Abstract
Boron carbide shows high thermoelectric power. Therefore, it is an interesting material for thermoelectric applications. In the past, there were already successful uses of boron carbide as a thermocouple material together with graphite. However, more reliable, cost-efficient, and long-term stable solutions are required for practical benefit. Boron carbide and hafnium boride composites were prepared by pressureless sintering of B4C and HfC powder mixtures. The effect of HfC addition on the sinterability of boron carbide was studied. Highly densified ceramic with a relative density of 95.4% was obtained at a sintering temperature of 2250 °C. The composition and the microstructure of the dense composites are characterized by means of X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX). In addition, the correlation between the composition, Seebeck coefficient, and the electrical conductivity was investigated. The Seebeck coefficient of the composite is decreased and the electrical conductivity is increased with the increasing addition of HfC, and a change in conduction behavior from semiconducting to a metallic mechanism is observed. Functional thermocouples based on the prepared composites were tested and showed potential for temperature measurement application.
1. Introduction
Accurate and reliable temperature measurement is crucial for the quality of many industrial processes. Thermocouples are frequently used temperature sensors in industry due to their robustness and simplicity. Depending on material combinations, technically sophisticated thermocouples are available for various temperature ranges up to 1800 °C, such as Cu-CuNi (max. 400 °C) [1], NiCr-CuNi (max. 900 °C) [2], NiCr-Ni (max. 1300 °C) [2], and PtRh30%-PtRh6% (max. 1800 °C) [3]. High-temperature processes are gaining in importance in the field of advanced technologies, such as crystal growth, material manufacturing, aerospace, and power generation. However, with the current temperature measurement technologies, a simple and accurate temperature detection above 1800 °C is still very challenging [4,5,6].
The only commercially available thermocouples for measurement above 1800 °C are based on tungsten-rhenium (W-Re) alloys. Under inert gases or in a vacuum they can be used for measurement up to 2300 °C [7,8]. A general problem of these thermocouples is that the metals are subject to rapid aging due to recrystallization at high temperatures, which leads to embrittlement and thermoelectric drift of the thermocouples. The accuracy of the W-Re thermocouples is therefore seriously limited by the effects of their inhomogeneity and uncontrollable drift [9]. In the temperature range of 2000–2300 °C, W-Re thermocouples can only be operated for a short time due to the above-mentioned reasons.
Alternatively, radiation pyrometers can also be used for measuring very high temperatures. This is a very reliable and widely used method to measure temperatures up to 3000 °C [10]. Preconditions for an exact measurement are the sufficient knowledge of emission coefficients of the measuring objects and the objects must be optically visible to the infrared thermometers. However, in many technical processes, there are unpredictable changes in the emission conditions during the measurements. In addition, process-related occurrence of high gas pressure (e.g., hot isostatic pressing (HIP)), smoke, dust, steam, or deposits can hinder the optical access, so that the measurement becomes inaccurate or technically impossible.
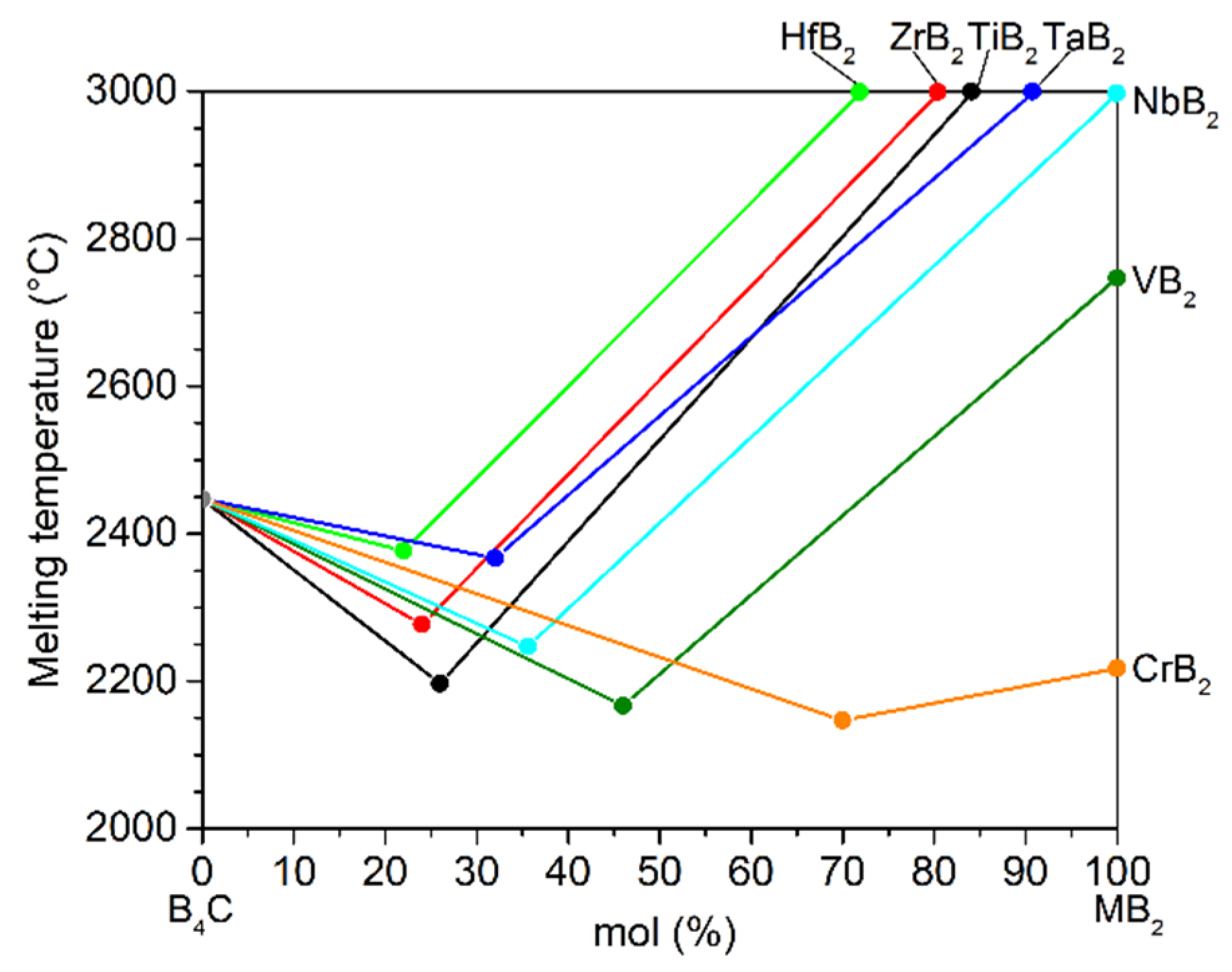
Therefore, the development of reliable temperature measurement technology for T > 1800 °C becomes increasingly important. In the 1990s, ceramic thermocouples based on boron carbide (B4C) and graphite/carbon were developed and showed potential applications for temperature measurements up to 2200 °C [11,12,13]. Materials used for such thermocouples should not only be stable at high temperatures in corresponding atmosphere but should also produce sufficient thermoelectric voltage when they are in junction with each other at one end and subjected to a temperature gradient. Boron carbide composites have been considered as candidate materials for high-temperature sensors due to their excellent high-temperature stability and high Seebeck coefficient with a level up to 300 μV/K [14,15]. Figure 1 shows a quasi-phase diagram scheme of promising material systems of boron carbide and metal boride (MB2, M = Hf, Zr, Ti, Ta, Nb, V, Cr) with respect to their eutectic temperatures above 2100 °C. In our previous works [15,16], it was found that the Seebeck coefficient of boron carbide could be tuned two orders of magnitude by the addition of different amounts of metal boride. Combining the two composite materials with only a difference in the metal boride compositions generates a thermocouple. Outstanding thermoelectric stability of the material system can be achieved due to the material similarity of the thermocouple components also at very high temperatures.
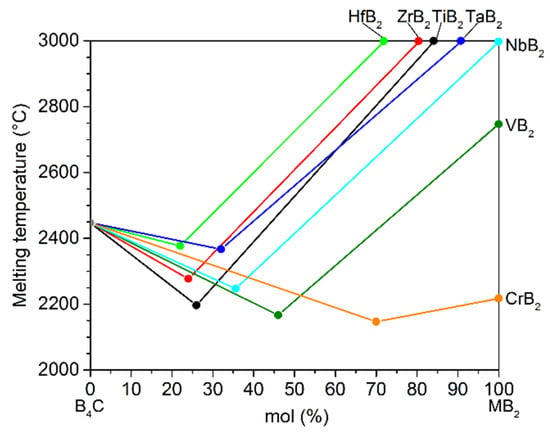
Figure 1.
Schematic illustration of eutectic melting points and single compounds in the quasi-phase diagram for the system of boron carbide and metal borides (B4C-MB2, M = Hf, Zr, Ti, Ta, Nb, V, Cr), showing the melting points of the single boron compounds and the corresponding eutectic points of their binary combinations [16,17,18,19].
In the present study, B4C-HfB2 composites were prepared via a direct reaction of B4C and HfC using the pressureless sintering technique, and the effects of HfC addition on sinterability, phase composition, microstructure, Seebeck coefficient, as well as electrical conductivity of the materials were studied. Moreover, the functionality of thermocouples based on B4C-HfB2 composites was examined.
2. Experimental Procedure
2.1. Sample Preparation
Commercially available B4C powder (grade HD20, d50 = 0.5 µm, H.C. Starck, Goslar, Germany) and HfC powder (d50 = 1.7 μm, ABCR GmbH, Karlsruhe, Germany) were used to produce B4C-HfB2 composites. Six batches of powder mixtures containing 2, 6, 10, 20, 30, and 40 wt.% HfC were prepared, and the corresponding products were denoted as BC2, BC6, BC10, BC20, BC30, and BC40, respectively. An overview of all produced compositions is presented in Table 1. The starting powders were firstly homogenized for 4 h by ball milling in ethanol using SiC milling balls. Organic additives (polyoxyethylene stearate, polyvinyl butyral resin, and PEG) with a total content of 3 wt.% were used as pressing agents. After drying in a vacuum rotary evaporator at 55 °C and oven-drying at 100 °C for 12 h, the powder mixtures were granulated through a sieve with a mesh size of 325 µm. Subsequently, different sizes of green bodies (discs: Ø 30 mm × 8 mm, and bars: 6 mm × 6 mm × 45 mm) were shaped by uniaxial pressing at 30 MPa followed by cold isostatic pressing (CIP) at 150 MPa.

Table 1.
Data of the composition, achieved density, and crystallographic phases for the prepared samples.
2.2. Pressureless Sintering
Prior to pressureless sintering, the organics were pyrolyzed at 800 °C for 30 min with a heating rate of 3 K/min in an argon atmosphere. Pressureless sintering of the composite samples was performed afterwards under argon in an electrical resistance furnace with graphite heating elements (model FSW 315/800-2600-PS; FCT Anlagenbau GmbH, Sonneberg, Germany). During the thermal treatment, the samples were firstly heated under vacuum to 1300 °C with a heating rate of 10 K/min for 30 min, to evaporate the surface oxide layer. After that dwell time, the samples were heated to 2250 °C with a heating rate of 5 K/min in flowing argon atmosphere and held at 2250 °C for 60 min.
2.3. Analytical Methods
To study the sintering behavior of the samples, high-temperature dilatometry experiments were carried out on pyrolyzed green bodies of BC10, BC20, BC30, and BC40 using a DIL 402ES/6 dilatometer (NETZSCH GmbH) in argon. The compacts were heated with 10 K/min up to 2250 °C and thereafter cooled down by 20 K/min to room temperature. Linear shrinkage (dl/l0) of the samples during sintering was calculated from the length displacement, dl, and starting length of the compacts, l0.
The density of the prepared composites was measured by the Archimedes method. The phase composition was determined by X-ray diffraction (XRD, Bruker D8) with Cu Kα radiation, and the microstructure was observed using field-emission scanning electron microscopy (FESEM, Gemini 982; CARL ZEISS) equipped with an energy-dispersive X-ray (EDX) detector. For microstructural characterization, the samples were prepared using a standard metallographic procedure with a final polish by 1 μm diamond paste. The Seebeck coefficients were measured using a specifically developed device (PhysTech GmbH and Fraunhofer IKTS, Hermsdorf, Germany) on sintered samples of 20 mm × 4 mm × 0.8 mm geometry. The thermoelectric voltage, ΔU, was measured between 2 contacts with a distance of 10 mm. The contacts were formed by silver brazing (CB 11; Umicore-BrazeTec, Hanau, Germany) on the 20 mm × 4 mm face and connected to a nanovoltmeter with gold wires using Ag/Pd paste. The thermoelectric power was obtained from the slope of the linear relationship between ΔU and the temperature gradient, ΔT. Electrical conductivity was measured by the 4-probe method from room temperature up to 1400 °C under Ar atmosphere. A Keithley 2050 multimeter was used to measure current and voltage.
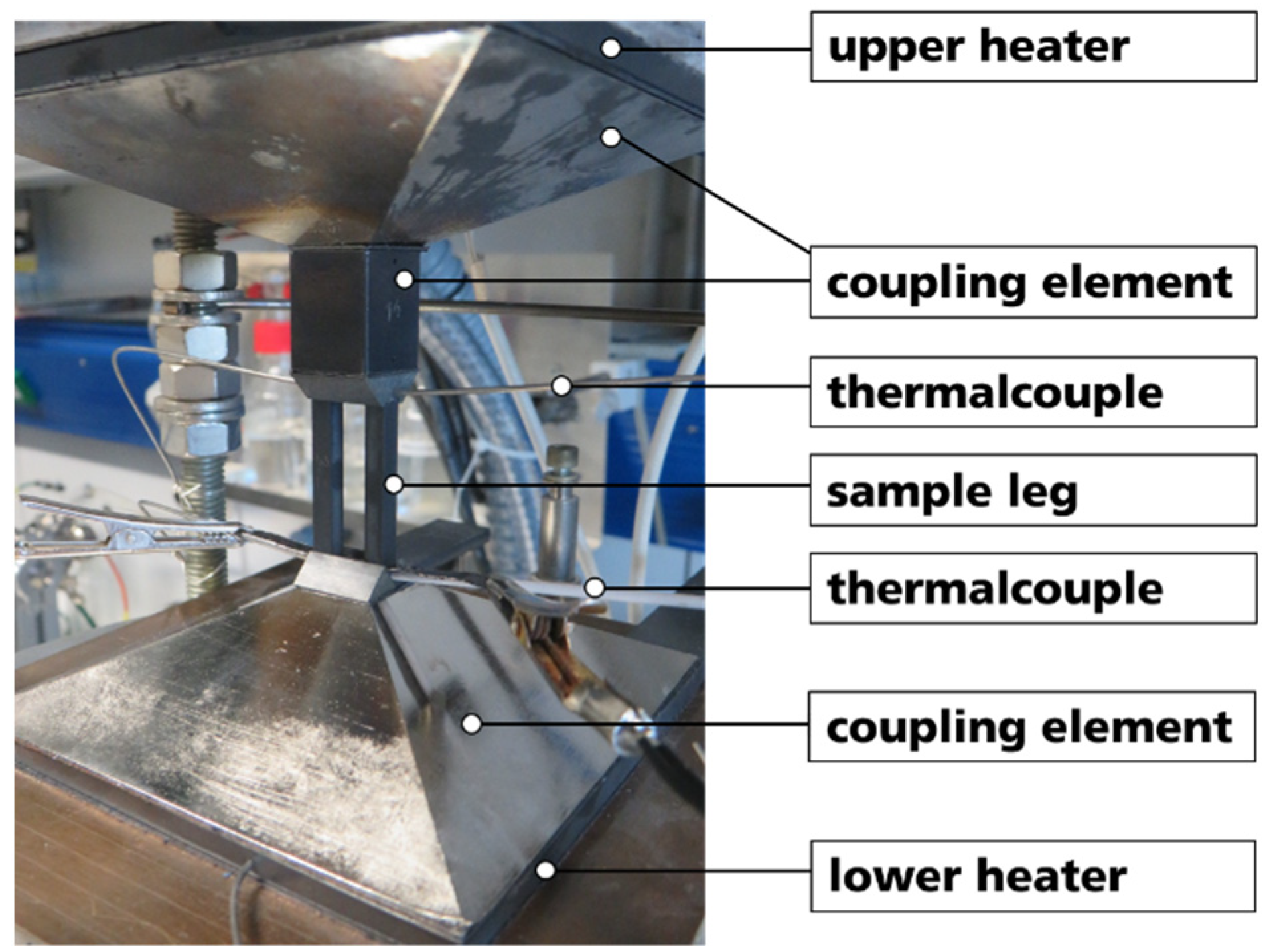
To evaluate the basic functionality of thermocouples based on the prepared B4C-HfB2 composites, thermoelectric voltage measurements were performed using a thermoelectric module-testing device (Mtec01-600, TEC COM GmbH, Kabelsketal, Germany) in N2 atmosphere. Two legs with dimensions of 4 mm × 4 mm × 30 mm of different combinations were fixed between two Si3N4-holders, and graphite foils were used for contacting the legs at the top end. The thermoelectric voltages (U) were measured by a multimeter up to a temperature difference ΔT = 502 K, and the hot-side temperature (Th) and cold-side temperature (Tc) of the legs were obtained by measuring the temperature directly at the samples. During testing, Tc was adjusted with a heat exchanger and kept at Tc = 45 °C. Experimental assembly of the test apparatus is shown in Figure 2.
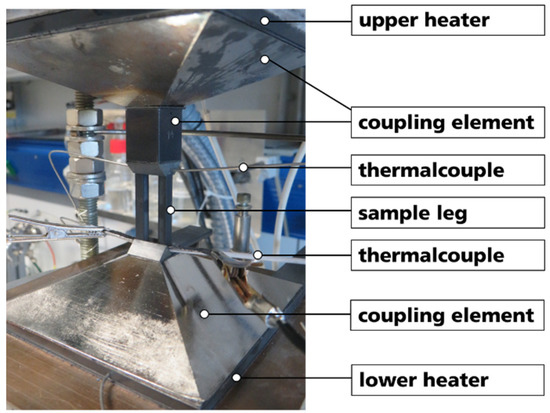
Figure 2.
Experimental assembly of test apparatus for performance evaluation of two sample legs as a thermocouple.
3. Results and Discussion
3.1. Thermodilatometry and Densification
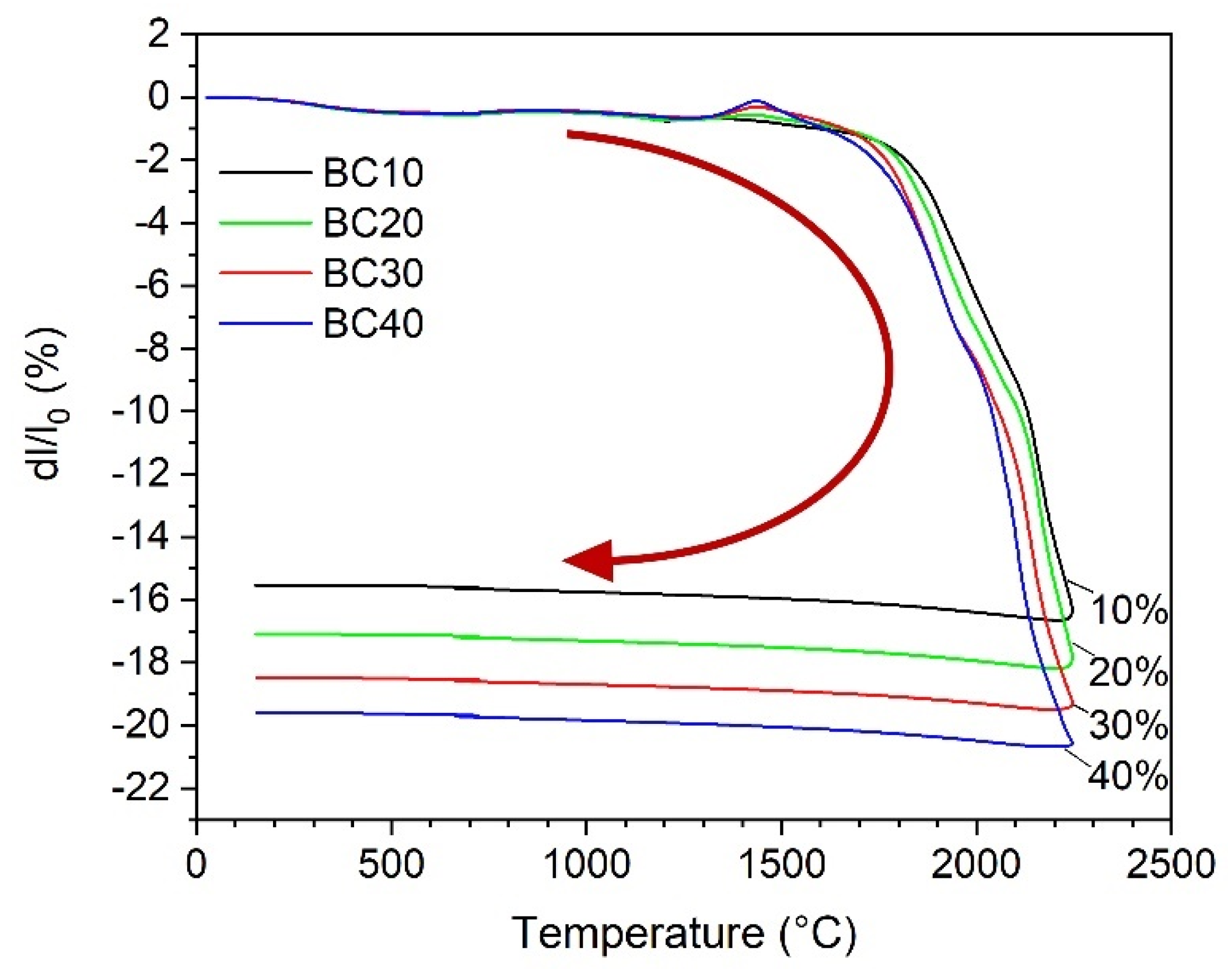
Full densification of boron carbide via pressureless sintering is still challenging due to its strong covalent bonding characteristics and low diffusion coefficients. It is well-known that the sinterability of boron carbide can be improved using metal carbide as a sintering aid [20,21]. As can be seen from the thermodilatometric curves in Figure 3, the onset temperature of the linear shrinkage decreases gradually from 1825 °C for B4C with 10 wt.% HfC (sample BC10) to 1775 °C for sample BC40 with 40 wt.% HfC addition. The final shrinkage increases from 15.5% for BC10 to 19.6% for BC40, respectively. This result is consistent with that of the samples after pressureless sintering at 2250 °C for 60 min in Table 1. The relative density of the samples increases from 88.6% for B4C with 2 wt.% HfC to 95.4% for the sample with 40 wt.% HfC addition. The presence of boron oxide in the starting B4C powder is the main reason for a retarded densification due to its high evaporation pressure at high temperatures in the process of pressureless sintering of boron carbide [22]. During the sintering procedure of B4C and HfC, a thermodynamically stable system of B4C-HfB2 is produced under the formation of free carbon according to Equation (1):
B4C + 2 HfC → 2 HfB2 + 3 C
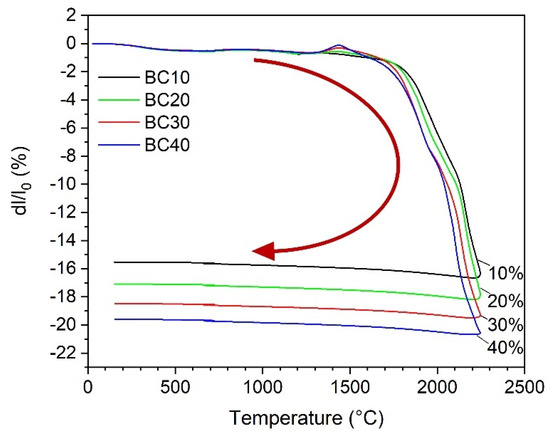
Figure 3.
Relative length change (dl/l0) up to 2250 °C of the composite samples with 10% (BC10), 20% (BC20), 30% (BC30), and 40% (BC40) HfC in initial powder mixture as a function of temperature. The arrow shows the sintering process from the beginning to the end.
The improvement of the sintering behavior of B4C with HfC addition can be attributed to the reduction of boron oxide impurities in B4C by carbon (Equation (2)):
2 B2O3 + 7 C → B4C + 6 CO
The free carbon may be fully consumed during the sintering process or stay in the composite if the oxide reduction is completed. Residual carbon at the grain boundaries can enhance the diffusion process of boron carbide, additionally facilitating accelerated solid-state sintering of B4C [21].
3.2. Phase Composition
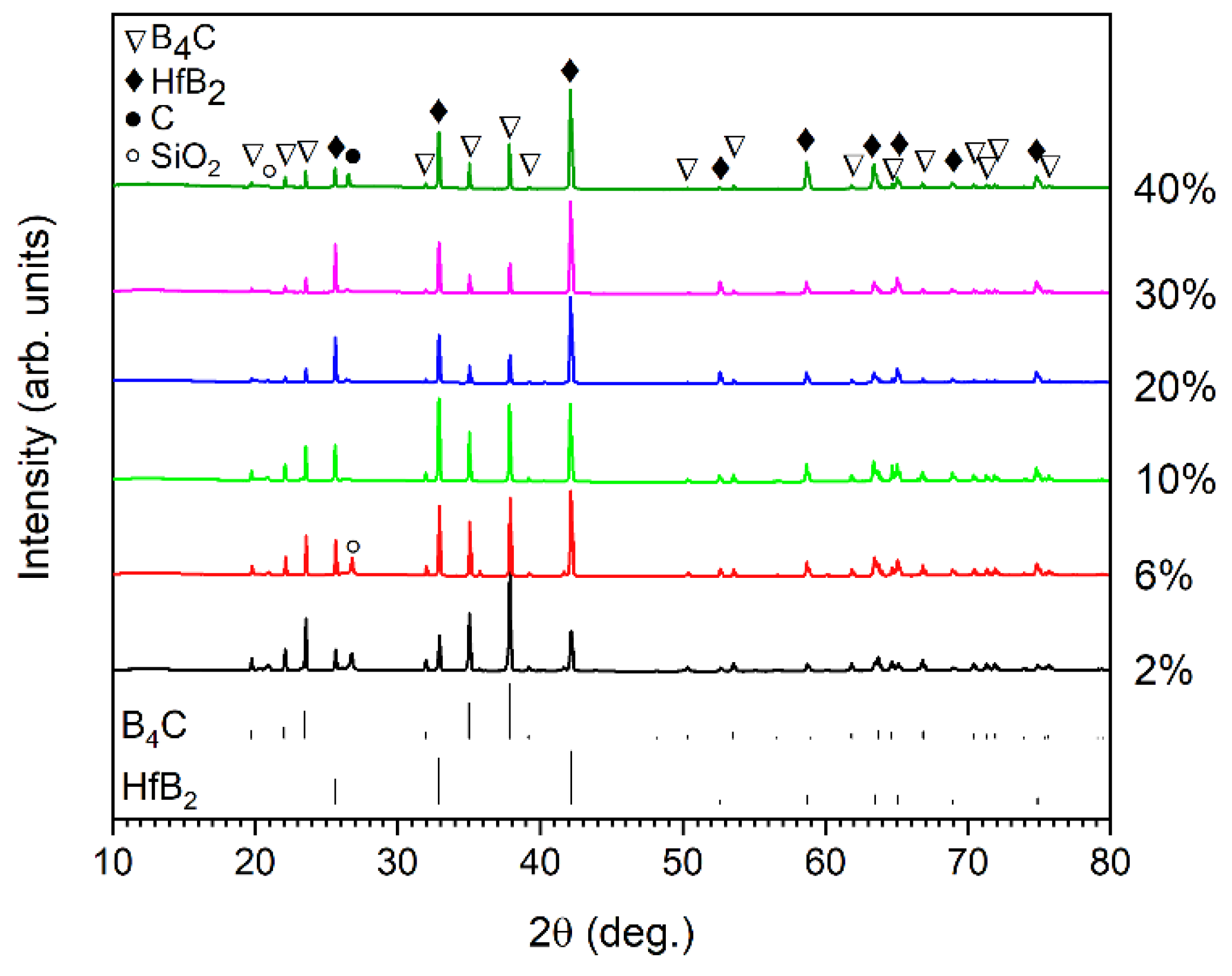
The XRD patterns of the sintered B4C-HfB2 composites are shown in Figure 4. As expected, the main phases of the composites are B4C and HfB2, and no residual hafnium carbide is detected, indicating a complete reaction of hafnium carbide according to Equation (1) after the sintering process. Free carbon assigned to graphite phase is detected for the samples with 10%, 20%, 30%, and 40% HfC addition. Meanwhile, a small amount of SiO2 impurity phase is also present, especially in the samples with relatively high porosity (BC2 and BC6). B4C and SiO2 are thermodynamically unstable at high temperatures, so it is unlikely that SiO2 would be present after sintering at 2250 °C. This SiO2 phase is presumably introduced in the composites during the sample preparation process for XRD, as SiO2-containing suspension was used for sample cutting.
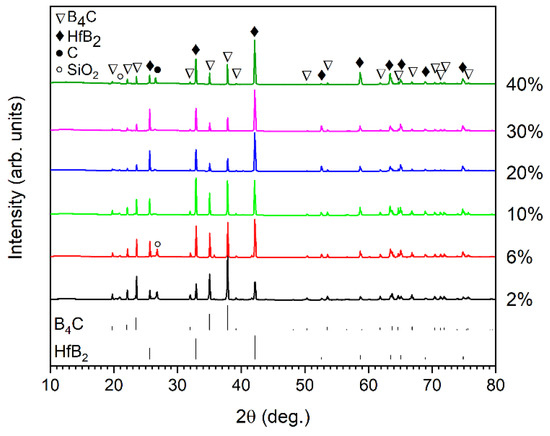
Figure 4.
XRD patterns of boron carbide-hafnium boride composites depending on the initial HfC content of the starting powder mixture.
3.3. Microstructure
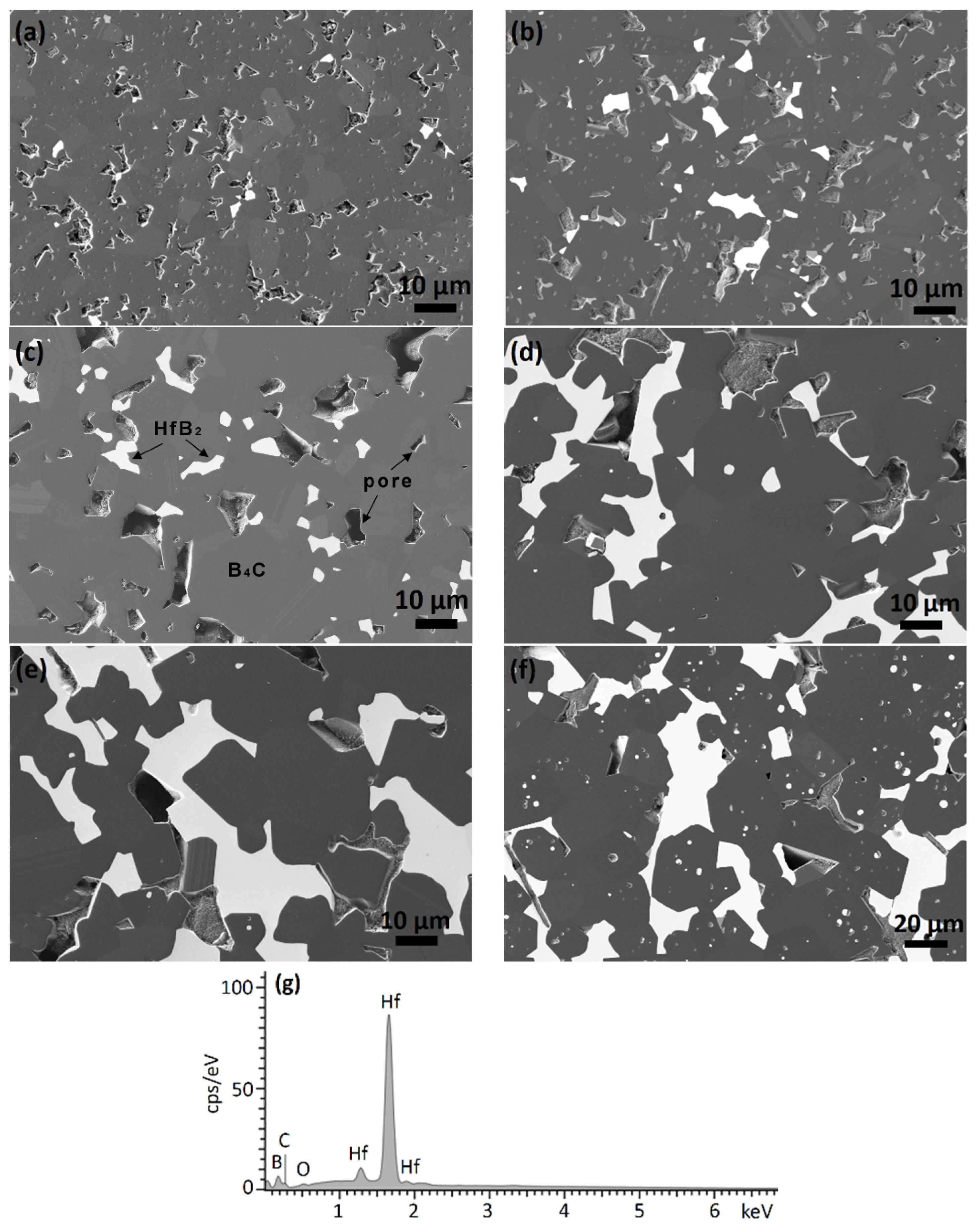
SEM micrographs of the densified composites are illustrated in Figure 5. As can be seen, the samples exhibit a similar microstructure with homogeneously distributed bright HfB2 phase in a grey B4C matrix. All phases are confirmed by EDX analysis. With increasing amounts of the hafnium carbide additive in the starting mixture, the hafnium boride forms irregular clusters of coarsened grains. Furthermore, huge grain growth of both HfB2 and B4C is observed in the BC20, BC30, and BC40 samples. The size of the larger grains in these samples reaches about 100 µm, whereas in the BC2, BC6, and BC10 samples, the grain size stays around 10 µm. The carbon components are preferentially removed during the polishing process, and therefore the structure appears to be more porous than it is.
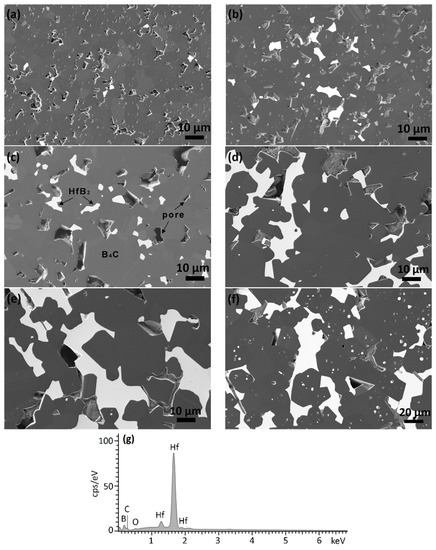
Figure 5.
FESEM images of boron carbide-hafnium boride composites with different initial HfC content in the starting powder mixture after sintering at 2250 °C: (a) 2%, BC2, (b) 6%, BC6, (c) 10%, BC10 (d) 20%, BC20, (e) 30%, BC30, (f) 40%, BC40, and (g) EDX spectrum of one of the bright grains in sample BC30.
3.4. Seebeck Coefficient
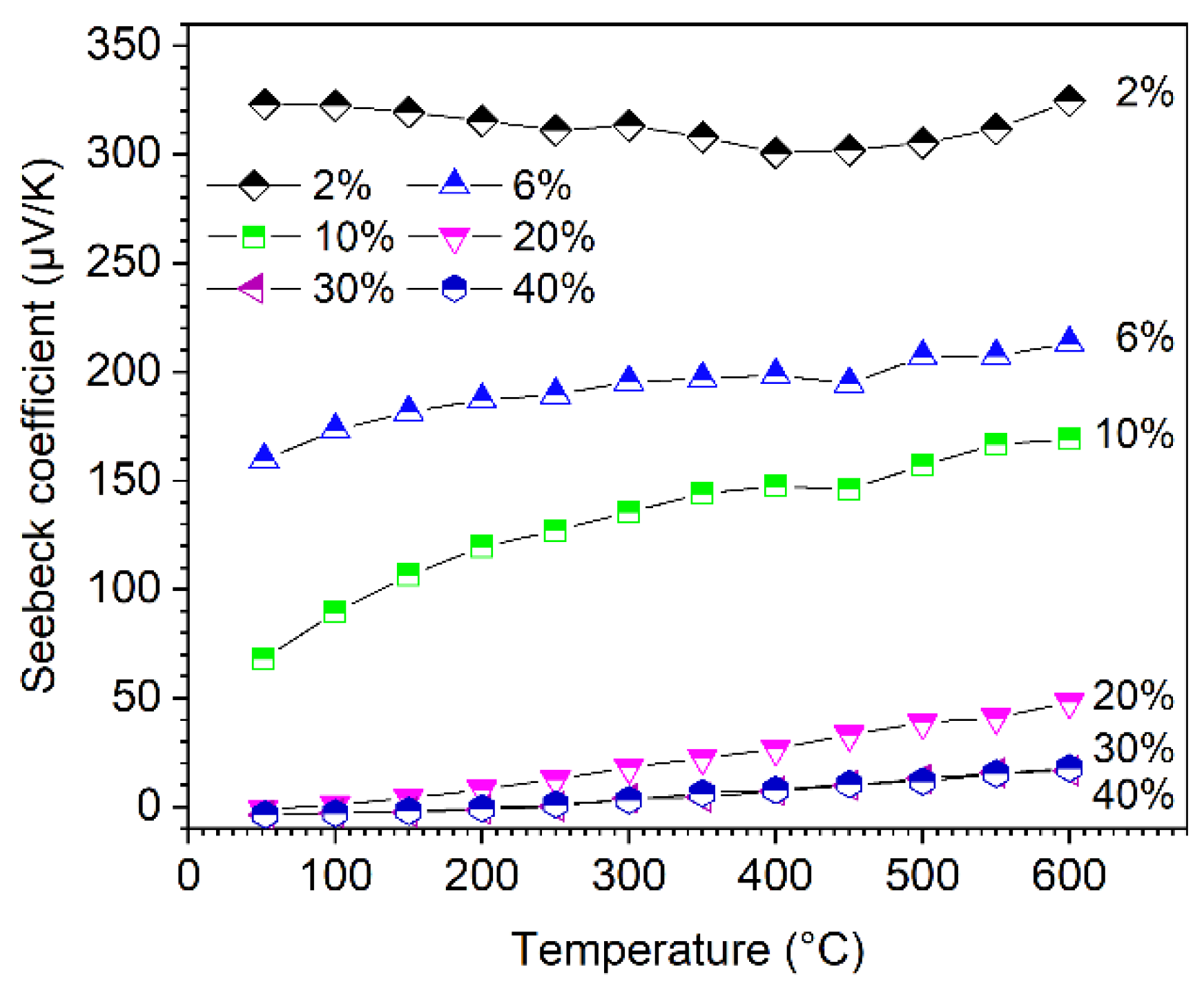
The temperature dependence of the Seebeck coefficient of the sintered composites is depicted in Figure 6. In the investigated temperature range, the Seebeck coefficients of the samples increase slightly with the increasing temperature, but they show a strong dependence on the amount of HfC in the starting powder mixture. As the amount of HfC increases, the Seebeck coefficients (S) sharply decrease. S can be adapted in a wide range, from few to 325 µV/K. Since HfB2 is an electrically conductive metal-like phase, it has negative Seebeck values in contrast to B4C. With increasing HfB2 content, the Seebeck contributions of B4C and HfB2 compensate each other in the composite samples, resulting in an overall reduction of the Seebeck coefficient [14,23]. The Seebeck coefficients show small negative values in the temperature range <200 °C, which clearly indicate the metallic-like behavior of this material, for the B4C with 30% and 40% HfC addition.
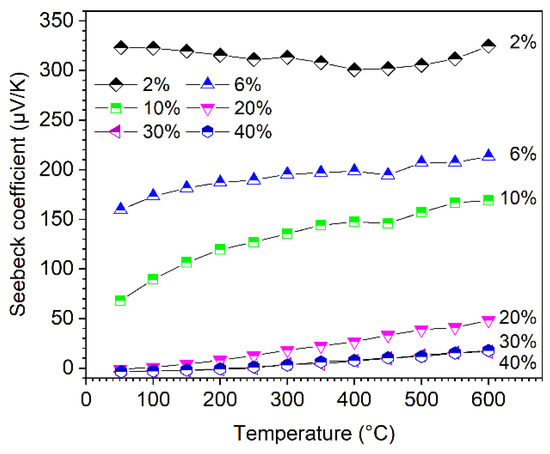
Figure 6.
Seebeck coefficients of boron carbide-hafnium boride composite depending on temperature and HfC content of the initial powder mixture.
3.5. Electrical Conductivity
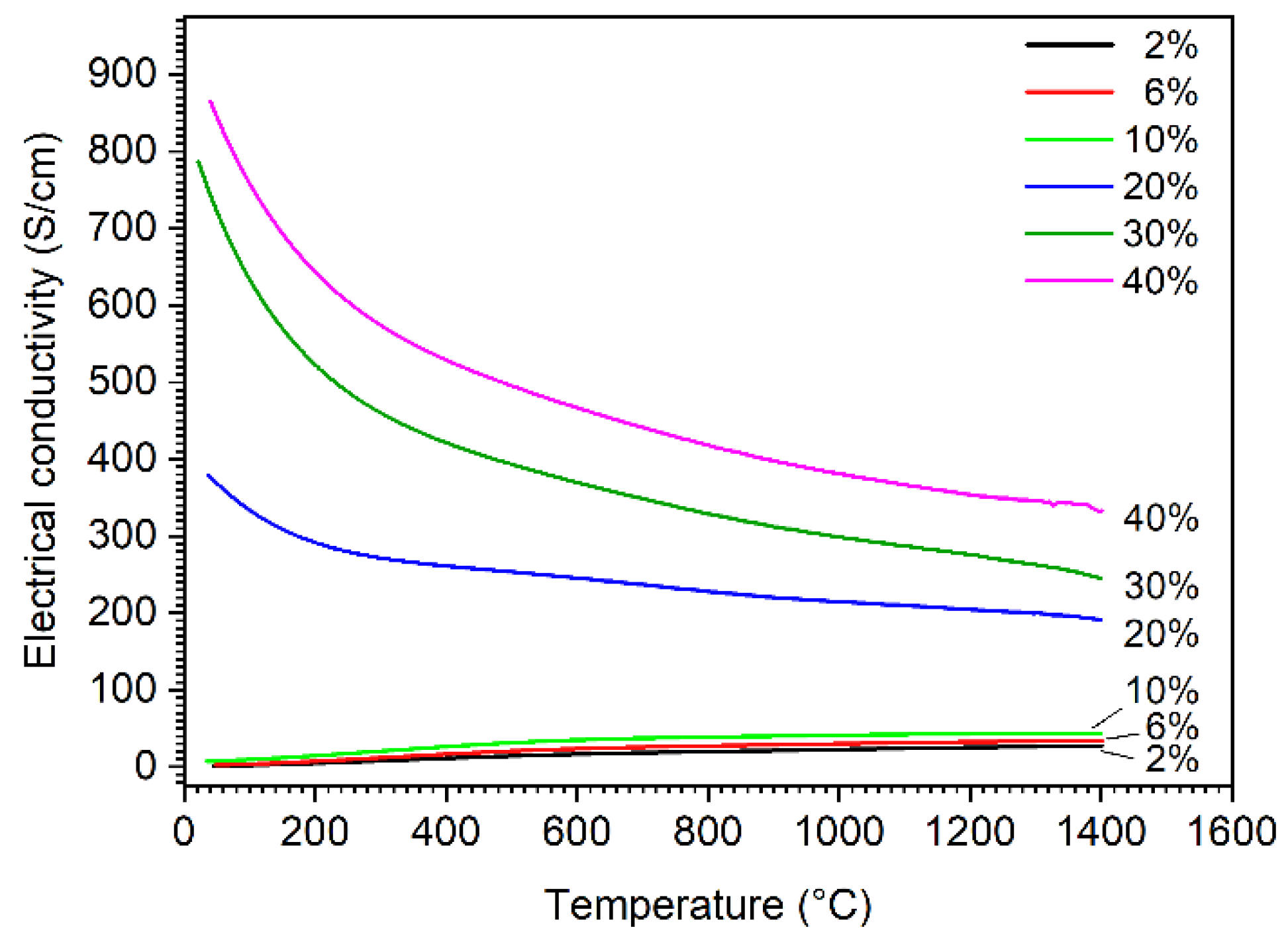
Figure 7 presents the electrical conductivities of the composites as a function of temperature. The addition of hafnium carbide leads to an increase of electrical conductivity compared with pure boron carbide, which has an electrical conductivity between 1 and 2 S/cm at room temperature and about 20 S/cm at 800 °C [24]. The conductivity of the composites increases as the amount of formed hafnium boride increases. HfB2 is a metal-like electrical conductor and boron carbide is an electrical semiconductor, so the increase in temperature leads to an increase in electrical conductivity as long as semi-conductivity is the dominant conduction mechanism of the material. It can be observed that the influence of the metallic conduction mechanism also increases with the increasing amount of HfB2. All samples show a metallic conduction behavior with additions of ≥20% HfC, and the electrical conductivity decreases with the increasing temperature along the investigated temperature range. This transition of conduction behavior could be attributed to the percolation effect, where the amount of highly conductive HfB2 phase in the composite is over its percolation threshold. The FESEM images (Figure 5d–f) clearly show that a conductive network of HfB2 was formed in the B4C matrix, resulting in a significant increase in the electrical conductivity. For samples with additions of 20% HfC and above, the electrical conductivity is about 2 orders of magnitude higher than that of samples with ≤10% additions at room temperature. A similar percolation effect in conduction has been observed in our previous study for a B4C-TiB2 system [15].
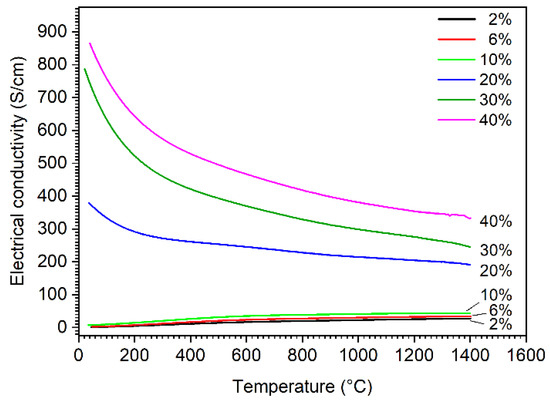
Figure 7.
Electrical conductivity of boron carbide-hafnium boride composite depending on temperature and HfC content of the initial powder mixture.
3.6. Thermoelectric Voltage Measurement
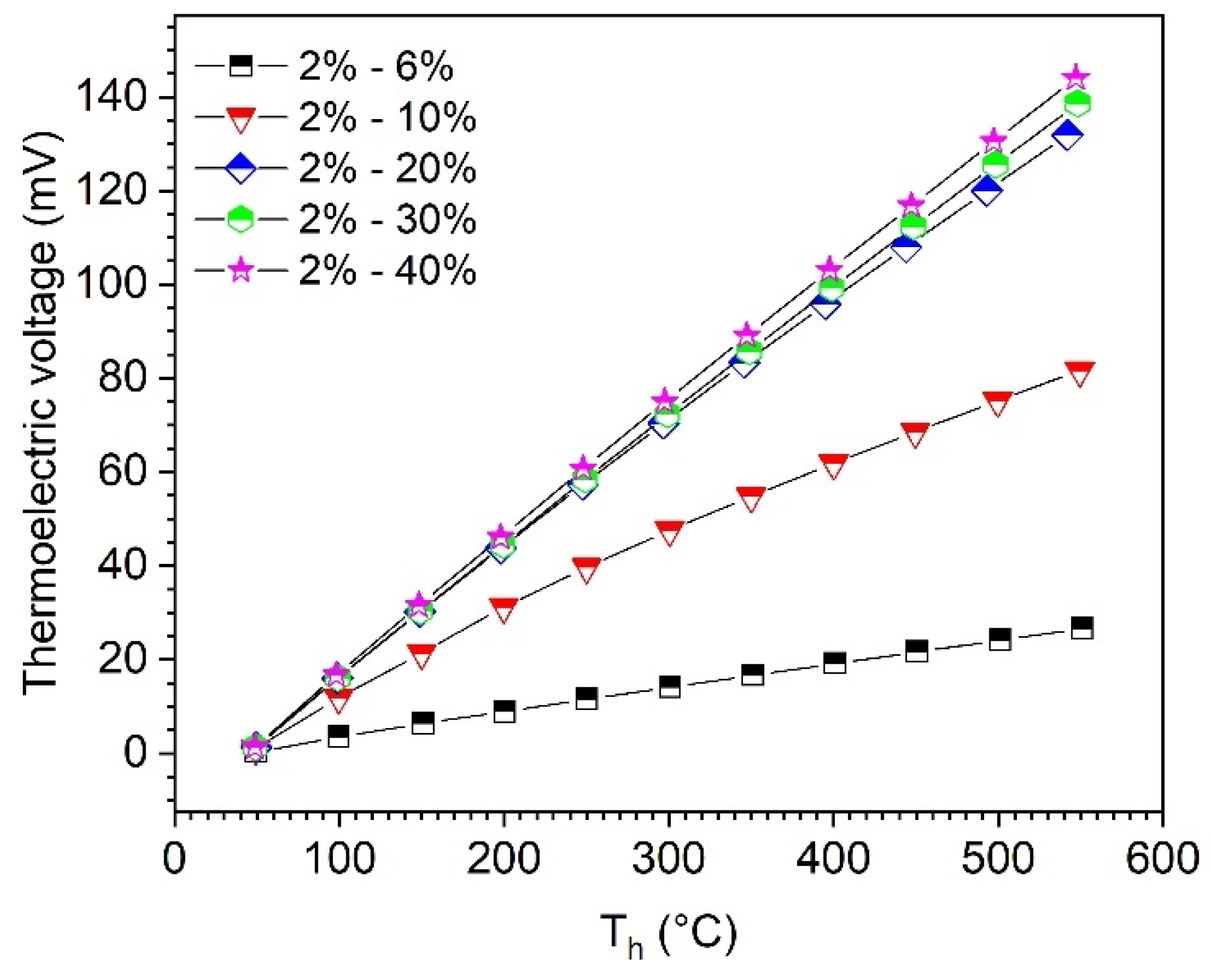
Quasi-thermocouples were manufactured using the sintered B4C-HfB2 composites with different Seebeck coefficients to evaluate the principal functionality. A total of 5 combinations of BC2 (2% HfC, leg 1) with more HfB2-containing sample (leg 2) in Table 1 were tested. During all measurements, the cold-side temperature, Tc, was kept at 45 °C, to reduce temperature reading delays of the thermocouples (Figure 2), and the measurements were carried out after 1 h of stabilization of the hot-side temperature, Th. However, contact resistance between the thermoelectric material and the electrode material (graphite foil) may cause testing errors. The measured thermoelectric voltages depending on the hot-side temperature, Th, are shown in Figure 8. Thermoelectric voltage increases almost linearly with the increasing temperature for all measurements; in addition, a high difference of Seebeck coefficients in the two legs results in high thermoelectric voltage of the thermocouples at the same temperature. For the combination of BC2 (2% HfC) and BC40 (40% HfC), a value of 144 mV was achieved at a temperature of 547 °C (temperature gradient ΔT = 502 K), compared to the tungsten-rhenium (W-Re5%-W-Re26%) thermocouple, which exhibits a thermoelectric voltage of 33 mV at 2000 °C [25]. A ΔT of 500 K produces a thermoelectric voltage of about 8.6 mV for the W-Re thermocouple. The significantly higher thermoelectric voltage of the B4C-HfB2 thermocouple may allow a better resolution of the measurement signal and improve the precision of the temperature detection. It is worth noting that the boron carbide-graphite combination in [11] tends to undergo a substantial alteration of the individual thermocouple components at high temperatures via diffusion, which leads to a shift of the detected signal. The main advantage of using B4C-HfB2 composites is that through suitable selection of the combination, e.g., BC2 (2% HfC) with BC6 (6% HfC), the substantial diffusion rate in the components is very low due to the material similarity and low concentration difference of the composites, so that a long-term stability of the B4C-HfB2 thermocouple for high-temperature applications can be expected.
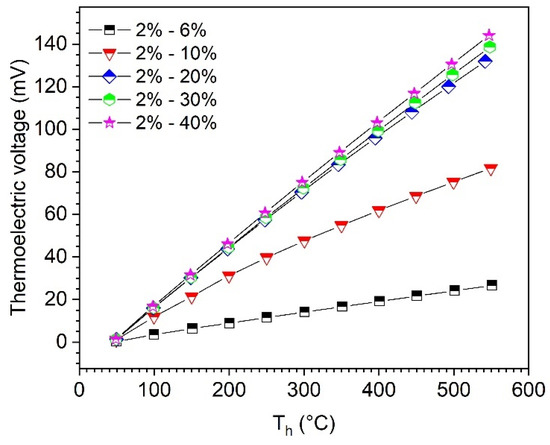
Figure 8.
Thermoelectric voltage of boron carbide-hafnium boride thermocouples depending on hot-side temperature, Th, measured with two legs of B4C-HfB2 composites. Cold-side temperature, Tc, was kept at 45 °C during all measurements.
4. Conclusions
B4C-HfB2 composites were successfully produced by pressureless sintering of boron carbide with the addition of hafnium carbide powder. The sintering of boron carbide was strongly promoted by the addition of HfC, which is attributed to the removal of the boron oxide impurity layer by the reaction of in situ-formed carbon. A dense composite with a densification of >95% was obtained at 2250 °C under argon by B4C with 40 wt.% HfC in the starting powder.
HfC addition to B4C was found to strongly affect the thermoelectric properties of boron carbide ceramic. More highly electrically conductive HfB2 phase was formed with increasing amounts of HfC, resulting in a decrease of the Seebeck coefficient and an increase of the electrical conductivity of the composites, simultaneously. A metallic conduction mechanism dominates for HfC additions ≥20 wt.% of the initial mixture.
Based on the sintered B4C-HfB2 composites, functional thermocouples were successfully tested at temperatures up to 547 °C (ΔT = 502 K). The produced high thermoelectric voltage of the thermocouples shows a promising potential of the composites for temperature measurement applications. Further tests up to 2000 °C will be carried out to evaluate the high-temperature functionality of the thermocouples in the future.
Author Contributions
B.F. contributed to the conceptualization, investigations, validation, methodology, visualization, and writing—original draft; H.-P.M. contributed to the conceptualization, funding acquisition, and writing—review and editing; A.M. supervised the research. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Federal Ministry of Education and Research BMBF/VIP+ program (Grant No: 03VP05250).
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the cooperation of our colleagues Lars Rebenklau and Paul Gierth in the development of this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yang, L.; Zhao, Y.; Feng, C.; Zhou, H. The Influence of Size Effect on Sensitivity of Cu/CuNi Thin- film Thermocouple. Phys. Procedia 2011, 22, 95–100. [Google Scholar] [CrossRef][Green Version]
- Childs, P.R.N.; Greenwood, J.R.; Long, C.A. Review of temperature measurement. Rev. Sci. Instrum. 2000, 71, 2959–2978. [Google Scholar] [CrossRef]
- The European Standard EN 60584-1; Thermocouples—Part 1: Reference Tables. Beuth Verlag: Berlin, Germany, 1995.
- Walker, B.E.; Ewing, C.T.; Miller, R.R. Instability of Refractory Metal Thermocouples. Rev. Sci. Instrum. 1965, 36, 816–825. [Google Scholar] [CrossRef]
- Edler, F. Material Problems in Using High-Temperature Thermocouples. Int. J. Thermophys. 2011, 32, 1418–1428. [Google Scholar] [CrossRef]
- Machin, G.; Anhalt, K.; Battuello, M.; Bourson, F.; Dekker, P.; Diril, A.; Edler, F.; Elliott, C.; Girard, F.; Greenen, A.; et al. The European project on high temperature measurement solutions in industry (HiTeMS)—A summary of achievements. Measurement 2016, 78, 168–179. [Google Scholar] [CrossRef]
- Elliott, C.J.; Large, M.J.; Pearce, J.V.; Machin, G. Compatibility of Materials for Use at High Temperatures with W–Re Thermocouples. Int. J. Thermophys. 2014, 35, 1202–1214. [Google Scholar] [CrossRef]
- Pearce, J.V.; Elliott, C.J.; Machin, G.; Ongrai, O. Self-validating type C thermocouples to 2300 °C using high temperature fixed points. AIP Conf. Proc. 2013, 1552, 595–600. [Google Scholar] [CrossRef]
- Ongrai, O.; Pearce, J.V.; Machin, G.; Sweeney, S. A miniature high-temperature fixed point for self-validation of type C thermocouples. Meas. Sci. Technol. 2011, 22, 105103. [Google Scholar] [CrossRef]
- Hollandt, J.; Hartmann, J.; Struß, O.; Gärtner, R. Radiometric temperature measurements: II. Applications. In Experimental Methods in the Physical Sciences; Zhang, Z.M., Tsai, B.K., Machin, G., Eds.; Elsevier Industrial Applications of Radiation Thermometry: Amsterdam, The Netherlands, 2010; Volume 43, pp. 1–56. [Google Scholar] [CrossRef]
- Hunold, K.; Lipp, A.; Reimuth, K.; Arnold, P. Thermoelement Comprising a Graphite/Boron Carbide Thermocouple. Patent U.S. 4,732,620, 22 March 1988. [Google Scholar]
- Hunold, K. A thermocouple for high temperatures. Adv. Mater. Process. 1986, 9, 4–5. [Google Scholar]
- Kanno, Y. Temperature control of inert gas furnace by tetraboron carbide/carbon thermocouple. Yogyo Kyokaishi 1986, 94, 449–451. [Google Scholar] [CrossRef]
- Feng, B.; Martin, H.-P.; Börner, F.-D.; Lippmann, W.; Schreier, M.; Vogel, K.; Lenk, A.; Veremchuk, I.; Dannowski, M.; Richter, C.; et al. Manufacturing and testing of thermoelectric modules consisting of BxC and TiOx elements. Adv. Eng. Mater. 2014, 16, 1252–1263. [Google Scholar] [CrossRef]
- Feng, B.; Martin, H.-P.; Michaelis, A. In situ preparation and thermoelectric properties of B4C1-x-TiB2 composites. J. Electron. Mater. 2013, 42, 2314–2319. [Google Scholar] [CrossRef]
- Beauvy, M. Stoichiometric limits of carbon-rich boron carbide phases. J. Less Common Met. 1983, 90, 169–175. [Google Scholar] [CrossRef]
- Gunjishima, I.; Akashi, T.; Goto, T. Characterization of directionally solidified B4C-TiB2 composites prepared by a floating zone method. Mater. Trans. 2002, 43, 712–720. [Google Scholar] [CrossRef]
- Demirskyi, D.; Sakka, Y.; Vasylkiv, O. High-Strength B4 C-TaB2 Eutectic Composites Obtained via In Situ by Spark Plasma Sintering. J. Am. Ceram. Soc. 2016, 99, 2436–2441. [Google Scholar] [CrossRef]
- Ordan’yan, S.S. Rules for the Reactions in B4C-MeIV–VIB2 Systems. Refractories 1993, 34, 268–271. [Google Scholar] [CrossRef]
- Radev, D.D. Pressureless Sintering of Boron Carbide-Based Superhard Materials. Solid State Phenom. 2010, 159, 145–148. [Google Scholar] [CrossRef]
- Suri, A.K.; Subramanian, C.; Sonber, J.K.; Murthy, T.S.R.C. Synthesis and consolidation of boron carbide: A review. Int. Mater. Rev. 2010, 55, 4–40. [Google Scholar] [CrossRef]
- Dole, S.L.; Prochazka, S.; Doremus, R.H. Microstructural Coarsening During Sintering of Boron Carbide. J. Am. Ceram. Soc. 1989, 72, 958–966. [Google Scholar] [CrossRef]
- Innocent, J.-L.; Portehault, D.; Gouget, G.; Maruyama, S.; Ohkubo, I.; Mori, T. Thermoelectric properties of boron carbide/HfB2 composites. Mater. Renew. Sustain. Energy 2017, 6, 6. [Google Scholar] [CrossRef]
- Roszeitis, S.; Feng, B.; Martin, H.-P.; Michaelis, A. Reactive sintering process and thermoelectric properties of boron rich boron carbides. J. Eur. Ceram. Soc. 2014, 34, 327–336. [Google Scholar] [CrossRef]
- Asamoto, R.R.; Novak, P.E. Tungsten-Rhenium Thermocouples for Use at High Temperatures. Rev. Sci. Instrum. 1967, 38, 1047–1052. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).