Enhanced Interfacial and Mechanical Properties of PBX Composites via Surface Modification on Energetic Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
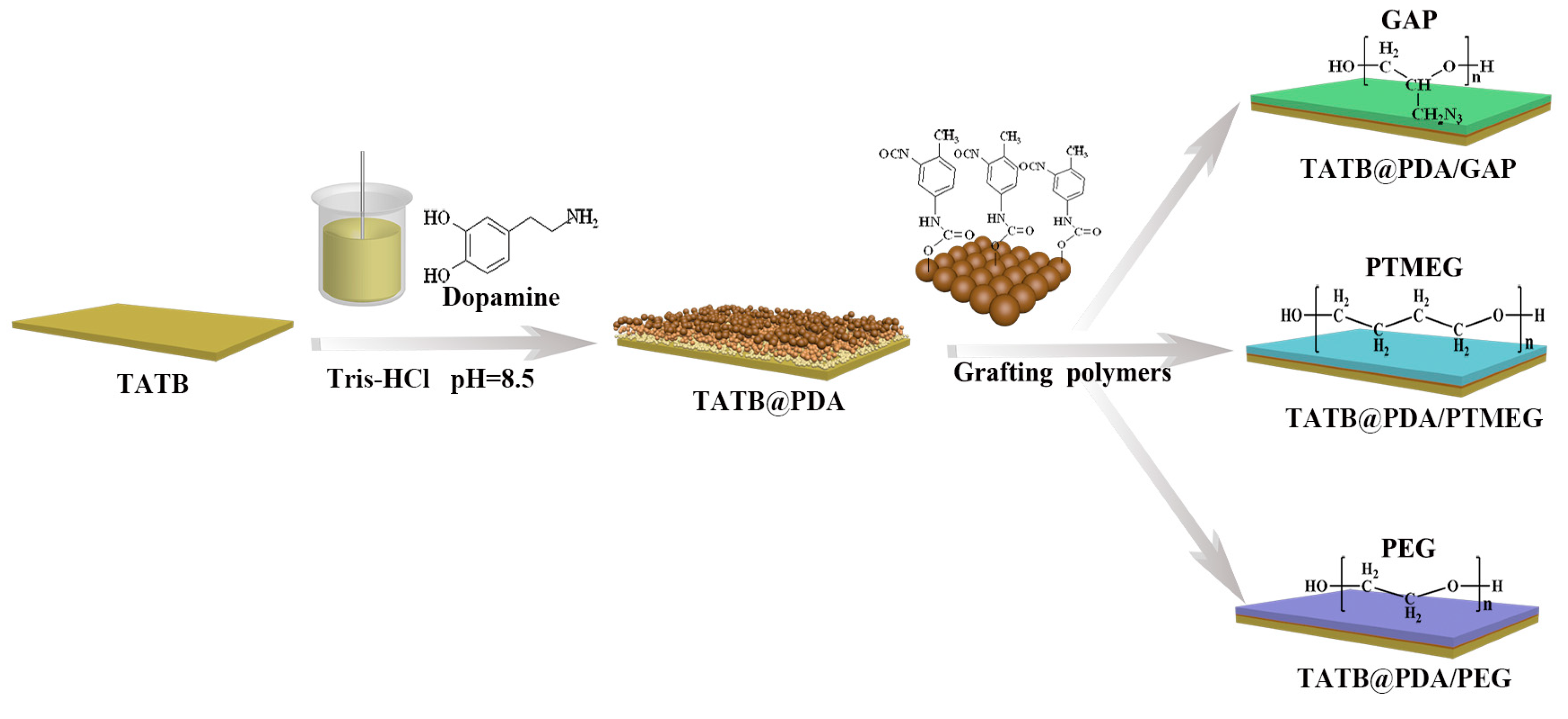
2.2. Coating of TATB with PDA and Interface-Reinforced Polymers
2.3. Preparation of TATB-Based PBX
2.4. Characterization Method
3. Results and Discussion
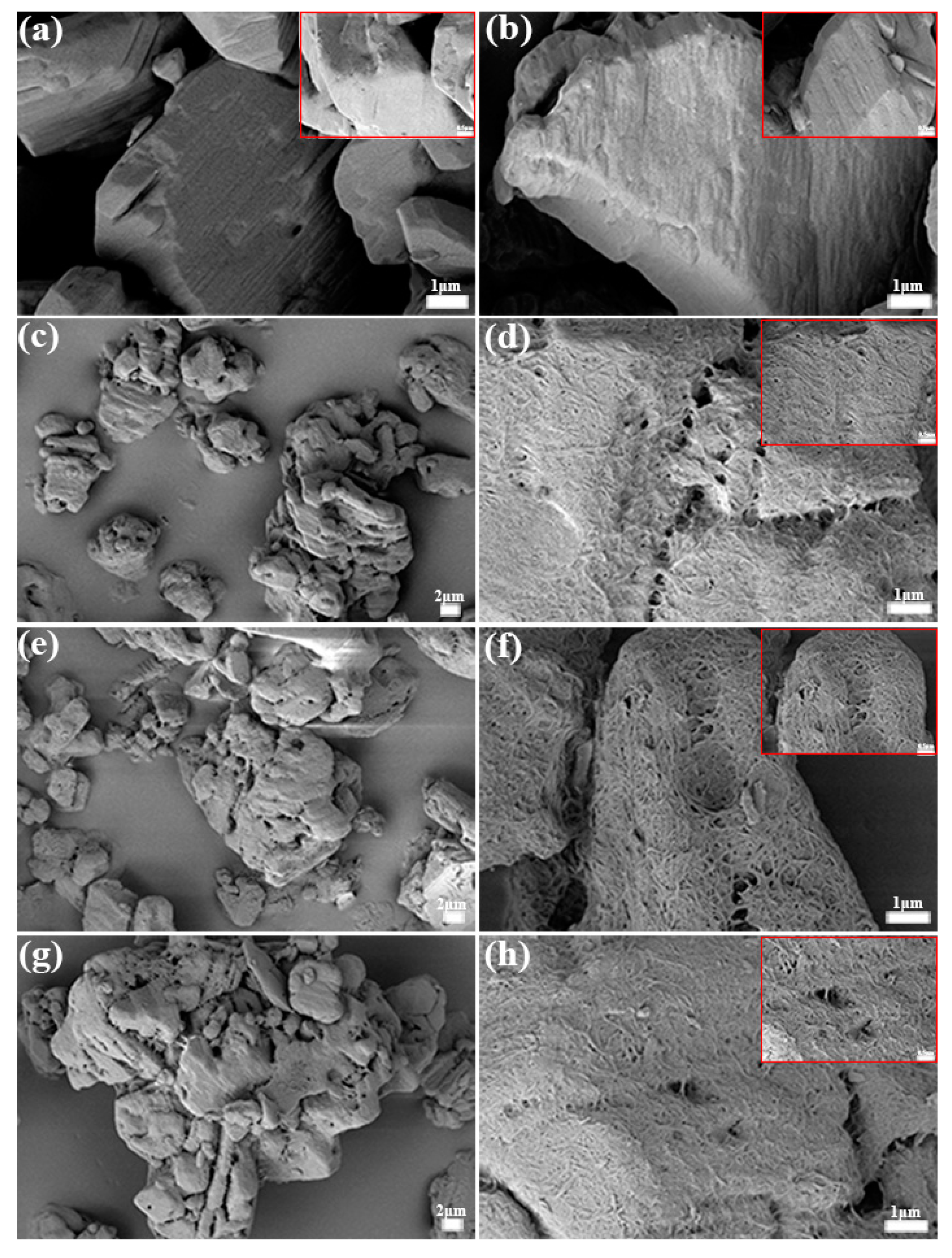
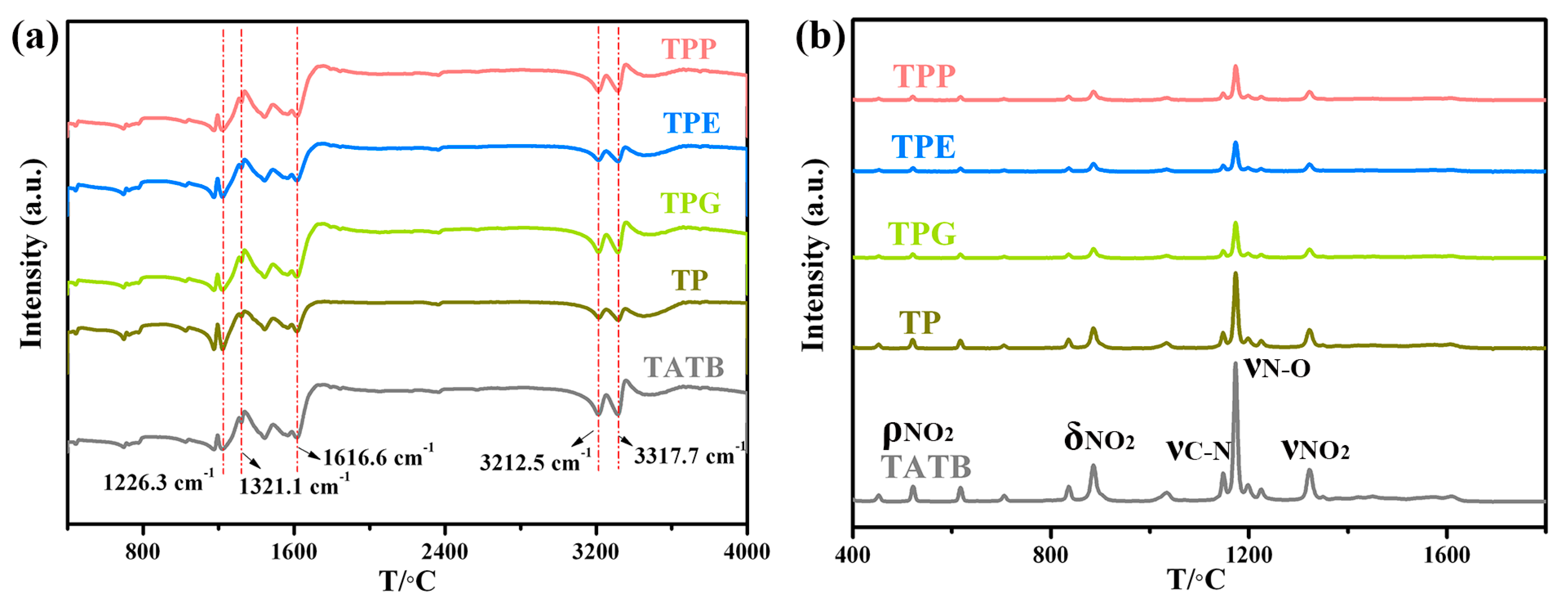
3.1. Morphology and Chemical Structure Characterization of Polymer-Grafted TATB
3.2. Mechanical Properties of Polymer-Grafted TATB
3.3. Surface and Thermodynamic Properties of Polymer-Grafted TATB
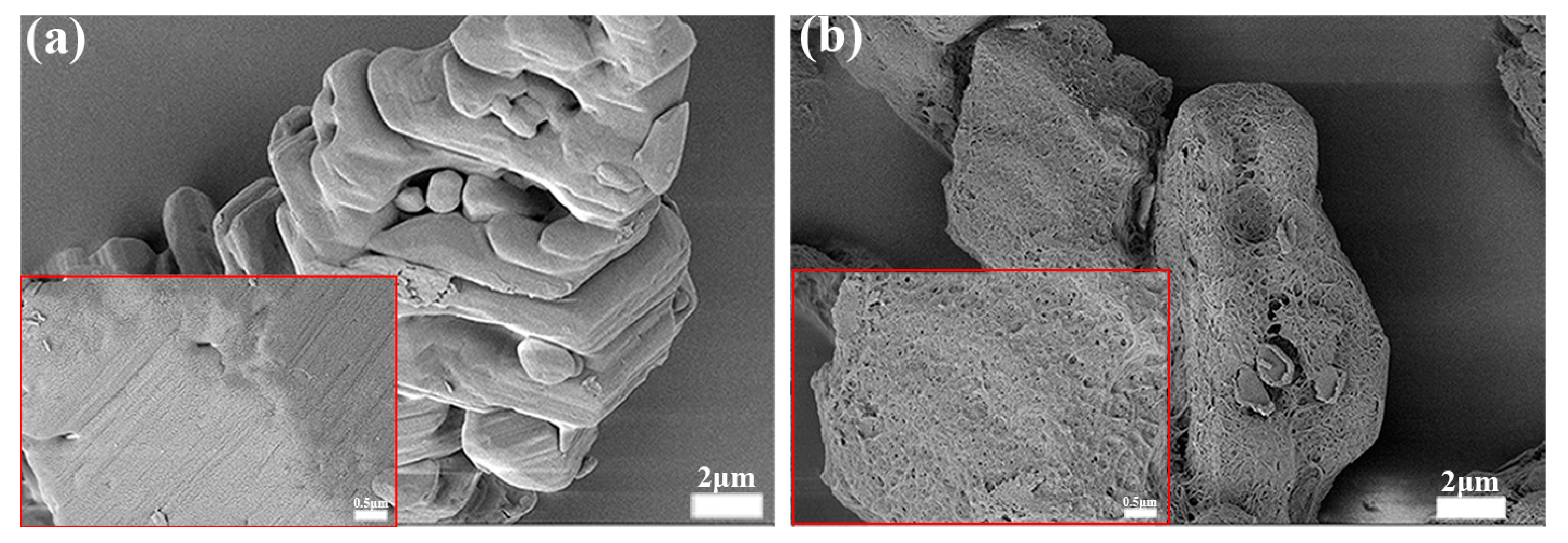
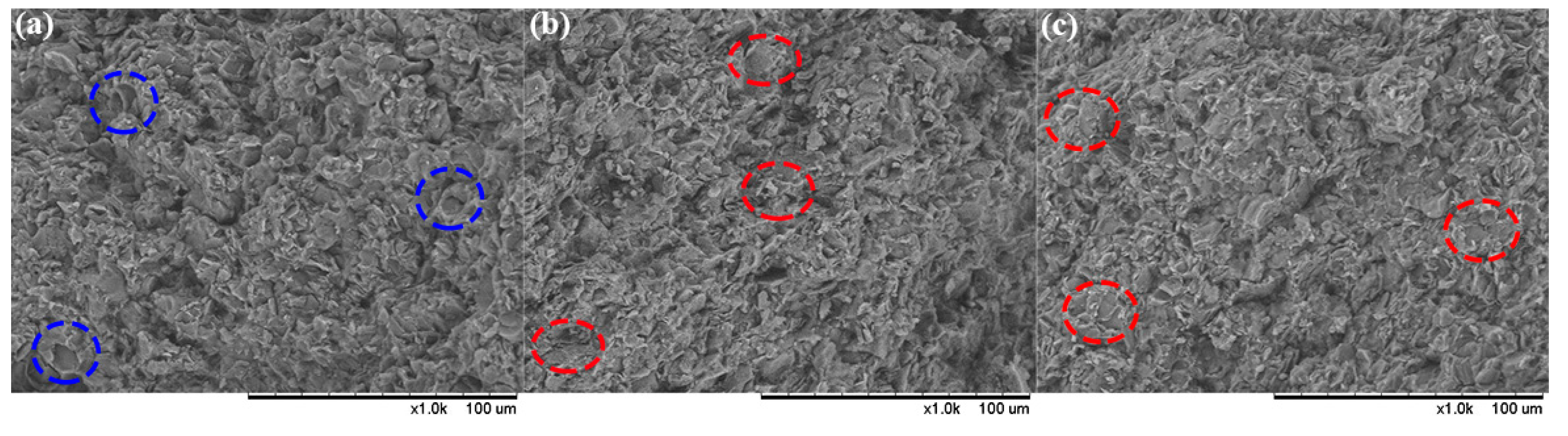
3.4. Morphology and Mechanical Characterization for PTMEG-Grafted TATB
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, C.; Gong, F.; Yang, Z.; Zhao, X.; Li, Y.; Zeng, C.; Li, J.; Guo, S. Core-Shell Structured HMX@Polydopamine Energetic Microspheres: Synergistically Enhanced Mechanical, Thermal, and Safety Performances. Polymers 2019, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.J.P.; Field, J.E.; Huntley, J.M. Deformation, Strengths and Strains to Failure of Polymer Bonded Explosives. Proc. R. Soc. A Math. Phys. Eng. Sci. 1993, 440, 399–419. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, B.; Chen, R.; Qu, K. Compression and Shear Experimental Study of PBX Explosive. Propellants Explos. Pyrotech. 2018, 43, 1245–1250. [Google Scholar] [CrossRef]
- Boddy, R.L.; Gould, P.J.; Jardine, A.P.; Williamson, D.M. Damage in Polymer Bonded Energetic Composites: Effect of Loading Rate. J. Dyn. Behav. Mater. 2016, 2, 157–165. [Google Scholar] [CrossRef][Green Version]
- Hashemi, R.; Spring, D.W.; Paulino, G.H. On Small Deformation Interfacial Debonding in Composite Materials Containing Multi-Coated Particles. J. Compos. Mater. 2018, 49, 3439–3455. [Google Scholar] [CrossRef]
- Liu, Y.L.; Cendón, D.A.; Chen, P.W.; Dai, K.D. Fracture of PBX notched Specimens: Experimental Research and Numerical Prediction. Theor. Appl. Fract. Mec. 2017, 90, 268–275. [Google Scholar] [CrossRef]
- Chen, P.W.; Huang, F.L.; Ding, Y.S. Microstructure, Deformation and Failure of Polymer Bonded Explosives. J. Mater. Sci. 2007, 42, 5272–5280. [Google Scholar] [CrossRef]
- Liu, J.H.; Yang, Z.J.; Liu, S.J.; Zhang, J.H.; Liu, Y.G. Effects of Fluoropolymer Binders on the Mechanical Properties of TATB-Based PBX. Propellants Explos. Pyrotech. 2018, 43, 664–670. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, P.; Duan, Z.; Huang, F. Study on Fracture Behaviour of a Polymer-Bonded Explosive Stimulant Subjected to Uniaxial Compression using Digital Image Correlation Method. Strain 2012, 48, 326–332. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ji, G.F.; Gong, Z.Z.; Wei, D.Q. New Coupling Mechanism of the Silane Coupling Agents in the TATB-based PBX. Mol. Simulat. 2013, 39, 423–427. [Google Scholar] [CrossRef]
- Kim, C.S.; Youn, H.; Nobel, P.N.; Gao, A. Development of Neutral Polymeric Bonding Agent for Propellant with Polarcomposite Filled with Organic Nitramine Crystals. Propellants Explos. Pyrotech. 1992, 17, 38–42. [Google Scholar] [CrossRef]
- Lin, C.; Liu, S.; He, G.; Liu, J. Enhanced Non-linear Viscoelastic Properties of Polymer Bonded Explosives Based on Graphene and a Neutral Polymeric Bonding Agent. Cent. Eur. J. Energ. Mater. 2017, 14, 788–805. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.S.; Tang, G.; Chiang, P.J.; Bettinger, C.J. Texture-Dependent Adhesion in Polydopamine Nanomembranes. ACS Appl. Mater. Interfaces 2018, 10, 7681–7687. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.; Israelachvili, J.; Waite, J. Mussel-Inspired Adhesives and Coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fang, Y.C.; Liu, X.L.; Wang, X.L.; Yang, B. Mussel-Inspired Modification of Carbon Fiber via Polyethyleneimine/Polydopamine Co-deposition for the Improved Interfacial Adhesion. Compos. Sci. Technol. 2017, 151, 164–173. [Google Scholar] [CrossRef]
- Gong, F.; Zhang, J.; Ding, L.; Yang, Z.; Liu, X. Mussel-inspired coating of energetic crystals: A compact core–shell structure with highly enhanced thermal stability. Chem. Eng. J. 2017, 309, 140–150. [Google Scholar] [CrossRef]
- He, G.S.; Zhang, J.H.; Liu, S.J.; Yan, Q.L.; Yang, Z.J.; Pan, L.P. Bioinspired interfacial reinforcement of polymer-based energetic composites with a high loading of solid explosive crystals. J. Mater. Chem. A 2017, 5, 13499–13510. [Google Scholar] [CrossRef]
- Owend, D.K. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar]
- Tran, N.T.; Flanagan, D.P.; Orlicki, J.A.; Lenhart, J.L.; Proctor, K.L.; Knorr, D.B. Polydopamine and Polydopamine–Silane Hybrid Surface Treatments in Structural Adhesive Applications. Langmuir 2018, 34, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Holy, J.A. Raman Spectroscopy of Aminated and Ultrafine 1,3,5-Triamino-2,4,6-trinitrobenzene and PBX 9502 as a Function of Pressing Pressure. J. Phys. Chem. B 2008, 112, 7489–7498. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zuo, X.; Xue, Y.; Zhou, Y.; Yang, Z.; Chuang, Y.C.; Chang, C.C.; Yuan, G.; Satija, S.K.; Gersappe, D.; et al. Enhancing Impact Resistance of Polymer Blends via Self-Assembled Nanoscale Interfacial Structures. Macromolecules 2018, 51, 3897–3910. [Google Scholar] [CrossRef]
- Qiu, M.; Wu, S.; Tang, Z.; Guo, B. Exchangeable interfacial crosslinks towards mechanically robust elastomer/carbon nanotubes vitrimers. Compos. Sci. Technol. 2018, 165, 24–30. [Google Scholar] [CrossRef]
- Lofthouse, M.G.; Burroughs, P. Materials testing by Dynamic mechanical analysis. J. Therm. Anal. Calorim. 1978, 13, 439–453. [Google Scholar] [CrossRef]
- Garcia, C.; Trendafilova, I.; De Villoria, R.G.; Del Río, J.S. Self-powered pressure sensor based on the triboelectric effect and its analysis using dynamic mechanical analysis. Nano Energy 2018, 50, 401–409. [Google Scholar] [CrossRef]
- Yeager, J.D.; Dattelbaum, A.M.; Orler, E.B.; Bahr, D.F.; Dattelbaum, D.M. Adhesive properties of some fluoropolymer binders with the insensitive explosive 1,3,5-triamino-2,4,6-trinitrobenzene (TATB). J. Colloid Interface Sci. 2010, 352, 535–541. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Qi, Z.; Tan, Y.; Wang, H.; Xu, T.; Wang, L.; Xiao, C. Effects of noncovalently functionalized multiwalled carbon nanotube with hyperbranched polyesters on mechanical properties of epoxy composites. Polym. Test. 2017, 64, 38–47. [Google Scholar] [CrossRef]
- Ran, J.; Xiao, L.; Wu, W.; Liu, Y.; Qiu, W.; Wu, J. Zeolitic Imidazolate Framework-8 (ZIF-8) as a Sacrificial Template: One-Pot Synthesis of Hollow poly(dopamine) Nanocapsules and Yolk-Structured Poly(dopamine) Nanocomposites. Nanotechnology 2017, 28, 055604. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloid Interfac. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, C.; Zhang, Y.; Zhou, G.; Zhang, S.; Wang, D. Micromechanism of Coal Dust Wettability and Its Effect on the Selection and Development of Dust Suppressants. Process Saf. Environ. 2017, 111, 726–732. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, F.; He, G.; Li, Y.; Ding, L.; Nie, F.; Huang, F. Perfect Energetic Crystals with Improved Performances Obtained by Thermally Metastable Interfacial Self-Assembly of Corresponding Nanocrystals. Cryst. Growth Des. 2018, 18, 1657–1665. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kim, W.S. High mechanical properties of super aligned carbon nanocomposite by polyurethane based crosslinking molecules. Compos. Sci. Technol. 2018, 161, 100–106. [Google Scholar] [CrossRef]
- Xiao, C.; Tan, Y.; Wang, X.; Gao, L.; Wang, L.; Qi, Z. Study on interfacial and mechanical improvement of carbon fiber/epoxy composites by depositing multi-walled carbon nanotubes on fibers. Chem. Phys. Lett. 2018, 703, 8–16. [Google Scholar] [CrossRef]





| Sample | Abbreviations for Grafting Powders | Sample | Abbreviations for Composites |
|---|---|---|---|
| TATB | TATB | TATB/F2314 | TF |
| TATB@PDA | TP | TATB@PDA/F2314 | TPF |
| (TATB@PDA)@GAP | TPG | (TATB@PDA)@GAP/F2314 | TPGF |
| (TATB@PDA)@PTMEG | TPP | (TATB@PDA)@PTMEG/F2314 | TPPF |
| (TATB@PDA)@PEG | TPE | (TATB@PDA)@PEG/F2314 | TPEF |
| Sample | Contact Angle (°) | Standard Deviation | Surface Energy (mN·m−2) | ||||
|---|---|---|---|---|---|---|---|
| Water | Diiodomethane | Water | Diiodomethane | γsp | γsd | γs | |
| TATB | 77.63 ± 0.77 | 24.10 ± 0.50 | 1.09 | 0.71 | 3.02 | 46.47 | 49.49 |
| TP | 72.05 ± 0.65 | 28.53 ± 0.58 | 0.92 | 0.81 | 5.25 | 44.82 | 50.07 |
| TPG | 80.95 ± 0.45 | 33.20 ± 0.80 | 0.64 | 1.13 | 2.62 | 42.85 | 45.47 |
| TPP | 84.60 ± 0.35 | 31.00 ± 0.30 | 0.49 | 0.42 | 1.56 | 43.80 | 45.36 |
| TPE | 78.30 ± 0.80 | 33.60 ± 0.40 | 1.13 | 0.57 | 3.46 | 42.67 | 46.13 |
| F2314 | 90.20 ± 0.45 | 45.00 ± 0.62 | 0.64 | 0.88 | 1.21 | 37.01 | 38.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, C.; Yang, Z.; Zhang, J.; Li, Y.; Lin, C.; He, G.; Zhao, X.; Liu, S.; Gong, F. Enhanced Interfacial and Mechanical Properties of PBX Composites via Surface Modification on Energetic Crystals. Polymers 2019, 11, 1308. https://doi.org/10.3390/polym11081308
Zeng C, Yang Z, Zhang J, Li Y, Lin C, He G, Zhao X, Liu S, Gong F. Enhanced Interfacial and Mechanical Properties of PBX Composites via Surface Modification on Energetic Crystals. Polymers. 2019; 11(8):1308. https://doi.org/10.3390/polym11081308
Chicago/Turabian StyleZeng, Chengcheng, Zhijian Yang, Jianhu Zhang, Yubin Li, Congmei Lin, Guansong He, Xu Zhao, Shijun Liu, and Feiyan Gong. 2019. "Enhanced Interfacial and Mechanical Properties of PBX Composites via Surface Modification on Energetic Crystals" Polymers 11, no. 8: 1308. https://doi.org/10.3390/polym11081308
APA StyleZeng, C., Yang, Z., Zhang, J., Li, Y., Lin, C., He, G., Zhao, X., Liu, S., & Gong, F. (2019). Enhanced Interfacial and Mechanical Properties of PBX Composites via Surface Modification on Energetic Crystals. Polymers, 11(8), 1308. https://doi.org/10.3390/polym11081308





