Syntheses and Solid-State Characterizations of N-Alkylated Glycine Derivatives
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-ray Diffraction Measurements
2.2. Preparation and Thermal Stability of N-Alkylated Glycine Derivatives
2.2.1. Synthesis of the N-Alkylglycinium Chlorides
2.2.2. Synthesis of the N-Alkylglycinium Nitrates and N-(n-Propyl)glycine Hydrate
3. Results and Discussion
3.1. Synthesis of N-Alkylated Glycine Derivatives
3.2. Crystal Structures of N-Alkylated Glycine Derivatives
3.2.1. Crystal Structures of the N-Alkylglycinium Chlorides
3.2.2. Crystal Structures of the N-Alkylglycinium Nitrates and Zwitterionic N-(n-Propyl)glycine Hydrate
3.2.3. Hirshfeld Surface Analysis of N-Alkylated Glycine Derivatives

3.2.4. Infrared Spectroscopy
3.2.5. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; W. H. Freeman: New York, NY, USA, 2012. [Google Scholar]
- Anioła, M.; Dega-Szafran, Z.; Katrusiak, A.; Szafran, M. NH⋯O and OH⋯O interactions of glycine derivatives with squaric acid. New J. Chem. 2014, 38, 3556–3568. [Google Scholar] [CrossRef]
- Hyslop, J.F.; Lovelock, S.L.; Watson, A.J.B.; Sutton, P.W.; Roiban, G.-D. N-Alkyl-α-amino acids in Nature and their biocatalytic preparation. J. Biotechnol. 2019, 293, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sagan, S.; Karoyan, P.; Lequin, O.; Chassaing, G.; Lavielle, S. N- and Cα-Methylationin Biologically Active Peptides: Synthesis, Structural and Functional Aspects. Curr. Med. Chem. 2004, 11, 2799–2822. [Google Scholar] [CrossRef] [PubMed]
- Mindt, M.; Hannibal, S.; Heuser, M.; Risse, J.M.; Sasikumar, K.; Nampoothiri, K.M.; Wendisch, V.F. Fermentative Production of N-Alkylated Glycine Derivatives by Recombinant Corynebacterium glutamicum Using a Mutant of Imine Reductase DpkA from Pseudomonas putida. Front. Bioeng. Biotechnol. 2019, 7, 232. [Google Scholar] [CrossRef]
- Waites, C.R.; Dominick, M.A.; Sanderson, T.P.; Schilling, B.E. Nonclinical Safety Evaluation of Muraglitazar, a Novel PPARα/γ Agonist. Toxicol. Sci. 2007, 100, 248–258. [Google Scholar] [CrossRef]
- Fandrick, D.R.; Reeves, J.T.; Bakonyi, J.M.; Nyalapatla, P.R.; Tan, Z.; Niemeier, O.; Akalay, D.; Fandrick, K.R.; Wohlleben, W.; Ollenberger, S.; et al. Zinc Catalyzed and Mediated Asymmetric Propargylation of Trifluoromethyl Ketones with a Propargyl Boronate. J. Org. Chem. 2013, 78, 3592–3615. [Google Scholar] [CrossRef]
- Santra, S.; Perez, J.M. Selective N -Alkylation of β-Alanine Facilitates the Synthesis of a Poly(amino acid)-Based Theranostic Nanoagent. Biomacromolecules 2011, 12, 3917–3927. [Google Scholar] [CrossRef]
- Li, Y.; Holmberg, K.; Bordes, R. Micellization of true amphoteric surfactants. J. Colloid Interface Sci. 2013, 411, 47–52. [Google Scholar] [CrossRef]
- Cleij, M.C.; Scrimin, P.; Tecilla, P.; Tonellato, U. Efficient and Highly Selective Copper(II) Transport across a Bulk Liquid Chloroform Membrane Mediated by Lipophilic Dipeptides. J. Org. Chem. 1997, 62, 5592–5599. [Google Scholar] [CrossRef]
- Headley, A.D.; Starnes, S.D. Conformational analysis of N-methylglycine and N,N-dimethylglycine by ab initio calculations. J. Mol. Struct. THEOCHEM 1996, 370, 147–155. [Google Scholar] [CrossRef]
- Werdehausen, R.; Mittnacht, S.; Bee, L.A.; Minett, M.S.; Armbruster, A.; Bauer, I.; Wood, J.N.; Hermanns, H.; Eulenburg, V. The lidocaine metabolite N-ethylglycine has antinociceptive effects in experimental inflammatory and neuropathic pain. Pain 2015, 156, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Görbitz, C.H.; Leirvåg, A.B.; Jacobsen, Ø. Hydrogen bond architecture in crystal structures of N -alkylated hydrophobic amino acids. CrystEngComm 2014, 16, 9631–9637. [Google Scholar] [CrossRef]
- Basolo, F.; Chen, Y.T. Steric Effects and the Stability of Complex Compounds. III. The Chelating Tendencies of N-Alkylglycines and N-Dialkylglycines with Copper(II) and Nickel(II) Ions 1. J. Am. Chem. Soc. 1954, 76, 953–955. [Google Scholar] [CrossRef]
- Paulić, N.; Raos, N. Review: The Chemistry of Chelates with N-Alkylated Amino Acids. J. Coord. Chem. 1994, 31, 181–190. [Google Scholar] [CrossRef]
- Yan, T.; Feringa, B.L.; Barta, K. Direct N-alkylation of unprotected amino acids with alcohols. Sci. Adv. 2017, 3, eaao6494. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Davies, J.S.; Mohammed, A.K. Assessment of racemisation in N-alkylated amino-acid derivatives during peptide coupling in a model dipeptide system. J. Chem. Soc. Perkin Trans. 1 1981, 2982. [Google Scholar] [CrossRef]
- Beltrán, H.I.; Abreu, A.; Zamudio-Rivera, L.S.; Mancilla, T.; Santillán, R.; Farfán, N. Síntesis y caracterización espectroscópica de N-(2-hidroxibencil)-α-aminoácidos. Rev. Soc. Quím. Méx. 2001, 45, 152–158. [Google Scholar]
- Dega-Szafran, Z.; Petryna, M.; Tykarska, E.; Szafran, M. Molecular structure of 1-piperidinium acid perchlorate studied by X-ray diffraction and FTIR spectroscopy. J. Mol. Struct. 2002, 643, 69–75. [Google Scholar] [CrossRef]
- Dega-Szafran, Z.; Dutkiewicz, G.; Kosturkiewicz, Z.; Petryna, M.; Szafran, M. Crystal and molecular structure of the 1:1 complex of 1-piperidineacetic acid with 2,6-dichloro-4-nitrophenol studied by X-ray diffraction and B3LYP calculation. J. Mol. Struct. 2005, 741, 115–120. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Zeng, Q. Chirality and absolute helicity in a pair of enantiomeric amino acid derivatives and their complexes: Structures, chiroptical, and photoluminescent properties. CrystEngComm 2013, 15, 3593. [Google Scholar] [CrossRef]
- Brancatelli, G.; Bruno, G.; Nicoló, F.; Canfora, L.; Ruisi, G. (S)-2-[(2-Hydroxybenzyl)azaniumyl]-4-(methylsulfanyl)butanoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o1366–o1367. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lu, L.-P. 3-Hydroxy-2-[(4-hydroxybenzyl)azaniumyl]propanoate monohydrate. IUCrData 2017, 2, x170271. [Google Scholar] [CrossRef]
- Canle, L.M.; Clegg, W.; Demirtas, I.; Elsegood, M.R.J.; Maskill, H. Preparations, X-ray crystal structure determinations, and base strength measurements of substituted tritylamines. J. Chem. Soc. Perkin Trans. 2 2000, 85–92. [Google Scholar] [CrossRef]
- Hosten, E.; Gerber, T.; Betz, R. 1-Carboxy-3-phenylpropan-2-aminium chloride. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o2947. [Google Scholar] [CrossRef]
- Vušak, D.; Smrečki, N.; Muratović, S.; Žilić, D.; Prugovečki, B.; Matković-Čalogović, D. Structural diversity in the coordination compounds of cobalt, nickel and copper with N-alkylglycinates: Crystallographic and ESR study in the solid state. RSC Adv. 2021, 11, 23779–23790. [Google Scholar] [CrossRef]
- Matkovic-Calogovic, D.; Vušak, D.; Smrecki, N.; Prugovecki, B. Interactions in copper(II), nickel(II) and cobalt(II) complexes with N-methyl-, N-ethyl- and N-propylglycine: Monomers, dimers and polymers. Acta Crystallogr. Sect. A 2019, 75, e596. [Google Scholar] [CrossRef]
- CrysAlisPRO Software System, Version 171.42.63a; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2018.
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Fischer, E. Synthese von Polypeptiden. Berichte Dtsch. Chem. Ges. 1903, 36, 2982–2992. [Google Scholar] [CrossRef]










| H2EtGlyCl | H2(i-PrGly)Cl | H2(n-PrGly)Cl | |
|---|---|---|---|
| Formula | C4H10NO2Cl | C5H12NO2Cl | C5H12NO2Cl |
| Formula weight | 139.58 | 153.61 | 153.61 |
| Crystal size/mm3 | 0.11 × 0.19 × 0.55 | 0.10 × 0.18 × 0.32 | 0.03 × 0.03 × 0.28 |
| Crystal system | orthorhombic | monoclinic | orthorhombic |
| Space group | Pnma | P21/n | Pnma |
| a/Å | 9.6643(6) | 11.42754(16) | 27.2385(7) |
| b/Å | 5.4868(3) | 5.76655(8) | 5.6178(1) |
| c/Å | 13.0554(7) | 24.2416(3) | 5.4562(1) |
| α/° | 90 | 90 | 90 |
| β/° | 90 | 90.2574(12) | 90 |
| γ/° | 90 | 90 | 90 |
| V/Å3 | 692.28(7) | 1597.44(4) | 834.91(3) |
| Z | 4 | 8 | 4 |
| Dcalc/g cm−3 | 1.339 | 1.277 | 1.222 |
| μ/mm−1 | 0.471 | 3.745 | 3.583 |
| F(000) | 296 | 656 | 328 |
| θ range/° | 4.5–27.0 | 3.6–77.5 | 3.2–79.2 |
| T/K | 150 | 298 | 293 |
| Radiation | MoKα | CuKα | CuKα |
| Range of h, k, l | −12–7, −7–6, −16–10 | −14–14, −7–7, −29–30 | −30–34, −7–7, −6–5 |
| Reflections collected | 2742 | 21184 | 4782 |
| Independent reflections | 828 | 3396 | 979 |
| Observed reflections [I ≥ 2σ(I)] (I ≥ 2σ) | 732 | 3189 | 925 |
| Rint | 0.020 | 0.047 | 0.026 |
| R 1, wR 2 [I ≥ 2σ(I)] | 0.0271, 0.0738 | 0.0465, 0.1288 | 0.0359, 0.1043 |
| Goodness-of-fit, S 3 | 1.05 | 1.05 | 1.09 |
| No. of parameters | 70 | 163 | 74 |
| Δρmin, Δρmax (e Å−3) | −0.34, 0.19 | −0.46, 0.69 | −0.22, 0.19 |
| CCDC no. | 2285208 | 2285209 | 2285213 |
| H2EtGlyNO3 | H2(i-PrGly)NO3 | H2(n-PrGly)NO3 | H(n-PrGly)·1/3H2O | |
|---|---|---|---|---|
| Formula | C4H10N2O5 | C5H12N2O5 | C5H12N2O5 | C15H35N3O7 |
| Formula weight | 166.14 | 180.17 | 180.17 | 369.46 |
| Crystal size/mm3 | 0.29 × 0.49 × 0.49 | 0.01 × 0.01 × 0.07 | 0.21 × 0.24 × 0.27 | 0.07 × 0.17 × 0.38 |
| Crystal system | orthorhombic | monoclinic | triclinic | orthorhombic |
| Space group | Pmn21 | P21/c | P | Pca21 |
| a/Å | 6.5719(1) | 5.6471(3) | 5.4914(1) | 16.2893(4) |
| b/Å | 5.0807(1) | 11.1482(8) | 11.6101(2) | 14.0655(3) |
| c/Å | 11.5516(2) | 13.5734(8) | 14.1453(4) | 8.8411(2) |
| α/° | 90 | 90 | 93.467(2) | 90 |
| β/° | 90 | 97.364(5) | 91.114(2) | 90 |
| γ/° | 90 | 90 | 93.998(1) | 90 |
| V/Å3 | 385.706(12) | 847.47(9) | 897.75(3) | 2025.65(8) |
| Z | 2 | 4 | 4 | 4 |
| Dcalc/g cm−3 | 1.431 | 1.412 | 1.333 | 1.212 |
| μ/mm−1 | 1.157 | 1.097 | 1.035 | 0.095 |
| F(000) | 176 | 384 | 384 | 808 |
| θ range/° | 6.8–62.5 | 5.2–76.4 | 3.8–79.8 | 4.3–27.0 |
| T/K | 298 | 170 | 298 | 295 |
| Radiation | CuKα | CuKα | CuKα | MoKα |
| Range of h, k, l | −6–8, −6–6, −14–14 | −7–6, −13–14, −16–16 | −4–6, −14–14, −17–17 | −20–20, −17–17, −11–11 |
| Reflections collected | 3149 | 4627 | 10962 | 32737 |
| Independent reflections | 883 | 1600 | 3730 | 4413 |
| Observed reflections [I ≥ 2σ(I)] (I ≥ 2σ) | 882 | 1223 | 3440 | 3687 |
| Rint | 0.027 | 0.044 | 0.012 | 0.043 |
| R 1, wR 2 [I ≥ 2σ(I)] | 0.0446, 0.1088 | 0.0469, 0.1382 | 0.0757, 0.2192 | 0.0403, 0.0914 |
| Goodness-of-fit, S 3 | 1.18 | 1.11 | 1.20 | 1.02 |
| No. of parameters | 76 | 157 | 217 | 258 |
| Δρmin, Δρmax (e Å−3) | −0.33, 0.29 | −0.37, 0.29 | −0.37, 0.68 | −0.13, 0.14 |
| CCDC no. | 2285211 | 2285210 | 2285214 | 2285212 |
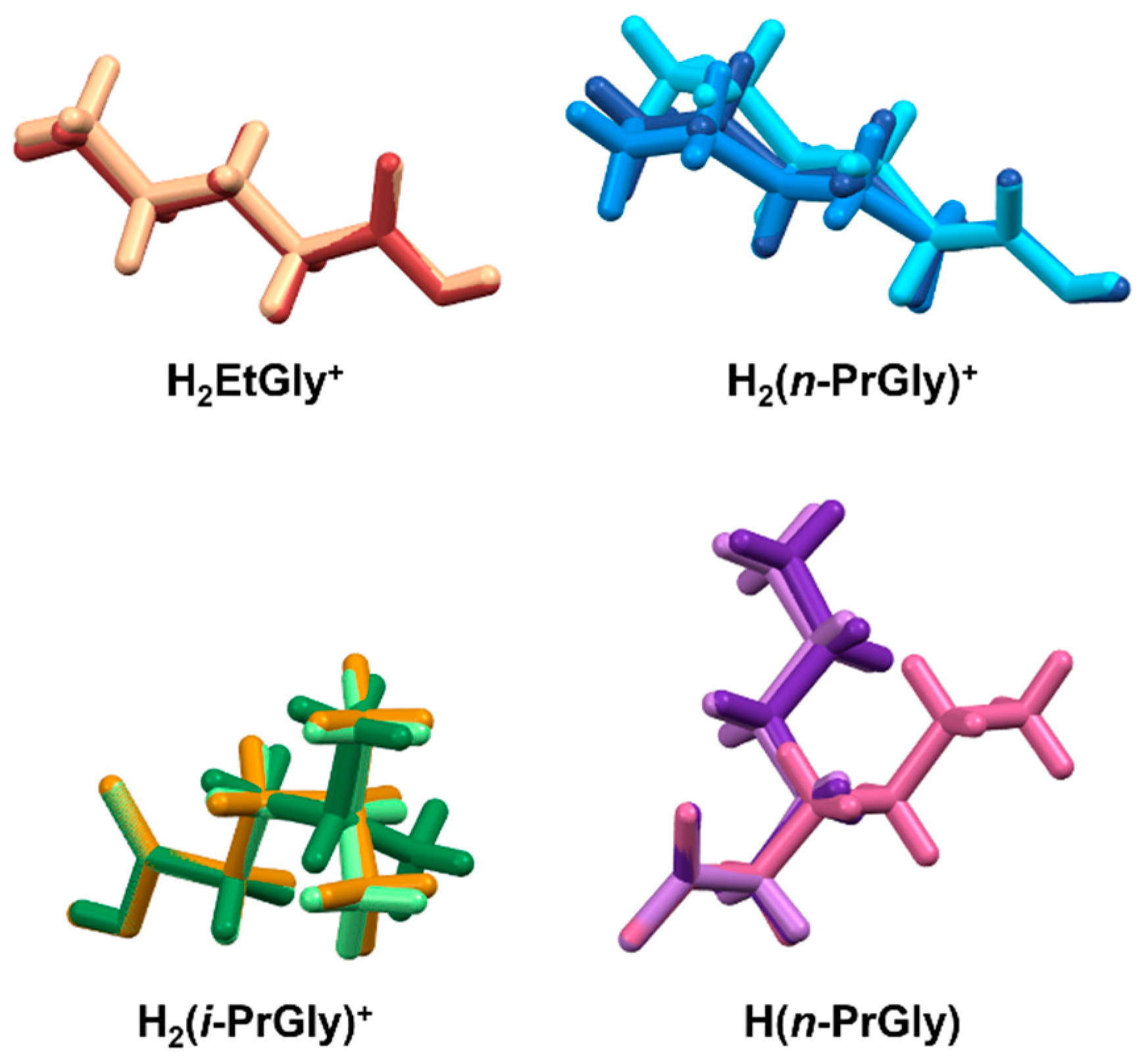
| Compound | ∠(C1–C2–N1–C3)/° | ∠(C2–N1–C3–C4(or C5)/° |
|---|---|---|
| H2EtGlyCl | 180 | 180 |
| H2(i-PrGly)Cl 1 | 170.62(15) −171.05(15) | 55.8(2) (or 178.25(16)) −55.7(2) (or −178.93(19)) |
| H2(n-PrGly)Cl | 180 | 180 |
| H2EtGlyNO3 | 180.00(1) | −180.00(1) |
| H2(i-PrGly)NO3 | −164.28(17) | 179.51(18) (or −57.2(2)) |
| H2(n-PrGly)NO3 1 | 174.2(2) 176.4(2) | 179.6(3) 178.5(2) |
| H(n-PrGly)·1/3H2O 2 | −67.0(3) −71.5(3) 179.7(2) | −174.7(2) −176.7(2) 178.7(2) |
| Compound | Melting Point/°C | Start of Decomposition/°C |
|---|---|---|
| H2EtGlyCl | 164.7 | 175 |
| H2(i-PrGly)Cl | 183.1 | 190 |
| H2(n-PrGly)Cl | 171.0 | 180 |
| H2EtGlyNO3 | 143.5 | 165 |
| H2(i-PrGly)NO3 | 112.5 | 170 |
| H2(n-PrGly)NO3 | 111.6 | 165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vušak, D.; Jurković, M.; Smrečki, N.; Prugovečki, B. Syntheses and Solid-State Characterizations of N-Alkylated Glycine Derivatives. Crystals 2023, 13, 1438. https://doi.org/10.3390/cryst13101438
Vušak D, Jurković M, Smrečki N, Prugovečki B. Syntheses and Solid-State Characterizations of N-Alkylated Glycine Derivatives. Crystals. 2023; 13(10):1438. https://doi.org/10.3390/cryst13101438
Chicago/Turabian StyleVušak, Darko, Mia Jurković, Neven Smrečki, and Biserka Prugovečki. 2023. "Syntheses and Solid-State Characterizations of N-Alkylated Glycine Derivatives" Crystals 13, no. 10: 1438. https://doi.org/10.3390/cryst13101438
APA StyleVušak, D., Jurković, M., Smrečki, N., & Prugovečki, B. (2023). Syntheses and Solid-State Characterizations of N-Alkylated Glycine Derivatives. Crystals, 13(10), 1438. https://doi.org/10.3390/cryst13101438







