Wafer-Scale ALD Synthesis of MoO3 Sulfurized to MoS2
Abstract
1. Introduction
Why 2D MoS2 Is of Interest?
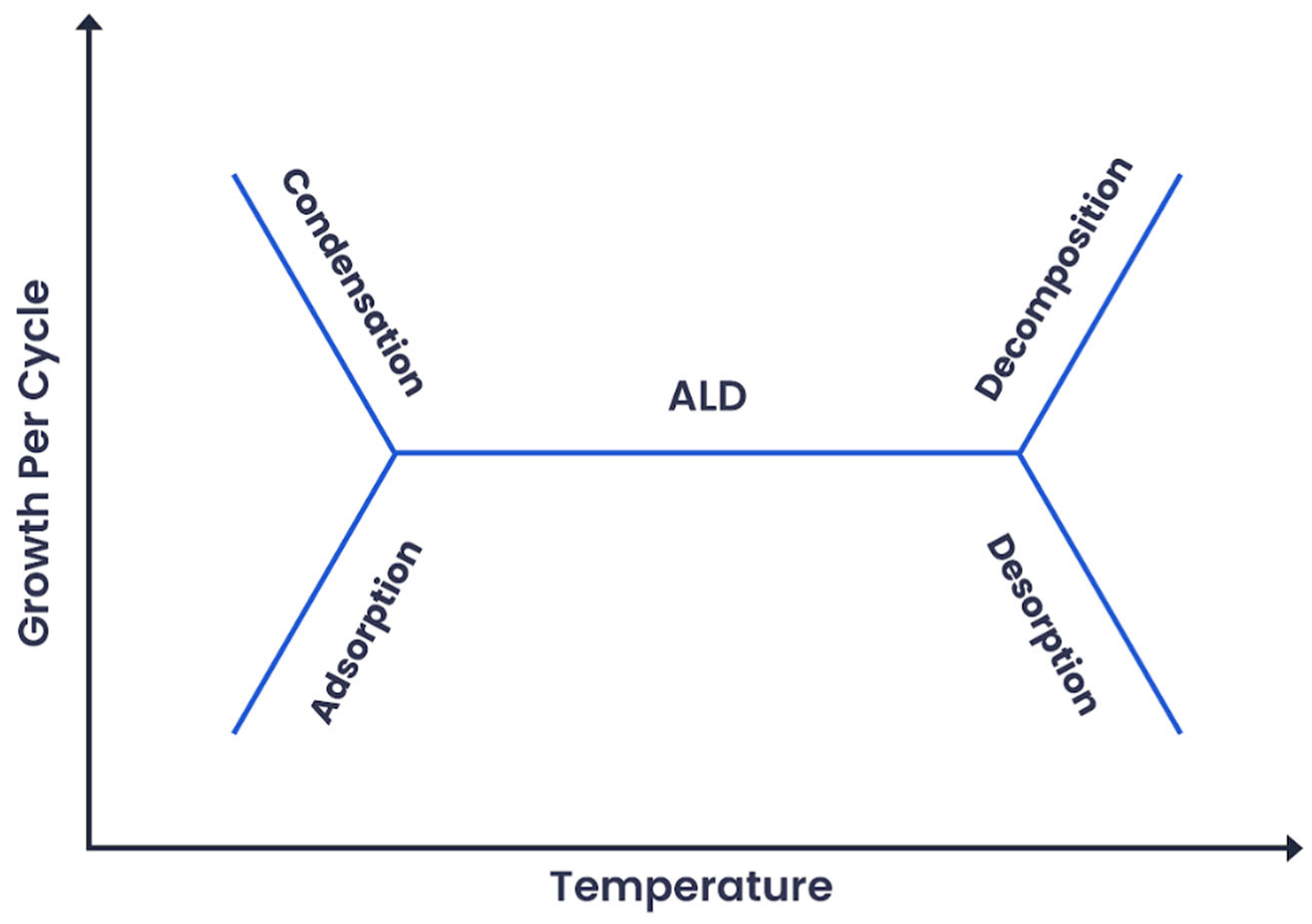
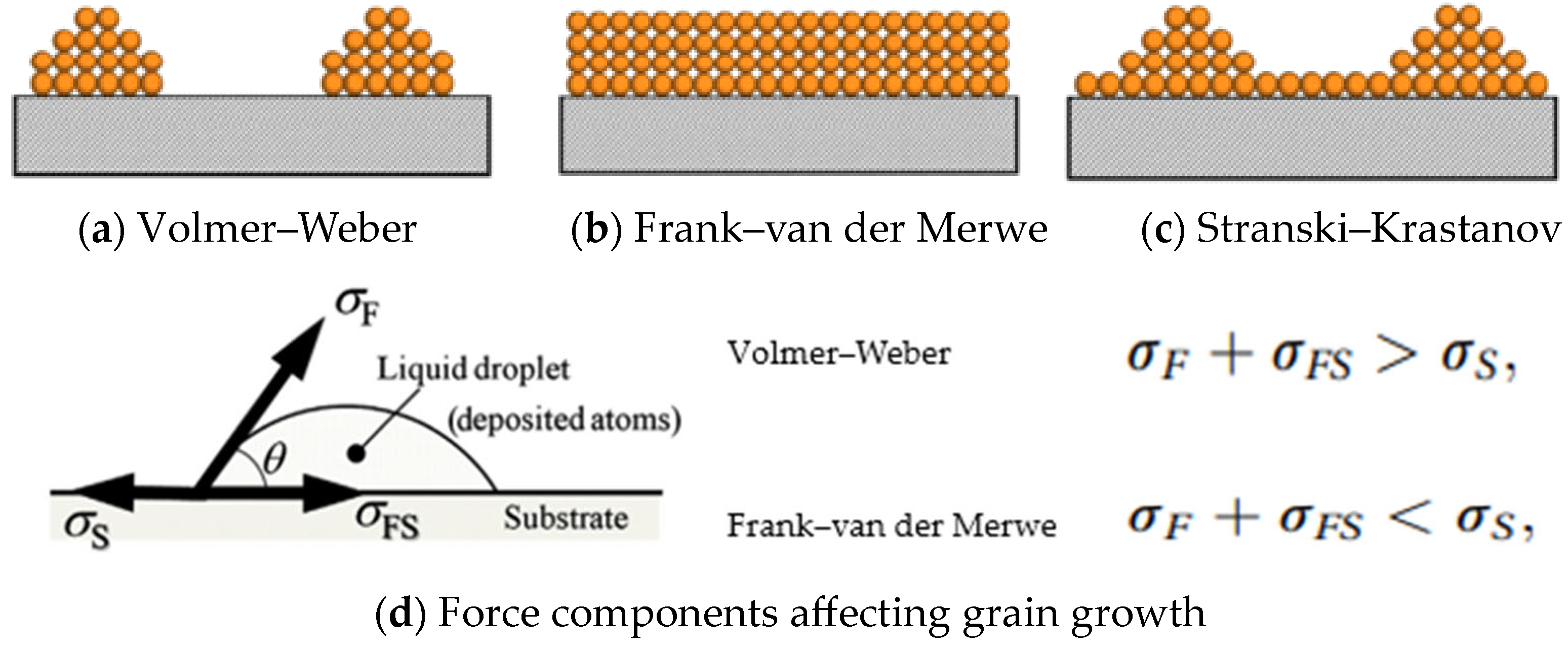
2. Fundamentals of MoO3 ALD Growth Recipe
3. Role of Activation Energy
4. Role of Gibbs Free Energy
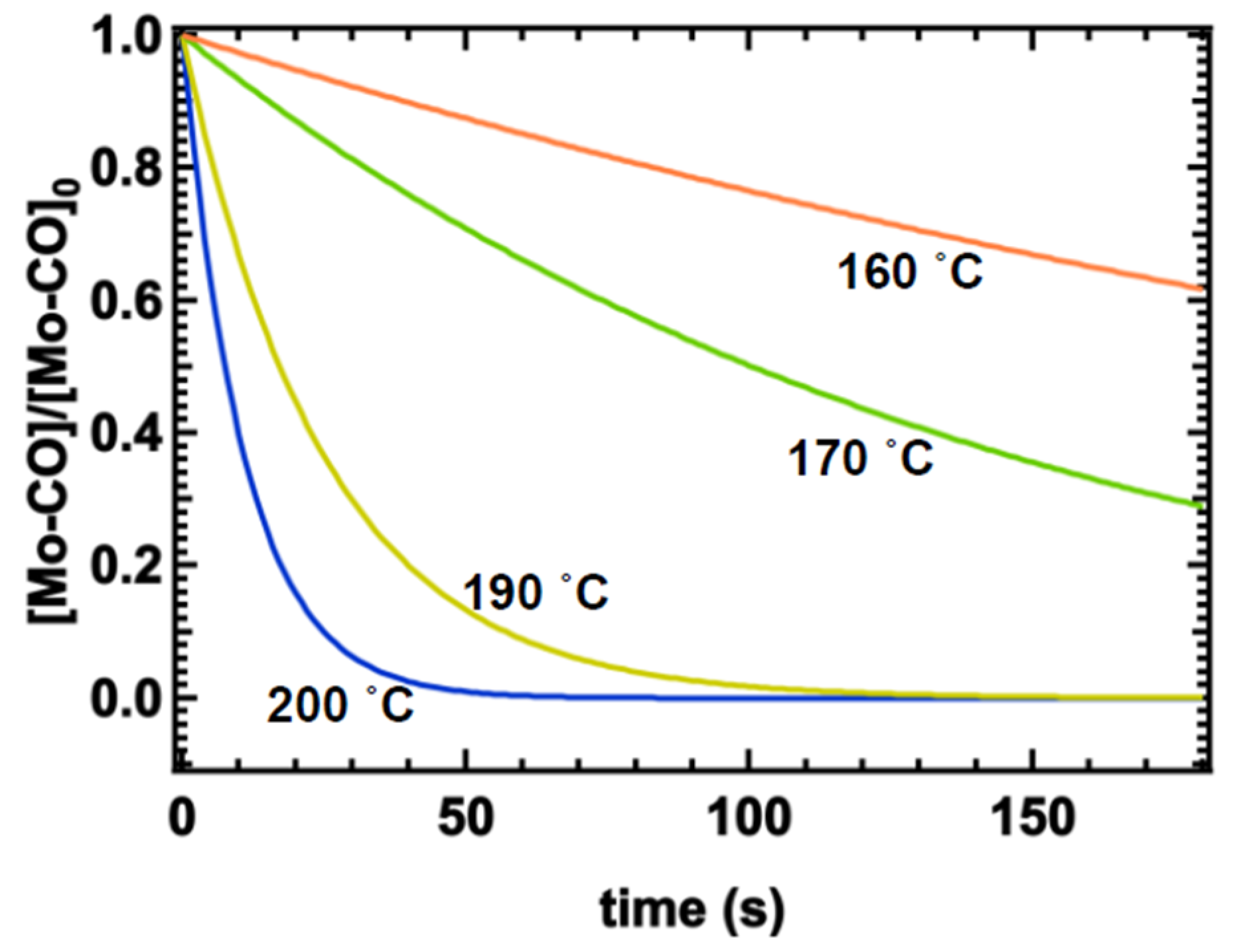
5. Determination of ALD Reactor Temperature and Time Based on the Reaction Kinetics
- k = reaction rate constant;
- A = pre-exponential factor;
- Ea = activation energy Kcal/moles (same unit as R × T);
- R = universal gas constant;
- T = absolute temperature (in Kelvin).
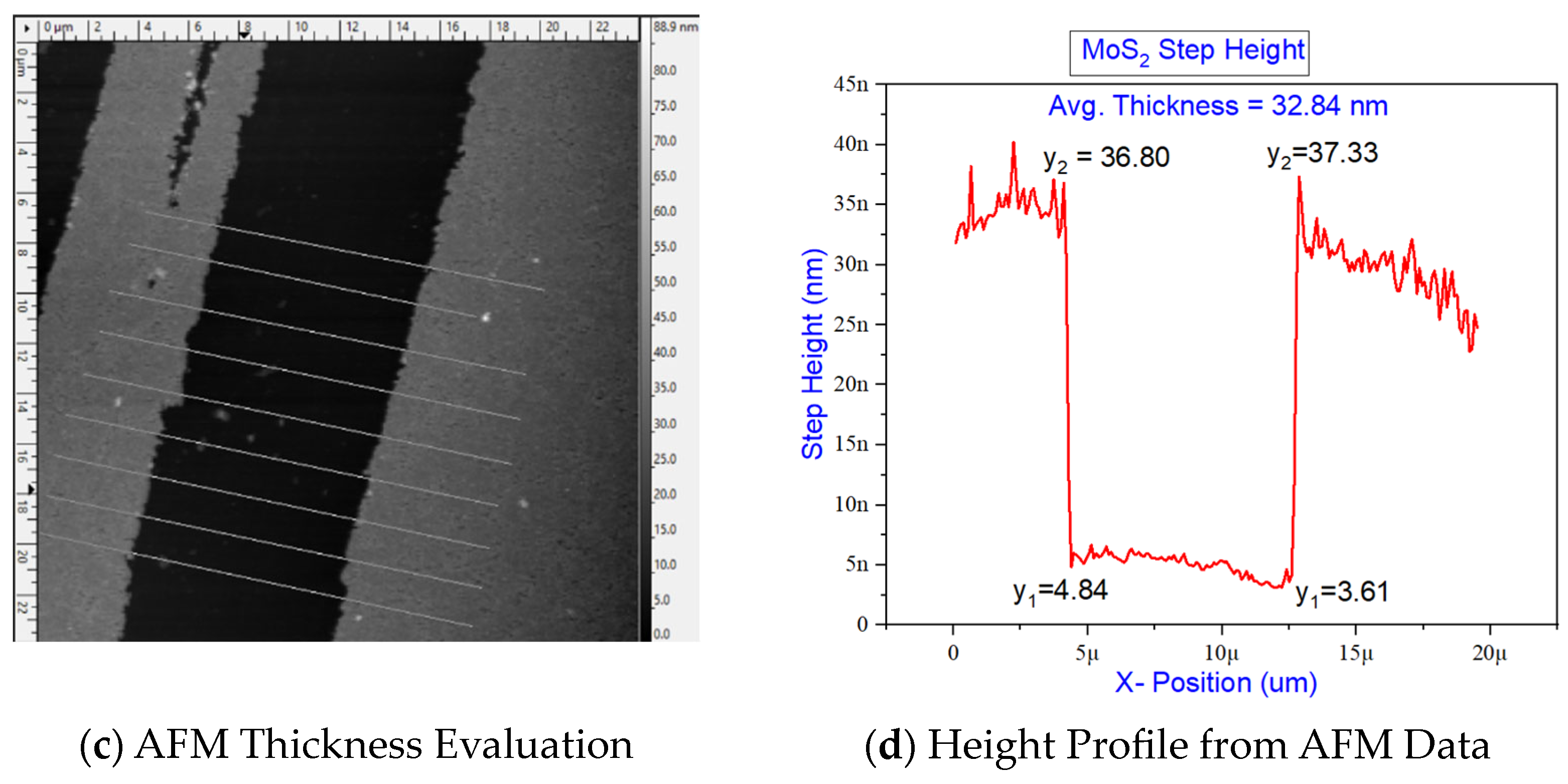
6. Results and Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Gu, F. A wafer-scale synthesis of monolayer MoS2 and their field-effect transistors toward practical applications. Nanoscale Adv. 2021, 3, 2117–2138. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, X.; Wei, J.; Wang, S.; Chen, S.; Wu, S.; Zhou, P. 12-inch growth of uniform MoS2 monolayer for integrated circuit manufacture. Nat. Mater. 2023, 22, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, M.; Odacı, C.; Shehu, Y.; Perkgöz, N.K.; Ay, F. Layer and size distribution control of CVD-grown 2D MoS2 using ALD-deposited MoO3 structures as the precursor. Mater. Sci. Semicond. Process. 2020, 108, 104880. [Google Scholar] [CrossRef]
- Romanov, R.I.; Kozodaev, M.G.; Myakota, D.I.; Chernikova, A.G.; Novikov, S.M.; Volkov, V.S.; Markeev, A.M. Synthesis of large area two-dimensional MoS2 films by sulfurization of atomic layer deposited MoO3 thin film for nanoelectronic applications. ACS Appl. Nano Mater. 2019, 2, 7521–7531. [Google Scholar] [CrossRef]
- Richey, N.E.; De Paula, C.; Bent, S.F. Understanding chemical and physical mechanisms in atomic layer deposition. J. Chem. Phys. 2020, 152, 040902. [Google Scholar] [CrossRef] [PubMed]
- Yanguas-Gil, A.; Elam, J.W. Self-Limited Reaction-Diffusion in Nanostructured Substrates: Surface Coverage Dynamics and Analytic Approximations to ALD Saturation Times. Chem. Vap. Depos. 2012, 18, 46–52. [Google Scholar] [CrossRef]
- Rothman, A.; Werbrouck, A.; Bent, S.F. Enhanced Growth in Atomic Layer Deposition of Ruthenium Metal: The Role of Surface Diffusion and Nucleation Sites. Chem. Mater. 2023, 36, 541–550. [Google Scholar] [CrossRef]
- Plummer, J.D.; Griffin, P.B. Material and process limits in silicon VLSI technology. Proc. IEEE 2001, 89, 240–258. [Google Scholar] [CrossRef]
- Schaller, R.R. Moore’s law: Past, present, and future. IEEE Spectr. 1997, 34, 52–59. [Google Scholar] [CrossRef]
- Borkar, S. Design challenges of technology scaling. IEEE Micro 1999, 19, 23–29. [Google Scholar] [CrossRef]
- Gargini, P. Roadmap evolution: From NTRS to ITRS, from ITRS 2.0 to IRDS. In Proceedings of the 2017 Fifth Berkeley Symposium on Energy Efficient Electronic Systems & Steep Transistors Workshop (E3S), Berkeley, CA, USA, 19–20 October 2017; IEEE: New York, NY, USA, 2017; pp. 1–62. [Google Scholar]
- Hills, G. Beyond-Silicon Computing: Nano-Technologies, Nano-Design, and Nano-Systems. In Emerging Computing: From Devices to Systems: Looking Beyond Moore and Von Neumann; Springer: Singapore, 2022; pp. 15–45. [Google Scholar]
- Das, S.; Sebastian, A.; Pop, E.; McClellan, C.J.; Franklin, A.D.; Grasser, T.; Singh, R. Transistors based on two-dimensional materials for future integrated circuits. Nat. Electron. 2021, 4, 786–799. [Google Scholar] [CrossRef]
- Balog, R.; Jørgensen, B.; Nilsson, L.; Andersen, M.; Rienks, E.; Bianchi, M.; Hornekær, L. Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat. Mater. 2010, 9, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Hung, T.Y.; Sathaiya, D.M.; Arutchelvan, G.; Hsu, C.F.; Su, S.K.; Chien, C.H. Comprehensive Study of Contact Length Scaling Down to 12 nm With Monolayer MoS2 Channel Transistors. IEEE Trans. Electron Devices 2023, 70, 6680–6686. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Wang, F. Emerging photoluminescence in monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Ye, P.D. Channel length scaling of MoS2 MOSFETs. ACS Nano 2012, 6, 8563–8569. [Google Scholar] [CrossRef] [PubMed]
- Molina-Sánchez, A.; Sangalli, D.; Hummer, K.; Marini, A.; Wirtz, L. Effect of spin-orbit interaction on the optical spectra of single-layer, double-layer, and bulk MoS2. Phys. Rev. B 2013, 88, 045412. [Google Scholar] [CrossRef]
- Yue, Q.; Kang, J.; Shao, Z.; Zhang, X.; Chang, S.; Wang, G.; Li, J. Mechanical and electronic properties of monolayer MoS2 under elastic strain. Phys. Lett. A 2012, 376, 1166–1170. [Google Scholar] [CrossRef]
- Akhter, M.J.; Kuś, W.; Mrozek, A.; Burczyński, T. Mechanical properties of monolayer MoS2 with randomly distributed defects. Materials 2020, 13, 1307. [Google Scholar] [CrossRef]
- Cao, Y. Roadmap and direction toward high-performance MoS2 hydrogen evolution catalysts. ACS Nano 2021, 15, 11014–11039. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Pang, H.; Xue, H.; Yu, Y. MoS2-based nanocomposites for electrochemical energy storage. Adv. Sci. 2017, 4, 1600289. [Google Scholar] [CrossRef] [PubMed]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Chhowalla, M. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef] [PubMed]
- Shokri, A.; Salami, N. Gas sensor based on MoS2 monolayer. Sens. Actuators B Chem. 2016, 236, 378–385. [Google Scholar] [CrossRef]
- Taffelli, A.; Dirè, S.; Quaranta, A.; Pancheri, L. MoS2 based photodetectors: A review. Sensors 2021, 21, 2758. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Singh, P.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Flexible molybdenum disulfide (MoS2) atomic layers for wearable electronics and optoelectronics. ACS Appl. Mater. Interfaces 2019, 11, 11061–11105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Park, Y.J.; Kang, M.; Kang, S.K.; Koo, J.; Shinde, S.M.; Ahn, J.H. CVD-grown monolayer MoS2 in bioabsorbable electronics and biosensors. Nat. Commun. 2018, 9, 1690. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Zhang, Y.; Liu, X.; Wang, Y.; Zhang, Q.; Yin, H. Theoretical limit of how small we can make MoS2 transistor channels. J. Phys. D: Appl. Phys. 2021, 55, 105304. [Google Scholar] [CrossRef]
- Jeon, J.; Jang, S.K.; Jeon, S.M.; Yoo, G.; Jang, Y.H.; Park, J.H.; Lee, S. Layer-controlled CVD growth of large-area two-dimensional MoS2 films. Nanoscale 2015, 7, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, T.H.; Huet, B.; Zhang, X.; Bansal, A.; Redwing, J.M. Scalable synthesis of 2D materials. In 2D Materials for Electronics, Sensors and Devices; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–54. [Google Scholar]
- Waite, A.R.; Pacley, S.; Glavin, N.R.; Voevodin, A.A.; Muratore, C. Tailoring ultra-thin MoS2 films via post-treatment of solid-state precursor phases. Thin Solid Film. 2018, 649, 177–186. [Google Scholar] [CrossRef]
- Zhan, L.; Wan, W.; Zhu, Z.; Shih, T.M.; Cai, W. MoS2 materials synthesized on SiO2/Si substrates via MBE. J. Phys. Conf. Ser. 2017, 864, 012037. [Google Scholar] [CrossRef]
- Serna, M.I.; Yoo, S.H.; Moreno, S.; Xi, Y.; Oviedo, J.P.; Choi, H.; Quevedo-Lopez, M.A. Large-area deposition of MoS2 by pulsed laser deposition with in situ thickness control. Acs Nano 2016, 10, 6054–6061. [Google Scholar] [CrossRef] [PubMed]
- George, A.S.; Mutlu, Z.; Ionescu, R.; Wu, R.J.; Jeong, J.S.; Bay, H.H.; Ozkan, C.S. Wafer-scale synthesis and high-resolution structural characterization of atomically thin MoS2 layers. Adv. Funct. Mater. 2014, 24, 7461–7466. [Google Scholar] [CrossRef]
- Liu, F.; Wu, W.; Bai, Y.; Chae, S.H.; Li, Q.; Wang, J.; Zhu, X.Y. Disassembling 2D van der Waals crystals into macroscopic monolayers and reassembling into artificial lattices. Science 2020, 367, 903–906. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-area atomically thin MoS2 nanosheets prepared using electrochemical exfoliation. ACS Nano 2014, 8, 6902–6910. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Sebastian, A.; Pendurthi, R.; Choudhury, T.H.; Redwing, J.M.; Das, S. Benchmarking monolayer MoS2 and WS2 field-effect transistors. Nat. Commun. 2021, 12, 693. [Google Scholar] [CrossRef]
- Tan, L.K.; Liu, B.; Teng, J.H.; Guo, S.; Low, H.Y.; Loh, K.P. Atomic layer deposition of a MoS2 film. Nanoscale 2014, 6, 10584–10588. [Google Scholar] [CrossRef]
- Diskus, M.; Nilsen, O.; Fjellvåg, H. Growth of thin films of molybdenum oxide by atomic layer deposition. J. Mater. Chem. 2011, 21, 705–710. [Google Scholar] [CrossRef]
- Cremers, V.; Puurunen, R.L.; Dendooven, J. Conformality in atomic layer deposition: Current status overview of analysis and modelling. Appl. Phys. Rev. 2019, 6, 021302. [Google Scholar] [CrossRef]
- Suntola, T. Atomic layer epitaxy. Mater. Sci. Rep. 1989, 4, 261–312. [Google Scholar] [CrossRef]
- Suntola, T. Surface chemistry of materials deposition at atomic layer level. Appl. Surf. Sci. 1996, 100, 391–398. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M. Atomic layer deposition. In Handbook of Thin Films; Academic Press: Cambridge, MA, USA, 2002; pp. 103–159. [Google Scholar]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.; Kessels WM, M.; Bol, A.A. Strategies to facilitate the formation of free standing MoS2 nanolayers on SiO2 surface by atomic layer deposition: A DFT study. APL Mater. 2018, 6, 111107. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Vega, A.; Liu, G.; Dezelah, C.L.; Kanjolia, R.K.; Chabal, Y.J. Role of initial precursor chemisorption on incubation delay for molybdenum oxide atomic layer deposition. Chem. Mater. 2016, 28, 8591–8597. [Google Scholar] [CrossRef]
- Paul, S.; Torsi, R.; Robinson, J.A.; Momeni, K. Effect of the substrate on MoS2 monolayer morphology: An integrated computational and experimental study. ACS Appl. Mater. Interfaces 2022, 14, 18835–18844. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi, M.; Mukherjee, R.; Sukotjo, C.; Diwekar, U.M.; Takoudis, C.G. Recent advances in theoretical development of thermal atomic layer deposition: A review. Nanomaterials 2022, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Diskus, M.; Balasundaram, M.; Nilsen, O.; Fjellvåg, H. Influence of precursors chemistry on ALD growth of cobalt–molybdenum oxide films. Dalton Trans. 2012, 41, 2439–2444. [Google Scholar] [CrossRef]
- Nandi, D.K.; Sarkar, S.K. Atomic layer deposition of molybdenum oxide for solar cell application. Appl. Mech. Mater. 2014, 492, 375–379. [Google Scholar] [CrossRef]
- Liu, H.; Yang, R.B.; Yang, W.; Jin, Y.; Lee, C.J. Atomic layer deposition and post-growth thermal annealing of ultrathin MoO3 layers on silicon substrates: Formation of surface nanostructures. Appl. Surf. Sci. 2018, 439, 583–588. [Google Scholar] [CrossRef]
- Dai, T.; Ren, Y.; Qian, L.; Liu, X. Characterization of molybdenum oxide thin films grown by atomic layer deposition. J. Electron. Mater. 2018, 47, 6709–6715. [Google Scholar] [CrossRef]
- Knoops, H.C.; Potts, S.E.; Bol, A.A.; Kessels WM, M. Atomic layer deposition. In Handbook of Crystal Growth; Elsevier: North-Holland, Amsterdam, The Netherlands, 2015; pp. 1101–1134. [Google Scholar]
- Shirazi, M.; Kessels WM, M.; Bol, A.A. Initial Stage of Atomic Layer Deposition of 2D-MoS2 on a SiO2 Surface. Phys. Chem. Chem. Phys. 2018, 20, 16861–16875. Available online: https://pure.tue.nl/ws/portalfiles/portal/102383306/M.Shirazi_W.M.M.Kessels_A.A.Bol.pdf (accessed on 6 July 2024). [CrossRef] [PubMed]
- Zeng, L.; Richey, N.E.; Palm, D.W.; Oh, I.K.; Shi, J.; Maclsaac, C.; Bent, S.F. Modified atomic layer deposition of MoS2 thin films. J. Vac. Sci. Technol. A 2020, 38, 060403. [Google Scholar] [CrossRef]
- Diware, M. Shraddha Ganorkar. In 2D Nanomaterials: Chemistry and Properties; CRC Press: Boca Raton, FL, USA, 2022; Volume 139. [Google Scholar]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in X-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Ganta, D.; Sinha, S.; Haasch, R.T. 2-D material molybdenum disulfide analyzed by XPS. Surf. Sci. Spectra 2014, 21, 19–27. [Google Scholar] [CrossRef]
- Weber, T.; Muijsers, J.C.; Van Wolput JH, M.C.; Verhagen CP, J.; Niemantsverdriet, J.W. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2, as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 1996, 100, 14144–14150. [Google Scholar] [CrossRef]
- van Esbroeck, N.M.; Sharma, A. Towards 2D-MoS2 Thin Films: Optimization of Thermal Sulfurization of ALD-Grown MoO3; Eindhoven University of Technology: Eindhoven, The Netherlands, 2016; Available online: https://pure.tue.nl/ws/portalfiles/portal/176691394/Esbroeck_BEP_verslag.pdf (accessed on 20 July 2024).
- Keller, B.D.; Bertuch, A.; Provine, J.; Sundaram, G.; Ferralis, N.; Grossman, J.C. Process control of atomic layer deposition molybdenum oxide nucleation and sulfidation to large-area MoS2 monolayers. Chem. Mater. 2017, 29, 2024–2032. [Google Scholar] [CrossRef]
- Pyeon, J.J.; Kim, S.H.; Jeong, D.S.; Baek, S.H.; Kang, C.Y.; Kim, J.S.; Kim, S.K. Wafer-scale growth of MoS2 thin films by atomic layer deposition. Nanoscale 2016, 8, 10792–10798. [Google Scholar] [CrossRef]
- Jin, Z.; Shin, S.; Han, S.J.; Min, Y.S. Novel chemical route for atomic layer deposition of MoS 2 thin film on SiO 2/Si substrate. Nanoscale 2014, 6, 14453–14458. [Google Scholar] [CrossRef]
- Available online: http://srdata.nist.gov/xps/SpectraIdentifier (accessed on 5 July 2024).
- Shendokar, S.; Aryeetey, F.; Hossen, M.F.; Ignatova, T.; Aravamudhan, S. Towards Low-Temperature CVD Synthesis and Characterization of Mono-or Few-Layer Molybdenum Disulfide. Micromachines 2023, 14, 1758. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Jurca, T.; Moody, M.J.; Henning, A.; Emery, J.D.; Wang, B.; Tan, J.M.; Marks, T.J. Low-temperature atomic layer deposition of MoS2 films. Angew. Chem. Int. Ed. 2017, 56, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, J.C.; Park, J.; Cho, D.Y.; Kim, W.H.; Shong, B.; Park, T.J. Wafer-scale growth of a MoS2 monolayer via one cycle of atomic layer deposition: An adsorbate control method. Chem. Mater. 2021, 33, 4099–4105. [Google Scholar] [CrossRef]
- Jang, Y.; Yeo, S.; Kim, H.; Kim, S.H. Wafer-scale, conformal and direct growth of MoS2 thin films by atomic layer deposition. Appl. Surf. Sci. 2016, 365, 160–165. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, M.; Sharma, R.K.; Reddy, G.B. An experimental study: Role of different ambient on sulfurization of MoO3 into MoS2. J. Alloys Compd. 2016, 671, 440–445. [Google Scholar] [CrossRef]
- Quilty, C.D.; Housel, L.M.; Bock, D.C.; Dunkin, M.R.; Wang, L.; Lutz, D.M.; Marschilok, A.C. Ex situ and operando XRD and XAS analysis of MoS2: A lithiation study of bulk and nanosheet materials. ACS Appl. Energy Mater. 2019, 2, 7635–7646. [Google Scholar] [CrossRef]
- Khan, M.; Kumar, S.; Mishra, A.; Sulania, I.; Tripathi, M.N.; Tripathi, A. Study of structural and electronic properties of few-layer MoS2 film. Mater. Today Proc. 2022, 57, 100–105. [Google Scholar] [CrossRef]






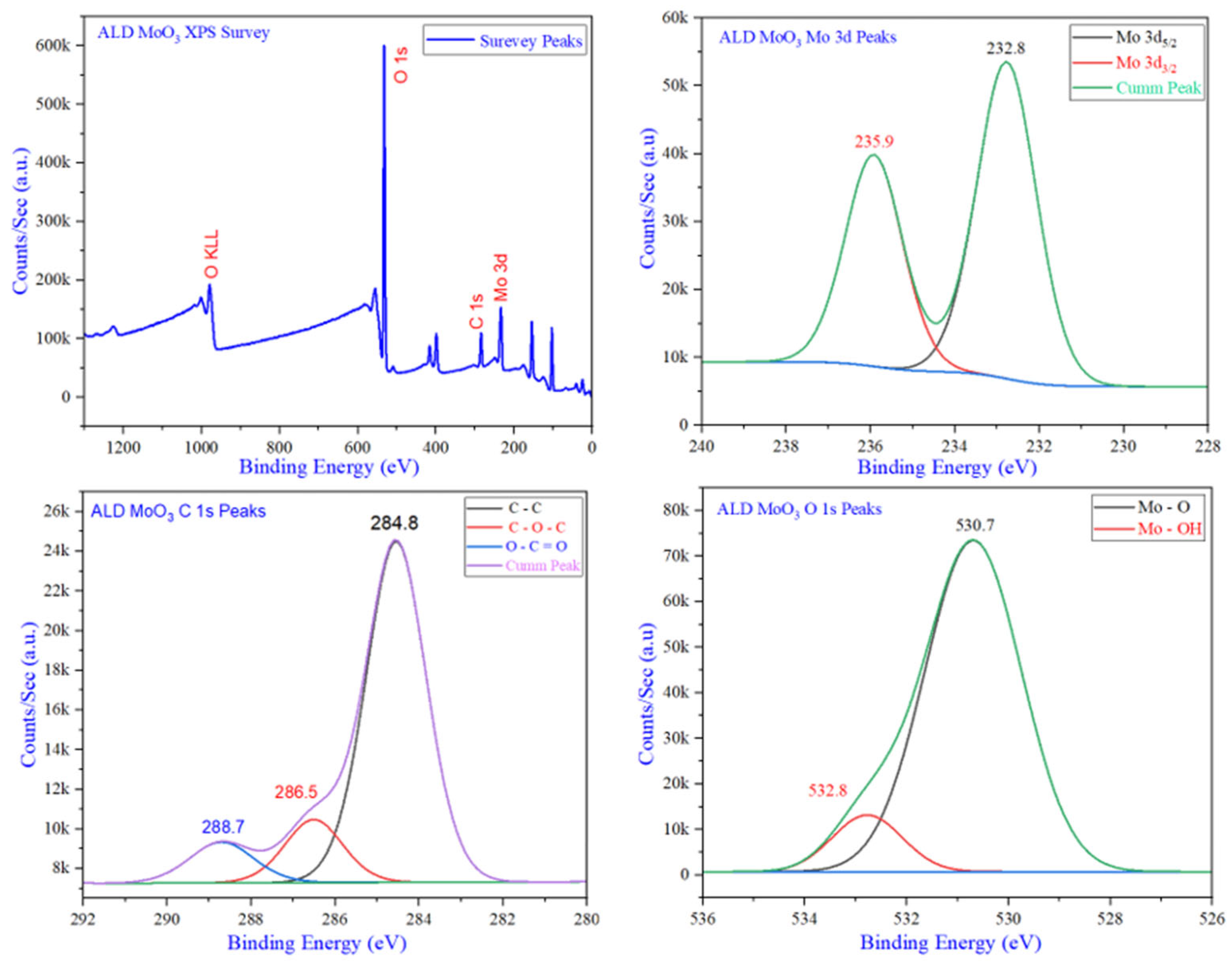
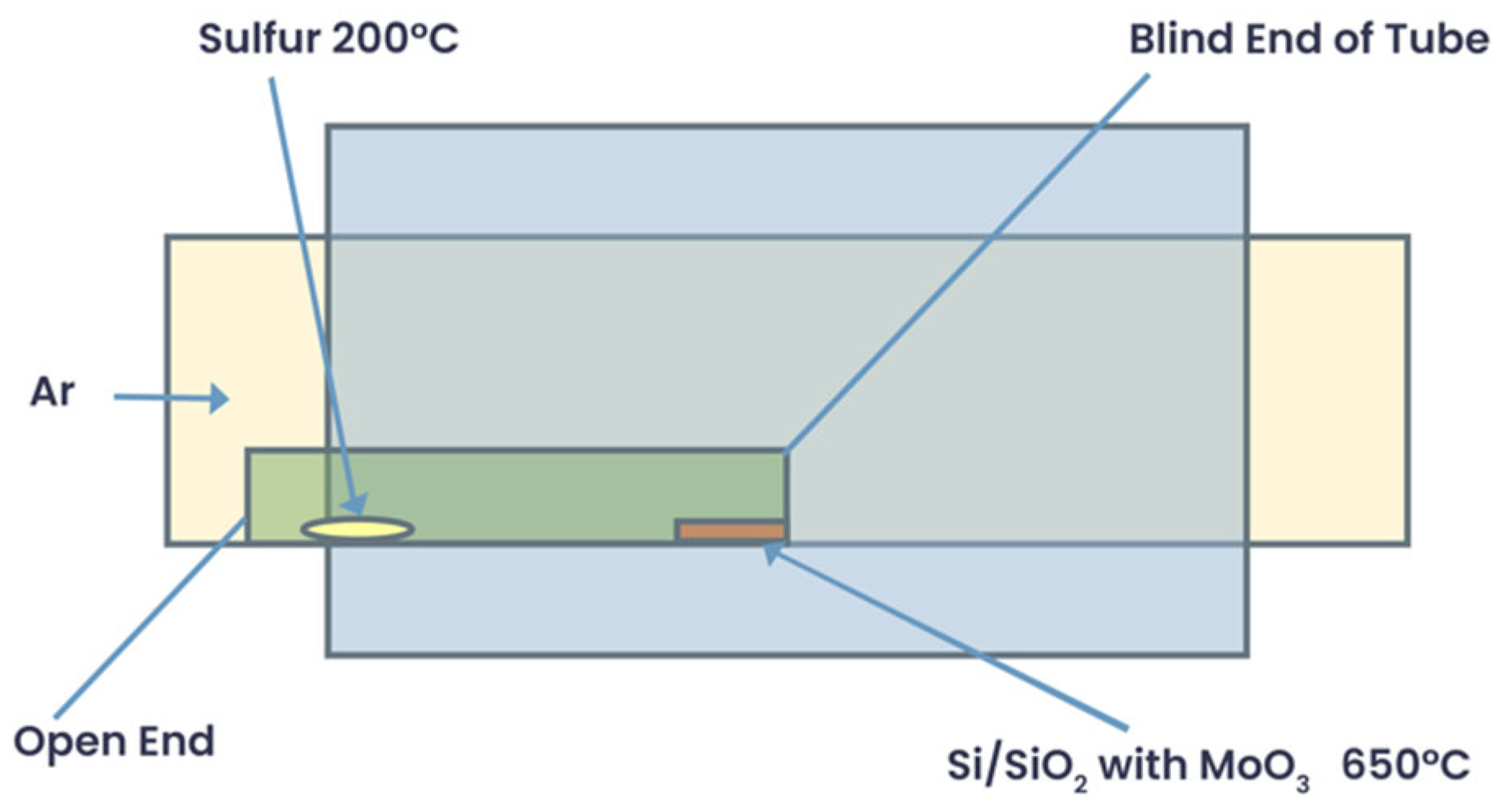
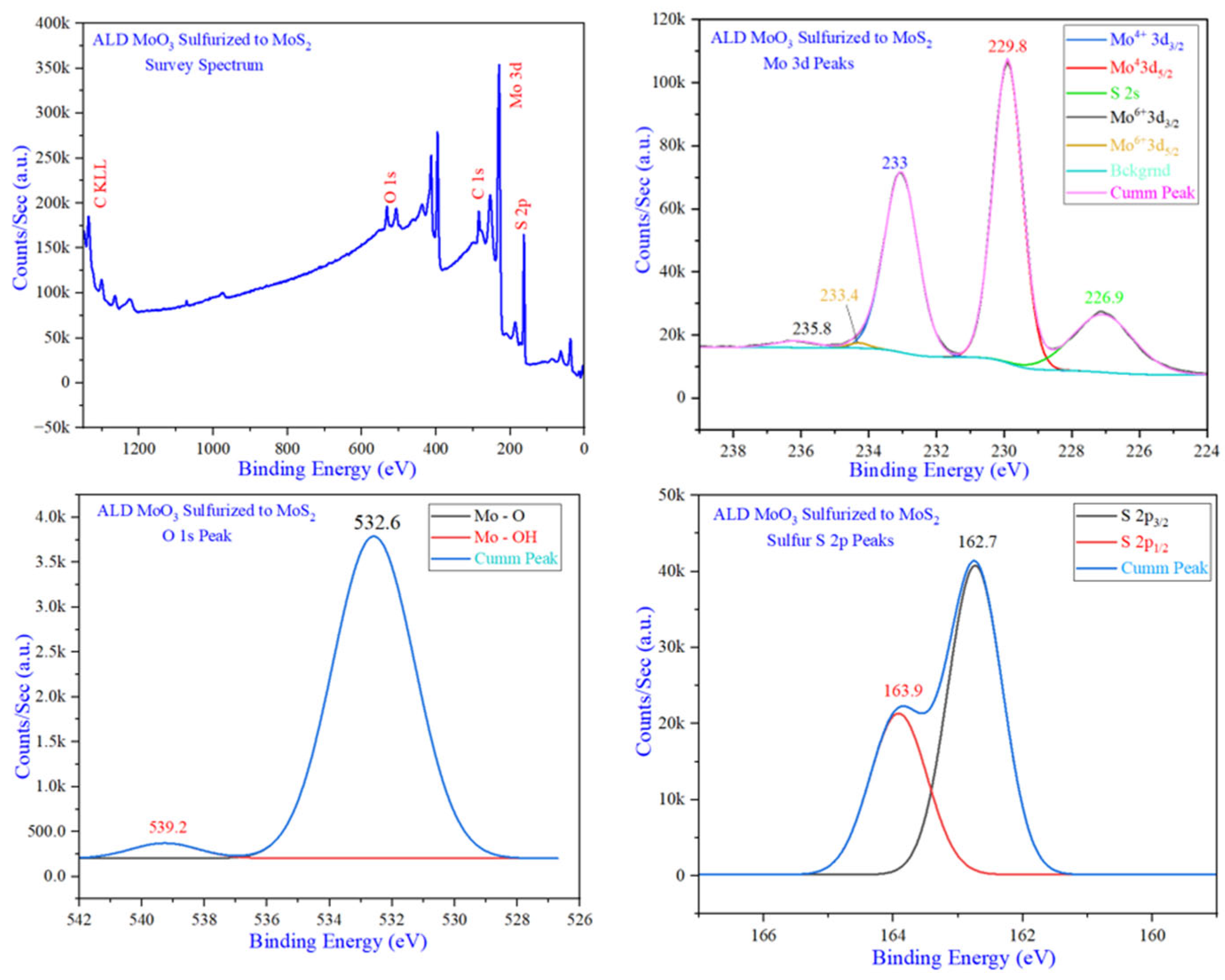



| Instruction | Channel | Values | Unit | |
|---|---|---|---|---|
| 0 | Wait | 5 | s | |
| 1 | Flow | 0 | 20 | sccm |
| 2 | Heater | 13 | 135 | °C |
| 3 | Heater | 8 | 167 | °C |
| 4 | Heater | 9 | 167 | °C |
| 5 | Heater | 10 | 150 | °C |
| 6 | Wait | 600 | s | |
| 7 | Ozone Flow | 1 | On | |
| 8 | Wait | 10 | s | |
| 9 | Ozone Flow | 1 | On | |
| 10 | Wait | 30 | s | |
| 11 | Pulse | 2 for Mo(CO)6 | 0.02 | s |
| 12 | Wait | 7 | s | |
| 13 | Pulse | 0 for Ozone | 0.02 | s |
| 14 | Wait | 7 | s | |
| 15 | Go To | 10 | 200 | Cycles |
| 16 | Ozone Flow | 0 | Off | |
| 17 | Wait | 20 | s | |
| 18 | Ozone Flow | 0 | Off | |
| 19 | Flow | 0 | 20 | sccm |
| Parameters—ALD of MoO3—No Sulfurization—Mo(CO)6 and Ozone (04) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Eqpt Type | Subs | Subs Condt | PreC1 | Pulse | PreC2 | Pulse | Purge | Cycles | R-T/P | VP-Heat | Post Processing | Issues/Remarks |
| 53 Diskus-2010 | F120-Electro By Suntola | Si(111) soda-LG | NM | Mo(CO)6 | 1 s | H2O + Ozone, 15%, 500 cm3/m | 3 s | 5 s, N2-300 cm3/min | 1000 | 152–172° | 3.5 mbar | Anneal—200–600 | Thk-35 to 70 nm for 1000 cyc. Combined Pulse H2O + Oz used |
| 54 Nandi-2014 | Custom built | NM | NM | Mo(CO)6 | 1 s | Ozone—1 gm/h | 1 s | 15 s | NM | 165–175° | 1 Torr | Ann—500 | QCM Studies. MoCO6- |
| 55 Hongfei Liu-2017 | Custom Built SS-Cross Flo | Si 1.0 cm2 | Ace/EtOh/DIW-Soni | Mo(CO)6 | 2 s | Ozone— O-D-P-M-D-P | 0.5 s | 2 s-Dwell 8 s-N2Purge | 50–200 | 120° | 1 Torr | 550–750 °C for 15min | GPC at 120 °C &150 °C are 0.9 ± 0.1Å/cyc & 3.4 ± 1.4Å/cyc |
| 56 Tinj Dai-2018 | PE-ALD | Si(111), Qrz, Si(100) | Ace/IPA/DIW | Mo(CO)6 | 4 s | O2–Plasma, 12 sccm, 180 W | 12 s | 60 s, 40 s | 1000 77n | 162° | 1.33 × 10−6 | Β-α 300–400 | 0.76 A/cyc; 13.56 MHz- RF-ICP, Procs Window-157-172 |
| Lattice Parameters | Pristine MoS2 | ALD MoS2 |
|---|---|---|
| a (Å) | 3.15792 | 3.13171 |
| b (Å) | 3.15792 | 3.13171 |
| c (Å) | 12.30628 | 12.53826 |
| α = β ° | 90 | 90 |
| γ ° | 120 | 120 |
| 2θ, °—1st/d (Å) | 14.39/6.1466 | 14.2/6.2 |
| 2θ, °—2nd/d (Å) | 28.99/3.0769 | 33/2.712 |
| 2θ, °—3rd/d (Å) | 44.11/2.05122 | 44.5/2.04 |
| 2θ, °—4th/d (Å) | 60.08/1.53871 | 69.17/1.357 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shendokar, S.; Hossen, M.F.; Aravamudhan, S. Wafer-Scale ALD Synthesis of MoO3 Sulfurized to MoS2. Crystals 2024, 14, 673. https://doi.org/10.3390/cryst14080673
Shendokar S, Hossen MF, Aravamudhan S. Wafer-Scale ALD Synthesis of MoO3 Sulfurized to MoS2. Crystals. 2024; 14(8):673. https://doi.org/10.3390/cryst14080673
Chicago/Turabian StyleShendokar, Sachin, Moha Feroz Hossen, and Shyam Aravamudhan. 2024. "Wafer-Scale ALD Synthesis of MoO3 Sulfurized to MoS2" Crystals 14, no. 8: 673. https://doi.org/10.3390/cryst14080673
APA StyleShendokar, S., Hossen, M. F., & Aravamudhan, S. (2024). Wafer-Scale ALD Synthesis of MoO3 Sulfurized to MoS2. Crystals, 14(8), 673. https://doi.org/10.3390/cryst14080673







