High-Pressure Laser Reactive Synthesis Within Diamond Anvil Cells of Carbon Allotropes from Methanol
Abstract
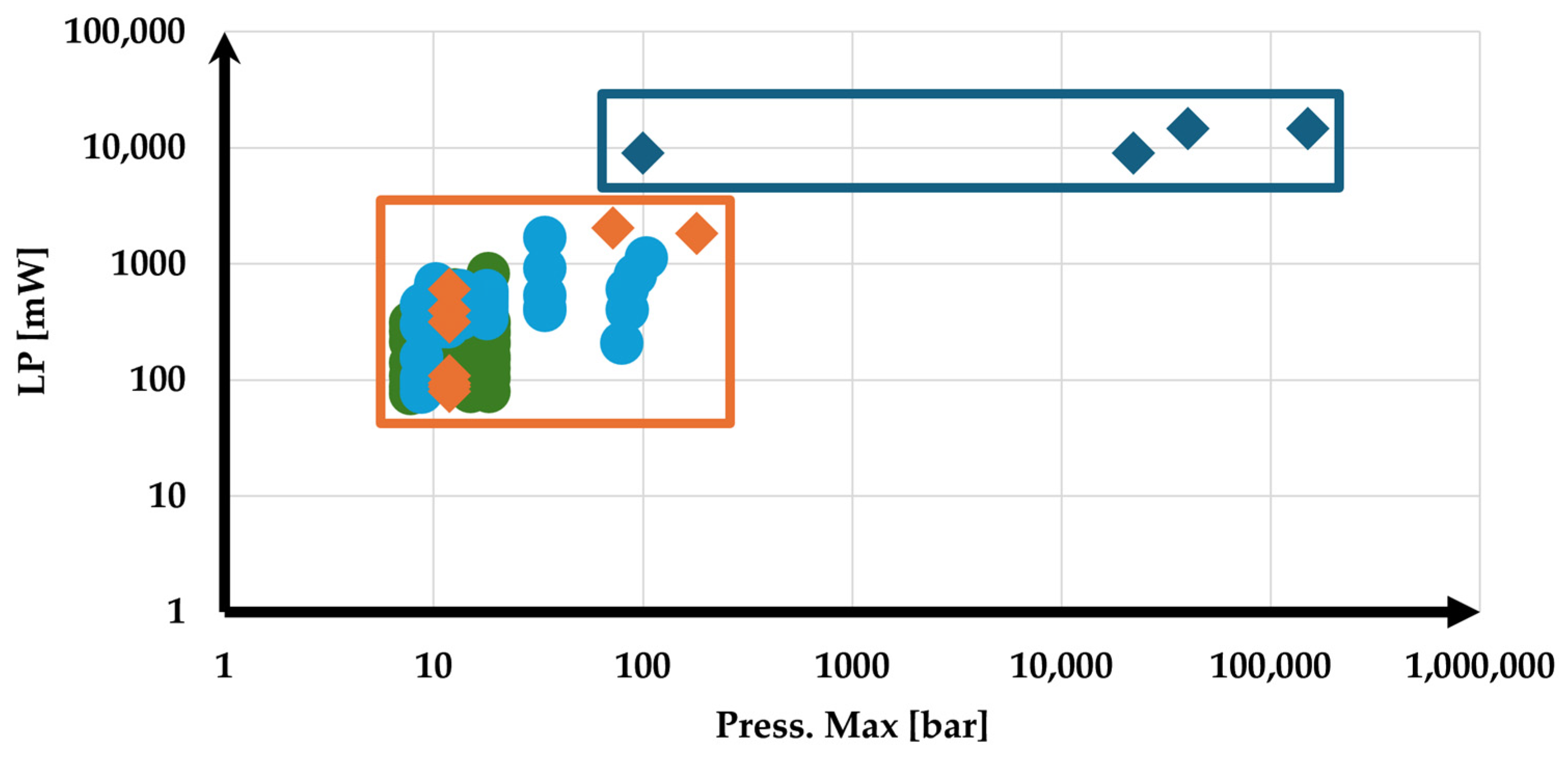
1. Introduction
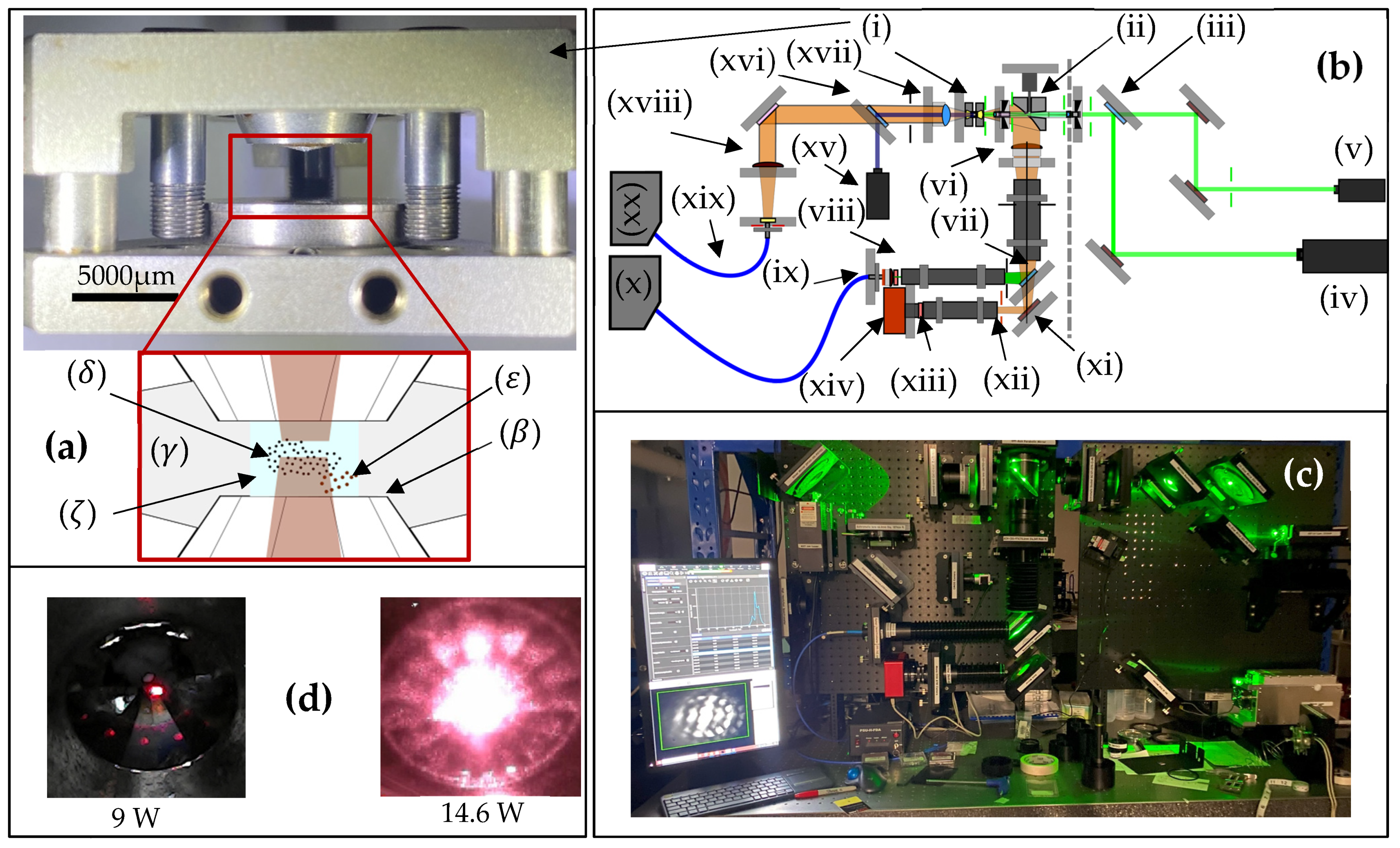
2. Materials and Methods
3. Results and Discussion
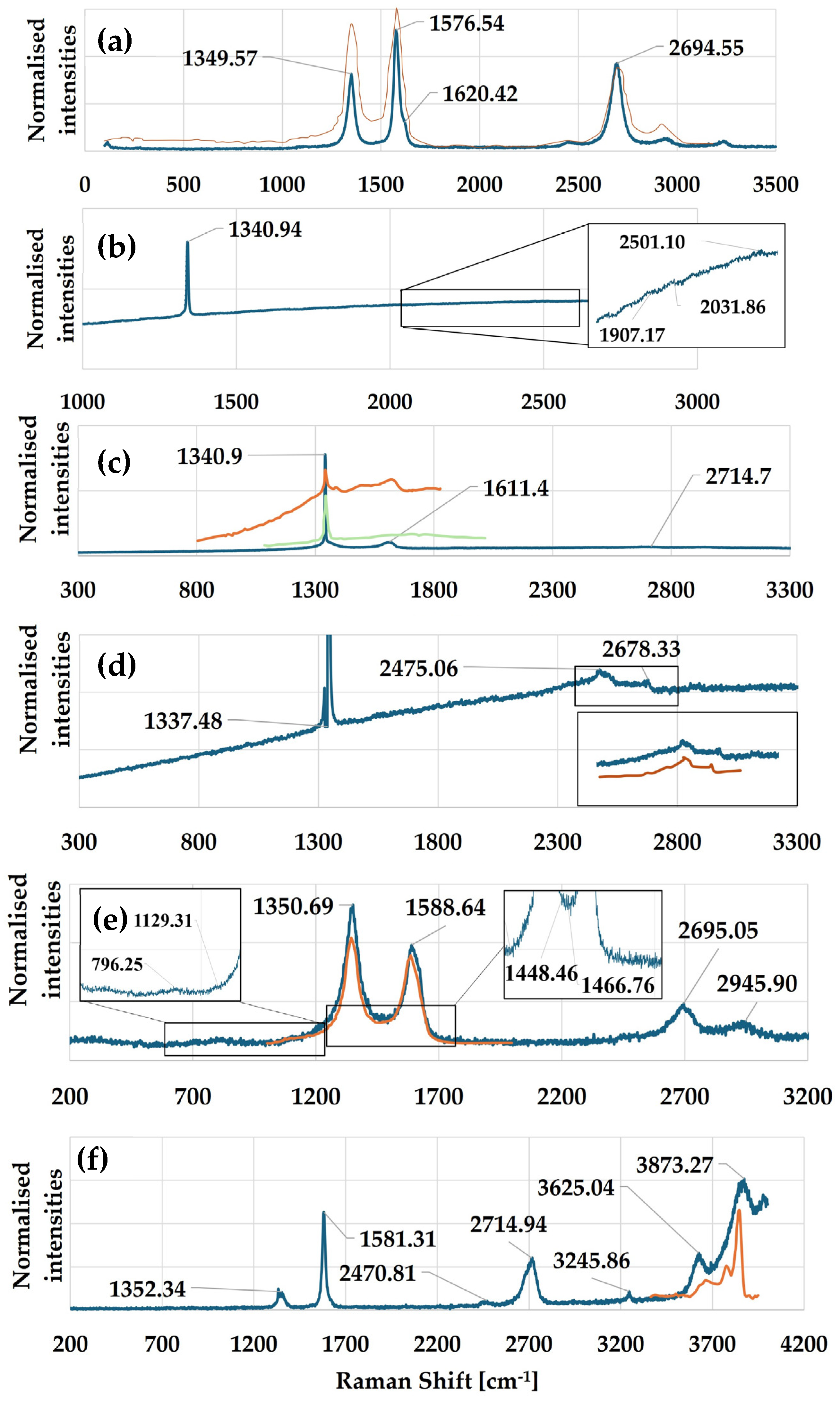
3.1. Multi-Walled Carbon Nanotubes
3.2. Continuous Diamond Films with Defects
3.3. Nanocrystalline Diamond
3.4. Microcrystalline Diamond with Defects
3.5. Amorphous Carbon
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorb, Y.A.; Bundy, F.P.; DeVries, R.C. Diamond: High-Pressure Synthesis. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bassett, W.A. Diamond anvil cell, 50th birthday. High Press. Res. 2009, 29, 163–186. [Google Scholar] [CrossRef]
- Berrada, M.; Hu, G.; Zhou, D.; Wang, S.; Nguyen, P.Q.H.; Zhang, D.; Prakapenka, V.; Chariton, S.; Chen, B.; Li, J.; et al. Detection of thin film phase transformations at high-pressure and high-temperature in a diamond anvil cell. Commun. Earth Environ. 2024, 5, 73. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, M.; Qian, C.; Li, C.; Yang, Y.; Du, X.; Dong, H.; Chen, B. Disordering of graphene nanoplatelet, carbon nanotube and C60 fullerene under shear stress. Carbon 2025, 232, 119802. [Google Scholar] [CrossRef]
- Geballe, Z.M.; Lai, J.; Walter, M.J. A broadband pulse amplifier for Joule heating experiments in diamond anvil cells. Rev. Sci. Instrum. 2024, 95, 053902. [Google Scholar] [CrossRef]
- Anzellini, S.; Boccato, S. A Practical Review of the Laser-Heated Diamond Anvil Cell for University Laboratories and Synchrotron Applications. Crystals 2020, 10, 459. [Google Scholar] [CrossRef]
- Bassett, W.A. The birth and development of laser heating in diamond anvil cells. Rev. Sci. Instrum. 2001, 72, 1270–1272. [Google Scholar] [CrossRef]
- Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells. J. Manuf. Mater. Process. 2022, 6, 111. [Google Scholar] [CrossRef]
- Yusa, H.; Takemura, K.; Matsui, Y.; Morishima, H.; Watanabe, K.; Yamawaki, H.; Aoki, K. Direct transformation of graphite to cubic diamond observed in a laser-heated diamond anvil cell. Appl. Phys. Lett. 1998, 72, 1843–1845. [Google Scholar] [CrossRef]
- Wei, B.; Lin, L.; Zhang, J.; Zhan, Z.; Cheng, Z.; Jiang, J. In Situ Measurement Techniques Using Diamond Anvil Cell at High Pressure–Temperature Conditions: A Review. Phys. Status Solidi (RRL) Rapid Res. Lett. 2024, 18, 2300469. [Google Scholar] [CrossRef]
- Utsumi, W.; Yamakata, M.; Yagi, T.; Shimomura, O. In situ X-ray diffraction study of the phase transition from graphite to hexagonal diamond under high pressures and high temperatures. AIP Conf. Proc. 1994, 309, 535–538. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Lin, Y.; Liu, J.; He, D.; Lei, L. LHDAC synthesis of nanopolycrystalline diamond from nano-flake-like graphite. Diam. Relat. Mater. 2025, 152, 111899. [Google Scholar] [CrossRef]
- Alabdulkarim, M.E.; Maxwell, W.D.; Thapliyal, V.; Maxwell, J.L. A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part III: Laser Reactive Synthesis within Diamond Anvil Cells. J. Manuf. Mater. Process. 2023, 7, 57. [Google Scholar] [CrossRef]
- Zhou, D.; Semenok, D.V.; Duan, D.; Xie, H.; Chen, W.; Huang, X.; Li, X.; Liu, B.; Oganov, A.R.; Cui, T. Superconducting praseodymium superhydrides. Sci. Adv. 2020, 6, eaax6849. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, M.; Wang, Y.; Kawaguchi, S.; Ohishi, Y.; Peng, F.; Liu, H.; Liu, G.; Wang, H.; Ma, Y. Experimental clathrate superhydrides EuH6 and EuH9 at extreme pressure conditions. Phys. Rev. Res. 2021, 3, 043107. [Google Scholar] [CrossRef]
- Struzhkin, V.V.; Mao, H.-K. Magnetic methods in studies of new superconducting hydrides in a diamond anvil cell. Natl. Sci. Rev. 2024, 11, nwae005. [Google Scholar] [CrossRef]
- Arpita Aparajita, A.N.; Sanjay Kumar, N.R.; Chandra, S.; Amirthapandian, S.; Shekar, N.V.C.; Sridhar, K. High-Pressure Synthesis of Manganese Monocarbide: A Potential Superhard Material. Inorg. Chem. 2018, 57, 14178–14185. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Arellano, E.A.; Winkler, B.; Friedrich, A.; Wilson, D.J.; Koch-Müller, M.; Knorr, K.; Vogel, S.C.; Wall, J.J.; Reiche, H.; Crichton, W.; et al. Reaction of rhenium and carbon at high pressures and temperatures. Z. Kristallogr.-Cryst. Mater. 2008, 223, 492–501. [Google Scholar] [CrossRef]
- Bhadram, V.S.; Kim, D.Y.; Strobel, T.A. High-Pressure Synthesis and Characterization of Incompressible Titanium Pernitride. Chem. Mater. 2016, 28, 1616–1620. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Serghiou, G.; Schwarz, M.; Kroke, E.; Riedel, R.; Fueß, H.; Kroll, P.; Boehler, R. Synthesis of cubic silicon nitride. Nature 1999, 400, 340–342. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Riedel, R. Synthesis of cubic zirconium and hafnium nitride having Th3P4 structure. Nat. Mater. 2003, 2, 185–189. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Serrano-Ruiz, M.; Morana, M.; Dziubek, K.; Scelta, D.; Garbarino, G.; Poręba, T.; Mezouar, M.; Bini, R.; Peruzzini, M. High-pressure and high-temperature synthesis of crystalline Sb3N5. Angew. Chem. 2024, 136, e202319278. [Google Scholar] [CrossRef]
- Ibragimova, O.; Vaquero, L.; Hussein, Z.; Drozd, V.; Chariton, S.; Prakapenka, V.; Chuvashova, I. The synthesis of novel lanthanum hydroxyborate at extreme conditions. Front. Chem. 2023, 11, 1259000. [Google Scholar] [CrossRef]
- Dewaele, A.; Worth, N.; Pickard, C.J.; Needs, R.J.; Pascarelli, S.; Mathon, O.; Mezouar, M.; Irifune, T. Synthesis and stability of xenon oxides Xe2O5 and Xe3O2 under pressure. Nat. Chem. 2016, 8, 784–790. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Lv, C.; Zhang, F.; Fu, S.; Prakapenka, V.B.; Wang, C.; Ho, K.; Lin, J.; Wentzcovitch, R.M. Iron-rich Fe–O compounds at Earth’s core pressures. Innovation 2023, 4, 100354. [Google Scholar] [CrossRef]
- Oka, K.; Mako, I.; Yoshiyuki, O.; Hirose, K. Leveraging oxide reactive sputtering for thermal insulation in laser-heated diamond anvil cell. High Press. Res. 2024, 44, 159–167. [Google Scholar] [CrossRef]
- Tamerius, A.D.; Clarke, S.M.; Gu, M.; Walsh, J.P.S.; Esters, M.; Meng, Y.; Hendon, C.H.; Rondinelli, J.M.; Jacobsen, S.D.; Freedman, D.E. Discovery of Cu3Pb. Angew. Chem. Int. Ed. 2018, 57, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.P.S.; Freedman, D.E. High-Pressure Synthesis: A New Frontier in the Search for Next-Generation Intermetallic Compounds. Acc. Chem. Res. 2018, 51, 1315–1323. [Google Scholar] [CrossRef]
- Hussein, Z.; Kazemiasl, N.; Hussaini, K.; Vaquero, L.; Barkova, O.; Drozd, V.; Chariton, S.; Prakapenka, V.; Chuvashova, I. High-pressure high-temperature synthesis of NdRe2. Front. Chem. 2024, 12, 1259032. [Google Scholar] [CrossRef]
- Park, S.; Abate, I.I.; Liu, J.; Wang, C.; Dahl, J.E.P.; Carlson, R.M.K.; Yang, L.; Prakapenka, V.B.; Greenberg, E.; Devereaux, T.P.; et al. Facile diamond synthesis from lower diamondoids. Sci. Adv. 2020, 6, eaay9405. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, L.; Zeng, Q.; Lou, H.; Sheng, H.; Wen, J.; Miller, D.J.; Meng, Y.; Yang, W.; Mao, W.L.; et al. Synthesis of quenchable amorphous diamond. Nat. Commun. 2017, 8, 322. [Google Scholar] [CrossRef]
- Saha, P.; Mukherjee, G.D. Temperature measurement in double-sided laser-heated diamond anvil cell and reaction of carbon. Indian J. Phys. 2021, 95, 621–628. [Google Scholar] [CrossRef]
- Maxwell, J.L.; Boman, M.; Springer, R.W.; Nobile, A.; DeFriend, K.; Espada, L.; Sandstrom, M.; Kommireddy, D.; Pegna, J.; Goodin, D. Process–Structure Map for Diamond-Like Carbon Fibers from Ethene at Hyperbaric Pressures. Adv. Funct. Mater. 2005, 15, 1077–1087. [Google Scholar] [CrossRef]
- Thapliyal, V.; Alabdulkarim, M.E.; Maxwell, J.L. Sustainable laser-induced synthesis of diamond-like-carbon and helical carbon allotropes from hyperbaric ethanol-water mixtures to 18 bar. Diam. Relat. Mater. 2025, 152, 111956. [Google Scholar] [CrossRef]
- Alabdulkarim, M.E.; Thapliyal, V.; Maxwell, J.L. Sustainable Synthesis of Diamond-like Carbon and Giant Carbon Allotropes from Hyperbaric Methanol–Water Mixtures Through the Critical Point. J. Manuf. Mater. Process. 2024, 8, 286. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Noda, H.; Nagai, H.; Shimakura, M.; Nawata, M. Diamond deposition from methanol–hydrogen–water mixed gas using a low pressure, radio frequency, inductively-coupled plasma. Thin Solid Film. 1998, 332, 136–140. [Google Scholar] [CrossRef]
- Matsushima, Y.; Naganuma, M.; Yamazaki, T.; Maeda, K.; Suzuki, T. Diamond synthesis under atmospheric pressure from ethanol-water solution using hot-filament chemical vapor deposition method assisted by electrospray. J. Appl. Phys. 2005, 98, 114902. [Google Scholar] [CrossRef]
- Simakov, S.K. Metastable nanosized diamond formation from a C−H−O fluid system. J. Mater. Res. 2010, 25, 2336–2340. [Google Scholar] [CrossRef]
- Ota, N.; Fujimori, N.; Watanabe, K. Laser CVD Method for Synthesizing Diamond. U.S. Patent 5,387,443, 7 February 1995. [Google Scholar]
- Fomin, Y.D.; Dzhavadov, L.N.; Tsiok, E.N.; Ryzhov, V.N.; Brazhkin, V.V. The thermodynamics of pressurized methanol: A simple hydrogen-bonded liquid as a touchstone for experiment and computer simulations. J. Chem. Phys. 2022, 157, 124503. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, C.; Guo, S.; Zhang, L.; Gao, J.; Peng, J.; Hu, T.; Wang, L. Novel Diamond Films Synthesis Strategy: Methanol and Argon Atmosphere by Microwave Plasma CVD Method Without Hydrogen. Nanoscale Res. Lett. 2016, 11, 415. [Google Scholar] [CrossRef]
- Tominaga, Y.; Uchida, A.; Hunge, Y.M.; Shitanda, I.; Itagaki, M.; Kondo, T.; Yuasa, M.; Uestuska, H.; Terashima, C. Enhanced growth rates of N-type phosphorus-doped polycrystalline diamond via in-liquid microwave plasma CVD. Solid State Sci. 2024, 155, 107650. [Google Scholar] [CrossRef]
- Riaz, B.; Thi, W.-F.; Machida, M.N. First observations of warm and cold methanol in Class 0/I proto-brown dwarfs. Mon. Not. R. Astron. Soc. 2023, 522, 4934–4954. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in Plant Life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Heikes, B.G.; Chang, W.; Pilson, M.E.Q.; Swift, E.; Singh, H.B.; Guenther, A.; Jacob, D.J.; Field, B.D.; Fall, R.; Riemer, D.; et al. Atmospheric methanol budget and ocean implication. Glob. Biogeochem. Cycles 2002, 16, 80-1–80-13. [Google Scholar] [CrossRef]
- Shin, K.; Udachin, K.A.; Moudrakovski, I.L.; Leek, D.M.; Alavi, S.; Ratcliffe, C.I.; Ripmeester, J.A. Methanol incorporation in clathrate hydrates and the implications for oil and gas pipeline flow assurance and icy planetary bodies. Proc. Natl. Acad. Sci. USA 2013, 110, 8437–8442. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.J.; Sephus, C.D.; Ziurys, L.M. Methanol at the Edge of the Galaxy: New Observations to Constrain the Galactic Habitable Zone. Astrophys. J. 2021, 922, 106. [Google Scholar] [CrossRef]
- Deschamps, F.; Mousis, O.; Sanchez-Valle, C.; Lunine, J.I. The Role of Methanol in the Crystallisation of Titan’s Primordial Ocean. Astrophys. J. 2010, 724, 887. [Google Scholar] [CrossRef]
- Hodyss, R.; Parkinson, C.D.; Johnson, P.V.; Stern, J.V.; Goguen, J.D.; Yung, Y.L.; Kanik, I. Methanol on Enceladus. Geophys. Res. Lett. 2009, 36, L17103. [Google Scholar] [CrossRef]
- Ghiringhelli, L.M.; Valeriani, C.; Los, J.H.; Meijer, E.J.; Fasolino, A.; Frenkel, D. State-of-the-art models for the phase diagram of carbon and diamond nucleation. Mol. Phys. 2008, 106, 2011–2038. [Google Scholar] [CrossRef]
- Yafei, Z.; Fangqing, Z.; Guanghua, C. A study of phase transformation between diamond and graphite in P-T diagram of carbon. Carbon 1994, 32, 1415–1418. [Google Scholar] [CrossRef]
- Zhou, S.; Zang, C.; Ma, H.; Li, X.; Zhang, H.; Jia, X. Study on growth of coarse grains of diamond with high quality under HPHT. Chin. Sci. Bull. 2009, 54, 163–167. [Google Scholar] [CrossRef]
- D’Haenens-Johansson, U.F.S.; Butler, J.E.; Katrusha, A.N. Synthesis of Diamonds and Their Identification. Rev. Mineral. Geochem. 2022, 88, 689–753. [Google Scholar] [CrossRef]
- Tessonnier, J.-P.; Su, D.S. Recent Progress on the Growth Mechanism of Carbon Nanotubes: A Review. ChemSusChem 2011, 4, 824–847. [Google Scholar] [CrossRef]
- Abdisaidov, I.J.; Gulomjanova, S.G.; Khudaykulov, I.K.; Ashurov, K.B. The Low-Temperature Growth of Carbon Nanotubes Using Nickel Catalyst. East Eur. J. Phys. 2024, 3, 355–358. [Google Scholar] [CrossRef]
- Li, Y.; Cui, R.; Ding, L.; Liu, Y.; Zhou, W.; Zhang, Y.; Jin, Z.; Peng, F.; Liu, J. How Catalysts Affect the Growth of Single-Walled Carbon Nanotubes on Substrates. Adv. Mater. 2010, 22, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Bleu, Y.; Bourquard, F.; Michalon, J.-Y.; Lefkir, Y.; Reynaud, S.; Loir, A.-S.; Barnier, V.; Garrelie, F.; Donnet, C. Transfer-free graphene synthesis by nickel catalyst dewetting using rapid thermal annealing. Appl. Surf. Sci. 2021, 555, 149492. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, Q.; Chang, J.; Teh, K.S.; Wei, M.; Zettl, A.; Lin, L. Synthesis of Single-Layer Graphene on Nickel Using a Droplet CVD Process. Adv. Mater. Interfaces 2017, 4, 1600783. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Kustov, A.L.; Stolbov, D.N.; Tedeeva, M.A.; Isaikina, O.Y.; Maslakov, K.I.; Usol’tseva, N.V.; Savilov, S.V. Chromium catalysts supported on carbon nanotubes and graphene nanoflakes for CO2-assisted oxidative dehydrogenation of propane. Appl. Surf. Sci. 2022, 578, 152099. [Google Scholar] [CrossRef]
- Abdulrazzak, F.; Abbas, A.; Hussein, F. Synthesis of few-wall carbon nanotubes using methanol/propanol mixture by chemical vapour deposition. Front. Nanosci. Nanotechnol. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Gamo, M.N.; Nakagawa, K.; Ando, T. Synthesis of aligned carbon nanotubes in organic liquids. J. Mater. Res. 2002, 17, 2457–2464. [Google Scholar] [CrossRef]
- Thapliyal, V.; Alabdulkarim, M.E.; Whelan, D.R.; Mainali, B.; Maxwell, J.L. A concise review of the Raman spectra of carbon allotropes. Diam. Relat. Mater. 2022, 127, 109180. [Google Scholar] [CrossRef]
- Botti, S.; Rufoloni, A.; Rindzevicius, T.; Schmidt, M.S. Surface-Enhanced Raman Spectroscopy Characterization of Pristine and Functionalized Carbon Nanotubes and Graphene. In Raman Spectroscopy; Do Nascimento, G.M., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Ivanova, M.; Lamprecht, C.; Loureiro, M.; Huzil, J.; Foldvari, M. Pharmaceutical characterization of solid and dispersed carbon nanotubes as nanoexcipients. Int. J. Nanomed. 2012, 7, 403–415. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Fortunato, W.; Chiquito, A.J.; Galzerani, J.C.; Moro, J.R. Crystalline quality and phase purity of CVD diamond films studied by Raman spectroscopy. J. Mater. Sci. 2007, 42, 7331–7336. [Google Scholar] [CrossRef]
- Klauser, F.; Steinmüller-Nethl, D.; Kaindl, R.; Bertel, E.; Memmel, N. Raman Studies of Nano- and Ultra-nanocrystalline Diamond Films Grown by Hot-Filament CVD. Chem. Vap. Depos. 2010, 16, 127–135. [Google Scholar] [CrossRef]
- Prawer, S.; Nemanich, R.J. Raman spectroscopy of diamond and doped diamond. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2537–2565. [Google Scholar] [CrossRef] [PubMed]
- Chou, I.M.; Anderson, A.J. Diamond dissolution and the production of methane and other carbon-bearing species in hydrothermal diamond-anvil cells. Geochim. Cosmochim. Acta 2009, 73, 6360–6366. [Google Scholar] [CrossRef]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef]
- Dychalska, A.; Popielarski, P.; Franków, W.; Fabisiak, K.; Paprocki, K.; Szybowicz, M. Study of CVD diamond layers with amorphous carbon admixture by Raman scattering spectroscopy. Mater. Sci.-Pol. 2015, 33, 799–805. [Google Scholar] [CrossRef]
- Bloch, J.; Zeiri, Y.; Lucchese, R.R. A theoretical study of laser-induced chemical vapor deposition. Surf. Sci. 1991, 257, 402–416. [Google Scholar] [CrossRef]
- Ceppatelli, M.; Fanetti, S.; Citroni, M.; Bini, R. Photoinduced Reactivity of Liquid Ethanol at High Pressure. J. Phys. Chem. B 2010, 114, 15437–15444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, K.; Men, Z.; Gao, S.; Li, Z.; Sun, C. Study of high-pressure Raman intensity behavior of aromatic hydrocarbons: Benzene, biphenyl and naphthalene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 526–531. [Google Scholar] [CrossRef]
- Sun, Q. The Raman OH stretching bands of liquid water. Vib. Spectrosc. 2009, 51, 213–217. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Han, L.; Xu, Y.; Ke, H.; Zhou, N.; Dong, H.; Liu, S.; Qiao, G. Near Infrared Spectroscopic Study of Trioctahedral Chlorites and Its Remote Sensing Application. Open Geosci. 2019, 11, 815–828. [Google Scholar] [CrossRef]
- Jha, P.K.; Halada, G.P. The catalytic role of uranyl in formation of polycatechol complexes. Chem. Cent. J. 2011, 5, 12. [Google Scholar] [CrossRef]
- Sun, J.; Deng, Y.; Li, J.; Wang, G.; He, P.; Tian, S.; Bu, X.; Di, Z.; Yang, S.; Ding, G.; et al. A New Graphene Derivative: Hydroxylated Graphene with Excellent Biocompatibility. ACS Appl. Mater. Interfaces 2016, 8, 10226–10233. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdulkarim, M.E.; Maxwell, J.L. High-Pressure Laser Reactive Synthesis Within Diamond Anvil Cells of Carbon Allotropes from Methanol. Crystals 2025, 15, 292. https://doi.org/10.3390/cryst15040292
Alabdulkarim ME, Maxwell JL. High-Pressure Laser Reactive Synthesis Within Diamond Anvil Cells of Carbon Allotropes from Methanol. Crystals. 2025; 15(4):292. https://doi.org/10.3390/cryst15040292
Chicago/Turabian StyleAlabdulkarim, Mohamad E., and James L. Maxwell. 2025. "High-Pressure Laser Reactive Synthesis Within Diamond Anvil Cells of Carbon Allotropes from Methanol" Crystals 15, no. 4: 292. https://doi.org/10.3390/cryst15040292
APA StyleAlabdulkarim, M. E., & Maxwell, J. L. (2025). High-Pressure Laser Reactive Synthesis Within Diamond Anvil Cells of Carbon Allotropes from Methanol. Crystals, 15(4), 292. https://doi.org/10.3390/cryst15040292







