. Synth. Cryst. 2020, 49, 1347–1360. [Google Scholar]
- Cao, D.; Peng, Q.; Du, S.; Xu, J.; Guo, Y.; Yang, J.; Bo, Y.; Zhang, J.; Cui, D.; Xu, Z. A 200 W diode-side-pumped CW 2 μm Tm:YAG laser with water cooling at 8 °C. Appl. Phys. B 2011, 103, 83–88. [Google Scholar] [CrossRef]
- Wang, S.Q.; Huang, H.T.; Chen, H.W.; Liu, X.; Liu, S.D.; Xu, J.L.; Shen, D.Y. High efficiency nanosecond passively Q-switched 2.3 μm Tm:YLF laser using a ReSe2-based saturable output coupler. OSA Contin. 2019, 2, 1676–1682. [Google Scholar] [CrossRef]
- Lancaster, D.G.; Gross, S.; Ebendorff-Heidepriem, H.; Fuerbach, A.; Withford, M.J.; Monro, T.M. 2.1 μm waveguide laser fabricated by femtosecond laser direct-writing in Ho3+, Tm3+:ZBLAN glass. Opt. Lett. 2012, 37, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Sottile, A.; Damiano, E.; Rabe, M.; Bertram, R.; Klimm, D.; Tonelli, M. Widely tunable, efficient 2 μm laser in monocrystalline Tm3+:SrF2. Opt. Express 2018, 26, 5368–5380. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.M.; Shen, Y.J.; Zhang, Z.; Su, L.B.; Dai, T.Y. A passively Q-switching of diode-pumped 2.08 µm Ho:CaF2 laser. Infrared Phys. Technol. 2019, 103, 103071. [Google Scholar] [CrossRef]
- Duan, X.M.; Shen, Y.J.; Yao, B.Q.; Wang, Y.Z. A 106 W Q-switched Ho:YAG laser with single crystal. Optik 2018, 169, 224–227. [Google Scholar] [CrossRef]
- Fan, M.Q.; Li, T.; Zhao, J.; Zhao, S.Z.; Li, G.Q.; Yang, K.J.; Su, L.B.; Ma, H.Y.; Kränkel, C. Continuous wave and ReS2 passively Q-switched Er:SrF2 laser at ∼3 μm. Opt. Lett. 2018, 43, 1726–1729. [Google Scholar] [CrossRef]
- Shen, B.J.; Kang, H.X.; Chen, P.; Liang, J.; Ma, Q.; Fang, J.; Sun, D.L.; Zhang, Q.L.; Yin, S.T.; Yan, X.P.; et al. Performance of continuous-wave laser-diode side-pumped Er:YSGG slab lasers at 2.79 μm. Appl. Phys. B 2015, 121, 511–515. [Google Scholar] [CrossRef]
- Jackson, S.D. Continuous wave 2.9 μm dysprosium-doped fluoride fiber laser. Appl. Phys. Lett. 2003, 83, 1316–1318. [Google Scholar] [CrossRef]
- Tsang, Y.H.; El-Taher, A.E. Efficient lasing at near 3 µm by a Dy-doped ZBLAN fiber laser pumped at ∼1.1 µm by an Yb fiber laser. Laser Phys. Lett. 2011, 8, 818–822. [Google Scholar] [CrossRef]
- Majewski, M.R.; Jackson, S.D. Highly efficient mid-infrared dysprosium fiber laser. Opt. Lett. 2016, 41, 2173–2176. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.I.; Majewski, M.R.; Bharathan, G.; Hudson, D.D.; Fuerbach, A.; Jackson, S.D. Watt-level dysprosium fiber laser at 3.15 µm with 73% slope efficiency. Opt. Lett. 2018, 43, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Djeu, N.; Hartwell, V.E.; Kaminskii, A.A.; Butashin, A.V. Room-temperature 3.4-µm Dy:BaYb2F8 laser. Opt. Lett. 1997, 22, 997–999. [Google Scholar] [CrossRef]
- Barnes, N.P.; Allen, R.E. Room temperature Dy:YLF laser operation at 4.34 µm. IEEE J. Quantum Electron. 1991, 27, 277–282. [Google Scholar] [CrossRef]
- Jelinkova, H.; Doroshenko, M.E.; Osiko, V.V.; Jelínek, M.; Šulc, J.; Němec, M.; Vyhlídal, D.; Badikov, V.V.; Badikov, D.V. Dysprosium thiogallate laser: Source of mid-infrared radiation at 2.4, 4.3, and 5.4 µm. Appl. Phys. A 2016, 122, 738. [Google Scholar] [CrossRef]
- Jelínková, H.; Doroshenko, M.E.; Jelínek, M.; Šulc, J.; Osiko, V.V.; Badikov, V.V.; Badikov, D.V. Dysprosium-doped PbGa2S4 laser generating at 4.3 µm directly pumped by 1.7 µm laser diode. Opt. Lett. 2013, 38, 3040–3043. [Google Scholar] [CrossRef]
- Nostrand, M.C.; Page, R.H.; Payne, S.A.; Krupke, W.F.; Schunemann, P.G. Room-temperature laser action at 4.3–4.4 µm in CaGa2S4:Dy3+. Opt. Lett. 1999, 24, 1215–1217. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, H.T.; Liu, J.; He, Y.F.; Jiang, D.P.; Tang, F.; Su, L.B. Tunable Yb:CaF2-SrF2 laser and femtosecond mode-locked performance based on semiconductor saturable absorber mirrors. Appl. Opt. 2016, 55, 8359–8362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.B.; Zheng, L.H.; Jiang, D.P.; Yin, H.D.; Zheng, J.G.; Yang, Q.H.; Cheng, G.F.; Su, L.B. Growth and optical properties of ytterbium and rare earth ions codoped CaF2-SrF2 eutectic solid-solution (RE = Y3+, Gd3+, La3+). J. Rare Earths 2021, 39, 390–397. [Google Scholar] [CrossRef]
- Ma, F.; Su, F.; Zhou, R.; Ou, Y.; Xie, L.; Liu, C.; Jiang, D.; Zhang, Z.; Wu, Q.; Su, L.; et al. The defect aggregation of RE3+ (RE = Y, La ∼ Lu) in MF2 (M = Ca, Sr, Ba) fluorites. Mater. Res. Bull. 2020, 125, 110788. [Google Scholar] [CrossRef]
- Ruan, F.F.; Yang, L.; Hu, G.; Wang, A.M.; Xue, Y.Y.; Yang, L.L.; Wang, Z.X.; Wu, S.H.; He, Z.L. Luminescence Properties of Dy3+ Doped Lanthanum Fluoride Crystal by Multi-crucible Temperature Gradient Technology. Chin. J. Lumin. 2021, 42, 158–164. [Google Scholar] [CrossRef]
- Renaud, E.; Robelin, C.; Heyrman, M.; Chartrand, P. Thermodynamic evaluation and optimization of the (LiF + NaF + KF + MgF2 + CaF2 + SrF2) system. J. Chem. Thermodyn. 2009, 41, 666–682. [Google Scholar] [CrossRef]
- Klimm, D.; Rabe, M.; Bertram, R.; Uecker, R.; Parthier, L. Phase diagram analysis and crystal growth of solid solutions Ca1-xSrxF2. J. Cryst. Growth 2008, 310, 152–155. [Google Scholar] [CrossRef] [Green Version]
- Karimov, D.N.; Komar’kova, O.N.; Sorokin, N.I.; Bezhanov, V.A.; Chernov, S.P.; Popov, P.A.; Sobolev, B.P. Growth of congruently melting Ca0.59Sr0.41F2 crystals and study of their properties. Crystallogr. Rep. 2010, 55, 518–524. [Google Scholar] [CrossRef]
- Xu, J.W.; Zhou, Y.Z.; Zhou, G.Q.; Xu, K.; Deng, P.Z.; Xu, J. Growth of large-sized sapphire boules by temperature gradient technique (TGT). J. Cryst. Growth 1998, 193, 123–126. [Google Scholar]
- Johnson, L.F.; Guggenheim, H.J. Laser emission at 3 μm from Dy3+ in BaY2F8. Appl. Phys. Lett. 1973, 23, 96–98. [Google Scholar] [CrossRef]




| Crystals | m1 (g) | m2 (g) | ρ (g/cm3) |
|---|---|---|---|
| 5% Dy:CaF2-SrF2 | 12.24010 | 9.20478 | 4.03256 |
| 10% Dy:CaF2-SrF2 | 14.00670 | 10.69937 | 4.23505 |
| 15% Dy:CaF2-SrF2 | 13.22491 | 10.28247 | 4.49454 |
| Crystal | (× 10−20 cm2) | |||
|---|---|---|---|---|
| 804 nm | 907 nm | 1094 nm | 1287 nm | |
| 5% Dy:CaF2-SrF2 | 0.176 | 0.223 | 0.335 | 0.211 |
| 10% Dy:CaF2-SrF2 | 0.147 | 0.184 | 0.293 | 0.185 |
| 15% Dy:CaF2-SrF2 | 0.150 | 0.187 | 0.315 | 0.205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Zhao, J.; Wang, Y.; Chen, W.; Ruan, F.; Lin, H.; Xue, Y.; Liu, J.; Liu, Y.; Yang, R.; et al. Mid-IR Optical Property of Dy:CaF2-SrF2 Crystal Fabricated by Multicrucible Temperature Gradient Technology. Crystals 2021, 11, 907. https://doi.org/10.3390/cryst11080907
Zheng L, Zhao J, Wang Y, Chen W, Ruan F, Lin H, Xue Y, Liu J, Liu Y, Yang R, et al. Mid-IR Optical Property of Dy:CaF2-SrF2 Crystal Fabricated by Multicrucible Temperature Gradient Technology. Crystals. 2021; 11(8):907. https://doi.org/10.3390/cryst11080907
Chicago/Turabian StyleZheng, Lihe, Jianbin Zhao, Yangxiao Wang, Weichao Chen, Fangfang Ruan, Hui Lin, Yanyan Xue, Jian Liu, Yang Liu, Ruiqin Yang, and et al. 2021. "Mid-IR Optical Property of Dy:CaF2-SrF2 Crystal Fabricated by Multicrucible Temperature Gradient Technology" Crystals 11, no. 8: 907. https://doi.org/10.3390/cryst11080907
APA StyleZheng, L., Zhao, J., Wang, Y., Chen, W., Ruan, F., Lin, H., Xue, Y., Liu, J., Liu, Y., Yang, R., Lu, H., Xu, X., & Su, L. (2021). Mid-IR Optical Property of Dy:CaF2-SrF2 Crystal Fabricated by Multicrucible Temperature Gradient Technology. Crystals, 11(8), 907. https://doi.org/10.3390/cryst11080907







