Ultraviolet Light-degradation Behavior and Antibacterial Activity of Polypropylene/ZnO Nanoparticles Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnO NPs
2.3. Preparation of PP/ZnO NPs Fiber
2.4. Characterization of ZnO NPs
2.5. Characterization of ZnO NPs-Filled PP Fibers
3. Results
3.1. Characterization of ZnO NPs
3.2. Preparation and Mechanical Properties of PP/ZnO Composites:
3.3. SEM Images of PP and ZnO NPs-Filled PP Fibers
3.4. Differential Scanning Calorimetry Results
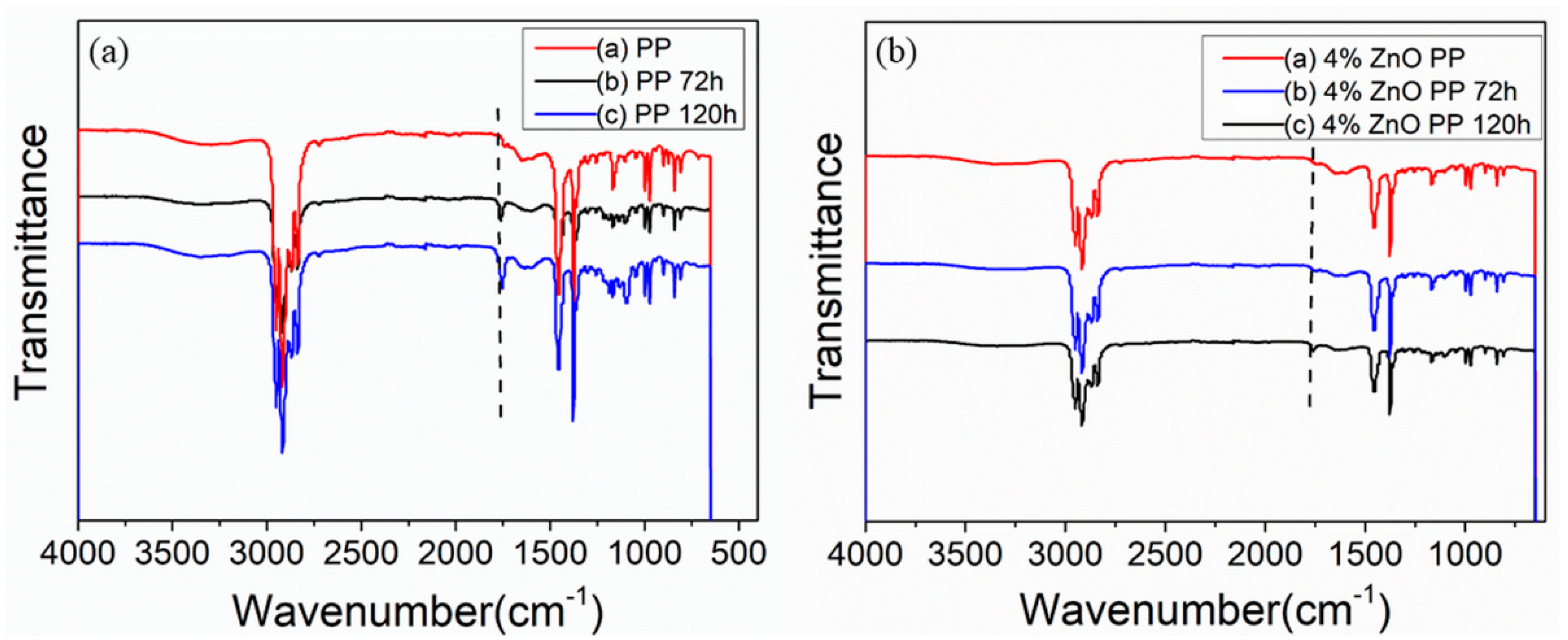
3.5. FTIR Analysis of PP and ZnO NPs Filled PP Fiber with UV Irradiation
3.6. Antibacterial Activity of ZnO NPs-Filled PP Fiber
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, H.; Li, R.K. A study on the photo-degradation of zinc oxide (ZnO) filled polypropylene nanocomposites. Polymer 2006, 47, 3207–3217. [Google Scholar] [CrossRef]
- Zapata, P.A.; Zenteno, A.; Amigó, N.; Rabagliati, F.M.; Sepúlveda, F.; Catalina, F.; Corrales, T. Study on the photodegradation of nanocomposites based on polypropylene and TiO2 nanotubes. Polym. Degrad. Stab. 2016, 133, 101–107. [Google Scholar] [CrossRef]
- Zaman, H.U.; Hun, P.D.; Khan, R.A.; Yoon, K.-B. Morphology, mechanical, and crystallization behaviors of micro-and nano-ZnO filled polypropylene composites. J. Reinf. Plast. Compos. 2012, 31, 323–329. [Google Scholar] [CrossRef]
- Aloui, F.; Ahajji, A.; Irmouli, Y.; George, B.; Charrier, B.; Merlin, A. Inorganic UV absorbers for the photostabilisation of wood-clearcoating systems: Comparison with organic UV absorbers. Appl. Surf. Sci. 2007, 253, 3737–3745. [Google Scholar] [CrossRef]
- Bojinov, V.B.; Georgiev, N.I.; Marinova, N.V. Design and synthesis of highly photostable fluorescence sensing 1,8-naphthalimide-based dyes containing s-triazine UV absorber and HALS units. Sens. Actuators B Chem. 2010, 148, 6–16. [Google Scholar] [CrossRef]
- Nasu, A.; Otsubo, Y. Rheology and UV-protecting properties of complex suspensions of titanium dioxides and zinc oxides. J. Colloid Interface Sci. 2007, 310, 617–623. [Google Scholar] [CrossRef]
- Li, T.; Li, B.; Ji, Y.; Wang, L. Luminescent and UV-Shielding ZnO Quantum Dots/Carboxymethylcellulose Sodium Nanocomposite Polymer Films. Polymers 2018, 10, 112. [Google Scholar] [CrossRef]
- Wang, Z. Foaming behaviour of microcellular foam short carbon fibres/polypropylene/nano-CaCO3composites. Plast. Rubber Compos. 2014, 43, 130–137. [Google Scholar]
- Pavasupree, S.; Dubas, S.T.; Rangkupan, R. Surface modification of polypropylene non-woven fibers with TiO2 nanoparticles via layer-by-layer self assembly method:Preparation and photocatalytic activity. J. Environ. Sci. 2015, 37, 59–66. [Google Scholar] [CrossRef]
- Mei, L.; Gu, L.; Jiang, J.; Zhang, Z.; Xin, D.; Mai, K. Ultraviolet Resistance and Antimicrobial Properties of ZnO in the Polypropylene Materials: A Review. J. Mater. Sci. Technol. 2015, 31, 331–339. [Google Scholar]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Zeng, A.; Zheng, Y.; Yong, G.; Qiu, S.; Lei, C. Effect of tetra-needle-shaped zinc oxide whisker (T-ZnOw) on mechanical properties and crystallization behavior of isotactic polypropylene. Mater. Des. 2012, 34, 691–698. [Google Scholar] [CrossRef]
- Erem, A.D.; Ozcan, G.; Skrifvars, M. In vitro assessment of antimicrobial polypropylene/zinc oxide nanocomposite fibers. Text. Res. J. 2013, 83, 2152–2163. [Google Scholar] [CrossRef]
- Karami, Z.; Youssefi, M.; Borhani, S. The effects of uv irradiation exposure on the structure and properties of polypropylene/zno nanocamposite fibers. Fibers Polym. 2013, 14, 1627–1634. [Google Scholar] [CrossRef]
- Esthappan, S.K.; Sinha, M.K.; Katiyar, P.; Srivastav, A.; Joseph, R. Polypropylene/zinc oxide nanocomposite fibers: Morphology and thermal analysis. J. Polym. Mater. 2013, 30, 79. [Google Scholar]
- Huang, Y.; Wang, T.; Zhao, X.; Wang, X.; Zhou, L.; Yang, Y.; Liao, F.; Ju, Y. Poly (lactic acid)/graphene oxide–ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. J. Chem. Technol. Biotechnol. 2015, 90, 1677–1684. [Google Scholar] [CrossRef]
- Silvestre, C.; Cimmino, S.; Pezzuto, M.; Marra, A.; Ambrogi, V.; Dexpertghys, J.; Verelst, M.; Augier, S.; Romano, I.; Duraccio, D. Preparation and characterization of isotactic polypropylene/zinc oxide microcomposites with antibacterial activity. Polym. J. 2013, 45, 938–945. [Google Scholar] [CrossRef]
- Ye, X.; Wang, H.; Zheng, K.; Wu, Z.; Zhou, H.; Tian, K.; Su, Z.; Tian, X. The interface designing and reinforced features of wood fiber/polypropylene composites: Wood fiber adopting nano-zinc-oxide-coating via ion assembly. Compos. Sci. Technol. 2016, 124, 1–9. [Google Scholar] [CrossRef]
- Rancourt, Y.D.; Couturaud, B.; Mas, A.; Robin, J.J. Synthesis of antibacterial surfaces by plasma grafting of zinc oxide based nanocomposites onto polypropylene. J. Colloid Interface Sci. 2013, 402, 320–326. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cao, Y.; Lan, T.; Chen, H.; Qin, Y. Effects of PLA film incorporated with ZnO nanoparticle on the quality attributes of fresh-cut apple. Nanomaterials 2017, 7, 207. [Google Scholar] [CrossRef]
- Lepot, N.; Bael, M.K.V.; Rul, H.V.D.; D’Haen, J.; Peeters, R.; Franco, D.; Mullens, J. Influence of incorporation of ZnO nanoparticles and biaxial orientation on mechanical and oxygen barrier properties of polypropylene films for food packaging applications. J. Appl. Polym. Sci. 2011, 120, 1616–1623. [Google Scholar] [CrossRef]
- Hu, G.; Ma, Y.; Wang, B. Mechanical properties and morphology of nylon 11/tetrapod-shaped zinc oxide whisker composite. Mater. Sci. Eng. A 2009, 504, 8–12. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Morikawa, H.; Chen, Y. Application of ZnO nanoparticles to enhance the antimicrobial activity; and ultraviolet protective property of bamboo pulp fabric. Cellulose 2013, 20, 1877–1884. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, G.; Ling, C.; Liao, Y.; Chen, Y.; Hong, L.; Morikawa, H. Synthesis of ZnO nanoparticles by PNP and its application on the functional finishing of cotton fabrics. Fibers Polym. 2014, 15, 1842–1849. [Google Scholar] [CrossRef]
- Saliba, S.; Valverde Serrano, C.; Keilitz, J.; Kahn, M.L.; Mingotaud, C.; Haag, R.; Marty, J.-D. Hyperbranched polymers for the formation and stabilization of ZnO nanoparticles. Chem. Mater. 2010, 22, 6301–6309. [Google Scholar] [CrossRef]
- Gao, H.; Yorifuji, D.; Wakita, J.; Jiang, Z.-H.; Ando, S. In situ preparation of nano ZnO/hyperbranched polyimide hybrid film and their optical properties. Polymer 2010, 51, 3173–3180. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Yi, Z.; Yi, Y.; Xu, X. Fabrication of pn heterostructure ZnO/Si moth-eye structures: Antireflection, enhanced charge separation and photocatalytic properties. Appl. Surf. Sci. 2018, 441, 40–48. [Google Scholar] [CrossRef]
- Wang, M.; Li, A.-D.; Kong, J.-Z.; Gong, Y.-P.; Zhao, C.; Tang, Y.-F.; Wu, D. Fabrication and characterization of ZnO nano-clips by the polyol-mediated process. Nanoscale Res. Lett. 2018, 13, 47. [Google Scholar] [CrossRef]
- Achehboune, M.; Khenfouch, M.; Boukhoubza, I.; Mothudi, B.; Zorkani, I.; Jorio, A. Structural and optical characterization of Holmium coated ZnO nanorods. J. Phys. Conf. Ser. 2018, 1, 012007. [Google Scholar] [CrossRef]
- Chandramouleeswaran, S.; Mhaske, S.T.; Kathe, A.A.; Varadarajan, P.V.; Prasad, V.; Vigneshwaran, N. Functional behaviour of polypropylene/ZnO–soluble starch nanocomposites. Nanotechnology 2007, 18, 385702. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nano composite coated paper for antimicrobial packaging application. LWT Food Sci. Technol. 2017, 78, 250–257. [Google Scholar] [CrossRef]








| Sample | Melting Temperature (Tm) (°C) | Melting Enthalpy (ΔHm) (Jg−1) | Crystalline Ratio (%) |
|---|---|---|---|
| PP | 162.16 | 90.14 | 43.75 |
| PP/ZnO 3% | 162.78 | 92.05 | 44.46 |
| PP/ZnO 4% | 162.25 | 92.38 | 44.62 |
| PP/ZnO 5% | 162.18 | 91.22 | 44.06 |
| Sample | S. aureus | E. coli | ||
|---|---|---|---|---|
| Surviving Cells (CFU/mL) | Reduction (%) | Surviving Cells (CFU/mL) | Reduction (%) | |
| Pure fiber | 1.47 × 106 | - | 2.3 × 105 | - |
| 1% ZnO filled fiber | 7.5 × 105 | 64.6 | 1.2 × 105 | 47.85 |
| 2% ZnO filled fiber | 1.1 × 105 | 92.51 | 2.6 × 104 | 88.7 |
| 3% ZnO filled fiber | 2.4 × 103 | 99.83 | 1.6 × 103 | 99.3 |
| 4% ZnO filled fiber | 7.2 × 102 | 99.9 | 6.5 × 102 | 99.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Xiao, Y.; Yan, J.; Xie, N.; Liu, R.; Zhang, Y. Ultraviolet Light-degradation Behavior and Antibacterial Activity of Polypropylene/ZnO Nanoparticles Fibers. Polymers 2019, 11, 1841. https://doi.org/10.3390/polym11111841
Zhang G, Xiao Y, Yan J, Xie N, Liu R, Zhang Y. Ultraviolet Light-degradation Behavior and Antibacterial Activity of Polypropylene/ZnO Nanoparticles Fibers. Polymers. 2019; 11(11):1841. https://doi.org/10.3390/polym11111841
Chicago/Turabian StyleZhang, Guangyu, Yao Xiao, Jiawei Yan, Ningwei Xie, Rong Liu, and Yu Zhang. 2019. "Ultraviolet Light-degradation Behavior and Antibacterial Activity of Polypropylene/ZnO Nanoparticles Fibers" Polymers 11, no. 11: 1841. https://doi.org/10.3390/polym11111841
APA StyleZhang, G., Xiao, Y., Yan, J., Xie, N., Liu, R., & Zhang, Y. (2019). Ultraviolet Light-degradation Behavior and Antibacterial Activity of Polypropylene/ZnO Nanoparticles Fibers. Polymers, 11(11), 1841. https://doi.org/10.3390/polym11111841






