Blue Light-Initiated Alcoholic RAFT Dispersion Polymerization of Benzyl Methacrylate: A Detailed Study
Abstract
1. Introduction
2. Results and Discussion
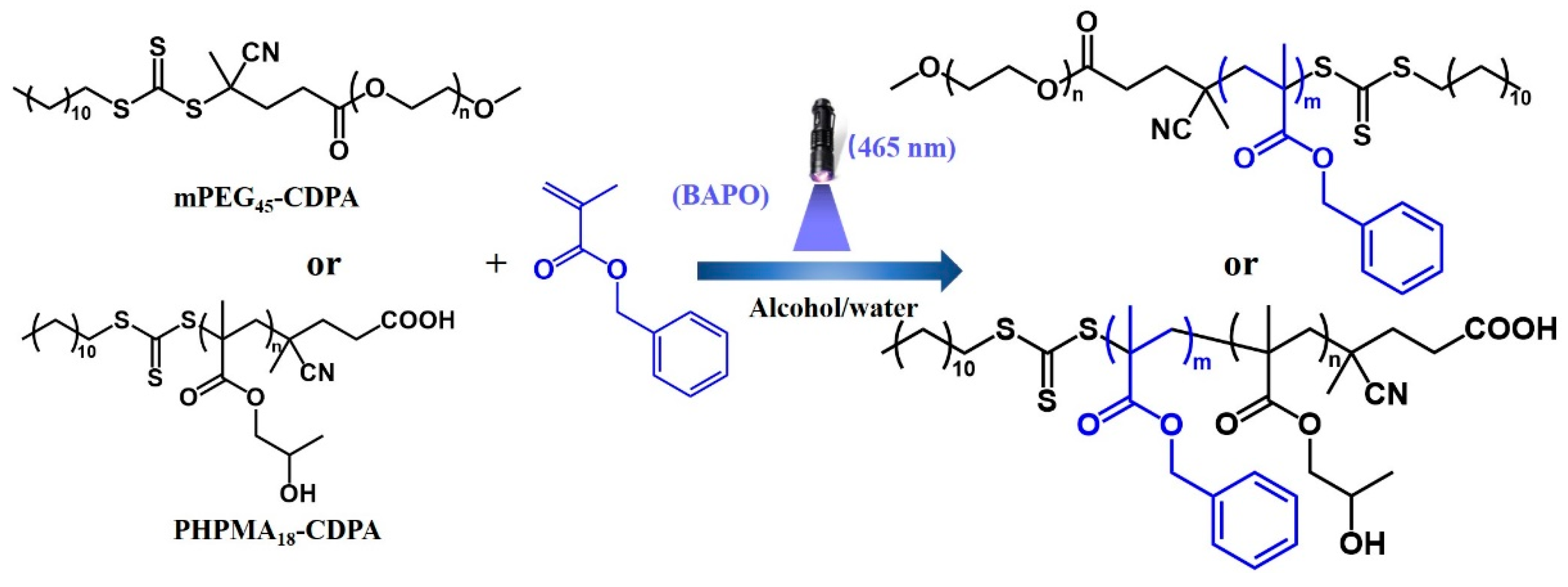
2.1. Synthesis of Macro-CTAs
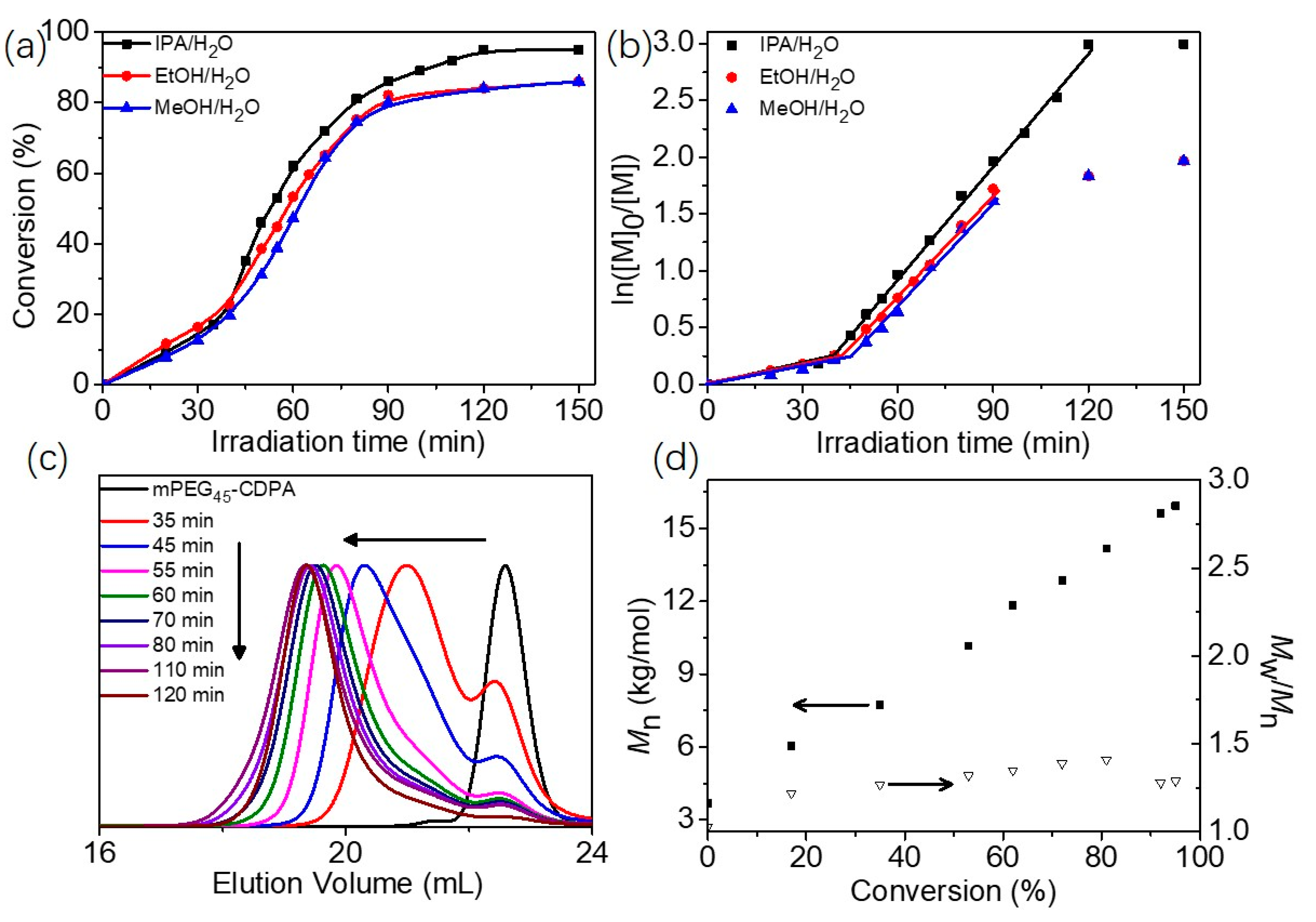
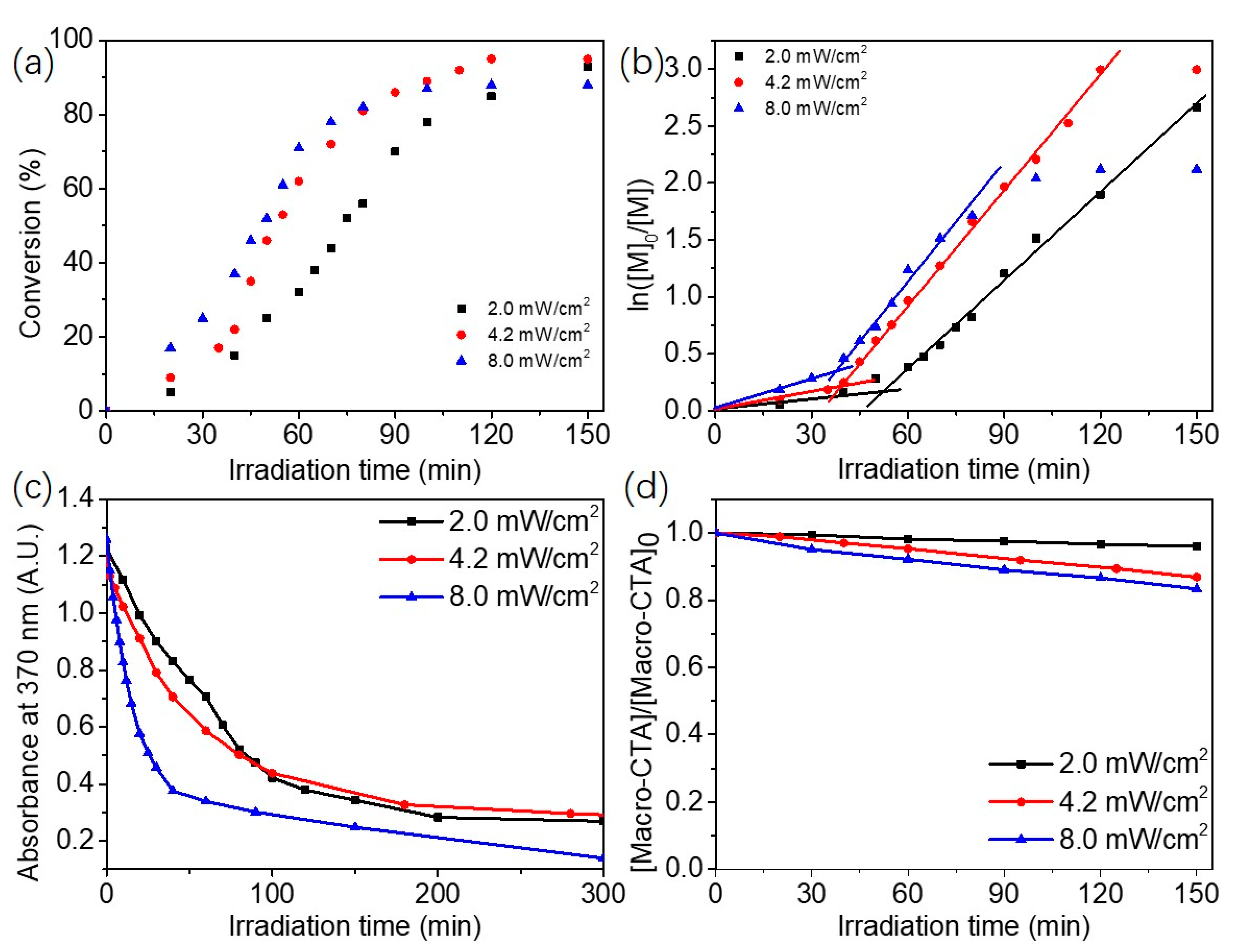
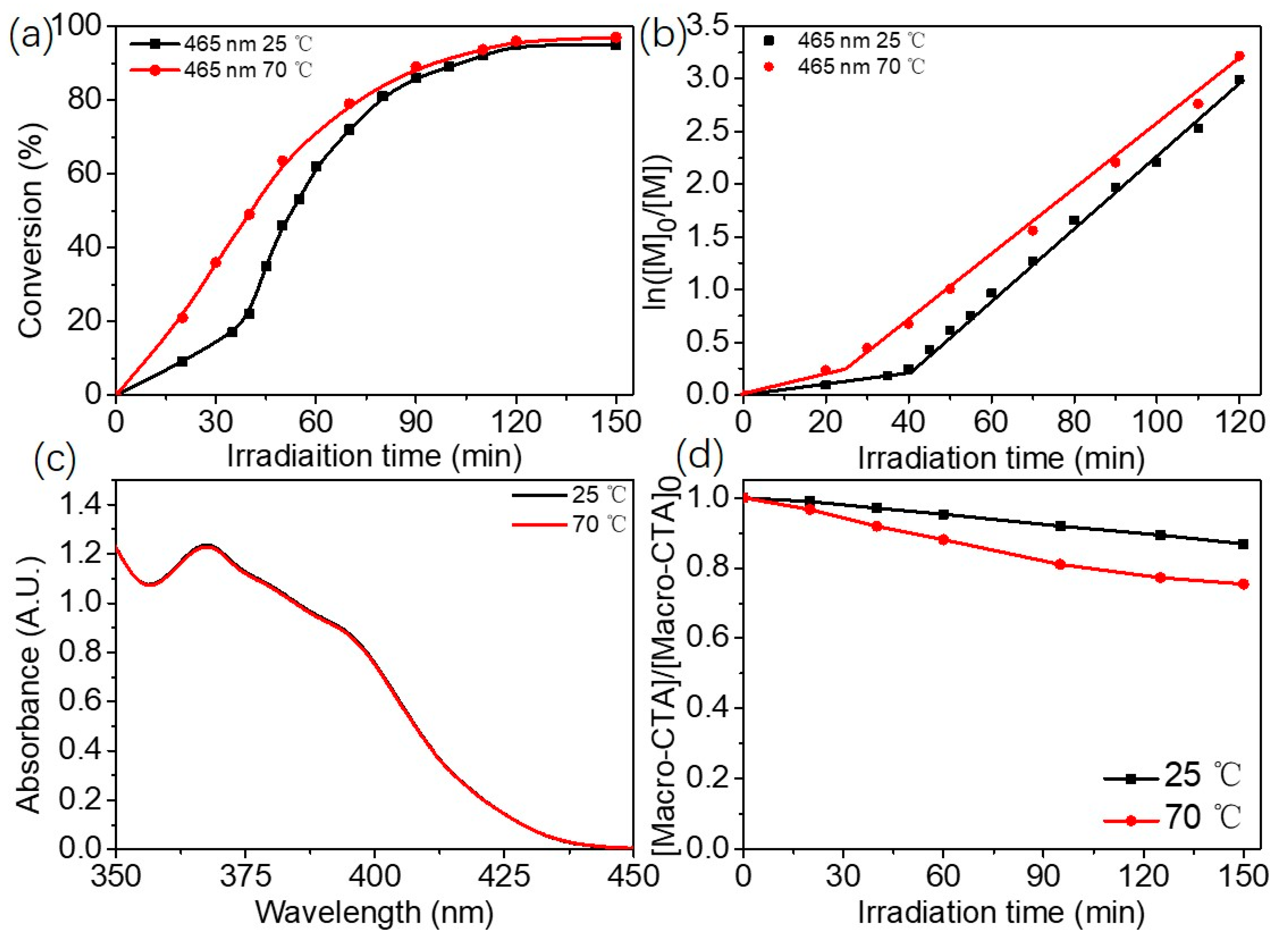
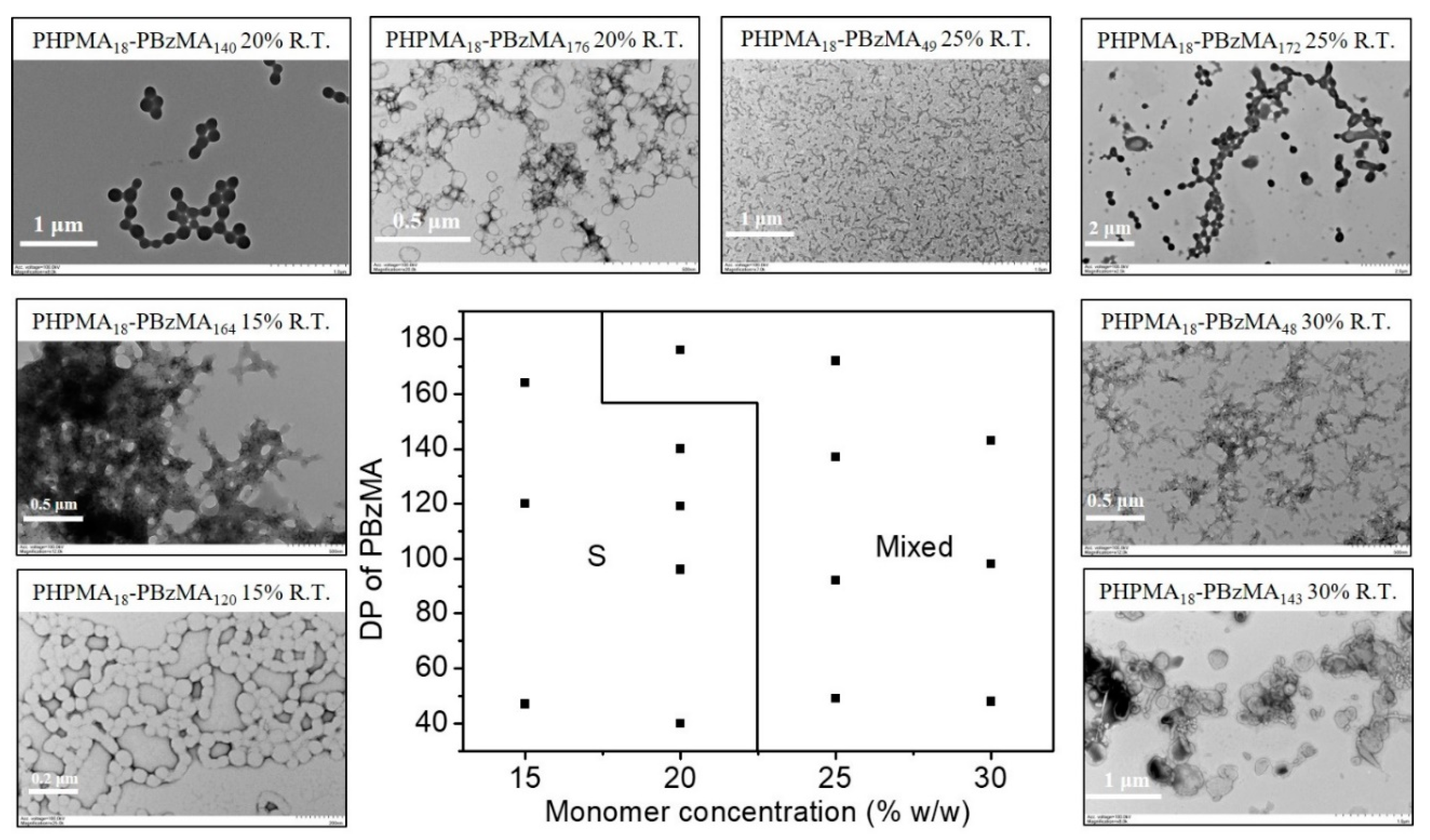
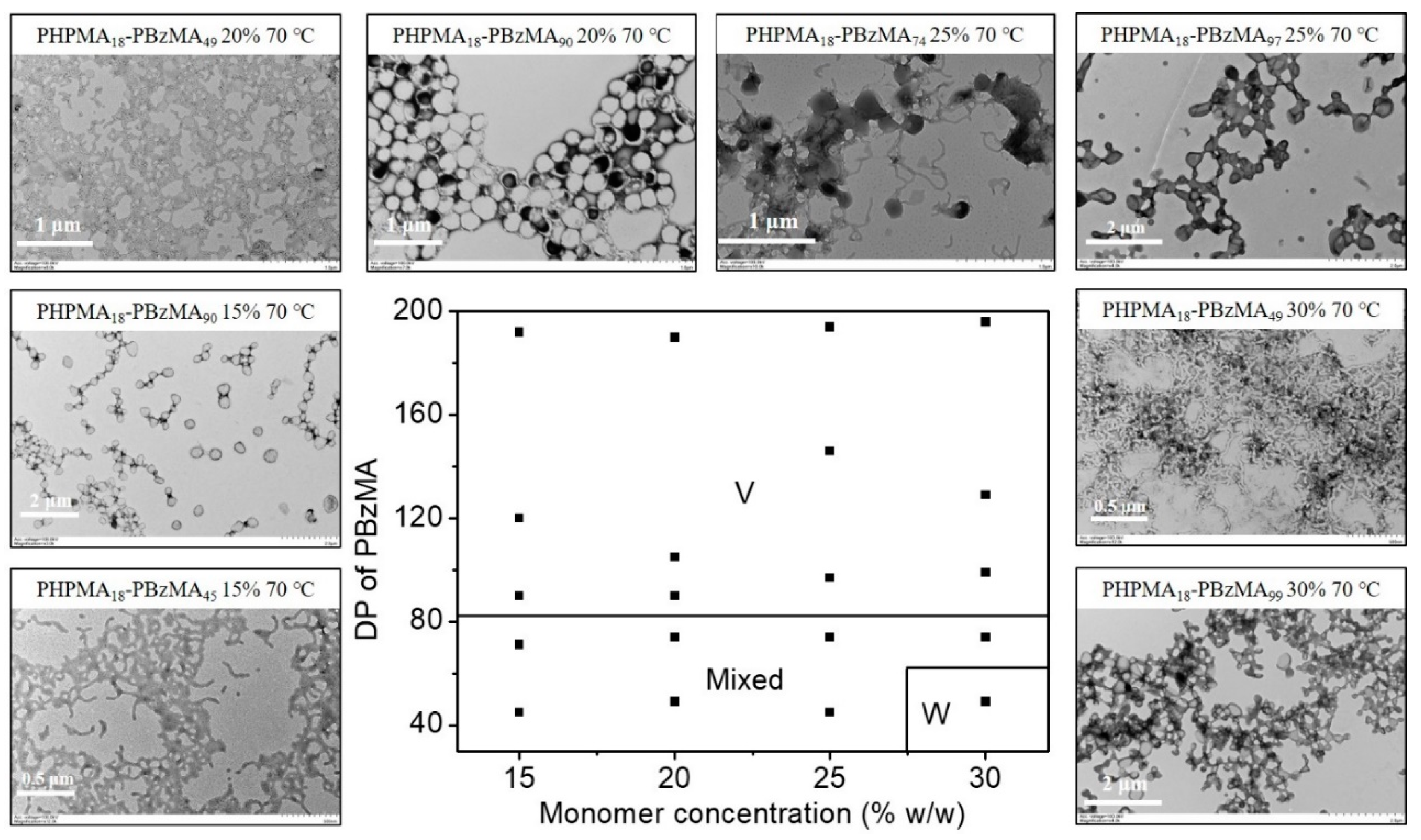
2.2. Blue Light-Initiated Alcoholic RAFT Dispersion Polymerization of BzMA
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Reynhout, I.C.; Cornelissen, J.J.L.M.; Nolte, R.J.M. Self-Assembled Architectures from Biohybrid Triblock Copolymers. J. Am. Chem. Soc. 2007, 129, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Kamps, A.C.; Cativo, M.H.M.; Fryd, M.; Park, S.J. Self-Assembly of Amphiphilic Conjugated Diblock Copolymers into One-Dimensional Nanoribbons. Macromolecules 2014, 47, 161–164. [Google Scholar] [CrossRef]
- Wang, Z.; Van Oers, M.C.M.; Rutjes, F.P.J.T.; Van Hest, J.C.M. Polymersome Colloidosomes for Enzyme Catalysis in a Biphasic System. Angew. Chem. Int. Ed. 2012, 51, 10746–10750. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, J.; Jin, Z.; Jin, F.; Liu, S. Two-Photon Ratiometric Fluorescent Mapping of Intracellular Transport Pathways of pH-Responsive Block Copolymer Micellar Nanocarriers. Adv. Healthc. Mater. 2013, 2, 1576–1581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Louzao, I.; Van Hest, J.C.M. Permeability Effects on the Efficiency of Antioxidant Nanoreactors. Biomacromolecules 2013, 14, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xu, Q.; Li, X.; He, J.; Zhang, Y.; Dai, X.; Yu, L.; Zeng, R.; Zhang, L. Enzyme-PISA: An Efficient Method for Preparing Well-Defined Polymer Nano-Objects under Mild Conditions. Macromol. Rapid Commun. 2018, 39, 1700871. [Google Scholar] [CrossRef] [PubMed]
- Warren, N.J.; Armes, S.P. Polymerization-Induced Self-Assembly of Block Copolymer Nano-Objects via RAFT Aqueous Dispersion Polymerization. J. Am. Chem. Soc. 2014, 136, 10174–10185. [Google Scholar] [CrossRef] [PubMed]
- Charleux, B.; Delaittre, G.; Rieger, J.; D’Agosto, F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. [Google Scholar] [CrossRef]
- Sun, J.T.; Hong, C.Y.; Pan, C.Y. Recent advances in RAFT dispersion polymerization for preparation of block copolymer aggregates. Polym. Chem. 2013, 4, 873–881. [Google Scholar] [CrossRef]
- Zhang, X.; Boissé, S.; Zhang, W.; Beaunier, P.; D’Agosto, F.; Rieger, J.; Charleux, B. Well-Defined Amphiphilic Block Copolymers and Nano-Objects Formed in Situ via RAFT-Mediated Aqueous Emulsion Polymerization. Macromolecules 2011, 44, 4149–4158. [Google Scholar] [CrossRef]
- Canning, S.L.; Smith, G.N.; Armes, S.P. A Critical Appraisal of RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2016, 49, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Qu, Q.; Xu, Y.; An, Z. Aqueous Polymerization-Induced Self-Assembly for the Synthesis of Ketone-Functionalized Nano-Objects with Low Polydispersity. ACS Macro Lett. 2015, 4, 495–499. [Google Scholar] [CrossRef]
- Blackman, L.D.; Doncom, K.E.B.; Gibson, M.I.; O’Reilly, R.K. Comparison of photo- and thermally initiated polymerization-induced self-assembly: A lack of end group fidelity drives the formation of higher order morphologies. Polym. Chem. 2017, 8, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, Y.; Li, X.; He, J.; Tan, J.; Zhang, L. Enzyme catalysis-induced RAFT polymerization in water for the preparation of epoxy-functionalized triblock copolymer vesicles. Polym. Chem. 2018, 9, 4908–4916. [Google Scholar] [CrossRef]
- Wan, W.M.; Hong, C.Y.; Pan, C.Y. One-pot synthesis of nanomaterials via RAFT polymerization induced self-assembly and morphology transition. Chem. Commun. 2009, 5883–5885. [Google Scholar] [CrossRef] [PubMed]
- Semsarilar, M.; Jones, E.R.; Blanazs, A.; Armes, S.P. Efficient Synthesis of Sterically-Stabilized Nano-Objects via RAFT Dispersion Polymerization of Benzyl Methacrylate in Alcoholic Media. Adv. Mater. 2012, 24, 3378–3382. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Huang, C.; Liu, D.; Zhang, X.; Bai, Y.; Zhang, L. Alcoholic Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA): A Fast Route toward Poly(isobornyl acrylate)-Based Diblock Copolymer Nano-Objects. ACS Macro Lett. 2016, 5, 894–899. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, H.; Qu, Y.; Wang, W.; Khan, H.; Zhang, W. In Situ Synthesis of Block Copolymer Nanoassemblies via Polymerization-Induced Self-Assembly in Poly(ethylene glycol). Macromolecules 2016, 49, 3789–3798. [Google Scholar] [CrossRef]
- Ding, Z.; Ding, M.; Gao, C.; Boyer, C.; Zhang, W. In Situ Synthesis of Coil—Coil Diblock Copolymer Nanotubes and Tubular Ag/Polymer Nanocomposites by RAFT Dispersion Polymerization in Poly(ethylene glycol). Macromolecules 2017, 50, 7593–7602. [Google Scholar] [CrossRef]
- Fielding, L.A.; Lane, J.A.; Derry, M.J.; Mykhaylyk, O.O.; Armes, S.P. Thermo-Responsive Diblock Copolymer Worm Gels in Non-Polar Solvents. J. Am. Chem. Soc. 2014, 136, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Fielding, L.A.; Derry, M.J.; Ladmiral, V.; Rosselgong, J.; Rodrigues, A.M.; Ratcliffe, L.P.D.; Sugihara, S.; Armes, S.P. RAFT dispersion polymerization in non-polar solvents: Facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chem. Sci. 2013, 4, 2081. [Google Scholar] [CrossRef]
- Pei, Y.; Thurairajah, L.; Sugita, O.R.; Lowe, A.B. RAFT Dispersion Polymerization in Nonpolar Media: Polymerization of 3-Phenylpropyl Methacrylate in Tetradecane with Poly(stearyl methacrylate) Homopolymers as Macro Chain Transfer Agents. Macromolecules 2015, 48, 236–244. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, S. Ionic Liquids: Versatile Media for Preparation of Vesicles from Polymerization-Induced Self-Assembly. ACS Macro Lett. 2015, 4, 755–758. [Google Scholar] [CrossRef]
- Jennings, J.; Beija, M.; Richez, A.P.; Cooper, S.D.; Mignot, P.E.; Thurecht, K.J.; Jack, K.S.; Howdle, S.M. One-Pot Synthesis of Block Copolymers in Supercritical Carbon Dioxide: A Simple Versatile Route to Nanostructured Microparticles. J. Am. Chem. Soc. 2012, 134, 4772–4781. [Google Scholar] [CrossRef]
- Lowe, A.B. RAFT alcoholic dispersion polymerization with polymerization-induced self-assembly. Polymer 2016, 106, 161–181. [Google Scholar] [CrossRef]
- Li, X.; Tan, J.; Xu, Q.; He, J.; Zhang, L. Photoinitiated Seeded RAFT Dispersion Polymerization: A Facile Method for the Preparation of Epoxy-Functionalized Triblock Copolymer Nano-Objects. Macromol. Rapid Commun. 2018, 39, 1800473. [Google Scholar] [CrossRef]
- Tan, J.; Li, X.; Zeng, R.; Liu, D.; Xu, Q.; He, J.; Zhang, Y.; Dai, X.; Yu, L.; Zeng, Z.; et al. Expanding the Scope of Polymerization-Induced Self-Assembly: Z-RAFT-Mediated Photoinitiated Dispersion Polymerization. ACS Macro Lett. 2018, 7, 255–262. [Google Scholar] [CrossRef]
- Tan, J.; Bai, Y.; Zhang, X.; Huang, C.; Liu, D.; Zhang, L. Low-Temperature Synthesis of Thermoresponsive Diblock Copolymer Nano-Objects via Aqueous Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA) Using Thermoresponsive Macro-RAFT Agents. Macromol. Rapid Commun. 2016, 37, 1434–1440. [Google Scholar] [CrossRef]
- Tan, J.; Liu, D.; Huang, C.; Li, X.; He, J.; Xu, Q.; Zhang, L. Photoinitiated Polymerization-Induced Self-Assembly of Glycidyl Methacrylate for the Synthesis of Epoxy-Functionalized Block Copolymer Nano-Objects. Macromol. Rapid Commun. 2017, 38, 1700195. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; He, J.; Li, X.; Xu, Q.; Huang, C.; Liu, D.; Zhang, L. Rapid synthesis of well-defined all-acrylic diblock copolymer nano-objects via alcoholic photoinitiated polymerization-induced self-assembly (photo-PISA). Polym. Chem. 2017, 8, 6853–6864. [Google Scholar] [CrossRef]
- Tan, J.; Xu, Q.; Zhang, Y.; Huang, C.; Li, X.; He, J.; Zhang, L. Room Temperature Synthesis of Self-Assembled AB/B and ABC/BC Blends by Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA) in Water. Macromolecules 2018, 51, 7396–7406. [Google Scholar] [CrossRef]
- He, J.; Xu, Q.; Tan, J.; Zhang, L. Ketone-Functionalized Polymer Nano-Objects Prepared via Photoinitiated Polymerization-Induced Self-Assembly (Photo-PISA) Using a Poly(diacetone acrylamide)-Based Macro-RAFT Agent. Macromol. Rapid Commun. 2019, 40, 1800296. [Google Scholar] [CrossRef]
- Tan, J.; Dai, X.; Zhang, Y.; Yu, L.; Sun, H.; Zhang, L. Photoinitiated Polymerization-Induced Self-Assembly via Visible Light-Induced RAFT-Mediated Emulsion Polymerization. ACS Macro Lett. 2019, 8, 205–212. [Google Scholar] [CrossRef]
- Yeow, J.; Xu, J.; Boyer, C. Polymerization-Induced Self-Assembly Using Visible Light Mediated Photoinduced Electron Transfer—Reversible Addition—Fragmentation Chain Transfer Polymerization. ACS Macro Lett. 2015, 4, 984–990. [Google Scholar] [CrossRef]
- Yeow, J.; Sugita, O.R.; Boyer, C. Visible Light-Mediated Polymerization-Induced Self-Assembly in the Absence of External Catalyst or Initiator. ACS Macro Lett. 2016, 5, 558–564. [Google Scholar] [CrossRef]
- Ng, G.; Yeow, J.; Xu, J.; Boyer, C. Application of oxygen tolerant PET-RAFT to polymerization-induced self-assembly. Polym. Chem. 2017, 8, 2841–2851. [Google Scholar] [CrossRef]
- Yeow, J.; Shanmugam, S.; Corrigan, N.; Kuchel, R.P.; Xu, J.; Boyer, C. A Polymerization-Induced Self-Assembly Approach to Nanoparticles Loaded with Singlet Oxygen Generators. Macromolecules 2016, 49, 7277–7285. [Google Scholar] [CrossRef]
- Ding, Y.; Cai, M.; Cui, Z.; Huang, L.; Wang, L.; Lu, X.; Cai, Y. Synthesis of Low-Dimensional Polyion Complex Nanomaterials via Polymerization-Induced Electrostatic Self-Assembly. Angew. Chem. Int. Ed. 2018, 57, 1053–1056. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, N.; Han, J.; Yu, Q.; Guo, L.; Gao, P.; Lu, X.; Cai, Y. The direct synthesis of interface-decorated reactive block copolymer nanoparticles via polymerisation-induced self-assembly. Polym. Chem. 2015, 6, 4955–4965. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, P.; Ding, Y.; Huang, L.; Wang, L.; Lu, X.; Cai, Y. Visible Light Initiated Thermoresponsive Aqueous Dispersion Polymerization-Induced Self-Assembly. Macromolecules 2019, 52, 1033–1041. [Google Scholar] [CrossRef]
- Blackman, L.D.; Varlas, S.; Arno, M.C.; Fayter, A.; Gibson, M.I.; O’Reilly, R.K. Permeable Protein-Loaded Polymersome Cascade Nanoreactors by Polymerization-Induced Self-Assembly. ACS Macro Lett. 2017, 6, 1263–1267. [Google Scholar] [CrossRef]
- Zaquen, N.; Azizi, W.A.A.W.; Yeow, J.; Kuchel, R.P.; Junkers, T.; Zetterlund, P.B.; Boyer, C. Alcohol-based PISA in batch and flow: Exploring the role of photoinitiators. Polym. Chem. 2019, 10, 2406–2414. [Google Scholar] [CrossRef]
- Corrigan, N.; Yeow, J.; Judzewitsch, P.; Xu, J.; Boyer, C. Seeing the Light: Advancing Materials Chemistry through Photopolymerization. Angew. Chem. Int. Ed. 2019, 58, 5170–5189. [Google Scholar] [CrossRef]
- Tan, J.; Huang, C.; Liu, D.; Li, X.; He, J.; Xu, Q.; Zhang, L. Polymerization-Induced Self-Assembly of Homopolymer and Diblock Copolymer: A Facile Approach for Preparing Polymer Nano-Objects with Higher-Order Morphologies. ACS Macro Lett. 2017, 6, 298–303. [Google Scholar] [CrossRef]
- Zhang, X.; Rieger, J.; Charleux, B. Effect of the solvent composition on the morphology of nano-objects synthesized via RAFT polymerization of benzyl methacrylate in dispersed systems. Polym. Chem. 2012, 3, 1502. [Google Scholar] [CrossRef]
- Zehm, D.; Ratcliffe, L.P.D.; Armes, S.P. Synthesis of Diblock Copolymer Nanoparticles via RAFT Alcoholic Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, Copolymer Concentration, and Solvent Type on the Final Particle Morphology. Macromolecules 2013, 46, 128–139. [Google Scholar] [CrossRef]
- Tan, J.; Liu, D.; Bai, Y.; Huang, C.; Li, X.; He, J.; Xu, Q.; Zhang, X.; Zhang, L. An insight into aqueous photoinitiated polymerization-induced self-assembly (photo-PISA) for the preparation of diblock copolymer nano-objects. Polym. Chem. 2017, 8, 1315–1327. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, H.; Liang, H.; Lu, J. Salicylaldehyde-functionalized block copolymer nano-objects: One-pot synthesis via polymerization-induced self-assembly and their simultaneous cross-linking and fluorescence modification. Polym. Chem. 2016, 7, 4761–4770. [Google Scholar] [CrossRef]
- Wenn, B.; Junkers, T. Continuous Microflow PhotoRAFT Polymerization. Macromolecules 2016, 49, 6888–6895. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zeng, R.; Sun, H.; Zhang, L.; Tan, J. Blue Light-Initiated Alcoholic RAFT Dispersion Polymerization of Benzyl Methacrylate: A Detailed Study. Polymers 2019, 11, 1284. https://doi.org/10.3390/polym11081284
Liu D, Zeng R, Sun H, Zhang L, Tan J. Blue Light-Initiated Alcoholic RAFT Dispersion Polymerization of Benzyl Methacrylate: A Detailed Study. Polymers. 2019; 11(8):1284. https://doi.org/10.3390/polym11081284
Chicago/Turabian StyleLiu, Dongdong, Ruiming Zeng, Hao Sun, Li Zhang, and Jianbo Tan. 2019. "Blue Light-Initiated Alcoholic RAFT Dispersion Polymerization of Benzyl Methacrylate: A Detailed Study" Polymers 11, no. 8: 1284. https://doi.org/10.3390/polym11081284
APA StyleLiu, D., Zeng, R., Sun, H., Zhang, L., & Tan, J. (2019). Blue Light-Initiated Alcoholic RAFT Dispersion Polymerization of Benzyl Methacrylate: A Detailed Study. Polymers, 11(8), 1284. https://doi.org/10.3390/polym11081284




