Simple Synthesis of 3D Ground-Moss-Shaped MnO@N-C Composite as Superior Anode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
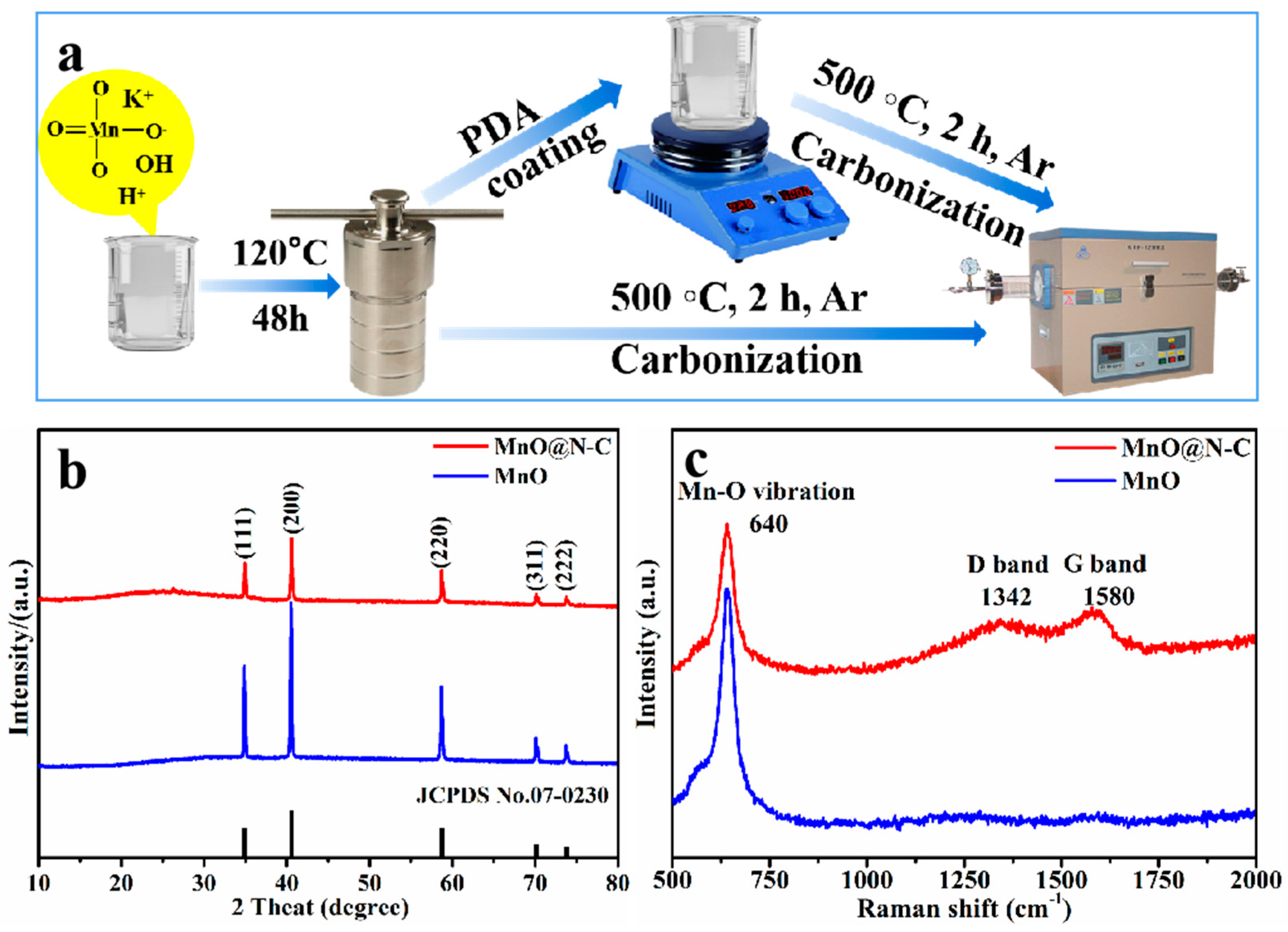
2.1. The Synthesis of MnO@N-C Composites with Ground-Moss Morphology
2.2. Materials Characterization
2.3. Electrochemical Measurements
3. Results
3.1. Composition and Microstructures of the Composite Materials
- D: crystallite size (nm)
- K: Scherrer constant (0.94)
- λ: wavelength of X-ray sources (0.15406 nm)
- β: full width at half maximum (FWHM, radians)
- θ: peak position (radians)
- d: interplanar spacing (nm)
- n: order of diffraction (1)
- λ and θ are the same as in Equation (1).
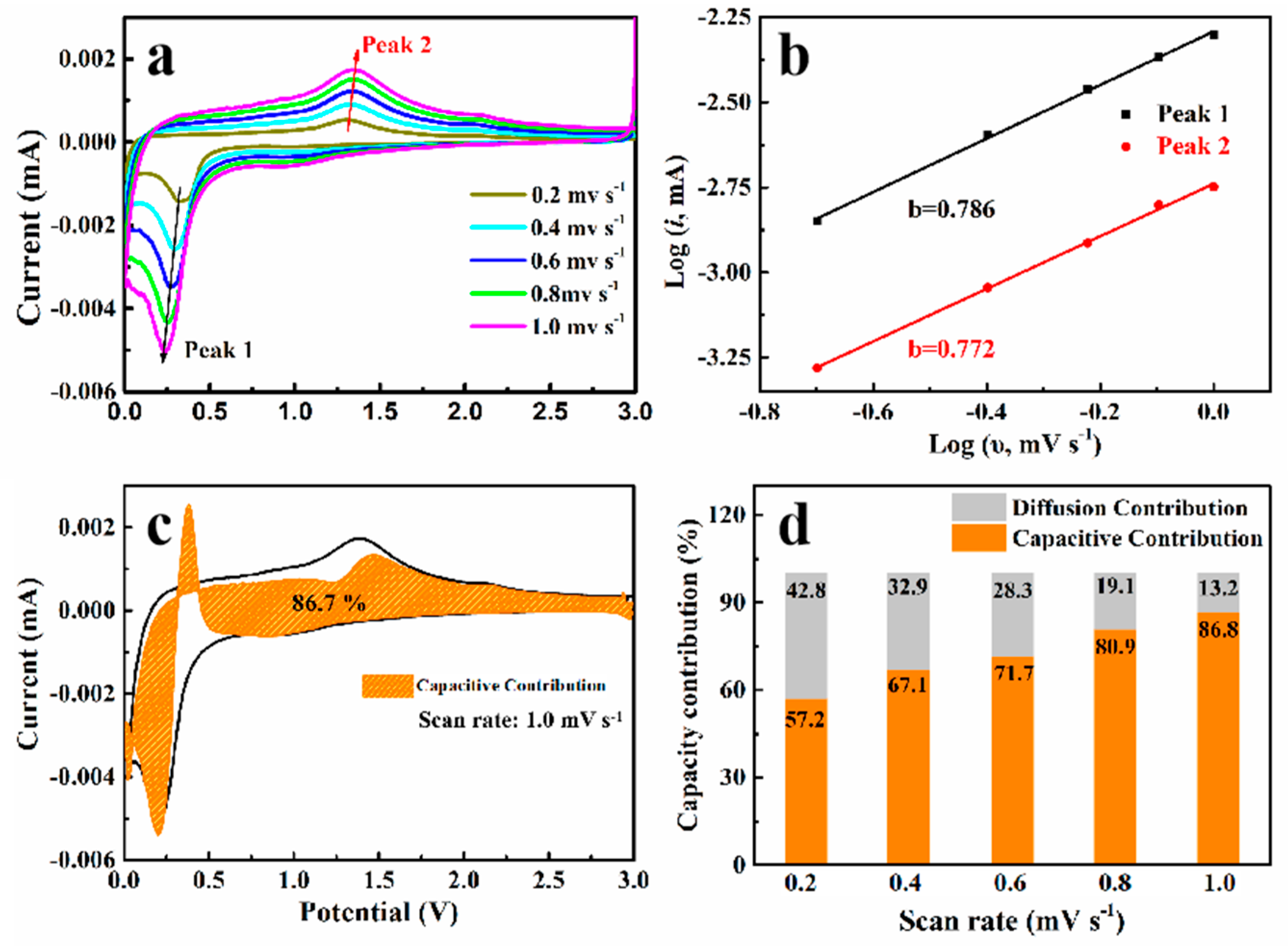
3.2. Electrochemical Properties in Half-Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, P.; Zhai, Y.; Wang, N.; Zhang, Y.; Dou, S. Selenium@Hollow Mesoporous Carbon Composites for High-Rate and Long-Cycling Lithium/Sodium-Ion Batteries. Chem. Eng. J. 2019, 392, 123676. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2020, 36, 186–212. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, K.; Zhao, G.; Chen, Z. Perspectives and challenges for future lithium-ion battery control and management. eTransportation 2023, 18, 100260. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Xu, R.; Gao, X.; Zhou, D.; Yuan, T.; Chen, Y.; Cui, L. In-situ embedding of ultrasmall MnO nanoparticles into pollen biomass-derived hollow porous N heteroatoms enriched carbon microspheres as high-performance anode for lithium-ion batteries. Chem. Eng. J. 2023, 473, 145117. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Z.; Yang, T.; Nie, C.; Xu, X.; Wang, Y.; Yang, J.; Dou, S.; Wang, N. Advances in high sulfur loading cathodes for practical lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Yang, Y.; Dong, R.; Cheng, H.; Wang, L.; Tu, J.; Zhang, S.; Zhao, S.; Zhang, B.; Pan, H.; Lu, Y. 2D Layered Materials for Fast-Charging Lithium-Ion Battery Anodes. Small 2023, 19, 2301574. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, Q.; Bi, Y.; Cai, M.; Dunn, B.; Glossmann, T.; Liu, J.; Osaka, T.; Sugiura, R.; Wu, B. Understanding and applying coulombic efficiency in lithium metal batteries. Nat. Energy 2020, 5, 561–568. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Cheng, X.; Gao, S.; Zhang, X.; Zhao, H.; Huo, L. Compounds, Coordination polymer-derived hierarchically structured MnO/NC composites as anode materials for high-performance lithium-ion batteries. J. Alloys Compd. 2023, 941, 168847. [Google Scholar] [CrossRef]
- Nzereogu, P.; Omah, A.; Ezema, F.; Iwuoha, E.; Nwanya, A. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Adelowo, E.; Drozd, V.; Shang, W.; Wang, C. High-performance red phosphorus-sulfurized polyacrylonitrile composite by electrostatic spray deposition for lithium-ion batteries. Electrochim. Acta 2020, 345, 136227. [Google Scholar] [CrossRef]
- Parekh, M.H.; Sediako, A.D.; Naseri, A.; Thomson, M.J.; Pol, V. In Situ Mechanistic Elucidation of Superior Si-C-Graphite Li-Ion Battery Anode Formation with Thermal Safety Aspects. Adv. Energy Mater. 2020, 10, 1902799. [Google Scholar] [CrossRef]
- Sarang, K.T.; Li, X.; Miranda, A.; Terlier, T.; Oh, E.-S.; Verduzco, R.; Lutkenhaus, J. Tannic acid as a small-molecule binder for silicon anodes. ACS Appl. Energy Mater. 2020, 3, 6985–6994. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From materials to cell: State-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 2021, 122, 903–956. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Zhang, Y. Fuels, A comprehensive review on metal-oxide nanocomposites for high-performance lithium-ion battery anodes. J. Energy Fuels 2021, 35, 6420–6442. [Google Scholar] [CrossRef]
- Hua, X.; Allan, P.K.; Gong, C.; Chater, P.A.; Schmidt, E.M.; Geddes, H.S.; Robertson, A.W.; Bruce, P.G.; Goodwin, A. Non-equilibrium metal oxides via reconversion chemistry in lithium-ion batteries. Nat. Commun. 2021, 12, 561. [Google Scholar] [CrossRef]
- Sheng, L.; Liang, S.; Wei, T.; Chang, J.; Jiang, Z.; Zhang, L.; Zhou, Q.; Zhou, J.; Jiang, L.; Fan, Z. Space-confinement of MnO nanosheets in densely stacked graphene: Ultra-high volumetric capacity and rate performance for lithium-ion batteries. Energy Storage Mater. 2018, 12, 94–102. [Google Scholar] [CrossRef]
- Yang, S.; Zou, Z.; Jiang, C. A ternary MnO/MnTiO3@C composite anode with greatly enhanced cycle stability for Li-ion batteries. Ceram. Int. 2023, 49, 8112–8120. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, Q.; Dong, H.; Li, J.; Tang, Z.; Wang, X.; Ouyang, J.; Liu, T. N-doped carbon coated porous hierarchical MnO microspheres as superior additive-free anode materials for lithium-ion batteries. Scr. Mater. 2022, 211, 114495. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, C.; Shi, J.; Huang, M.; Liu, S.; Shi, Z.; Wang, H. Compounds, One-pot synthesis of nanosized MnO incorporated into N-doped carbon nanosheets for high performance lithium storage. J. Alloys Compd. 2022, 902, 163827. [Google Scholar] [CrossRef]
- He, C.; Li, J.; Zhao, X.; Peng, X.; Lin, X.; Ke, Y.; Xiao, X.; Zuo, X.; Nan, J. In situ anchoring of MnO nanoparticles into three-dimensional nitrogen-doped porous carbon framework as a stable anode for high-performance lithium storage. Appl. Surf. Sci. 2023, 614, 156217. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhao, K.; He, Z.; Liu, S. Preparation of N-doped porous carbon coated MnO nanospheres through solvent-free in-situ growth of ZIF-8 on ZnMn2O4 for high-performance lithium-ion battery anodes. Electrochim. Acta 2018, 266, 254–262. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, S.; Han, J.; Gao, C.; Fan, L.; Guo, R. Multi-yolk–shell MnO@carbon nanopomegranates with internal buffer space as a lithium ion battery anode. Langmuir 2021, 37, 2195–2204. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Yu, N.; Sun, H.; Geng, B. Constructing an interspace in MnO@ NC microspheres for superior lithium ion battery anodes. Chem. Commun. 2021, 57, 10951–10954. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, X.; Liang, L.; Li, R.; Fan, P.; Li, J.; Zhao, H. Compounds, Double-network aerogel-derived spongy 3D porous MnO/C composite for lithium-ion batteries with increasing capacity. J. Alloys Compd. 2023, 948, 169799. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Xu, G.; Liu, X.; Zhang, Q.; Yang, L.; Cao, J.; Wei, X. Porous N-doped carbon sheets wrapped MnO in 3D carbon networks as high-performance anode for Li-ion batteries. Electrochim. Acta 2020, 342, 136115. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ju, Z.; Yu, X.; Wang, Y.; Du, Y.; Bai, Z.; Dou, S.; Yu, G. Thickness-independent scalable high-performance Li-S batteries with high areal sulfur loading via electron-enriched carbon framework. Nat. Commun. 2021, 12, 4519. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; You, F.; He, B.; Wu, Y.; Wang, D.; Zhou, W.; Qian, C.; Yang, G.; Liu, G.; Wang, H. Directing the architecture of surface-clean Cu2O for CO electroreduction. J. Am. Chem. Soc. 2022, 144, 12410–12420. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, B.; Chang, S.; Yang, C.; Hu, Y.; Cao, S.; Lu, J.; Zhang, L.; Ye, H. Technology, Effects of annealing temperature on electrochemical performance of SnSx embedded in hierarchical porous carbon with N-carbon coating by in-situ structural phase transformation as anodes for lithium ion batteries. J. Mater. Sci. Technol. 2021, 84, 191–199. [Google Scholar] [CrossRef]
- Ko, I.-H.; Jin, A.; Kim, M.K.; Park, J.-H.; Kim, H.S.; Yu, S.-H.; Sung, Y.-E. Compounds, The keys for effective distribution of intergranular voids of peapod-like MnO@C core-shell for lithium ion batteries. J. Alloys Compd. 2020, 817, 152760. [Google Scholar] [CrossRef]
- Kitz, P.G.; Lacey, M.J.; Novák, P.; Berg, E. Operando investigation of the solid electrolyte interphase mechanical and transport properties formed from vinylene carbonate and fluoroethylene carbonate. J. Power Sources 2020, 477, 228567. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene carbonate as an important component for the formation of an effective solid electrolyte interphase on anodes and cathodes for advanced Li-ion batteries. ACS Energy Lett. 2017, 2, 1337–1345. [Google Scholar] [CrossRef]
- Jiao, R.; Zhao, L.; Zhou, S.; Zhai, Y.; Wei, D.; Zeng, S.; Zhang, X.J.N. Effects of carbon content and current density on the Li+ storage performance for MnO@C nanocomposite derived from Mn-based complexes. Nanomaterials 2020, 10, 1629. [Google Scholar] [CrossRef]
- Wang, S.; Dong, Y.; Cao, F.; Li, Y.; Zhang, Z.; Tang, Z.J. Conversion-Type MnO Nanorods as a Surprisingly Stable Anode Framework for Sodium-Ion Batteries. Adv. Funct. Mater. 2020, 30, 2001026. [Google Scholar] [CrossRef]
- Sheng, L.; Jiang, H.; Liu, S.; Chen, M.; Wei, T.; Fan, Z. Nitrogen-doped carbon-coated MnO nanoparticles anchored on interconnected graphene ribbons for high-performance lithium-ion batteries. J. Power Sources 2018, 397, 325–333. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, Z.; Ye, L.; Cui, Y.; Zheng, K.; Zhao, L. Raspberry-shaped nickel-enhanced MnO-based carbon-containing nanostructures as anode materials for Li-ion batteries. ACS Appl. Nano Mater. 2021, 4, 7925–7934. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-pot hydrothermal synthesis of Nitrogen-doped graphene as high-performance anode materials for lithium ion batteries. Sci. Rep. 2016, 6, 26146. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, J.-S.; Baek, K.W.; Saroha, R.; Yang, S.H.; Kang, Y.C.; Cho, J. Coral-like porous microspheres comprising polydopamine-derived N-doped C-coated MoSe2 nanosheets composited with graphitic carbon as anodes for high-rate sodium-and potassium-ion batteries. Chem. Eng. J. 2023, 456, 141118. [Google Scholar] [CrossRef]
- Jia, J.; Hu, X.; Wen, Z. Robust 3D network architectures of MnO nanoparticles bridged by ultrathin graphitic carbon for high-performance lithium-ion battery anodes. Nano Res. 2018, 11, 1135–1145. [Google Scholar] [CrossRef]
- Lin, J.; Yu, L.; Sun, Q.; Wang, F.; Cheng, Y.; Wang, S.; Zhang, X. Multiporous core-shell structured MnO@N-Doped carbon towards high-performance lithium-ion batteries. Int. J. Hydrogen Energy 2020, 45, 1837–1845. [Google Scholar] [CrossRef]
- Xiao, T.; Zhang, W.; Xu, T.; Wu, J.; Wei, M.J.E.A. Hollow SiO2 microspheres coated with nitrogen doped carbon layer as an anode for high performance lithium-ion batteries. Electrochim. Acta 2019, 306, 106–112. [Google Scholar] [CrossRef]
- Pei, X.-Y.; Mo, D.-C.; Lyu, S.-S.; Zhang, J.-H.; Fu, Y.-X. Facile preparation of N-doped MnO/rGO composite as an anode material for high-performance lithium-ion batteries. Appl. Surf. Sci. 2019, 465, 470–477. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhang, W.; Wei, W.; Liao, R.; Shi, J.; Huang, M.; Liu, S.; Shi, Z. High potassium ion storage capacity with long cycling stability of sustainable oxygen-rich carbon nanosheets. Nanoscale 2021, 13, 2389–2398. [Google Scholar] [CrossRef]
- Zhang, K.; Han, P.; Gu, L.; Zhang, L.; Liu, Z.; Kong, Q.; Zhang, C.; Dong, S.; Zhang, Z.; Yao, J. Synthesis of nitrogen-doped MnO/graphene nanosheets hybrid material for lithium ion batteries. ACS Appl. Mater. 2012, 4, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Mao, B.; Cao, M. In situ coupling of MnO and Co@N-doped graphite carbon derived from prussian blue analogous achieves high-performance reversible oxygen electrocatalysis for Zn–Air batteries. Inorg. Chem. 2021, 60, 10340–10349. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Q.; Xu, C.; Zou, R.; Zhang, J.; Zhang, W.; Guan, G.; Hu, J.; Sun, Y. A new strategy to effectively alleviate volume expansion and enhance the conductivity of hierarchical MnO@C nanocomposites for lithium ion batteries. J. Mater. Chem. A 2017, 5, 21699–21708. [Google Scholar] [CrossRef]
- Zhan, D.; Wen, T.; Li, Y.; Zhu, Y.; Liu, K.; Cui, P.; Jia, Z.; Liu, H.; Lei, K.; Xiao, Z. Using peanut shells to construct a porous MnO/C composite material with highly improved lithium storage performance. ChemElectroChem 2020, 7, 347–354. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, K.; Zhang, F.; Zhou, H.; Qi, L. Hierarchical MnO@C hollow nanospheres for advanced lithium-ion battery anodes. ACS Appl. Nano Mater. 2018, 2, 429–439. [Google Scholar] [CrossRef]
- Lei, D.; Hou, Z.; Li, N.; Cao, Y.; Ren, L.; Liu, H.; Zhang, Y.; Wang, J.-G. A homologous N/P-codoped carbon strategy to streamline nanostructured MnO/C and carbon toward boosted lithium-ion capacitors. Carbon 2023, 201, 260–268. [Google Scholar] [CrossRef]
- Parekh, M.H.; Palanisamy, M.; Pol, V. Reserve lithium-ion batteries: Deciphering in situ lithiation of lithium-ion free vanadium pentoxide cathode with graphitic anode. Carbon 2023, 203, 561–570. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Xu, Z.; Wang, W.; Wen, J.; Bai, X. Rate mechanism of vanadium oxide coated tin dioxide nanowire electrode for lithium ion battery. Nano Energy 2017, 42, 294–299. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Shi, L.; Li, J.; Zhang, G. Rational design of hierarchical nanotubes through encapsulating CoSe2 nanoparticles into MoSe2/C composite shells with enhanced lithium and sodium storage performance. ACS Appl. Mater. 2018, 10, 20635–20642. [Google Scholar] [CrossRef] [PubMed]
- Park, G.D.; Park, J.-S.; Kim, J.K.; Kang, Y. Metal sulfoselenide solid solution embedded in porous hollow carbon nanospheres as effective anode material for potassium-ion batteries with long cycle life and enhanced rate performance. Chem. Eng. J. 2022, 428, 131051. [Google Scholar] [CrossRef]
- Chen, Z.; Song, J.; Zhang, B.; Wu, Z.; Mandler, D.; Lei, W.; Hao, Q. Double-carbon coated MnO nanoparticles as high-performance anode materials for lithium-ion storage. Ionics 2023, 29, 483–496. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, Y.; Zheng, C.; Wang, N.; Wang, D.; Tian, F.; Bai, Z.; Yang, J.; Qian, Y.; Dou, S. Intermolecular Cross-Linking Reinforces Polymer Binders for Durable Alloy-Type Anode Materials of Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2202939. [Google Scholar] [CrossRef]
- Gong, Y.; Sun, L.; Si, H.; Zhang, Y.; Shi, Y.; Wu, L.; Gu, J.; Zhang, Y. MnO nanorods coated by Co-decorated N-doped carbon as anodes for high performance lithium ion batteries. Appl. Surf. Sci. 2020, 504, 144479. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Gai, L.; Gao, Y.; Tong, Z.; Wang, W.; Cao, H.; Zeng, S.; Qu, K.; Bai, Z.; Tian, G.; et al. Simple Synthesis of 3D Ground-Moss-Shaped MnO@N-C Composite as Superior Anode Material for Lithium-Ion Batteries. Crystals 2023, 13, 1420. https://doi.org/10.3390/cryst13101420
Zhai Y, Gai L, Gao Y, Tong Z, Wang W, Cao H, Zeng S, Qu K, Bai Z, Tian G, et al. Simple Synthesis of 3D Ground-Moss-Shaped MnO@N-C Composite as Superior Anode Material for Lithium-Ion Batteries. Crystals. 2023; 13(10):1420. https://doi.org/10.3390/cryst13101420
Chicago/Turabian StyleZhai, Yanjun, Longhui Gai, Yingjian Gao, Ziwei Tong, Wenlin Wang, Huimei Cao, Suyuan Zeng, Konggang Qu, Zhongchao Bai, Gang Tian, and et al. 2023. "Simple Synthesis of 3D Ground-Moss-Shaped MnO@N-C Composite as Superior Anode Material for Lithium-Ion Batteries" Crystals 13, no. 10: 1420. https://doi.org/10.3390/cryst13101420




