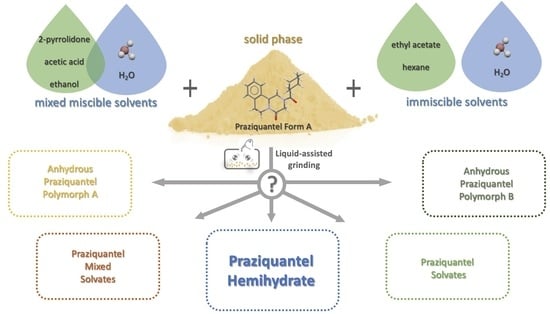
Mechanochemical Synthesis of Praziquantel Hemihydrate in the Presence of Five Solvents with Different Water Miscibility †
Abstract
:1. Introduction
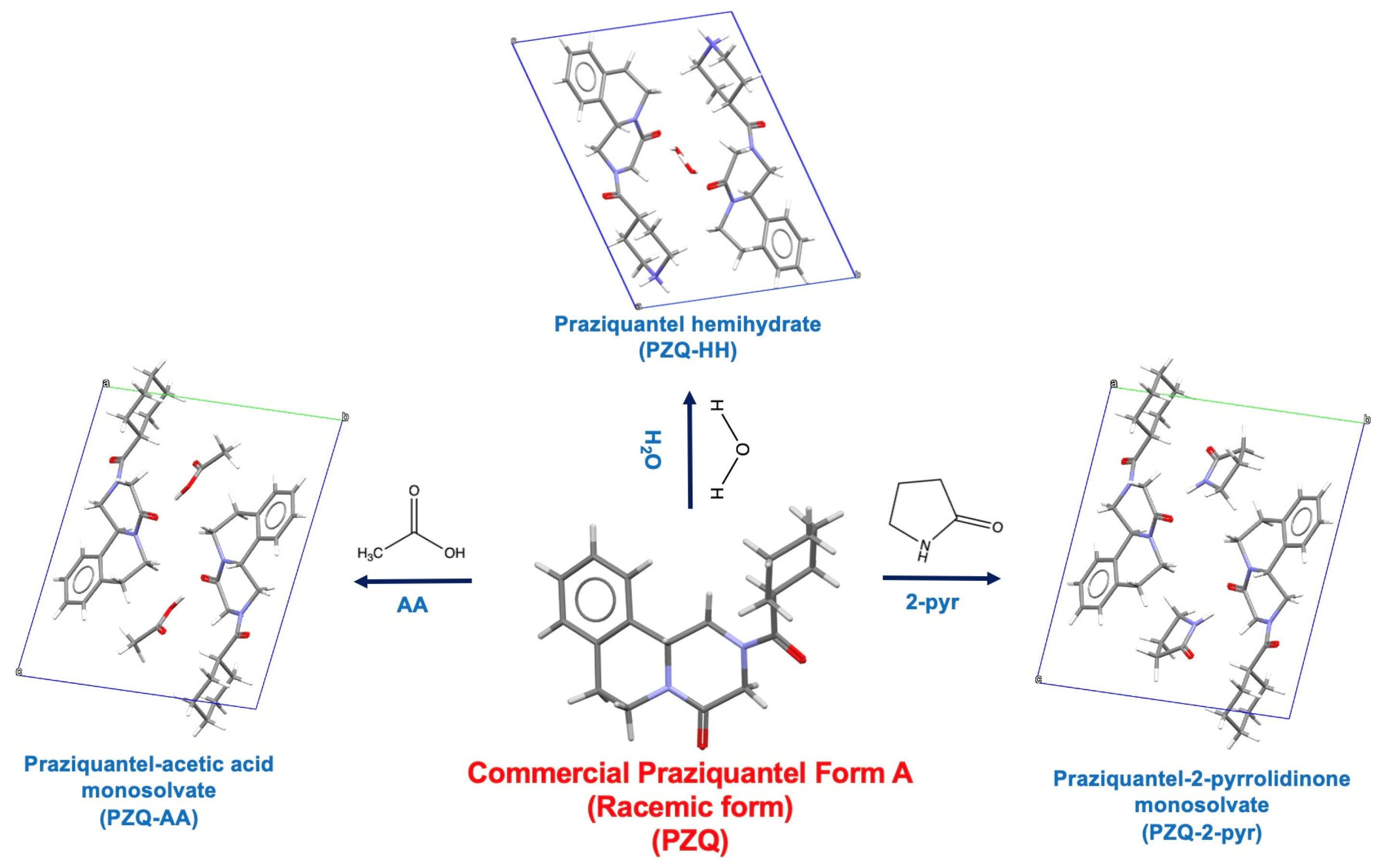
- Two solvents able to form solvates with PZQ, one miscible and one less miscible, such as AA and 2-pyr, respectively;
- Two solvents not forming PZQ solvate, one miscible and one slightly miscible, i.e., EtOH and EA, respectively;
- One solvent that does not form PZQ solvate and is immiscible, such as HXN.
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Milling Experiments
2.2.2. Solvent Properties
2.2.3. Mechanochemical Interconversion Experiments
2.3. Sample Characterization
2.3.1. Powder X-ray Diffraction (PXRD)
2.3.2. Differential Scanning Calorimetry (DSC)
3. Results
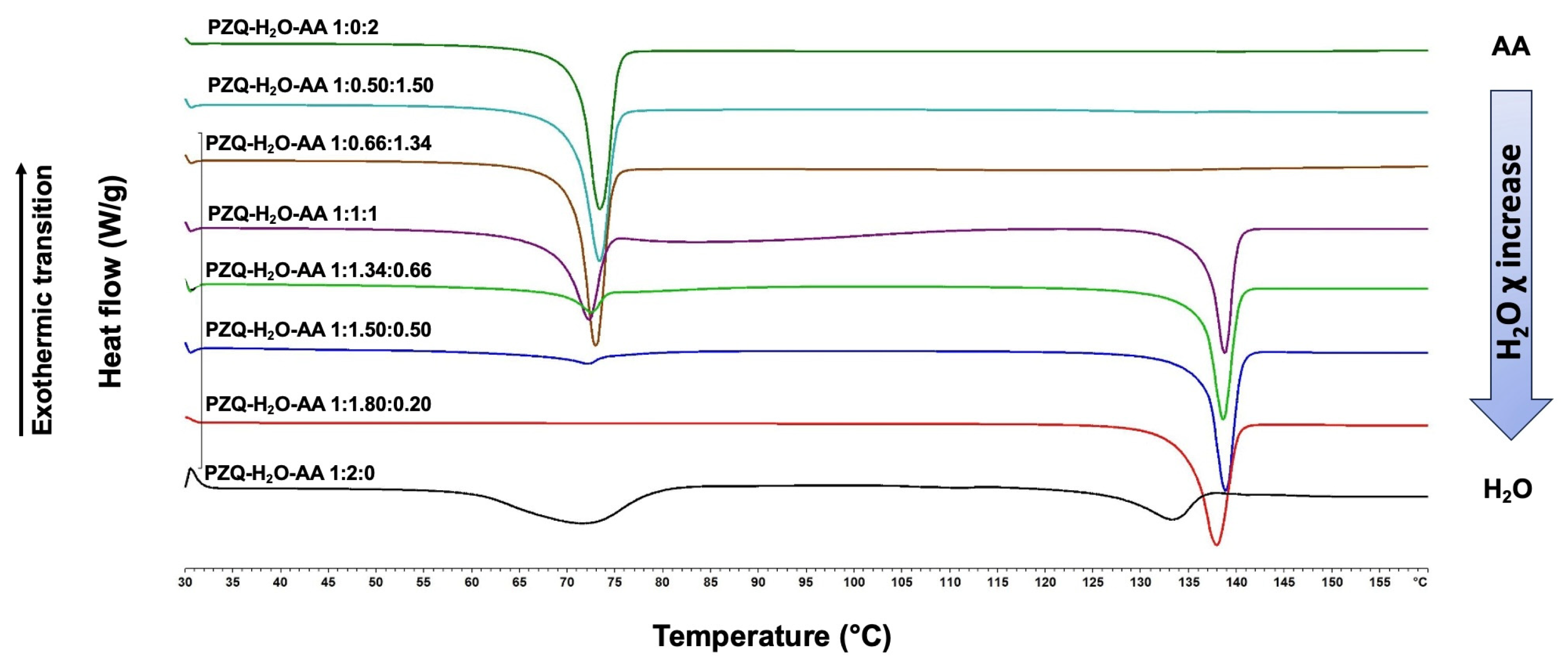
3.1. Grinding Tests in the Presence of H2O-AA Mixture
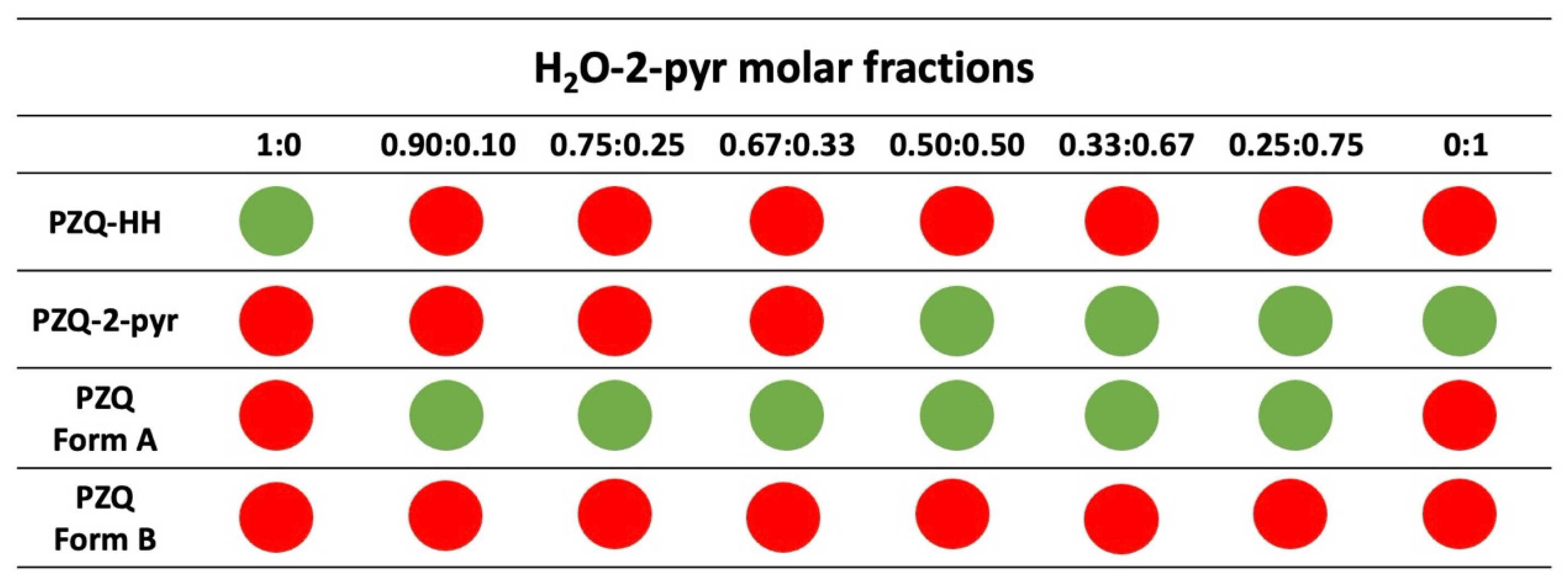
3.2. Grinding Tests in the Presence of H2O-2-pyr Mixture
3.3. Grinding Tests in the Presence of H2O-EtOH Mixture
3.4. Grinding Tests in the Presence of H2O-EA Mixtures
3.5. Grinding Tests in the Presence of H2O-HXN Mixtures
3.6. Mechanochemical Interconversion Experiments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Griesser, U.J. The Importance of Solvates. In Polymorphism; Wiley: Hoboken, NJ, USA, 2006; pp. 211–233. [Google Scholar]
- ICH Q3C (R8) Residual Solvents—Scientific Guideline|European Medicines Agency. Available online: https://www.ema.europa.eu/en/ich-q3c-r8-residual-solvents-scientific-guideline (accessed on 29 February 2024).
- Papadakis, E.; Tula, A.K.; Gani, R. Solvent selection methodology for pharmaceutical processes: Solvent swap. Chem. Eng. Res. Des. 2016, 115, 443–461. [Google Scholar] [CrossRef]
- D’Abbrunzo, I.; Spadaro, M.; Arhangelskis, M.; Zingone, G.; Hasa, D.; Perissutti, B. Competitive Mechanochemical Solvate Formation of Theophylline in the Presence of Miscible Liquid Mixtures. Cryst. Growth Des. 2023, 23, 8094–8102. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis Control: Praziquantel Forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Cioli, D.; Pica-Mattoccia, L. Praziquantel. Parasitol. Res. 2003, 90, S3–S9. [Google Scholar] [CrossRef]
- Cedillo-Cruz, A.; Aguilar, M.I.; Flores-Alamo, M.; Palomares-Alonso, F.; Jung-Cook, H. A Straightforward and Efficient Synthesis of Praziquantel Enantiomers and Their 4′-Hydroxy Derivatives. Tetrahedron Asymmetry 2014, 25, 133–140. [Google Scholar] [CrossRef]
- Meister, I.; Ingram-Sieber, K.; Cowan, N.; Todd, M.; Robertson, M.N.; Meli, C.; Patra, M.; Gasser, G.; Keiser, J. Activity of Praziquantel Enantiomers and Main Metabolites against Schistosoma Mansoni. Antimicrob. Agents Chemother. 2014, 58, 5466–5472. [Google Scholar] [CrossRef] [PubMed]
- Kovač, J.; Vargas, M.; Keiser, J. In Vitro and in Vivo Activity of R- and S- Praziquantel Enantiomers and the Main Human Metabolite Trans-4-Hydroxy-Praziquantel against Schistosoma Haematobium. Parasit. Vectors 2017, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Bagchus, W.M.; Bezuidenhout, D.; Harrison-Moench, E.; Kourany-Lefoll, E.; Wolna, P.; Yalkinoglu, O. Relative Bioavailability of Orally Dispersible Tablet Formulations of Levo- and Racemic Praziquantel: Two Phase I Studies. Clin. Transl. Sci. 2019, 12, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Shu-Hua, X.; Catto, B.A. Comparative in Vitro and in Vivo Activity of Racemic Praziquantel and Its Levorotated Isomer on Schistosoma Mansoni. J. Infect. Dis. 1989, 159, 589–592. [Google Scholar] [CrossRef]
- WHO Model List of Essential Medicines—22nd List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02 (accessed on 18 September 2023).
- WHO Model List of Essential Medicines for Children—8th List. 2021. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.03 (accessed on 18 September 2023).
- FDA Biltricide (Praziquantel) Tablets Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018714s012lbl.pdf (accessed on 4 November 2023).
- Zanolla, D.; Perissutti, B.; Passerini, N.; Chierotti, M.R.; Hasa, D.; Voinovich, D.; Gigli, L.; Demitri, N.; Geremia, S.; Keiser, J.; et al. A New Soluble and Bioactive Polymorph of Praziquantel. Eur. J. Pharm. Biopharm. 2018, 127, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, D.; Perissutti, B.; Vioglio, P.C.; Chierotti, M.R.; Gigli, L.; Demitri, N.; Passerini, N.; Albertini, B.; Franceschinis, E.; Keiser, J.; et al. Exploring Mechanochemical Parameters Using a DoE Approach: Crystal Structure Solution from Synchrotron XRPD and Characterization of a New Praziquantel Polymorph. Eur. J. Pharm. Sci. 2019, 140, 105084. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.; Seidel-Morgenstern, A.; Lorenz, H. Role of Mechanochemistry in Solid Form Selection and Identification of the Drug Praziquantel. Cryst. Growth Des. 2021, 21, 5854–5861. [Google Scholar] [CrossRef]
- de Moraes, M.G.F.; Barreto, A.G., Jr.; Secchi, A.R.; de Souza, M.B., Jr.; Lage, P.L.D.C.; Myerson, A.S. Polymorphism of Praziquantel: Role of Cooling Crystallization in Access to Solid Forms and Discovery of New Polymorphs. Cryst. Growth Des. 2023, 23, 1247–1258. [Google Scholar] [CrossRef]
- Meyer, T.; Sekljic, H.; Fuchs, S.; Bothe, H.; Schollmeyer, D.; Miculka, C. Taste, A New Incentive to Switch to (R)-Praziquantel in Schistosomiasis Treatment. PLoS Negl. Trop. Dis. 2009, 3, e357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Wang, J.-K.; Ching, C.B. Investigation of the Phase Diagrams of Chiral Praziquantel. Chirality 2006, 18, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, D.; Hasa, D.; Arhangelskis, M.; Schneider-Rauber, G.; Chierotti, M.R.; Keiser, J.; Voinovich, D.; Jones, W.; Perissutti, B. Mechanochemical Formation of Racemic Praziquantel Hemihydrate with Improved Biopharmaceutical Properties. Pharmaceutics 2020, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- MacEachern, L.; Kermanshahi-Pour, A.; Mirmehrabi, M. Transformation under Pressure: Discovery of a Novel Crystalline Form of Anthelmintic Drug Praziquantel Using High-Pressure Supercritical Carbon Dioxide. Int. J. Pharm. 2022, 619, 121723. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Rojas, D.; Maggio, R.M.; Kaufman, T.S. Preparation and Characterization of a New Solid Form of Praziquantel, an Essential Anthelmintic Drug. Praziquantel Racemic Monohydrate. Eur. J. Pharm. Sci. 2020, 146, 105267. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, D.; Gigli, L.; Hasa, D.; Chierotti, M.R.; Arhangelskis, M.; Demitri, N.; Jones, W.; Voinovich, D.; Perissutti, B. Mechanochemical Synthesis and Physicochemical Characterization of Previously Unreported Praziquantel Solvates with 2-Pyrrolidone and Acetic Acid. Pharmaceutics 2021, 13, 1606. [Google Scholar] [CrossRef] [PubMed]
- D’Abbrunzo, I.; Bianco, E.; Gigli, L.; Demitri, N.; Birolo, R.; Chierotti, M.R.; Škorić, I.; Keiser, J.; Häberli, C.; Voinovich, D.; et al. Praziquantel Meets Niclosamide: A Dual-Drug Antiparasitic Cocrystal. Int. J. Pharm. 2023, 644, 123315. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Lara, J.C.; Guzman-Villanueva, D.; Arenas-García, J.I.; Herrera-Ruiz, D.; Rivera-Islas, J.; Román-Bravo, P.; Morales-Rojas, H.; Höpfl, H. Cocrystals of Active Pharmaceutical Ingredients—Praziquantel in Combination with Oxalic, Malonic, Succinic, Maleic, Fumaric, Glutaric, Adipic, and Pimelic Acids. Cryst. Growth Des. 2013, 13, 169–185. [Google Scholar] [CrossRef]
- Cappuccino, C.; Spoletti, E.; Renni, F.; Muntoni, E.; Keiser, J.; Voinovich, D.; Perissutti, B.; Lusi, M. Co-Crystalline Solid Solution Affords a High-Soluble and Fast-Absorbing Form of Praziquantel. Mol. Pharm. 2023, 20, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, D.; Chen, T.; Zhang, B.; Xing, C.; Zhang, L.; Lu, Y.; Du, G. Insights into the Solubility and Structural Features of Four Praziquantel Cocrystals. Cryst. Growth Des. 2021, 21, 6321–6331. [Google Scholar] [CrossRef]
- Yang, D.; Cao, J.; Heng, T.; Xing, C.; Yang, S.; Zhang, L.; Lu, Y.; Du, G. Theoretical Calculation and Structural Analysis of the Cocrystals of Three Flavonols with Praziquantel. Cryst. Growth Des. 2021, 21, 2292–2300. [Google Scholar] [CrossRef]
- Cugovčan, M.; Jablan, J.; Lovrić, J.; Cinčić, D.; Galić, N.; Jug, M. Biopharmaceutical Characterization of Praziquantel Cocrystals and Cyclodextrin Complexes Prepared by Grinding. J. Pharm. Biomed. Anal. 2017, 137, 42–53. [Google Scholar] [CrossRef]
- Devogelaer, J.J.; Charpentier, M.D.; Tijink, A.; Dupray, V.; Coquerel, G.; Johnston, K.; Meekes, H.; Tinnemans, P.; Vlieg, E.; Ter Horst, J.H.; et al. Cocrystals of Praziquantel: Discovery by Network-Based Link Prediction. Cryst. Growth Des. 2021, 21, 3428–3437. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.G.; Chaves, I.d.S.; Melo, N.F.S.; Jesus, M.B.; Fraceto, L.F.; Fernandes, S.A.; Paula, E.; de Freitas, M.P.; Pinto, L.d.M.A. Computational Analysis and Physico-Chemical Characterization of an Inclusion Compound between Praziquantel and Methyl-β-Cyclodextrin for Use as an Alternative in the Treatment of Schistosomiasis. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 19–28. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, C.; Salas-Zúñiga, R.; Obdulia Sánchez-Guadarrama, M.; Delgado-Díaz, A.; Herrera-Ruiz, D.; Morales-Rojas, H.; Höpfl, H. Structural, Physicochemical, and Biopharmaceutical Properties of Cocrystals with RS- and R-Praziquantel—Generation and Prolongation of the Supersaturation State in the Presence of Cellulosic Polymers. Cryst. Growth Des. 2022, 22, 6023–6038. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Q.; Ji, W.; An, Q.; Song, J.; Xing, C.; Yang, D.; Zhang, L.; Lu, Y.; Du, G. Cocrystals of Praziquantel with Phenolic Acids: Discovery, Characterization, and Evaluation. Molecules 2022, 27, 2022. [Google Scholar] [CrossRef] [PubMed]
- Rapeenun, P.; Gerard, C.J.J.; Cartigny, Y.; Tinnemans, P.; De Gelder, R.; Flood, A.E.; Ter Horst, J.H. Searching for Conglomerate Cocrystals of the Racemic Compound Praziquantel. Cryst. Growth Des. 2024, 24, 490. [Google Scholar] [CrossRef]
- Garrido, C.C.; Vandooren, M.; Robeyns, K.; Debecker, D.P.; Luis, P.; Leyssens, T. Combining a Drug and a Nutraceutical: A New Cocrystal of Praziquantel and Curcumin. Crystals 2024, 14, 181. [Google Scholar] [CrossRef]
- D’Abbrunzo, I.; Procida, G.; Perissutti, B. Praziquantel Fifty Years on: A Comprehensive Overview of Its Solid State. Pharmaceutics 2023, 16, 27. [Google Scholar] [CrossRef]
- Tabasum, F.; Sowmyalatha, T.; Omar, M.; Raja Reddy, R. Design of Experiment (Doe) of a New Formulation of Praziquantel by Using Microcrystalline Depolymerized Cellulose. IJAMSCR 2023, 11, 463–470. [Google Scholar] [CrossRef]
- WHO Part 6. 2018. Available online: https://extranet.who.int/prequal/sites/default/files/whopar_files/NT004part6v1.pdf (accessed on 9 April 2024).
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, E.; Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M.; Zielińska-Pisklak, M. Pharmaceutical Hydrates Analysis—Overview of Methods and Recent Advances. Pharmaceutics 2020, 12, 959. [Google Scholar] [CrossRef]
- Bērziņš, A.; Skarbulis, E.; Rekis, T.; Actiņš, A. On the Formation of Droperidol Solvates: Characterization of Structure and Properties. Cryst. Growth Des. 2014, 14, 2654–2664. [Google Scholar] [CrossRef]
- Caira, M.R.; Nassimbeni, L.R.; Toda, F.; Vujovic, D. Inclusion of Aminobenzonitrile Isomers by a Diol Host Compound: Structure and Selectivity. J. Am. Chem. Soc. 2000, 122, 9367–9372. [Google Scholar] [CrossRef]
- Friščić, T.; Halasz, I.; Strobridge, F.C.; Dinnebier, R.E.; Stein, R.S.; Fábián, L.; Curfs, C. A Rational Approach to Screen for Hydrated Forms of the Pharmaceutical Derivative Magnesium Naproxen Using Liquid-Assisted Grinding. CrystEngComm 2011, 13, 3125. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Jones, W.; Motherwell, W.D.S. Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-Assisted Grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hasa, D.; Pastore, M.; Arhangelskis, M.; Gabriele, B.; Cruz-Cabeza, A.J.; Rauber, G.S.; Bond, A.D.; Jones, W. On the Kinetics of Solvate Formation through Mechanochemistry. CrystEngComm 2019, 21, 2097–2104. [Google Scholar] [CrossRef]
- Bingham, A.L.; Hughes, D.S.; Hursthouse, M.B.; Lancaster, R.W.; Tavener, S.; Threlfall, T.L. Over One Hundred Solvates of Sulfathiazole. Chem. Comumn. 2001, 7, 603–604. [Google Scholar] [CrossRef]
- Campeta, A.M.; Chekal, B.P.; Abramov, Y.A.; Meenan, P.A.; Henson, M.J.; Shi, B.; Singer, R.A.; Horspool, K.R. Development of a Targeted Polymorph Screening Approach for a Complex Polymorphic and Highly Solvating API. J. Pharm. Sci. 2010, 99, 3874–3886. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Loots, L.; Friščić, T. Towards Medicinal Mechanochemistry: Evolution of Milling from Pharmaceutical Solid Form Screening to the Synthesis of Active Pharmaceutical Ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.D.; Priotti, J.; Orlandi, S.; Leonardi, D.; Lamas, M.C.; Nunes, T.G.; Diogo, H.P.; Salomon, C.J.; Ferreira, M.J. Unexpected Solvent Impact in the Crystallinity of Praziquantel/Poly(Vinylpyrrolidone) Formulations. A Solubility, DSC and Solid-State NMR Study. Int. J. Pharm. 2016, 511, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. Handbook of Organic Solvents; Routledge: London, UK; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Altshuller, A.P.; Everson, H.E. The Solubility of Ethyl Acetate in Water. J. Am. Chem. Soc. 1953, 75, 1727. [Google Scholar] [CrossRef]
- Carl Roth GmbH. Safety Data Sheet: 2-Pyrrolidone. Available online: https://www.carlroth.com/medias/SDB-8779-GB-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyNDk5ODB8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oMmYvaDVmLzkwNzQ1MTQ1NTkwMDYucGRmfDhhZjU0NWVjZGFhMThlNTY2NmFiMGY2OGQxNThiYTU5MDBiMTc2NzU5NjU2YjllYWUwMWE0NjE4OGY2NzE1MzY (accessed on 29 February 2024).
- McNaught, A.D.; Wilkinson, A. The IUPAC Compendium of Chemical Terminology, 2nd ed.; Gold, V., Ed.; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 1997. [Google Scholar]
- Carl Roth GmbH. Safety Data Sheet: N-Hexane. Available online: https://www.carlroth.com/medias/SDB-CP47-IE-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wzMDYwMTN8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oNjYvaDE2LzkwNjAzNzY4MDU0MDYucGRmfGU3NjdjNGYxZjI0ZTNjMDhjNTU4NGRiYjZkMTc3MWNmNjRlNmQxNWFmYWZjZmMyMTMwNTA3NjU2ODJhNjQ5OGU (accessed on 29 February 2024).
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 1986. [CrossRef]
- Hasa, D.; Voinovich, D.; Perissutti, B.; Grassi, G.; Fiorentino, S.; Farra, R.; Abrami, M.; Colombo, I.; Grassi, M. Reduction of Melting Temperature and Enthalpy of Drug Crystals: Theoretical Aspects. Eur. J. Pharm. Sci. 2013, 50, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Hasa, D.; Jones, W. Screening for New Pharmaceutical Solid Forms Using Mechanochemistry: A Practical Guide. Adv. Drug Deliv. Rev. 2017, 117, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Dávila, M.J.; Alcalde, R.; Aparicio, S. Pyrrolidone Derivatives in Water Solution: An Experimental and Theoretical Perspective. Ind. Eng. Chem. Res. 2009, 48, 1036–1050. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.; Gong, N.; Cao, X.; Wang, S.; Sun, C. Study of Molecular Association in Acetic Acid-Water Binary Solution by Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Pezzotti, S.; Das Mahanta, D.; Schulke, S.; Schwaab, G.; Havenith, M. From Local Hydration Motifs in Aqueous Acetic Acid Solutions to Macroscopic Mixing Thermodynamics: A Quantitative Connection from THz-Calorimetry. J. Phys. Chem. B 2023, 127, 9204–9210. [Google Scholar] [CrossRef] [PubMed]

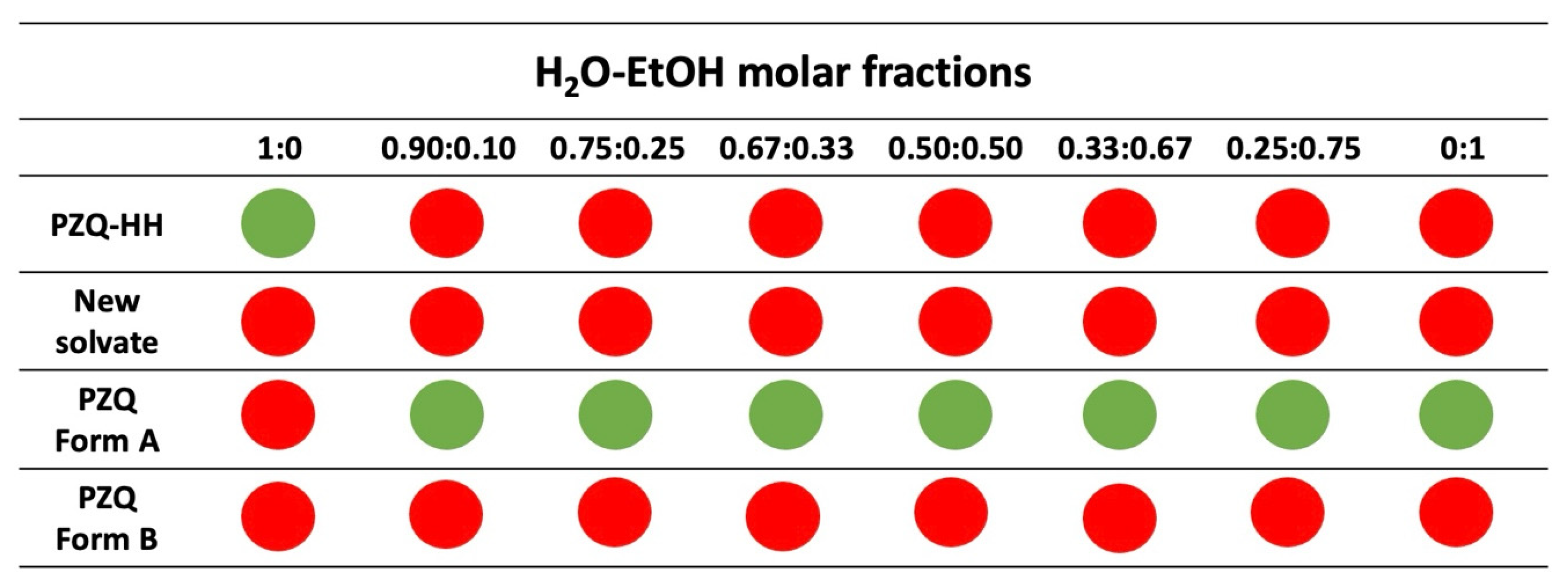
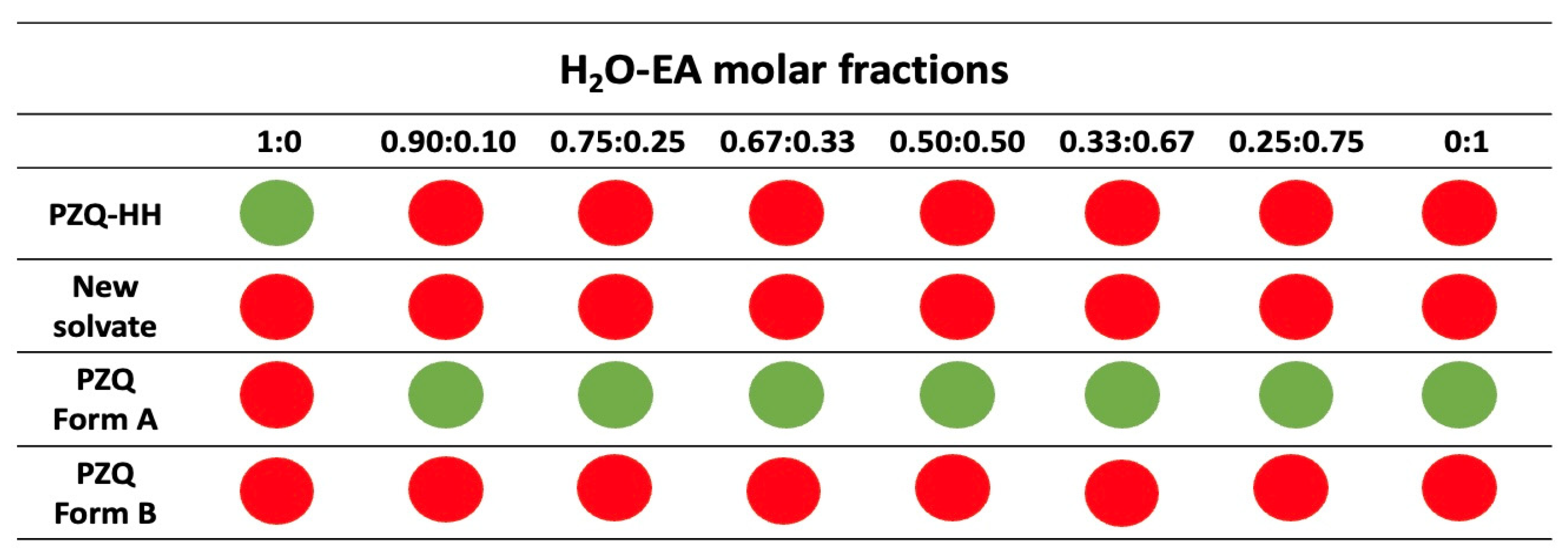
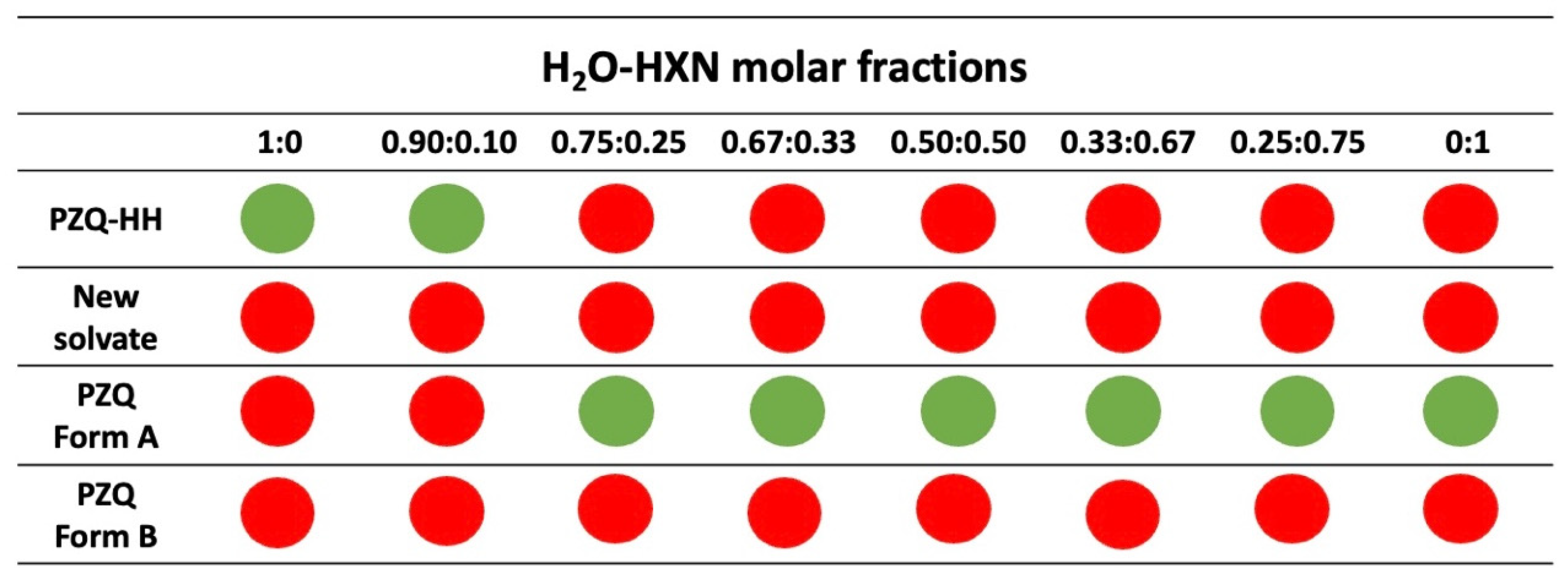
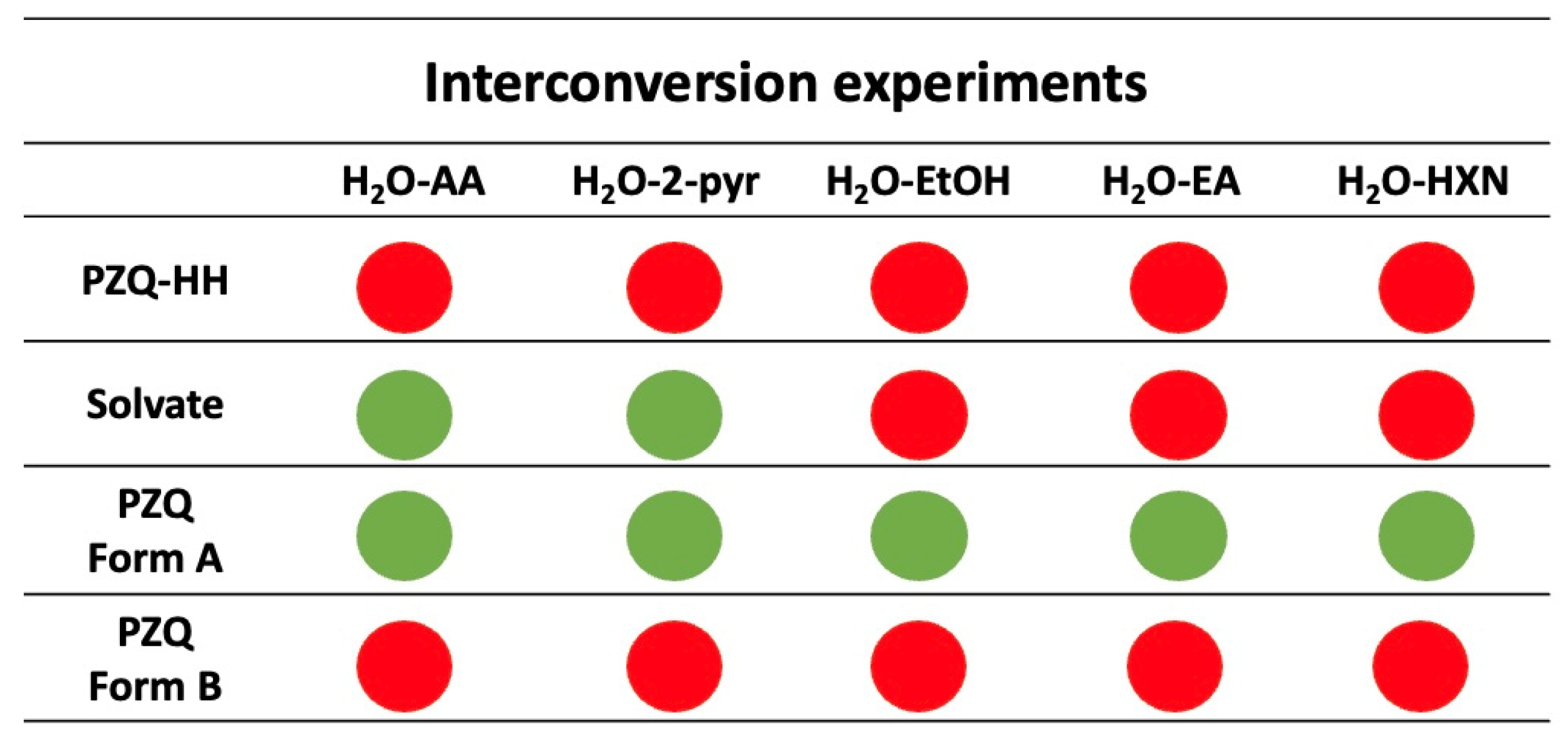
| Solvent Molar Fractions | ||||||||
|---|---|---|---|---|---|---|---|---|
| H2O | 0 | 0.25 | 0.33 | 0.50 | 0.67 | 0.75 | 0.90 * | 1 |
| Second Solvent ** | 1 | 0.75 | 0.67 | 0.50 | 0.33 | 0.25 | 0.10 * | 0 |
| Solvents | AA | 2-pyr | EtOH | EA | HXN |
|---|---|---|---|---|---|
| Known PZQ solvates [18,21,22,23,24,31] | Yes | Yes | No | No | No |
| Miscibility with H2O [51,52,53,54,55,56] | Miscible 1050 g/L | Miscible >65 g/L | Miscible 100 g/L | Slightly miscible 0.8 g/L | Immiscible <0.1 g/L |
| Starting Solid (PZQ-HH) | AA | 2-pyr | EtOH | EA | HXN |
|---|---|---|---|---|---|
| 411.5 mg | 73 μL | 98 μL | 75 μL | 125 μL | 167 μL |
| Initial Polymorph | Method/Technique | Condition/Duration | Outcome |
|---|---|---|---|
| PZQ Form A | LAG with H2O | NG+LAG | PZQ-HH |
| PZQ Form A | LAG with H2O | Di-LAG | PZQ Form A |
| PZQ Form A | Slurry [21] | 7 days | PZQ Form A |
| PZQ Form B | Slurry [21] | 3 days | PZQ-HH |
| PZQ-HH | NG [21] | 60 min | PZQ Form B |
| PZQ Form A | LAG with H2O-EtOH | NG+LAG or Di-LAG | PZQ Form A |
| PZQ Form A | LAG with H2O-EA | NG+LAG or Di-LAG | PZQ Form A |
| PZQ Form A | LAG with 0.9 H2O–0.1 HXN | NG+LAG | PZQ-HH |
| PZQ Form A | LAG with H2O-HXN | NG+LAG or Di-LAG | PZQ Form A |
| PZQ Form A | LAG with H2O-AA (≥0.5AA) | NG+LAG or Di-LAG | PZQ-AA |
| PZQ Form A | LAG with H2O-2-pyr (≥0.5AA) | NG+LAG or Di-LAG | PZQ-2-pyr |
| PZQ Form A | LAG with H2O-AA (<0.5AA) | NG+LAG or Di-LAG | PZQ Form A |
| PZQ Form A | LAG with H2O-2-pyr (<0.5AA) | NG+LAG or Di-LAG | PZQ Form A |
| PZQ-HH | LAG with AA | Di-LAG | PZQ-AA |
| PZQ Form A | LAG with 2-pyr | Di-LAG | PZQ-2-pyr |
| PZQ Form A | LAG with EtOH or EA or HXN | Di-LAG | PZQ Form A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Abbrunzo, I.; Voinovich, D.; Perissutti, B. Mechanochemical Synthesis of Praziquantel Hemihydrate in the Presence of Five Solvents with Different Water Miscibility. Crystals 2024, 14, 374. https://doi.org/10.3390/cryst14040374
D’Abbrunzo I, Voinovich D, Perissutti B. Mechanochemical Synthesis of Praziquantel Hemihydrate in the Presence of Five Solvents with Different Water Miscibility. Crystals. 2024; 14(4):374. https://doi.org/10.3390/cryst14040374
Chicago/Turabian StyleD’Abbrunzo, Ilenia, Dario Voinovich, and Beatrice Perissutti. 2024. "Mechanochemical Synthesis of Praziquantel Hemihydrate in the Presence of Five Solvents with Different Water Miscibility" Crystals 14, no. 4: 374. https://doi.org/10.3390/cryst14040374
APA StyleD’Abbrunzo, I., Voinovich, D., & Perissutti, B. (2024). Mechanochemical Synthesis of Praziquantel Hemihydrate in the Presence of Five Solvents with Different Water Miscibility. Crystals, 14(4), 374. https://doi.org/10.3390/cryst14040374