Optical Properties of CaNb2O6 Single Crystals Grown by OFZ*
Abstract
:1. Introduction
2. Materials and Methods
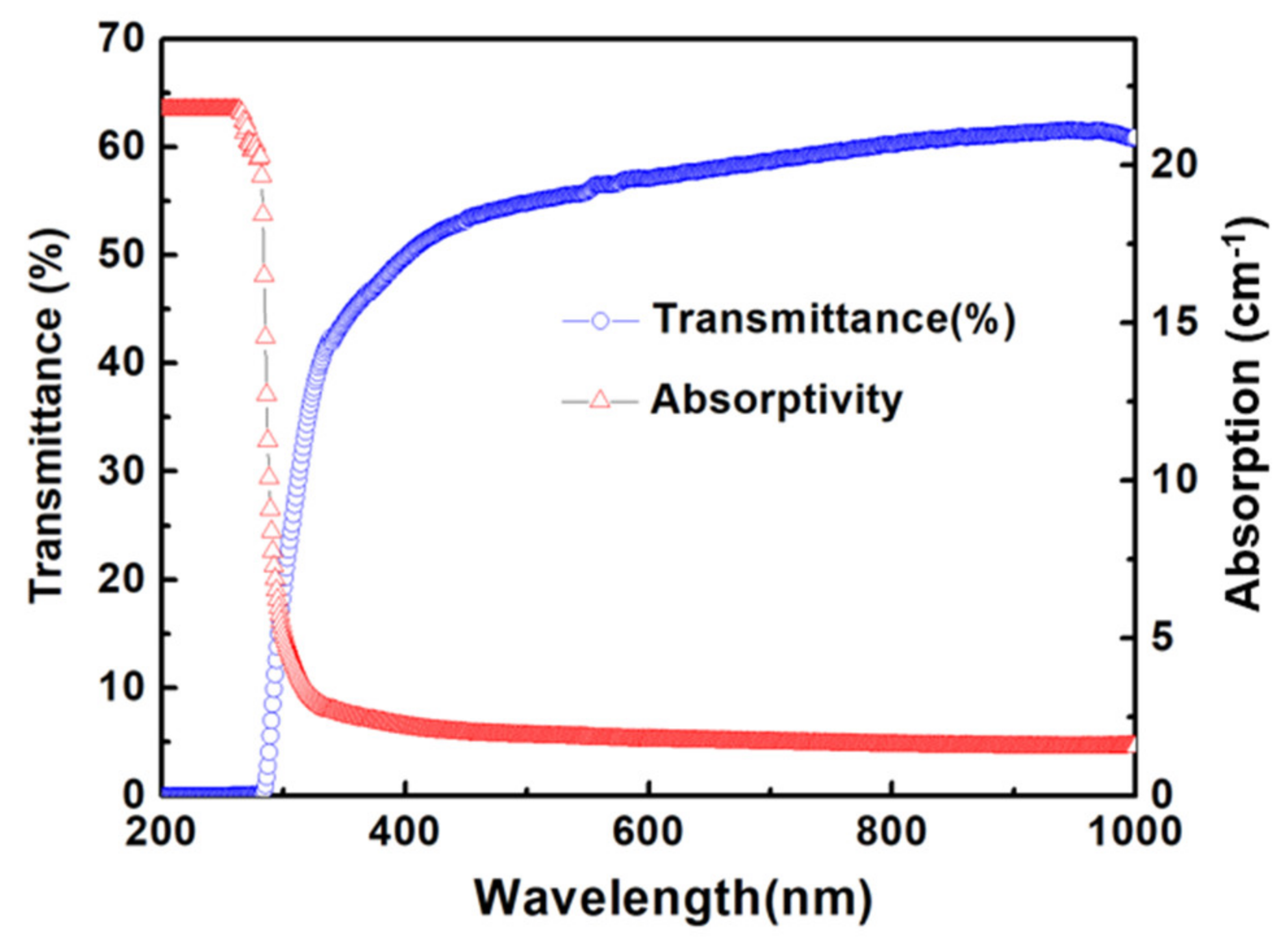
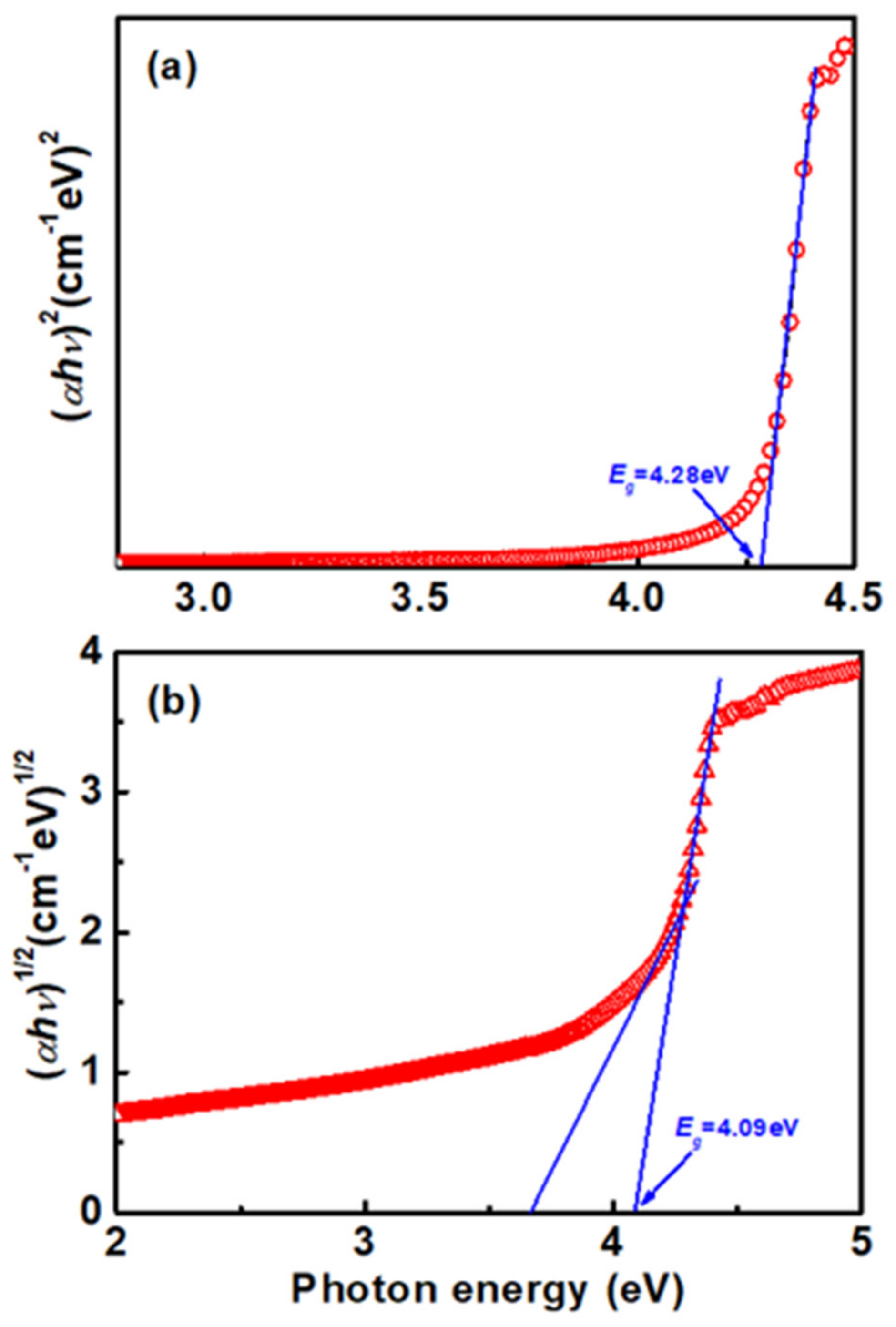
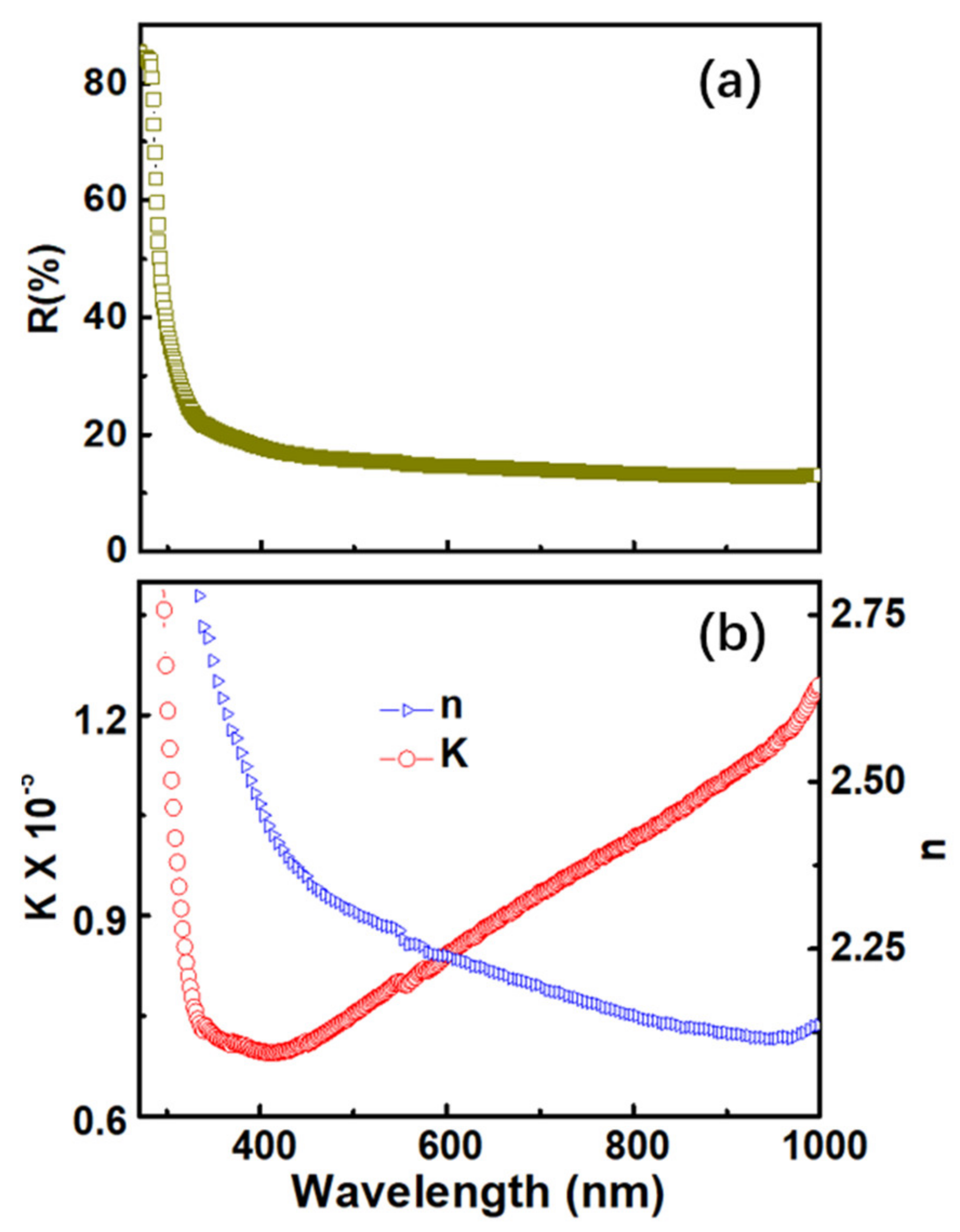
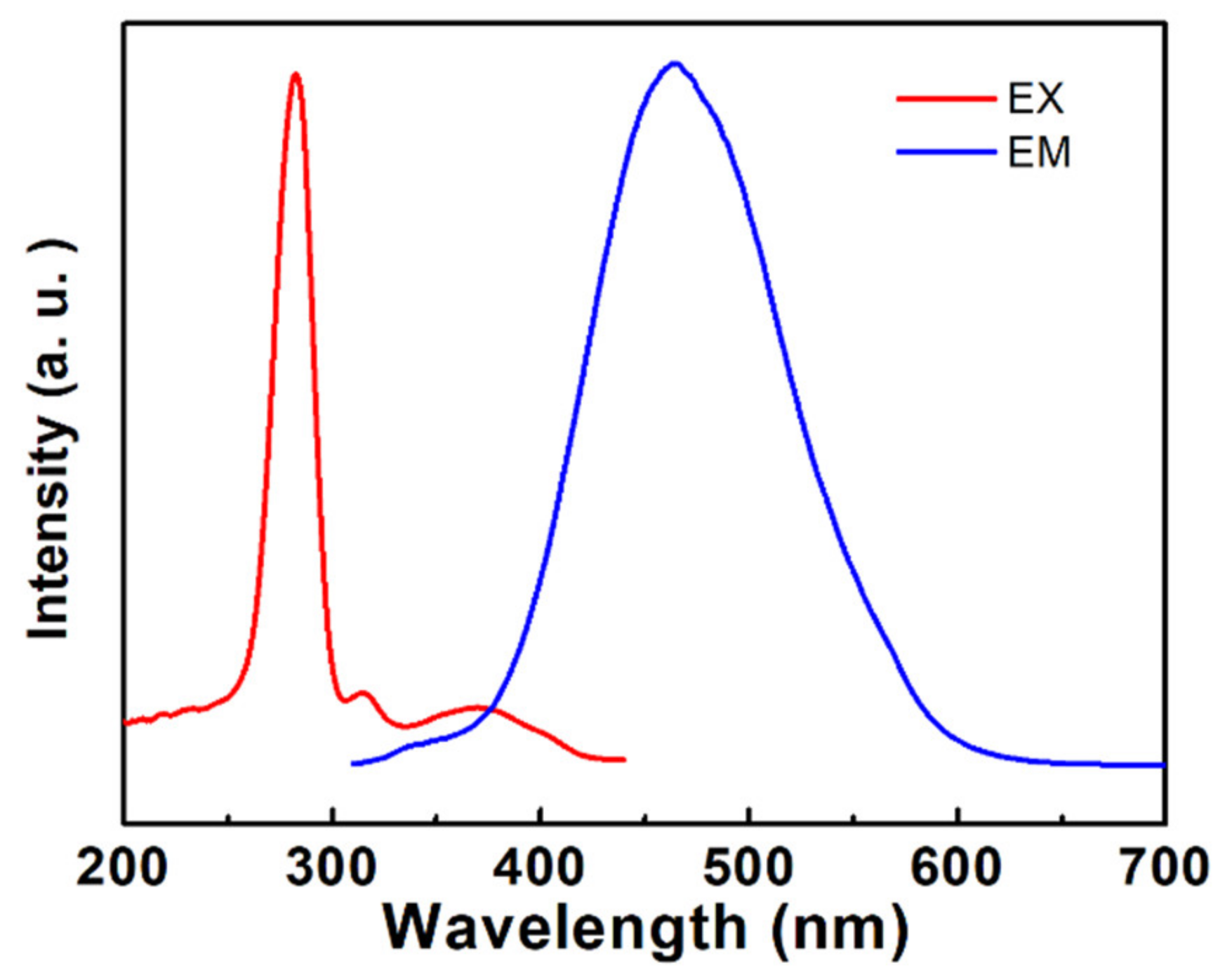
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Johnson, J.C.; Yan, H.; Schaller, R.D.; Petersen, P.B.; Yang, P.; Saykally, R.J. Near-field imaging of nonlinear optical mixing in single zinc oxide nanowires. Nano Lett. 2002, 2, 279–283. [Google Scholar] [CrossRef]
- Xiao, H.-M.; Fu, S.-Y.; Zhu, L.-P.; Li, Y.-Q.; Yang, G. Controlled synthesis and characterization of CuO nanostructures through a facile hydrothermal route in the presence of sodium citrate. Eur. J. Inorg. Chem. 2007, 1966–1971. [Google Scholar] [CrossRef]
- Yucai, H.J. Hydrothermal Synthesis of Nano Ce-Zr-Y Oxide Solid Solution for Automotive Three-Way Catalyst. Am. Ceram. Soc. 2006, 89, 2949. [Google Scholar] [CrossRef]
- Cao, A.M.; Hu, J.S.; Liang, H.P.; Wan, L.J. Self-Assembled Vanadium Pentoxide (V2O5) Hollow Microspheres from Nanorods and Their Application in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2005, 44, 4391–4395. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science 2001, 293, 2227–2231. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 9, 69–96. [Google Scholar] [CrossRef]
- Wu, T.; Lin, T.; Zhao, J.; Hidaka, H.; Serpone, N. TiO2-assisted photodegradation of dyes. 9. Photooxidation of a squarylium cyanine dye in aqueous dispersions under visible light irradiation. Environ. Sci. Technol. 1999, 33, 1379. [Google Scholar] [CrossRef]
- Cho, I.S.; Bae, S.T.; Yim, D.K.; Kim, D.W.; Hong, K.S. Preparation, characterization, and photocatalytic properties of CaNb2O6 nanoparticles. J. Am. Ceram. Soc. 2009, 92, 506–510. [Google Scholar] [CrossRef]
- Miseki, Y.; Kato, H.; Kudo, A. Water splitting into H2 and O2 over Ba5Nb4O15 photocatalysts with layered perovskite structure prepared by polymerizable complex method. Chem. Lett. 2006, 35, 1052–1053. [Google Scholar] [CrossRef]
- Maeda, K.; Eguchi, M.; Youngblood, W.J.; Mallouk, T.E. Niobium oxide nanoscrolls as building blocks for dye-sensitized hydrogen production from water under visible light irradiation. Chem. Mater. 2008, 20, 6770–6778. [Google Scholar] [CrossRef]
- Zou, Z.G.; Arakawa, H. Direct water splitting into H2 and O2 under visible light irradiation with a new series of mixed oxide semiconductor photocatalysts. J. Photochem. Photobiol. A Chem. 2013, 158, 145. [Google Scholar] [CrossRef]
- Yoshino, M.; Kakihana, M. Polymerizable Complex Synthesis of Pure Sr2NbxTa2-xO7 Solid Solutions with High Photocatalytic Activities for Water Decomposition into H2 and O2. Chem. Mater. 2020, 14, 3369–3376. [Google Scholar] [CrossRef]
- Hsiaoa, Y.J.; Liu, C.W.; Daia, B.T.; Chang, Y.H. Sol–gel synthesis and the luminescent properties of CaNb2O6 phosphor powders. J. Alloys Compd. 2009, 475, 698–701. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Qiu, Z.F.; Lu, M.K.; Ma, Q.; Zhang, A.Y.; Zhou, G.J.; Zhang, H.P.; Yang, Z.S. Photoluminescence characteristics of pure and Dy-doped ZnNb2O6 nanoparticles prepared by a combustion method. J. Phys. Chem. C 2007, 111, 10190–10193. [Google Scholar] [CrossRef]
- Cho, I.-S.; Kim, D.W.; Cho, C.M.; An, J.-S.; Roh, H.-S.; Hong, K.S. Synthesis, characterization and photocatalytic properties of CaNb2O6 with ellipsoid-like plate morphology. Solid Stat. Sci. 2010, 12, 982–988. [Google Scholar] [CrossRef]
- Cho, I.-S.; Bae, S.T.; Kim, D.H.; Hong, K.S. Effects of crystal and electronic structures of ANb2O6 (A=Ca, Sr, Ba) metaniobate compounds on their photocatalytic H2 evolution from pure water. Inter. J. Hyd. Energy 2010, 35, 12954. [Google Scholar] [CrossRef]
- Emmenegger, P. Preparation of single crystals of CaNb2O6 by chemical transport. J. Cryst. Growth 1968, 2, 109–110. [Google Scholar] [CrossRef]
- Oishi, S.; Nagai, Y.; Ishizawa, N. Flux Growth of CaNb2O6 Crystals. Nippon Kagaku Kaishi 1998, 9, 598. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.d.; de Camargo, A.S.S.; Cusatis, C.; Nunes, L.A.O.; Andreeta, J.P. Growth and characterization of columbite CaNb2O6 high quality single crystal fiber. J. Cry Grow. 2004, 262, 246–250. [Google Scholar] [CrossRef]
- Pless, J.D.; Erdman, N.; Ko, D.; Marks, L.D.; Stair, P.C.; Poeppelmeier, K.R. Single-crystal growth of magnesium orthovanadate, Mg3(VO4)2, by the optical floating zone technique. Cryst. Growth Des. 2003, 3, 615–619. [Google Scholar] [CrossRef]
- Majchrowski, A.; Swirkowicz, M.; Jaroszewicz, L.; Piasecki, M.; Kityk, I.V.; Brik, M.G. Crystal growth and spectroscopic studies of novel Yb-doped K5Nd (MoO4)4 single crystals. Mater. Lett. 2010, 64, 2363–2365. [Google Scholar] [CrossRef]
- Otani, S.; Aizawa, T. Floating-zone growth of CrB2 single crystals. J. Alloys Compd. 2008, 454, 147–149. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Wondre, F.R.; Boothroyd, A.T. Preparation of large single crystals of ANb2O6 (A= Ni, Co, Fe, Mn) by the floating-zone method. J. Cryst. Growth 2003, 250, 72–76. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Wang, D.; Feng, G.; Li, B.; Gao, Z.; Xu, D.; Ding, Z.; Liu, X. Large-size and high-quality Zn2TiO4 single crystal grown by the optical floating zone method. J. Cryst. Growth 2010, 312, 3561. [Google Scholar] [CrossRef]
- Islam, A.T.M.N.; Pieper, O.; Lake, B.; Siemensmeyer, K. Optical Floating-Zone Growth of Large Single Crystal of Spin Half Dimer Sr3Cr2O8. Cryst. Growth Des. 2010, 10, 465. [Google Scholar] [CrossRef]
- Yu, P.; Wu, A.; Su, L.; Guo, X.; Wang, Y.; Zhao, H.; Yang, Y.; Yang, Q.; Xu, J. Crystal growth and spectroscopic properties of MoO3 and WO3 doped Bi4Ge3O12 by optical floating zone method. J. Alloy. Compd. 2010, 503, 380–383. [Google Scholar] [CrossRef]
- Feng, H.; Ding, D.; Li, H.; Lu, S.; Pan, S.; Chen, X.; Ren, G. Growth and luminescence characteristics of cerium-doped yttrium pyrosilicate single crystal. J. Alloy. Compd. 2010, 489, 645–649. [Google Scholar] [CrossRef]
- Phan, M.H.; Franco, V.; Bingham, N.S.; Srikanth, H.; Hur, N.H.; Yu, S.C. Tricritical point and critical exponents of La0.7Ca0.3-xSrxMnO3 (x = 0, 0.05, 0.1, 0.2, 0.25) single crystals. J. Alloy. Compd. 2010, 508, 238–244. [Google Scholar] [CrossRef]
- Rudajevová, A.; Pospísil, J. Influence of anisotropy, the latent heat and the thermal history of alloy on martensitic transformation strain in Ni3Ta single crystal. J. Alloy. Compd. 2011, 509, 5500–5505. [Google Scholar] [CrossRef]
- Husson, E.; Dao, N.Q. Etude du Spectre Raman Polarise du Niobate de Calcium. C. R. Acad. Sci. C 1974, 279, 141. [Google Scholar]
- Husson, E.; Repelin, Y.; Dao, N.Q.; Brusset, H. Etude par spectrophotométries d’absorption infrarouge et de diffusion Raman des niobates de structure columbite. Spectrochim. Acta A 1977, 33, 995–1001. [Google Scholar] [CrossRef]
- Husson, E.; Repelin, Y.; Dao, N.Q.; Brusset, H. Normal coordinate analysis for CaNb2O6 of columbite structure. J. Chem. Phys. 1997, 66, 5173–5180. [Google Scholar] [CrossRef]
- Javed, A.; Hidetoshi, M.; Sher, A.; Jianding, Y.; Yasutomo, A.; Hiromoto, U. Optical Transmittance and Band Gap of Ferroelectric BaTi2O5 Bulk Glass. Chin Phys. Lett. 2008, 25, 4421. [Google Scholar]
- El-Korashy, A.; Abu El-Fadl, A. Temperature dependence of the optical band gap of nearly perfect K2ZnCl4 single crystals in the ferroelectric phase. Phys. B 1999, 271, 205. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Pang, G.; Jiao, S.; Zhu, S.; Wang, D.; Liang, D.; Feng, S. Hydrothermal Synthesis of a CaNb2O6 Hierarchical Micro/Nanostructure and Its Enhanced Photocatalytic Activity. Eur. J. Inorg. Chem. 2010, 2010, 1275. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, S.; Xiao, D.; Wang, X.; Xu, G. Optical absorption properties of doped lithium niobate crystals. J. Phys. Condens. Matter. 1992, 4, 2977. [Google Scholar]
- Matsumoto, Y.; Omae, M.; Watanabe, I.; Sato, E.I. Photoelectrochemical properties of the Zn-Ti-Fe spinel oxides. J. Electrochem. Soc. 1986, 133, 711. [Google Scholar] [CrossRef]
- Blasse, G. The luminescence of closed-shell transition-metal complexes. New developments. Struct. Bond 1980, 42, 1. [Google Scholar]
- Cao, J.; Ji, Y.; Li, J.; Zhu, Z.; You, Y.W.Z.; Tu, C. Crystal growth, Judd–Ofelt analysis and fluorescence properties of Tm3+: CaNb2O6 crystal. J. Lumin. 2011, 131, 1350–1354. [Google Scholar] [CrossRef]
- Li, L.; Feng, G.; Wang, D.; Yang, H.; Gao, Z.; Li, B.; Xu, D.; Ding, Z.; Liu, X. Optical floating zone method growth and photoluminescence property of MgNb2O6 crystal. J. Alloy. Compd. 2011, 509, L263–L266. [Google Scholar] [CrossRef]
- Srivastava, A.M.; Ackerman, J.F.; Beers, W.W. On the luminescence of Ba5M4O15 (M= Ta5+, Nb5+). J. Solid State Chem. 1997, 134, 187–191. [Google Scholar] [CrossRef]
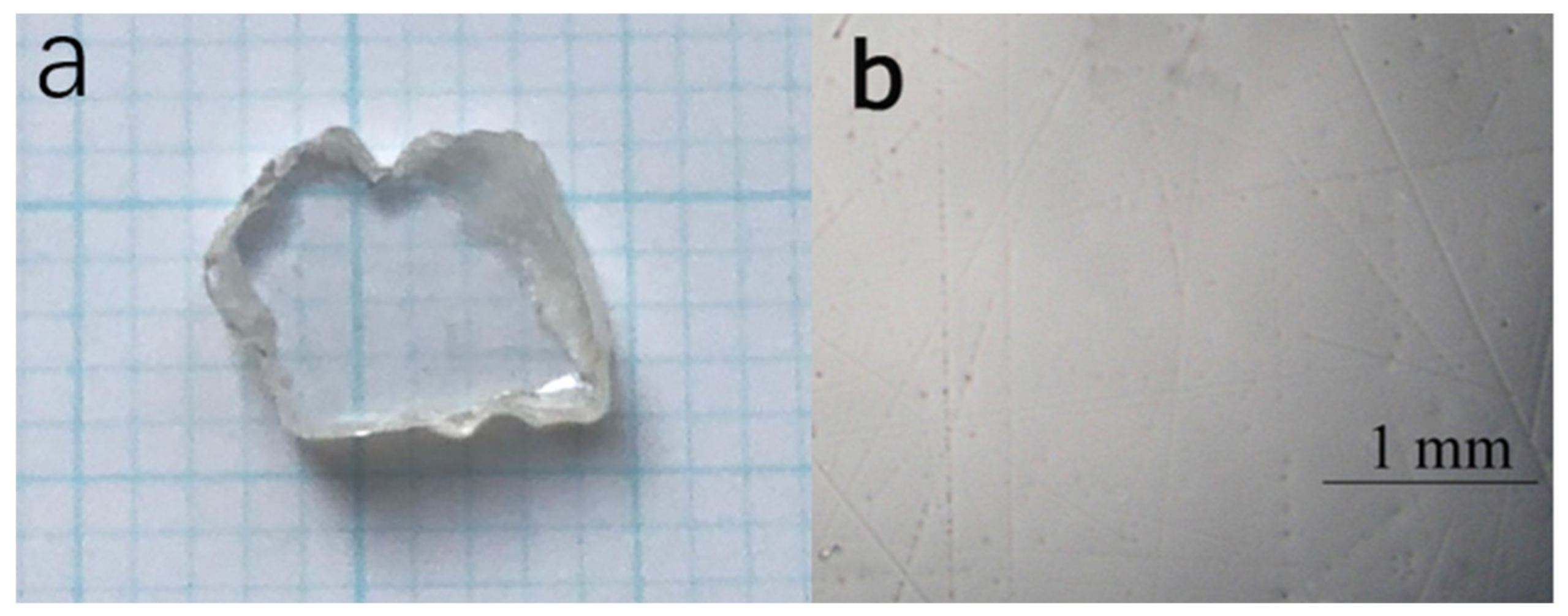
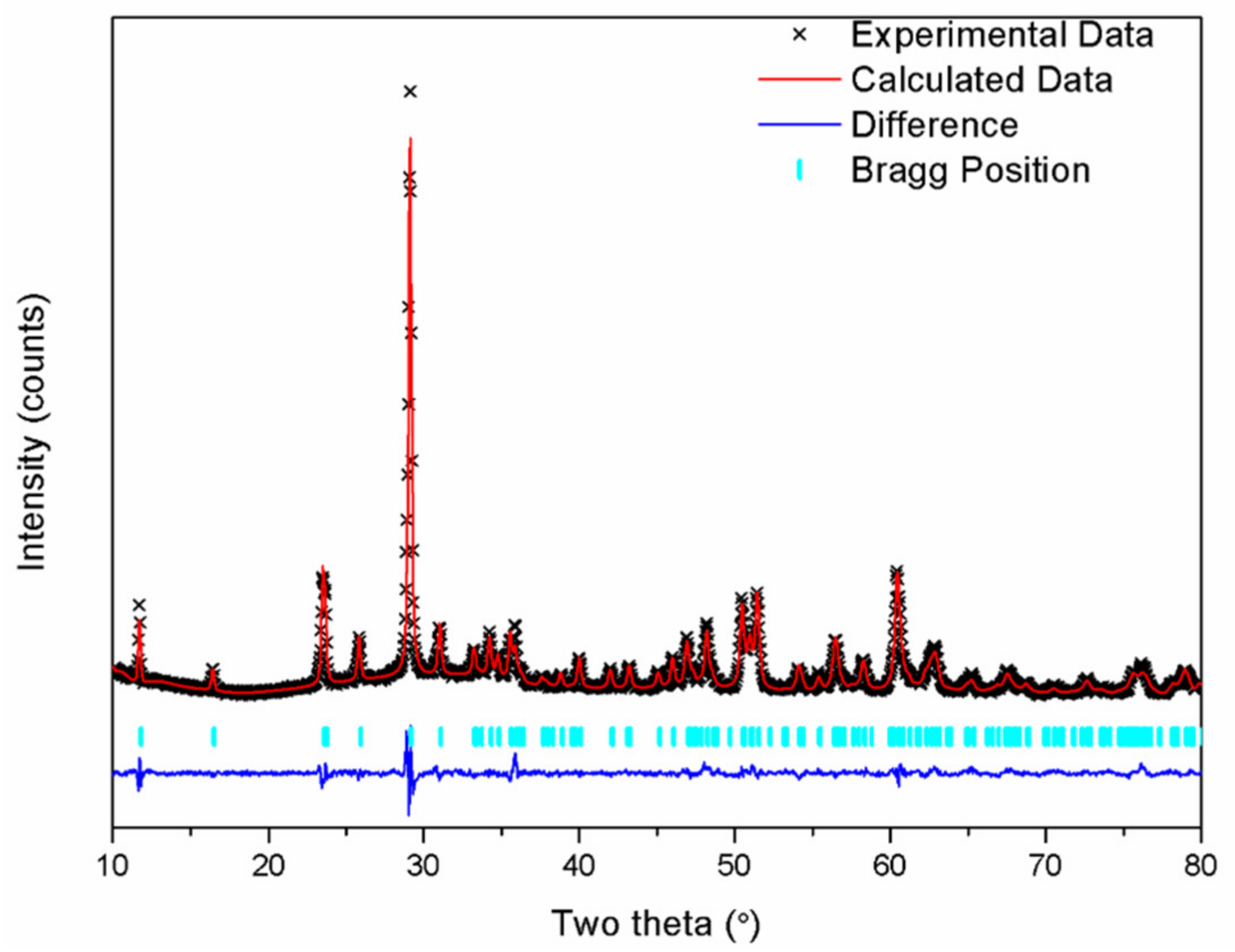
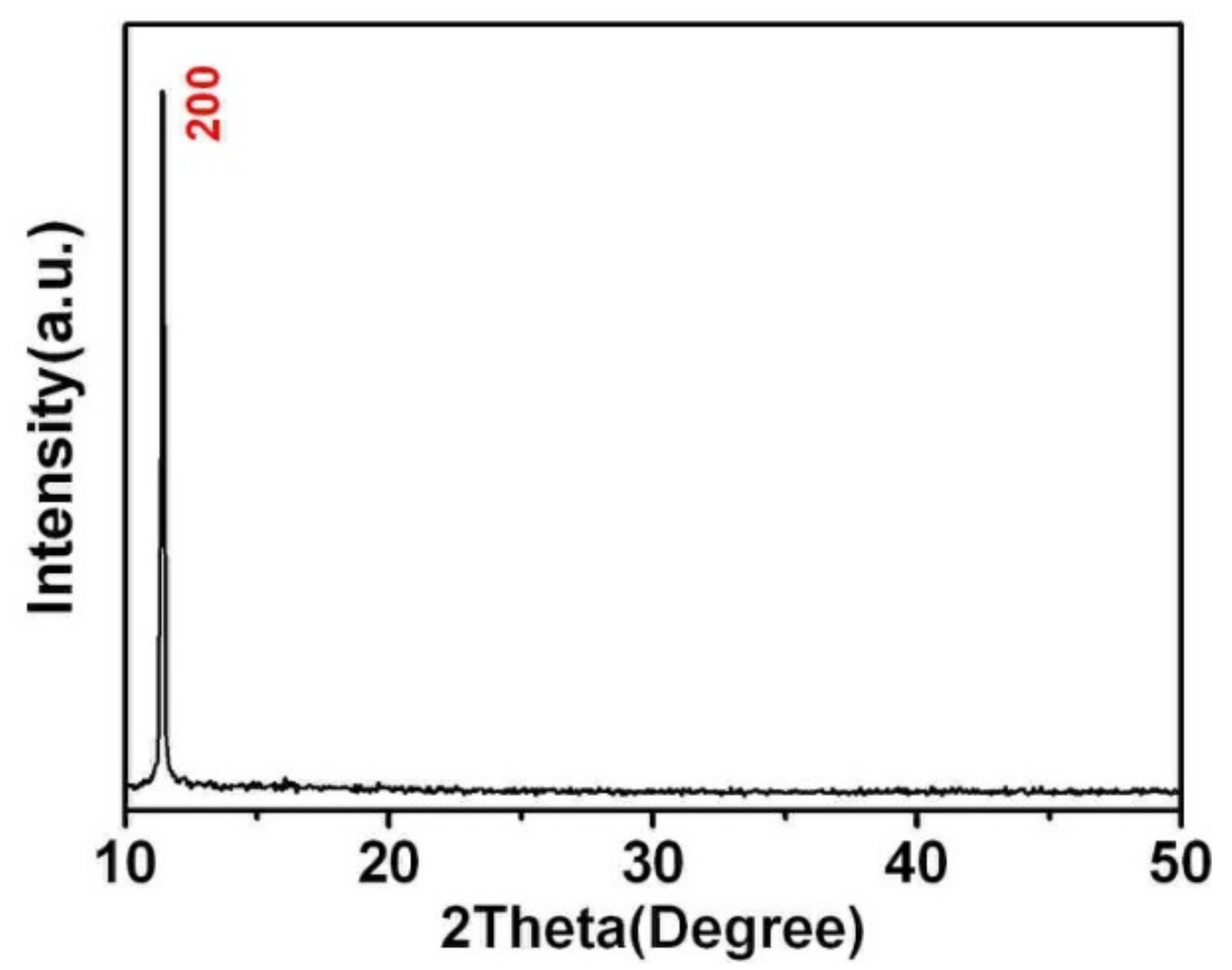
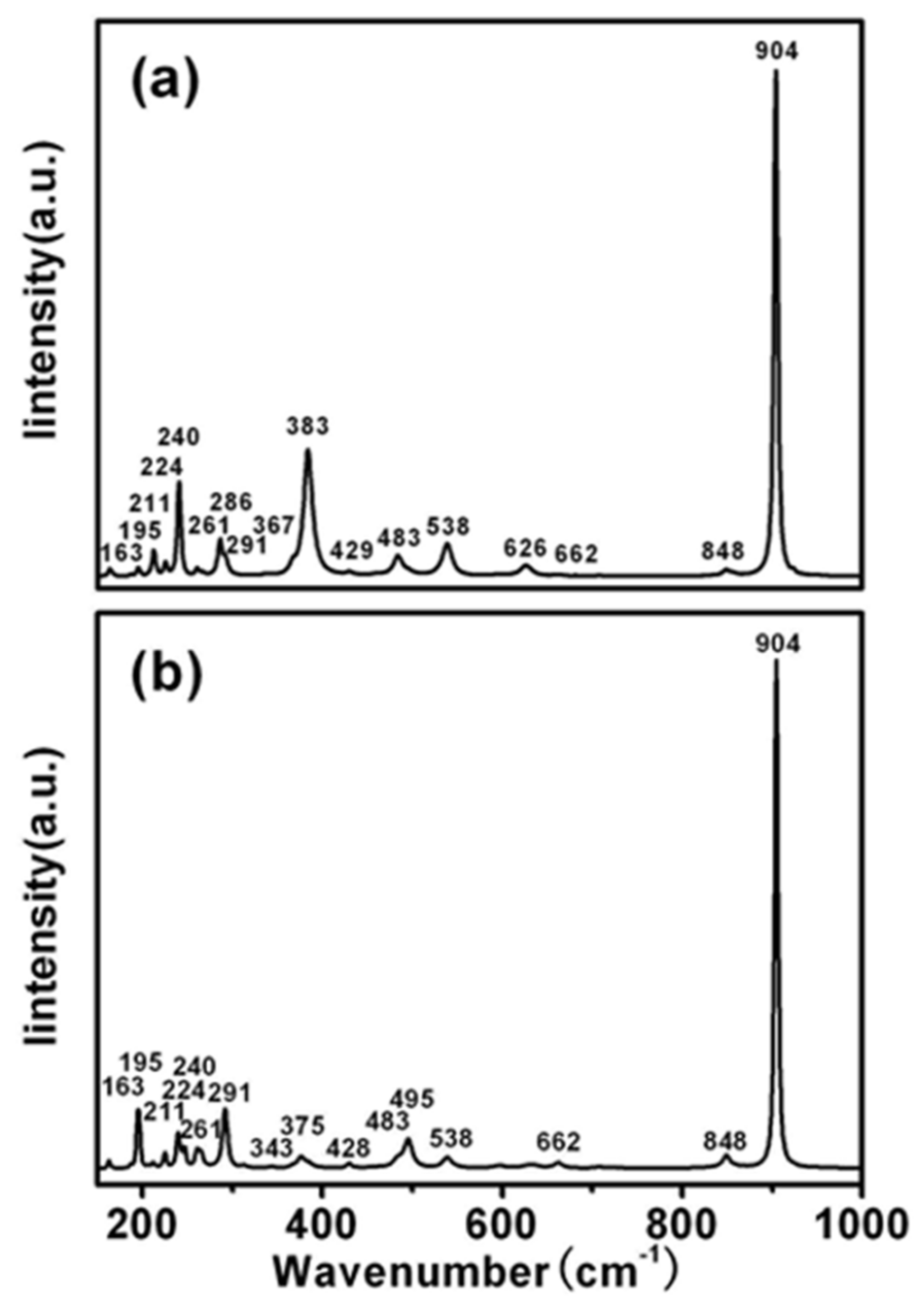
| Sample | CaNb2O6 |
|---|---|
| Space group | Pbcn |
| a (Å) | 14.9861 |
| b (Å) | 5.7482 |
| c (Å) | 5.2253 |
| Volume (Å3) | 450.130 |
| Density (g/cm3) | 4.709 |
| Rp | 0.0430 |
| Rwp | 0.0566 |
| X2 | 6.644 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Li, L.; Xu, D. Optical Properties of CaNb2O6 Single Crystals Grown by OFZ*. Crystals 2021, 11, 928. https://doi.org/10.3390/cryst11080928
Feng G, Li L, Xu D. Optical Properties of CaNb2O6 Single Crystals Grown by OFZ*. Crystals. 2021; 11(8):928. https://doi.org/10.3390/cryst11080928
Chicago/Turabian StyleFeng, Guanlin, Liang Li, and Dapeng Xu. 2021. "Optical Properties of CaNb2O6 Single Crystals Grown by OFZ*" Crystals 11, no. 8: 928. https://doi.org/10.3390/cryst11080928
APA StyleFeng, G., Li, L., & Xu, D. (2021). Optical Properties of CaNb2O6 Single Crystals Grown by OFZ*. Crystals, 11(8), 928. https://doi.org/10.3390/cryst11080928






