The Chemical Compatibility and Adhesion of Energetic Materials with Several Polymers and Binders: An Experimental Study
Abstract
:1. Introduction
2. Materials and methods
2.1. Materials
2.2. Experimental techniques and methods
2.2.1. Chemical compatibility test
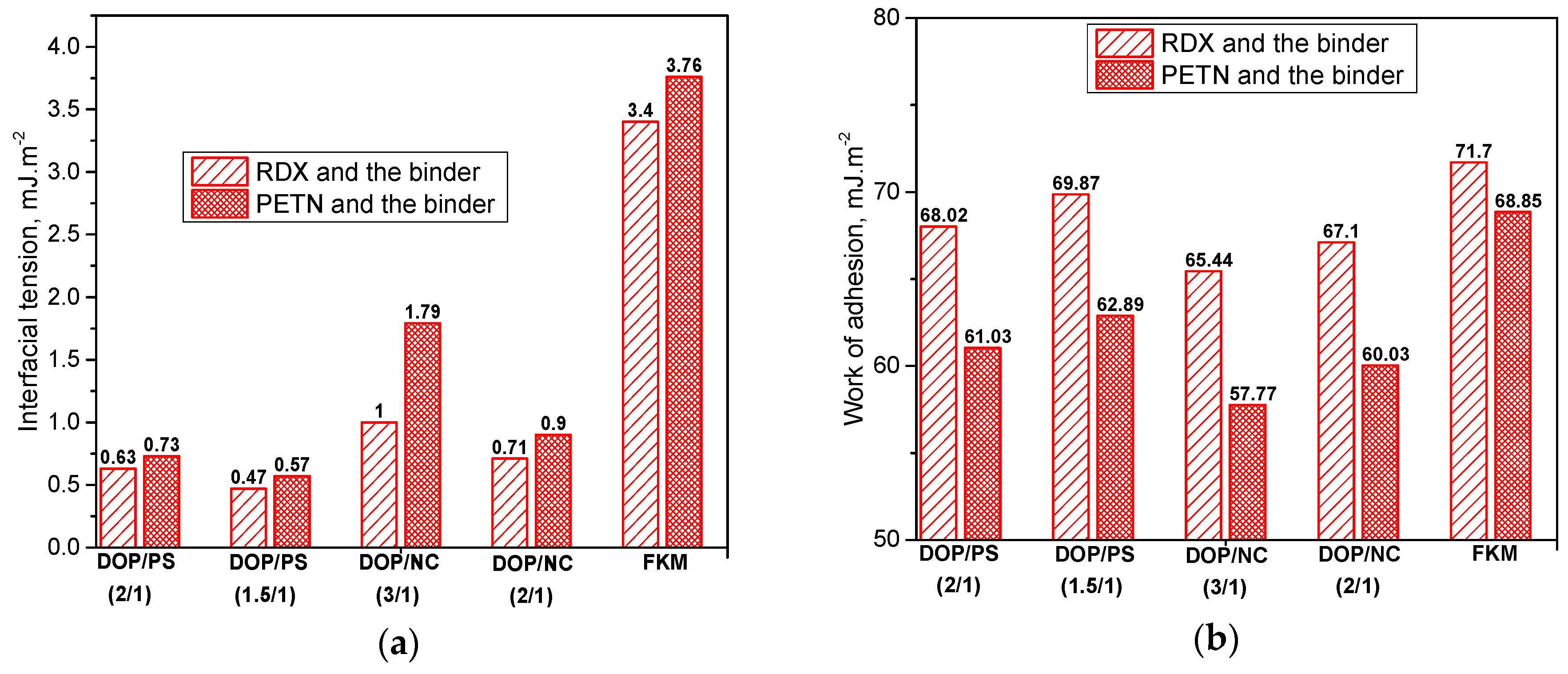
2.2.2. Surface parameters calculation
2.2.3. Determination of the Resistance to Compression of PBX
3. Results and discussion
3.1. The Chemical Compatibility
3.1.1. VST Results
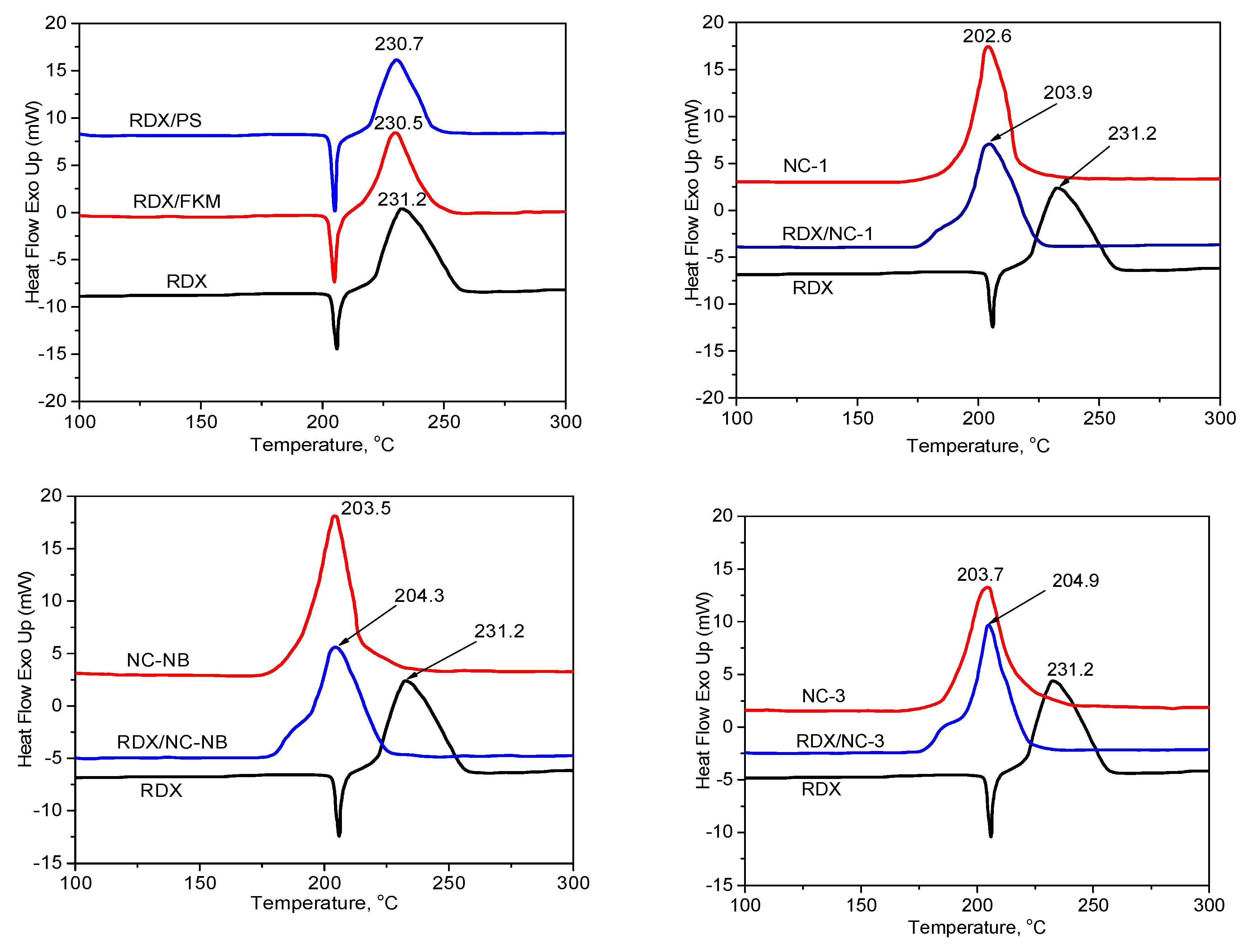
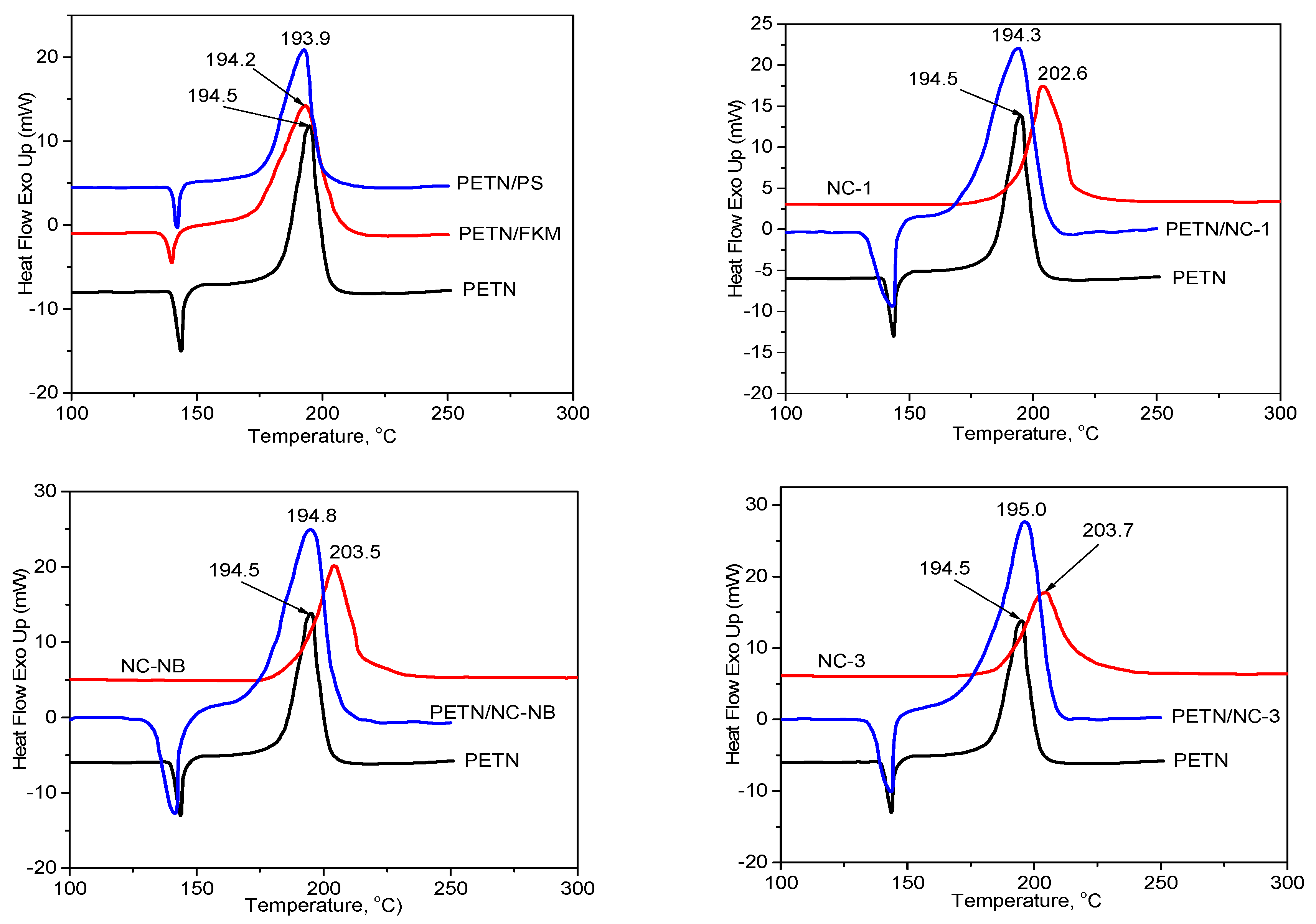
3.1.2. DSC results
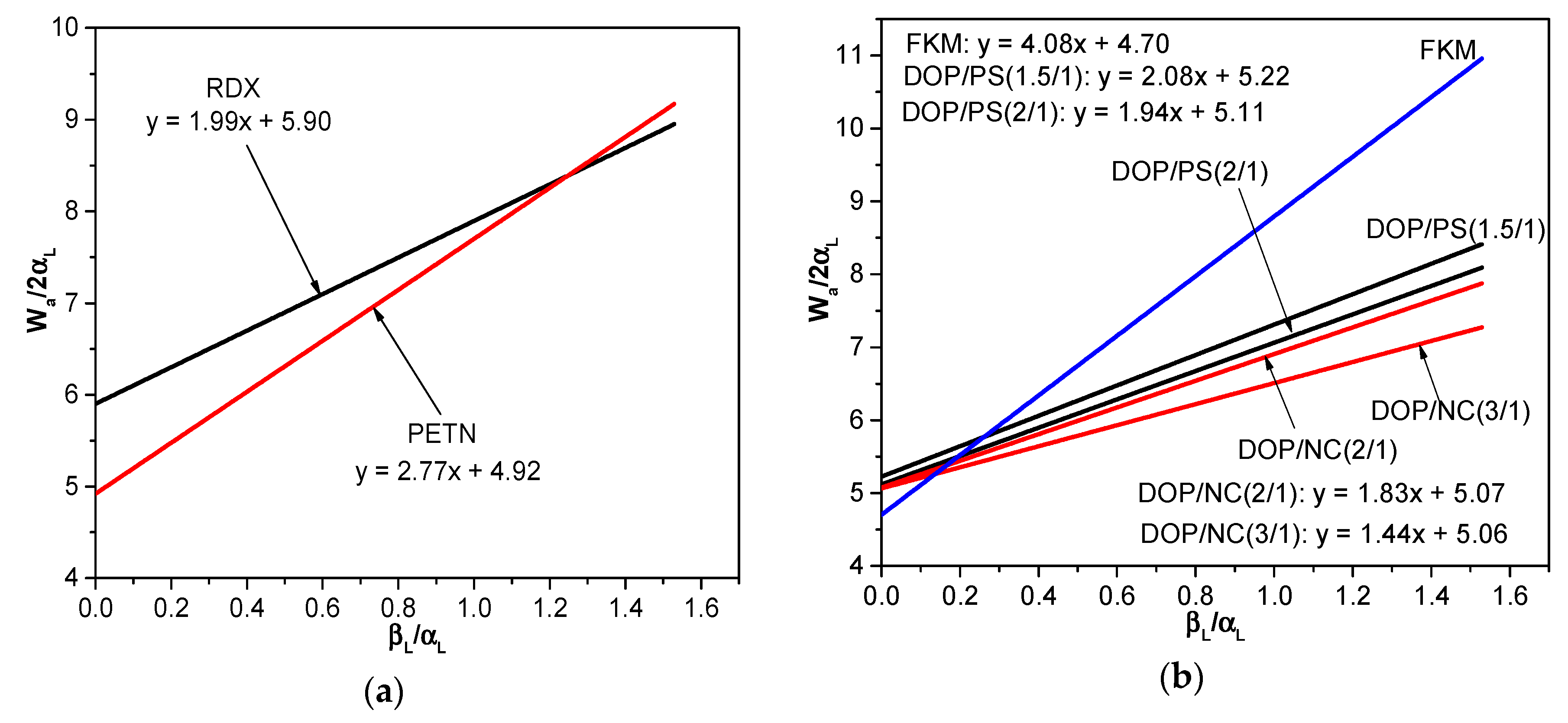
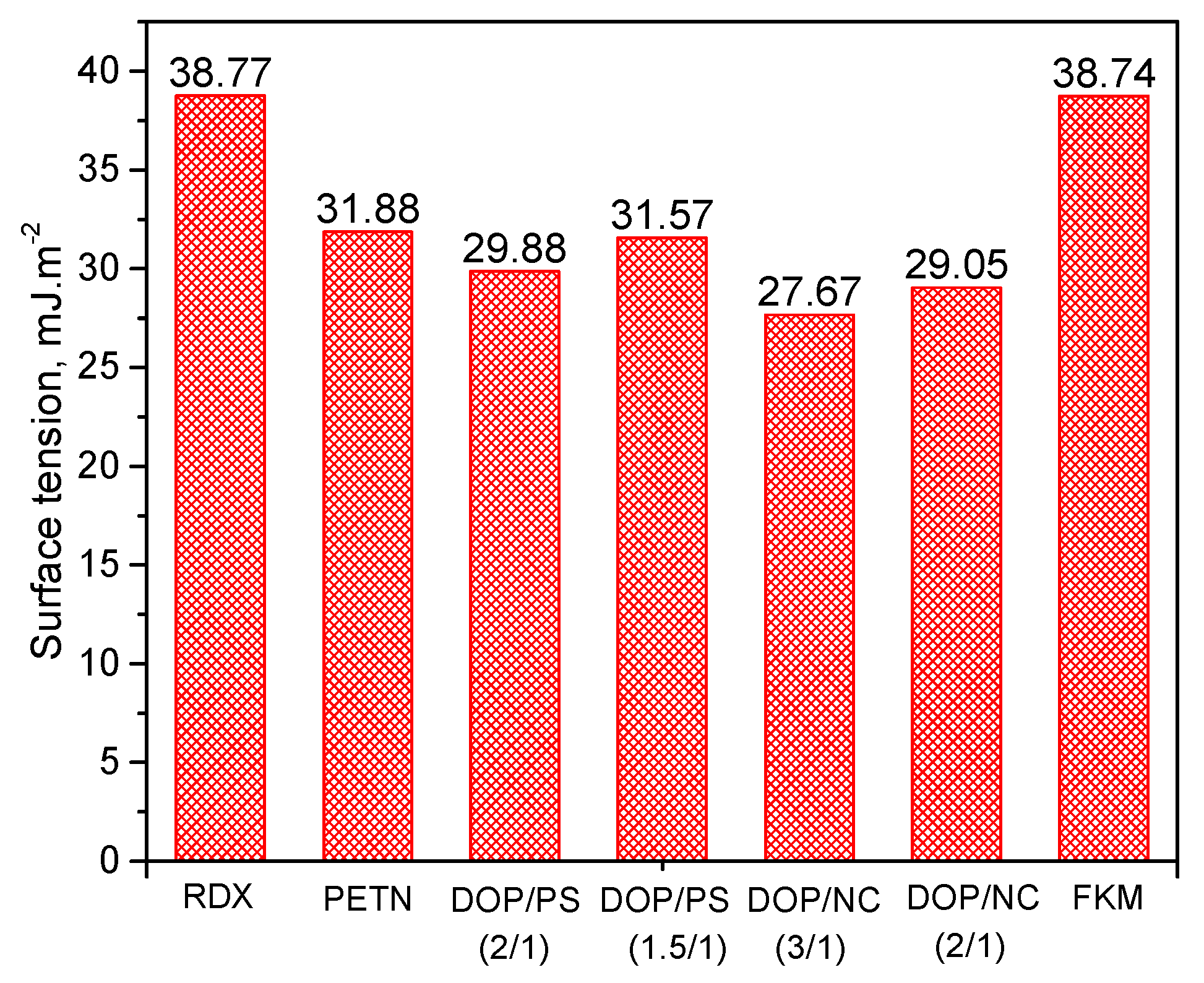
3.2. The Interfacial Parameters
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Elbeih, A.; Zeman, S.; Jungová, M.; Vávra, P. Attractive nitramines and related PBXs. Propellants Explos. Pyrotech. 2013, 38, 379–385. [Google Scholar] [CrossRef]
- Yeager, J.D. Microstructural Characterization of Simulated Plastic-Bonded Explosives. Ph.D. Thesis, Washington State University, Washington, DC, USA, 2011. [Google Scholar]
- Elbeih, A.; Zeman, S.; Jungova, M.; Akstein, Z. Effect of different polymeric matrices on the sensitivity and performance of interesting cyclic nitramines. Cent. Eur. J. Energ. Mater. 2012, 9, 131–138. [Google Scholar]
- Elbeih, A.; Mokhtar Mohamed, M.; Wafy, T. Sensitivity and detonation characteristics of selected nitramines bonded by Sylgard binder. Propellants Explos. Pyrotech. 2016, 41, 1044–1049. [Google Scholar] [CrossRef]
- Provatas, A. Energetic Polymers and Plasticizers for Explosive Formulations-A Review of Recent Advances; Defence Science and Technology Organisation Melbourne: Melbourne, Australia, 2000. [Google Scholar]
- Vogelsanger, B. Chemical stability, compatibility and shelf life of explosives. CHIMIA Int. J. Chem. 2004, 58, 401–408. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, H.S.; Lee, K.D.; Kim, J.K. A study on the interfacial characteristics of nitramine explosive-polymer binder. In Proceedings of the 2004 Annual Conference of AIChE, Austin, TX, USA, 7–12 November 2004. [Google Scholar]
- Mazzeu, M.A.C.; Mattos, E.D.C.; Iha, K. Studies on compatibility of energetic materials by thermal methods. J. Aerosp. Technol. Manag. 2010, 2, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Stanković, M.; Dimić, M.; Blagojević, M.; Petrović, S.; Mijin, D. Compatibility examination of explosive and polymer materials by thermal methods. Sci.-Tech. Rev. 2003, 53, 25–29. [Google Scholar]
- Martin, E.C.; Yee, R.Y. Effects of Surface Interactions and Mechanical Properties of PBXs (Plastic Bonded Explosives) on Explosive Sensitivity (No. NWC-TP-6560); Naval Weapons Center China Lake: China Lake, CA, USA, 1984. [Google Scholar]
- NATO. Chemical Compatibility of Ammunition Components with Explosives (Non-Nuclear Applications); STANAG-4147; NATO Standardization Agreements: Washington, DC, USA, 2001. [Google Scholar]
- Mittal, K.L. Advances in Contact Angle, Wettability and Adhesion; Scrivener Publishing LLC-Wiley: Hoboken, NJ, USA, 2018; Volume 3. [Google Scholar]
- Neumann, A.W.; Kwok, D. Contact Angles and Surface Energetics. In Horizons 2000–Aspects of Colloid and Interface Science at the Turn of the Millennium; Springer: Berlin, Germany, 1998; pp. 170–184. [Google Scholar]
- NATO. Explosives: Vacuum Stability Test; STANAG-4556; NATO Standardization Agreements: Washington, DC, USA, 1999. [Google Scholar]
- Huang, C.C.; Ger, M.D.; Chen, S.I. Study on thermal decomposition of Pentolites by modified vacuum stability apparatus and differential scanning calorimetry. Propellants Explos. Pyrotech. 1992, 17, 254–259. [Google Scholar] [CrossRef]
- Jizhen, L.; Xuezhong, F.; Xiping, F.; Fengqi, Z.; Rongzu, H. Compatibility study of 1,3,3-trinitroazetidine with some energetic components and inert materials. J. Therm. Anal. Calorim. 2006, 85, 779–784. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.L.; Lin, Q.H.; Chen, L.P. Compatibility study of DNTF with some insensitive energetic materials and inert materials. J. Energ. Mater. 2016, 34, 409–415. [Google Scholar] [CrossRef]
- Liao, L.Q.; Wei, H.J.; Li, J.Z.; Fan, X.Z.; Zheng, Y.; Ji, Y.P.; Fu, X.L.; Zhang, Y.J.; Liu, F.L. Compatibility of PNIMMO with some energetic materials. J. Therm. Anal. Calorim. 2011, 109, 1571–1576. [Google Scholar] [CrossRef] [Green Version]
- Hoss, D.J.; Knepper, R.; Hotchkiss, P.J.; Tappan, A.S.; Boudouris, B.W.; Beaudoin, S.P. An evaluation of complementary approaches to elucidate fundamental interfacial phenomena driving adhesion of energetic materials. J. Colloid Interface Sci. 2016, 473, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.; Kim, H.; Lee, K. The Analysis of surface free energy of RDX/EVA from contact angle measurements. J. Korea Inst. Mil. Sci. Technol. 2000, 3, 219–230. [Google Scholar]
- Teipel, U. Energetic Materials: Particle Processing and Characterization; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Haye, K.-L.; de Klerk, W.; Miszczak, M.; Szymanowski, J. Compatibility testing of energetic materials at TNO-PML and MIAT. J. Therm. Anal. Calorim. 2003, 72, 931–942. [Google Scholar] [CrossRef]
- Myburgh, A. Standardization on stanag test methods for ease of compatibility and thermal studies. J. Therm. Anal. Calorim. 2006, 85, 135–139. [Google Scholar] [CrossRef]

| Compositions | Content of Material, wt % | |||
|---|---|---|---|---|
| RDX | PETN | DOP/PS (2/1) | DOP/NC-NB (2/1) | |
| PBXR-85-1 | 85 | - | 15 | - |
| PBXR-85-2 | 85 | - | - | 15 |
| PBXR-90-1 | 90 | - | 10 | - |
| PBXR-90-2 | 90 | - | - | 10 |
| PBXP-85-1 | - | 85 | 15 | - |
| PBXP-85-2 | - | 85 | - | 15 |
| PBXP-90-1 | - | 90 | 10 | - |
| PBXP-90-2 | - | 90 | - | 10 |
| Sample | VR, mL | ||||
|---|---|---|---|---|---|
| PS | FKM | NC-1 | NC-NB | NC-3 | |
| RDX | 0.03 ± 0.01 | 0.09 ± 0.02 | 0.90 ± 0.04 | 0.27 ± 0.03 | 0.13 ± 0.02 |
| PETN | 0.05 ± 0.02 | 0.13 ± 0.02 | 1.00 ± 0.07 | 0.42 ± 0.04 | 0.20 ± 0.04 |
| Samples | The Exothermic Temperature Peak | , °C | ||
|---|---|---|---|---|
| Mixture Systems (1/1) | Single System | , °C | , °C | |
| RDX/FKM | RDX | 231.2 | 230.5 | 0.7 |
| RDX/PS | RDX | 231.2 | 230.7 | 0.5 |
| RDX/NC-1 | NC-1 | 202.6 | 203.9 | -1.3 |
| RDX/NC-NB | NC-NB | 203.5 | 204.3 | -0.8 |
| RDX/NC-3 | NC-3 | 203.7 | 204.9 | -1.2 |
| PETN/FKM | PETN | 194.5 | 194.2 | 0.3 |
| PETN/PS | PETN | 194.5 | 193.9 | 0.6 |
| PETN/NC-1 | PETN | 194.5 | 194.3 | 0.2 |
| PETN/NC-NB | PETN | 194.5 | 194.8 | -0.3 |
| PETN/NC-3 | PETN | 194.5 | 195.0 | -0.5 |
| Solvent | |||||
|---|---|---|---|---|---|
| Water | 72.8 | 21.8 | 51.0 | 4.67 | 7.14 |
| Glycerol | 63.1 | 37.0 | 26.1 | 6.08 | 5.11 |
| Ethylene glycol | 44.4 | 31.7 | 12.7 | 5.63 | 3.56 |
| Chloroform | 27.2 | 27.2 | 0 | 5.21 | 0 |
| Sample | Water | Glycerol | Ethylene Glycol | Chloroform |
|---|---|---|---|---|
| RDX | 81.50 ± 1.91 | 62.51 ± 2.03 | 35.32 ± 1.32 | Spread * |
| PETN | 79.45 ± 1.42 | 69.20 ± 2.35 | 42.41 ± 2.74 | Spread * |
| DOP/PS = 2/1 | 88.68 ± 1.10 | 72.49 ± 1.82 | 47.00 ± 1.42 | 26.20 ± 2.40 |
| DOP/PS = 1.5/1 | 86.05 ± 2.31 | 70.60 ± 2.07 | 43.02 ± 1.54 | 18.32 ± 2.37 |
| DOP/NC = 3/1 | 94.64 ± 1.89 | 79.54 ± 2.76 | 50.83 ± 1.52 | 30.36 ± 1.66 |
| DOP/NC = 2/1 | 89.76 ± 1.03 | 76.72 ± 2.20 | 46.50 ± 2.09 | 27.23 ± 1.25 |
| FKM | 66.78 ± 2.23 | 50.02 ± 2.17 | 39.24 ± 1.98 | Spread * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Phan, D.N.; Nguyen, D.C.; Do, V.T.; Bach, L.G. The Chemical Compatibility and Adhesion of Energetic Materials with Several Polymers and Binders: An Experimental Study. Polymers 2018, 10, 1396. https://doi.org/10.3390/polym10121396
Nguyen TT, Phan DN, Nguyen DC, Do VT, Bach LG. The Chemical Compatibility and Adhesion of Energetic Materials with Several Polymers and Binders: An Experimental Study. Polymers. 2018; 10(12):1396. https://doi.org/10.3390/polym10121396
Chicago/Turabian StyleNguyen, Trung Toan, Duc Nhan Phan, Duy Chinh Nguyen, Van Thom Do, and Long Giang Bach. 2018. "The Chemical Compatibility and Adhesion of Energetic Materials with Several Polymers and Binders: An Experimental Study" Polymers 10, no. 12: 1396. https://doi.org/10.3390/polym10121396





