Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Electrochemical Measurements
2.3. Computational Details
3. Results and Discussion
3.1. Corrosion Inhibition Activities of Expired Glucosamine Drugs on Carbon Steel
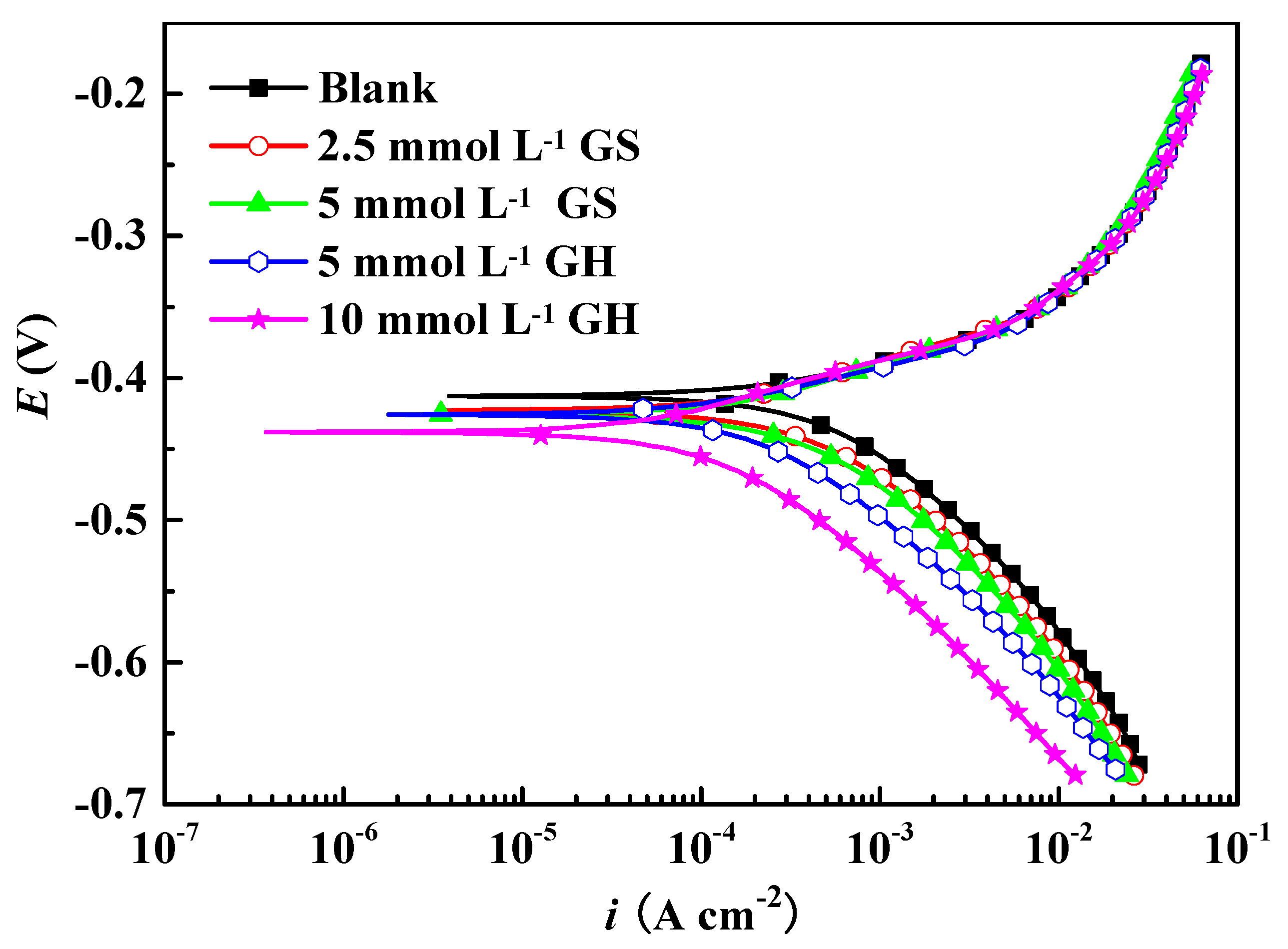
3.1.1. PDP
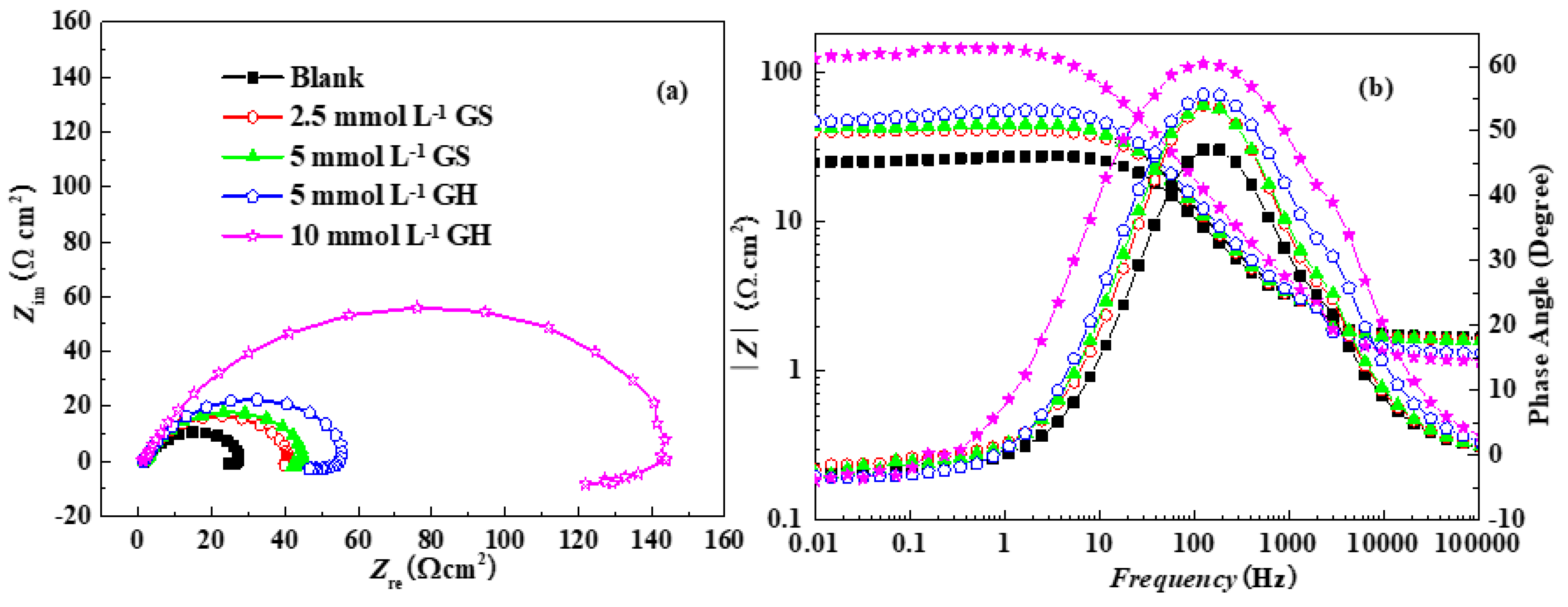
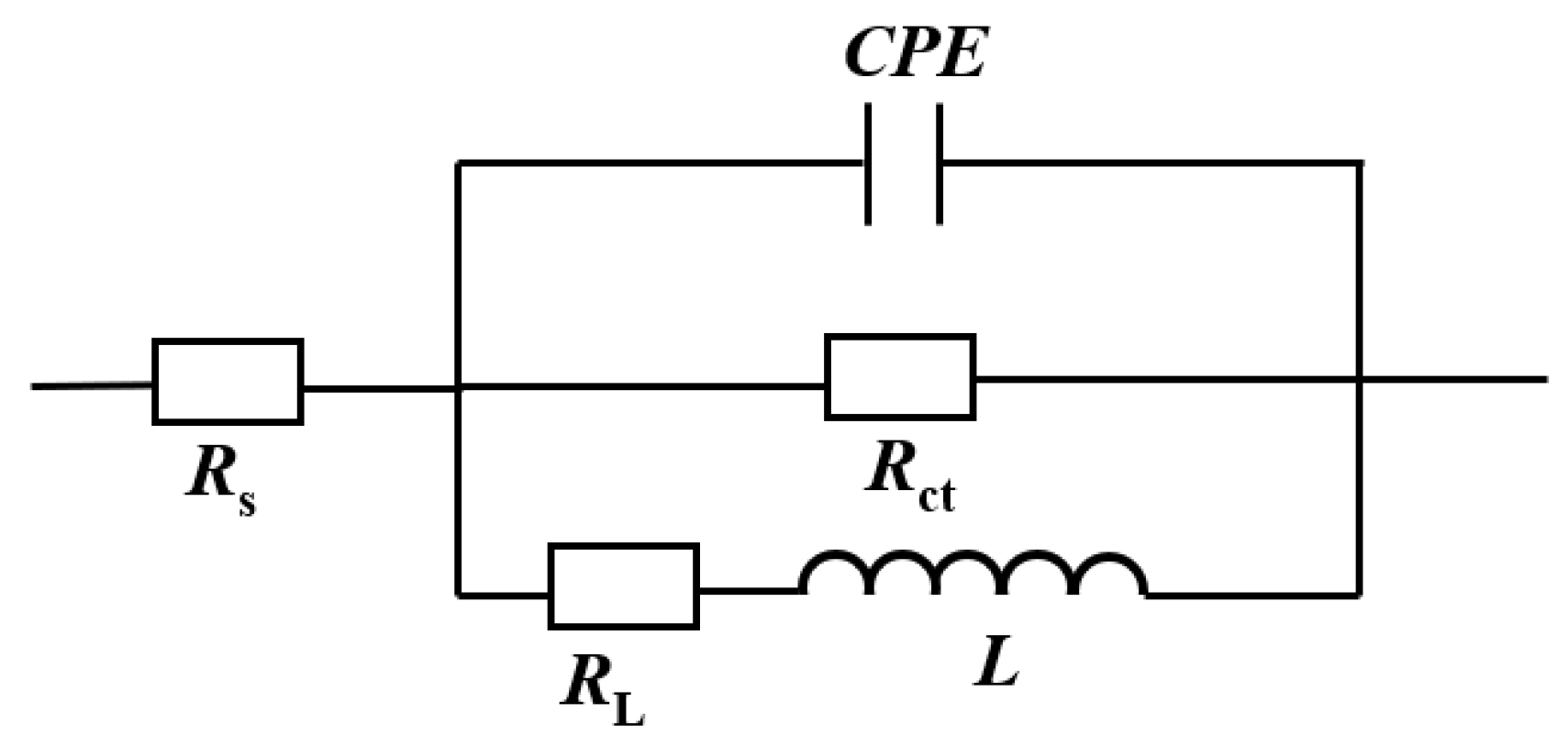
3.1.2. EIS
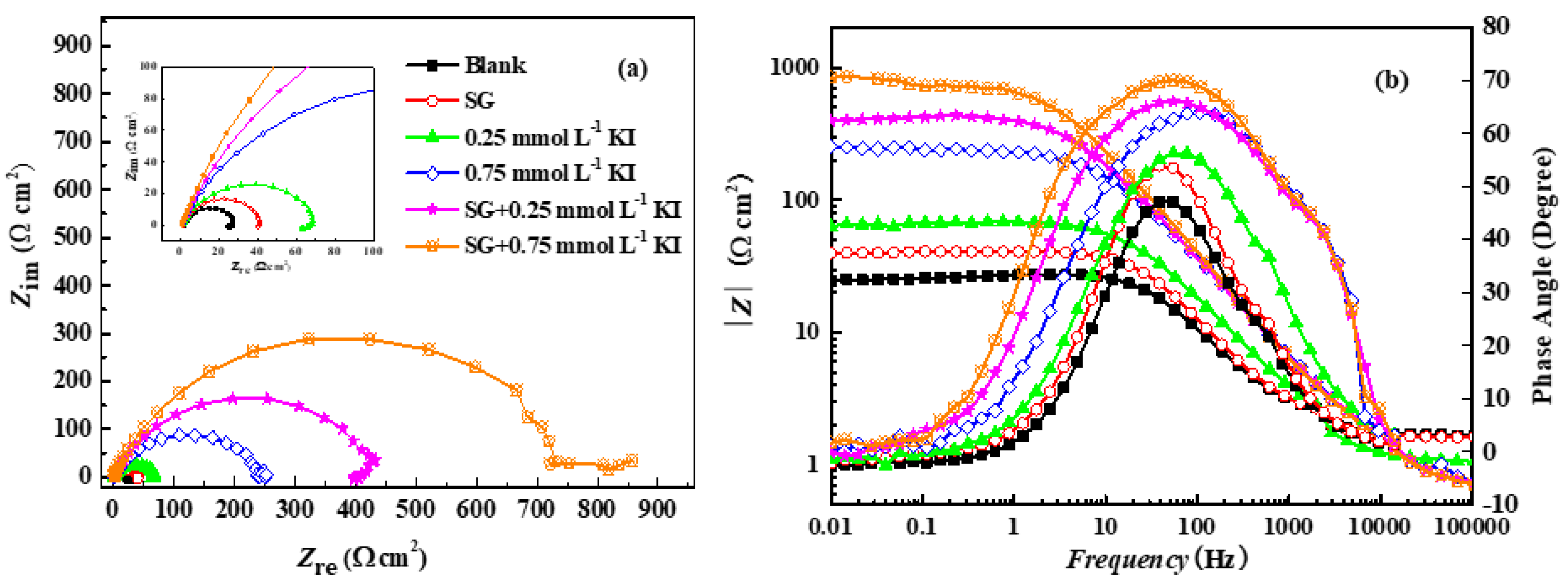
3.2. Synergistic Corrosion Inhibition Effect of GS with KI
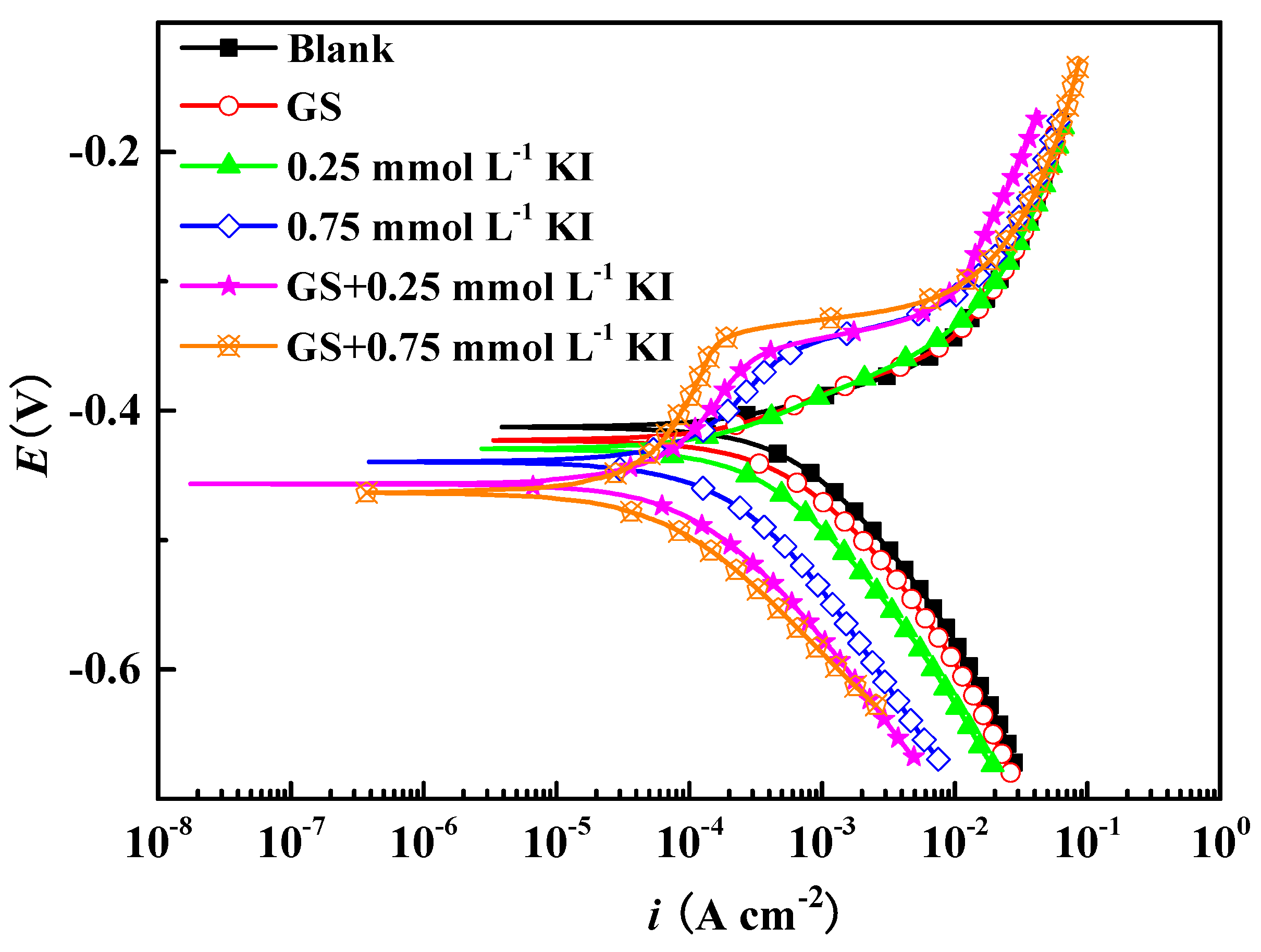
3.2.1. PDP
3.2.2. EIS
3.3. Quantum Chemical Calculations
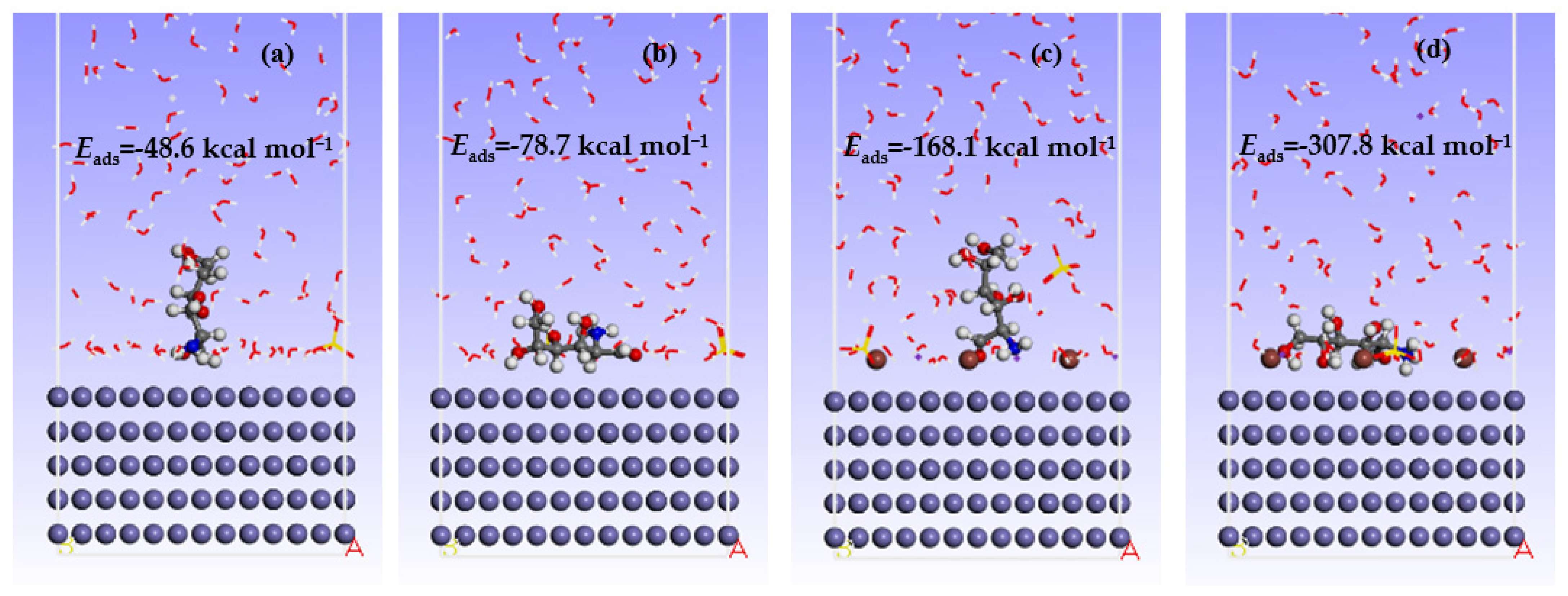
3.4. Molecular Dynamics Simulations (MDS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eshwaran, R.; Kolibabka, M.; Poschet, G.; Jainta, G.; Zhao, D.; Teuma, L.; Murillo, K.; Hammes, H.P.; Schmidt, M.; Wieland, T.; et al. Glucosamine protects against neuronal but not vascular damage in experimental diabetic retinopathy. Mol. Metab. 2021, 54, 101333. [Google Scholar] [CrossRef] [PubMed]
- Maruccia, E.; Lourenço, M.A.O.; Priamushko, T.; Bartoli, M.; Bocchini, S.; Pirri, F.C.; Saracco, G.; Kleitz, F.; Gerbaldi, C. Nanocast nitrogen-containing ordered mesoporous carbons from glucosamine for selective CO2 capture. Mater. Today Sustain. 2022, 17, 100089. [Google Scholar] [CrossRef]
- Mir, J.M.; Malik, B.A.; Khan, M.W. Glucosamine and maltol anchored Zinc(II) complex of COVID-19 health supplement relevance: DFT collaborated spectroscopic formulation with profound biological implications. J. Indian Chem. Soc. 2022, 99, 100743. [Google Scholar] [CrossRef]
- Shintani, H.; Ashida, H.; Shintani, T. Shifting the focus of d-glucosamine from a dietary supplement for knee osteoarthritis to a potential anti-aging drug. Hum. Nutr. Metab. 2021, 26, 200134. [Google Scholar] [CrossRef]
- Almashhadani, H.A.; Alshujery, M.K.; Khalil, M.; Kadhem, M.M.; Khadom, A.A. Corrosion inhibition behavior of expired diclofenac Sodium drug for Al 6061 alloy in aqueous media: Electrochemical, morphological, and theoretical investigations. J. Mol. Liq. 2021, 343, 117656. [Google Scholar] [CrossRef]
- Tanwer, S.; Shukla, S.K. Recent advances in the applicability of drugs as corrosion inhibitor on metal surface: A review. Curr. Res. Green Sustain. Chem. 2022, 5, 100227. [Google Scholar] [CrossRef]
- Wang, Y.; Qiang, Y.; Zhi, H.; Ran, B.; Zhang, D. Evaluating the synergistic effect of maple leaves extract and iodide ions on corrosion inhibition of Q235 steel in H2SO4 solution. J. Ind. Eng. Chem. 2023, 117, 422–433. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Ahmed, A.H. Alleviation of Iron Corrosion in Chloride Solution by N,N′-bis[2-Methoxynaphthylidene]amino]oxamide as a Corrosion Inhibitor. Crystals 2021, 11, 1516. [Google Scholar] [CrossRef]
- Zakaria, K.; Abbas, M.A.; Bedair, M.A. Herbal expired drug bearing glycosides and polysaccharides moieties as green and cost-effective oilfield corrosion inhibitor: Electrochemical and computational studies. J. Mol. Liq. 2022, 352, 118689. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Hao, L.; Du, H.; Pan, R.; Huang, G.; Liu, H. Cucumber (Cucumis sativus L.) Leaf Extract as a Green Corrosion Inhibitor for Carbon Steel in Acidic Solution: Electrochemical, Functional and Molecular Analysis. Molecules 2022, 27, 3826. [Google Scholar] [CrossRef]
- Haruna, K.; Saleh, T.A.; Quraishi, M.A. Expired metformin drug as green corrosion inhibitor for simulated oil/gas well acidizing environment. J. Mol. Liq. 2020, 315, 113716. [Google Scholar] [CrossRef]
- Sherif, E.S.M.; Ahmed, A.H.; Abdo, H.S.; DefAllah, M.N. Impediment of Iron Corrosion by N,N′-Bis[2-hydroxynaphthylidene]amino]oxamide in 3.5% NaCl Solution. Crystals 2021, 11, 1263. [Google Scholar] [CrossRef]
- Chandrika, K.V.S.M.; Prathyusha, V. d-glucose and its analogue, d-glucosamine, as potential hydrogen storage materials: A quantum mechanical study. Int. J. Hydrogen Energy 2022, 47, 24014–24025. [Google Scholar] [CrossRef]
- Tang, M.; Li, X.; Deng, S.; Lei, R. Synergistic inhibition effect of Mikania micrantha extract with KI on steel corrosion in H2SO4 solution. J. Mol. Liq. 2021, 344, 117926. [Google Scholar] [CrossRef]
- Jeyaprabha, C.; Sathiyanarayanan, S.; Venkatachari, G. Influence of halide ions on the adsorption of diphenylamine on iron in 0.5M H2SO4 solutions. Electrochim. Acta 2006, 51, 4080–4088. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, K.; Yan, L.; Pang, X. Inhibition of the corrosion of X70 and Q235 steel in CO2-saturated brine by imidazoline-based inhibitor. Electroanal. Chem. 2017, 791, 83–94. [Google Scholar] [CrossRef]
- Ai, J.Z.; Guo, X.P.; Chen, Z.Y. The adsorption behavior and corrosion inhibition mechanism of anionic inhibitor on galvanic electrode in 1% NaCl solution. Appl. Surf. Sci. 2006, 253, 683–688. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Wang, F. An investigation of benzimidazole derivative as corrosion inhibitor for mild steel in different concentration HCl solutions. Corros. Sci. 2011, 53, 113–121. [Google Scholar] [CrossRef]
- Liu, S.; Xu, N.; Duan, J.; Zeng, Z.; Feng, Z.; Xiao, R. Corrosion inhibition of carbon steel in tetra-n-butylammonium bromide aqueous solution by benzotriazole and Na3PO4. Corros. Sci. 2009, 51, 1356–1363. [Google Scholar] [CrossRef]
- Anaee, R.A.; Tomi, I.H.R.; Abdulmajeed, M.H.; Naser, S.A.; Kathem, M.M. Expired Etoricoxib as a corrosion inhibitor for steel in acidic solution. J. Mol. Liq. 2019, 279, 594–602. [Google Scholar] [CrossRef]
- Rakhymbay, G.; Jumanova, R.; Avchukir, K.; Bakhytzhan, Y.; Argimbayeva, A.; Burkitbayeva, B.; Turmukhanova, M.; Vacandio, F.; Adeloye, A. Synthesis and evaluation of corrosion inhibitory and adsorptive properties of N-(beta-ethoxypropionitrile-N,N-bis(2-hydroxyethylethoxy) fatty amide. R. Soc. Open Sci. 2021, 8, 211066. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, H.; Wang, F. Investigation on the inhibition behavior of a pentaerythritol glycoside for carbon steel in 3.5% NaCl saturated Ca(OH)2 solution. Corros. Sci. 2012, 54, 193–200. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, J.; Bi, Y.; Lu, X.; Tu, X.; Yang, J. Insight into synergistic corrosion inhibition of 3-amino-1,2,4-triazole-5-thiol (ATT) and NaF on magnesium alloy: Experimental and theoretical approaches. Corros. Sci. 2022, 208, 110618. [Google Scholar] [CrossRef]
- Usman, B.J.; Umoren, S.A.; Gasem, Z.M. Inhibition of API 5L X60 steel corrosion in CO2-saturated 3.5% NaCl solution by tannic acid and synergistic effect of KI additive. J. Mol. Liq. 2017, 237, 146–156. [Google Scholar] [CrossRef]
- Sorkh Kaman Zadeh, A.; Shahidi Zandi, M.; Kazemipour, M. Corrosion protection of carbon steel in acidic media by expired bupropion drug; experimental and theoretical study. J. Indian Chem. Soc. 2022, 99, 100522. [Google Scholar] [CrossRef]
- Tajabadipour, H.; Mohammadi-Manesh, H.; Shahidi-Zandi, M. Experimental and theoretical studies of carbon steel corrosion protection in phosphoric acid solution by expired lansoprazole and rabeprazole drugs. J. Indian Chem. Soc. 2022, 99, 100285. [Google Scholar] [CrossRef]
- Dohare, P.; Chauhan, D.S.; Sorour, A.A.; Quraishi, M.A. DFT and experimental studies on the inhibition potentials of expired Tramadol drug on mild steel corrosion in hydrochloric acid. Mater. Discov. 2017, 9, 30–41. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, X.; Li, X.; Wang, J.; Hu, Z.; Ma, X. 2-aminobenzimidazole derivative with surface activity as corrosion inhibitor of carbon steel in HCl: Experimental and theoretical study. J. Mol. Liq. 2020, 297, 111720. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, J.; Wang, M.; Liu, Q.; Wang, J.; Chong, Y.; Jia, G. Synergistic corrosion inhibition effects of quaternary ammonium salt cationic surfactants and thiourea on Q235 steel in sulfuric acid: Experimental and theoretical research. Corros. Sci. 2022, 199, 110199. [Google Scholar] [CrossRef]
- Ali, S.M.; Al Lehaibi, H.A. Control of zinc corrosion in acidic media: Green fenugreek inhibitor. Trans. Nonferrous Met. Soc. China 2016, 26, 3034–3045. [Google Scholar] [CrossRef]
- Mohamed, K.E.M.; Ibrahim, O.H.; El-Bedawy, M.E.; Ali, A.H. Synergistic effect of different Zn salts with sodium octanoate on the corrosion inhibition of carbon steel in cooling water. J. Radiat. Res. Appl. Sci. 2020, 13, 276–287. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Synergistic effect of iodide ions on the corrosion inhibition of mild steel in 1 M HCl by Cardaria Draba leaf extract. Results Chem. 2022, 4, 100668. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Chen, X.; Zhang, Z.; Wang, B.; Li, H.J.; Wu, Y.C. Synthesis and evaluation of omeprazole-based derivatives as eco-friendly corrosion inhibitors for Q235 steel in hydrochloric acid. J. Environ. Chem. Eng. 2022, 10, 108674. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Wang, F. Experimental and theoretical studies for corrosion inhibition of carbon steel by imidazoline derivative in 5% NaCl saturated Ca(OH)2 solution. Electrochim. Acta 2011, 58, 427–436. [Google Scholar] [CrossRef]
- Obot, I.B.; Kaya, S.; Kaya, C.; Tüzün, B. Density Functional Theory (DFT) modeling and Monte Carlo simulation assessment of inhibition performance of some carbohydrazide Schiff bases for steel corrosion. Phys. E Low Dimens. Syst. Nanostruct. 2016, 80, 82–90. [Google Scholar] [CrossRef]
- Xu, X.; Singh, A.; Sun, Z.; Ansari, K.R.; Lin, Y. Theoretical, thermodynamic and electrochemical analysis of biotin drug as an impending corrosion inhibitor for mild steel in 15% hydrochloric acid. R. Soc. Open Sci. 2017, 4, 170933. [Google Scholar] [CrossRef] [PubMed]
- Olasunkanmi, L.O.; Obot, I.B.; Kabanda, M.M.; Ebenso, E.E. Some Quinoxalin-6-yl Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies. J. Phys. Chem. C 2015, 119, 16004–16019. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Cui, X.; Chen, D.; Li, G. Experimental and theoretical investigation on corrosion inhibitive properties of steel rebar by a newly designed environmentally friendly inhibitor formula. RSC Adv. 2018, 8, 6507–6518. [Google Scholar] [CrossRef]
- El-Hendawy, M.M.; Kamel, A.M.; Mohamed, M.M.A. The anti-corrosive behavior of benzo-fused N-heterocycles: An in silico study toward developing organic corrosion inhibitors. Phys. Chem. Chem. Phys. 2022, 24, 743–756. [Google Scholar] [CrossRef]
- Song, Z.; Cai, H.; Liu, Q.; Liu, X.; Pu, Q.; Zang, Y.; Xu, N. Numerical Simulation of Adsorption of Organic Inhibitors on C-S-H Gel. Crystals 2020, 10, 742. [Google Scholar] [CrossRef]
- Lgaz, H.; Chaouiki, A.; Chafiq, M.; Salghi, R.; Tachallait, H.; Bougrin, K.; Chi, H.Y.; Kwon, C.; Chung, I.M. Evaluating the corrosion inhibition properties of novel 1,2,3-triazolyl nucleosides and their synergistic effect with iodide ions against mild steel corrosion in HCl: A combined experimental and computational exploration. J. Mol. Liq. 2021, 338, 116522. [Google Scholar] [CrossRef]


| Content | C | Mn | P | S | Si | Fe |
|---|---|---|---|---|---|---|
| Amount (wt%) | 0.16 | 0.45 | 0.04 | 0.05 | 0.03 | Balance |
| Inhibitor | βa (mV dec−1) | −βc (mV dec−1) | icorr (mA cm−2) | Ecorr (mV) | IE (%) |
|---|---|---|---|---|---|
| Blank | 76 | 121 | 0.667 | −413 | / |
| 2.5 mmol L−1 GS | 74 | 120 | 0.427 | −423 | 36.0 |
| 5 mmol L−1 GS | 75 | 122 | 0.370 | −426 | 44.5 |
| 5 mmol L−1 GH | 74 | 122 | 0.298 | −426 | 55.3 |
| 10 mmol L−1 GH | 72 | 119 | 0.119 | −438 | 82.2 |
| Inhibitor | Rs(Ω cm2) | CPE−Y (μ Ω−1 cm−2 Sn) | CPE−n | Rct (Ω cm2) | RL (Ω cm2) | L (H cm2) |
|---|---|---|---|---|---|---|
| Blank | 1.67 | 643 | 0.76 | 30.5 | 121 | 7.68 |
| 2.5 mmol L−1 GS | 1.64 | 535 | 0.77 | 45.1 | 142 | 10.1 |
| 5 mmol L−1 GS | 1.61 | 482 | 0.78 | 51.7 | 186 | 11.9 |
| 5 mmol L−1 GH | 1.31 | 415 | 0.78 | 60.5 | 233 | 75.5 |
| 10 mmol L−1 GH | 1.14 | 352 | 0.77 | 167.8 | 699 | 128 |
| Inhibitor Formulation | βa (mV dec−1) | −βc (mV dec−1) | icorr (mA cm−2) | Ecorr (mV) | IE (%) | S |
|---|---|---|---|---|---|---|
| Blank | 76 | 121 | 0.667 | −413 | / | / |
| GS | 74 | 120 | 0.427 | −423 | 36.0 | / |
| 0.25 mmol L−1 KI | 78 | 119 | 0.304 | −429 | 54.4 | / |
| 0.75 mmol L−1 KI | 90 | 118 | 0.109 | −439 | 83.7 | / |
| GS + 0.25 mmol L−1 KI | 154 | 118 | 0.081 | −457 | 87.8 | 1.24 |
| GS + 0.75 mmol L−1 KI | 257 | 119 | 0.059 | −464 | 91.2 | 1.02 |
| Inhibitor Formulation | Rs (Ω cm2) | CPE−Y (μ Ω−1 cm−2 Sn) | CPE−n | Rct (Ω cm2) | RL (Ω cm2) | L (H cm2) |
|---|---|---|---|---|---|---|
| Blank | 1.67 | 643 | 0.76 | 30.5 | 121 | 7.68 |
| GS | 1.64 | 535 | 0.77 | 45.1 | 142 | 10.1 |
| 0.25 mmol L−1 KI | 0.82 | 511 | 0.75 | 74.6 | 553 | 132 |
| 0.75 mmol L−1 KI | 0.78 | 174 | 0.80 | 246 | / | / |
| GS + 0.25 mmol L−1 KI | 0.74 | 161 | 0.78 | 449 | 1.43 × 104 | 5.61 × 103 |
| GS + 0.75 mmol L−1 KI | 0.74 | 135 | 0.80 | 795 | / | / |
| Compound | EHOMO (eV) | ELUMO (eV) | η (eV) | x (eV) | σ (eV−1) | ω (eV) | ΔN |
|---|---|---|---|---|---|---|---|
| Neutral Glucosamine | −6.91 | −1.51 | 2.70 | 4.21 | 0.37 | 3.28 | 0.113 |
| Protonated Glucosamine | −7.72 | −1.94 | 2.89 | 4.83 | 0.35 | 4.04 | −0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Zhang, S.; Zhou, Y.; Pan, R.; Du, H.; Liu, F.; Yang, Y. Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations. Crystals 2023, 13, 205. https://doi.org/10.3390/cryst13020205
Feng L, Zhang S, Zhou Y, Pan R, Du H, Liu F, Yang Y. Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations. Crystals. 2023; 13(2):205. https://doi.org/10.3390/cryst13020205
Chicago/Turabian StyleFeng, Lijuan, Shanshan Zhang, Yan Zhou, Rongkai Pan, Hongchen Du, Fangfang Liu, and Yongqi Yang. 2023. "Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations" Crystals 13, no. 2: 205. https://doi.org/10.3390/cryst13020205
APA StyleFeng, L., Zhang, S., Zhou, Y., Pan, R., Du, H., Liu, F., & Yang, Y. (2023). Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations. Crystals, 13(2), 205. https://doi.org/10.3390/cryst13020205






