pH Behavior of Polymer Complexes between Poly(carboxylic acids) and Poly(acrylamide derivatives) Using a Fluorescence Label Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of VDP-Labeled Polymers
2.3. Fluorescence Measurements
2.4. Fraction of Dissociated Carboxyl Group (α) of Poly(Carboxylic Acids)
3. Results and Discussion
3.1. pH Responsive Behavior of VDP-Labeled Polymers
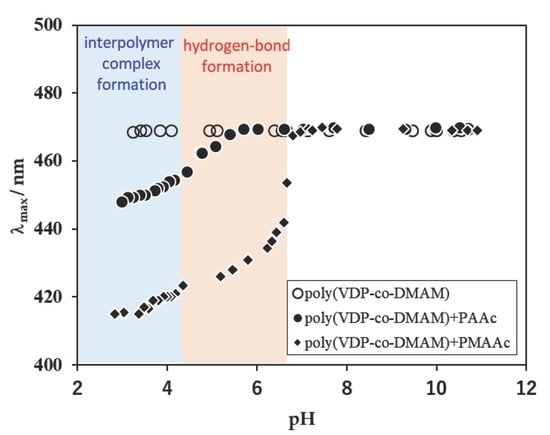
3.2. Complexation Between PolyDMAM With Poly(Carboxylic Acids)
3.3. Complexation Between PolyAAc and VDP-Labeled Poly(Acrylamide Derivatives)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsuchida, E.; Abe, K. Interactions between macromolecules in solution and intermacromolecular complexes. Adv. Polym. Sci. 1982, 45, 1–99. [Google Scholar]
- Khutoryanskiy, V.V. Hydrogen-bonded interpolymer complexes as materials for pharmaceutical applications. Int. J. Pharm. 2007, 334, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte Complexes: A Review of their Applicability in Drug Delivery Technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rubio, L.; Laza, J.M.; Pérez, L.; Rioja, N.; Bilbao, E. Polymer–polymer complexes of poly(N-isopropylacrylamide) and poly(N,N-diethylacrylamide) with poly(carboxylic acids): A comparative study. Colloid Polym. Sci. 2014, 292, 423–430. [Google Scholar] [CrossRef]
- Winnik, F.M.; Regismond, S.T.A. Fluorescence methods in the study of the interactions of surfactants with polymers. Colloids Surf. A Physicochem. Eng. Asp. 1996, 118, 1–39. [Google Scholar] [CrossRef]
- Matsumura, Y.; Iwai, K. Synthesis and thermo-responsive behavior of fluorescent labeled microgel particles based on poly(N-isopropylacrylamide) and its related polymers. Polymer 2005, 46, 10027–10034. [Google Scholar] [CrossRef]
- Uchiyama, S.; Matsumura, Y.; De Silva, A.P.; Iwai, K. Fluorescent molecular thermometers based on polymers showing temperature-induced phase transitions and labeled with polarity-responsive Benzofurazans. Anal. Chem. 2003, 75, 5926–5935. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pérez, L.; Pryke, A.; Sommer, M.; Battaglia, G.; Soutar, I.; Swanson, L.; Geoghegan, M. Conformation of Poly(methacrylic acid) Chains in Dilute Aqueous Solution. Macromolecules 2008, 4, 2203–2211. [Google Scholar] [CrossRef]
- Laguecir, A.; Ulrich, S.; Labille, J.; Fatin-Rouge, N.; Stoll, S.; Buffle, J. Size and pH effect on electrical and conformational behavior of poly(acrylic acid): Simulation and experiment. Eur. Polym. J. 2006, 42, 1135–1144. [Google Scholar] [CrossRef]
- Wang, Y.; Morawetz, H. Fluorescence study of the complexation of poly(acrylic acid) with poly(N,N-dimethylacrylamide-co-acrylamide). Macromolecules 1989, 22, 164–167. [Google Scholar] [CrossRef]
- Aoki, T.; Kawashima, M.; Katono, H.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-Responsive Interpenetrating Polymer Networks Constructed with Poly(acrylic acid) and Poly(N,N-dimethylacrylamide). Macromolecules 1994, 27, 947–952. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Y.; Gao, G.; Liu, M.; Hu, D. Fluorescence probe studies on the complexation between poly(methacrylic acid) and poly(N,N-diethylacrylamide). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, N.L. Technique of electroorganic synthesis Part II. In Techniques of Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1975; p. 668. [Google Scholar]






| Polymer | Solvent | Precipitant | Reaction Time (h) | mol% of VDP Monomer | Yield (%) | |
|---|---|---|---|---|---|---|
| In Feed | In Polymer | |||||
| PolyAAc | methanol | ethyl acetate | 6 | 0 | 0 | 72.7 |
| Poly(VDP-co-AAc) | methanol | ethyl acetate | 6 | 0.1 | 0.12 | 74.1 |
| PolyMAAc | methanol | diethyl ether | 6 | 0 | 0 | 68.0 |
| Poly(VDP-co-MAAc) | methanol | diethyl ether | 6 | 0.1 | 0.15 | 61.0 |
| PolyDMAM | benzene | n-hexane | 1 | 0 | 0 | 52.9 |
| Poly(VDP-co-DMAM) | benzene | n-hexane | 1 | 0.1 | 0.17 | 50.5 |
| Poly(VDP-co-EMAM) 2 | benzene | n-hexane | 1 | 0.1 | 0.14 | 47.4 |
| Poly(VDP-co-DEAM) | benzene | n-hexane | 1 | 0.1 | 0.15 | 57.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumura, Y.; Iwai, K. pH Behavior of Polymer Complexes between Poly(carboxylic acids) and Poly(acrylamide derivatives) Using a Fluorescence Label Technique. Polymers 2019, 11, 1196. https://doi.org/10.3390/polym11071196
Matsumura Y, Iwai K. pH Behavior of Polymer Complexes between Poly(carboxylic acids) and Poly(acrylamide derivatives) Using a Fluorescence Label Technique. Polymers. 2019; 11(7):1196. https://doi.org/10.3390/polym11071196
Chicago/Turabian StyleMatsumura, Yuriko, and Kaoru Iwai. 2019. "pH Behavior of Polymer Complexes between Poly(carboxylic acids) and Poly(acrylamide derivatives) Using a Fluorescence Label Technique" Polymers 11, no. 7: 1196. https://doi.org/10.3390/polym11071196
APA StyleMatsumura, Y., & Iwai, K. (2019). pH Behavior of Polymer Complexes between Poly(carboxylic acids) and Poly(acrylamide derivatives) Using a Fluorescence Label Technique. Polymers, 11(7), 1196. https://doi.org/10.3390/polym11071196