Development of Covalent Triazine Frameworks as Heterogeneous Catalytic Supports
Abstract
:1. Introduction to Covalent Triazine Frameworks (CTFs)
2. Design and Synthesis Methods of CTFs
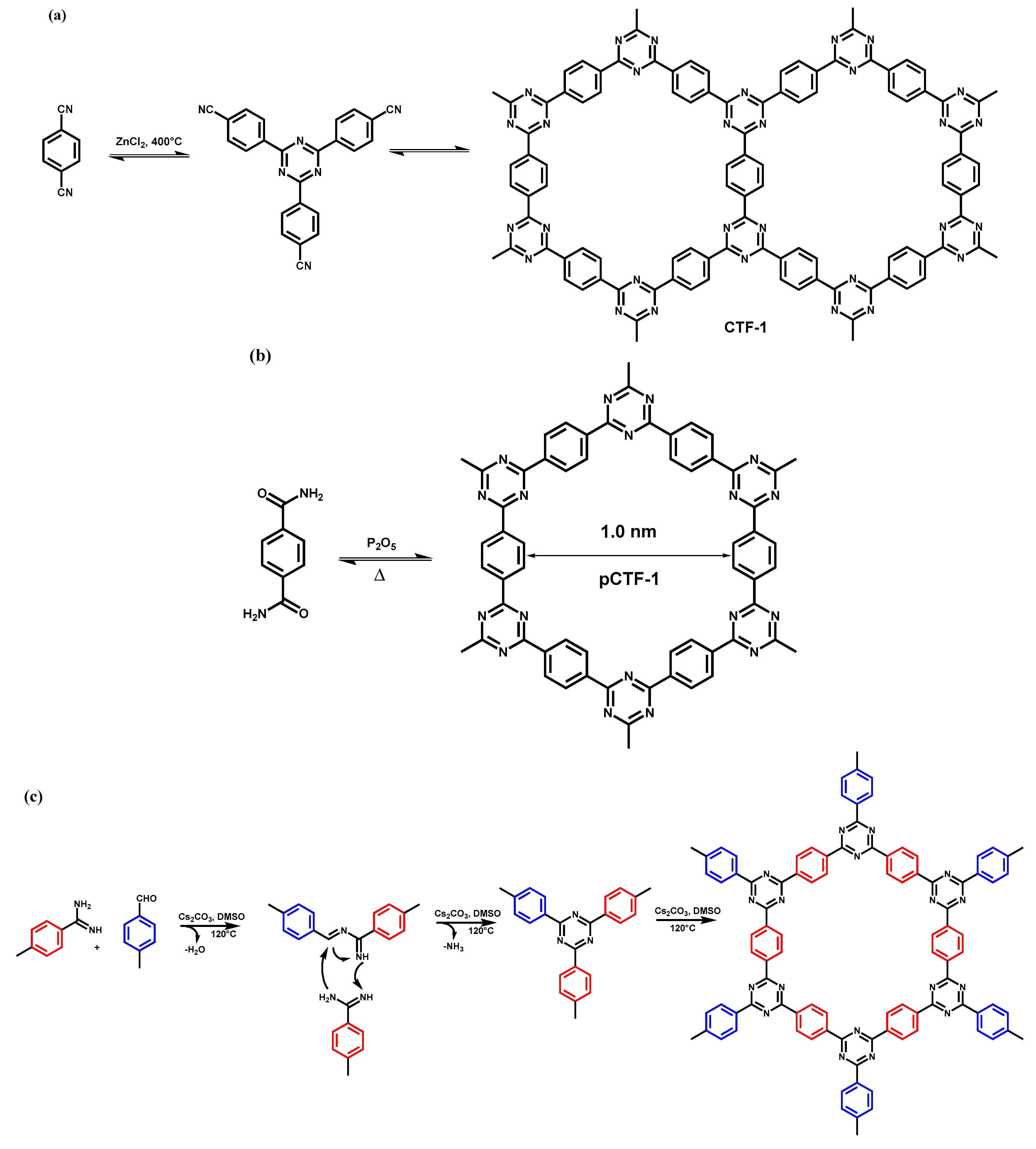
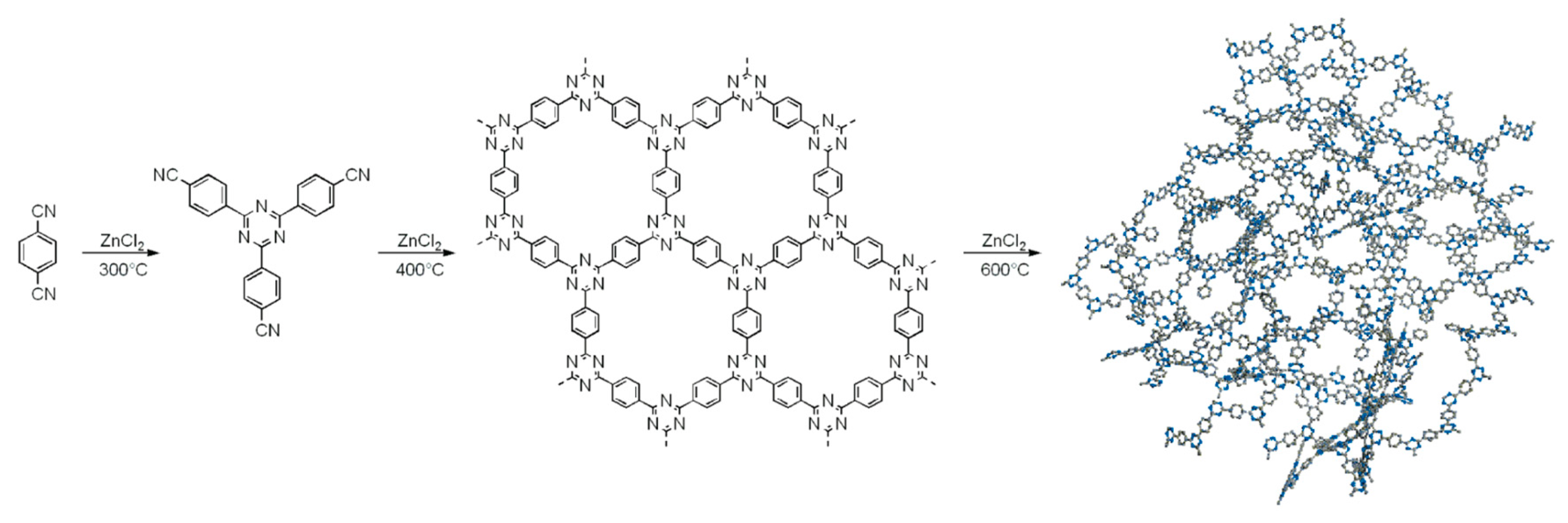
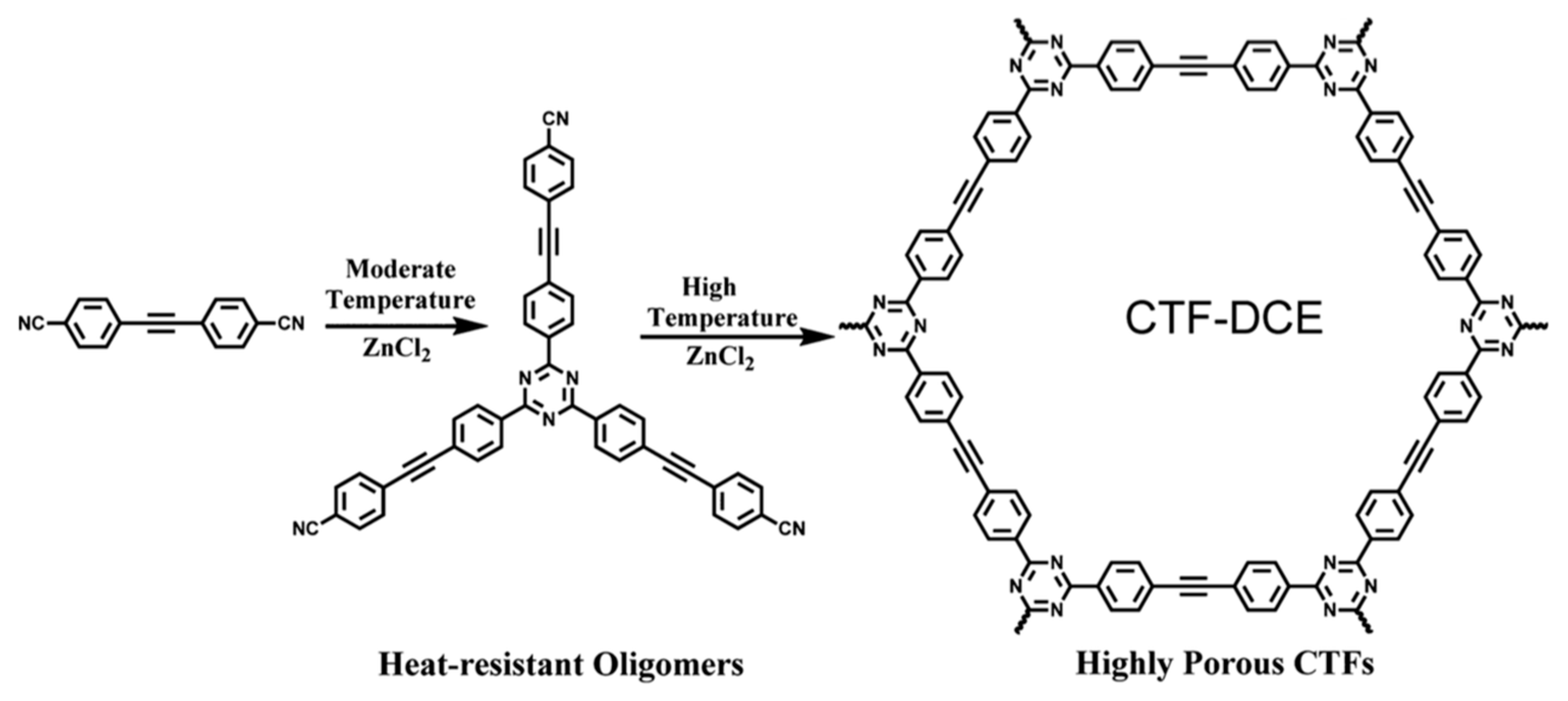
2.1. Trimerization of Aromatic Nitriles
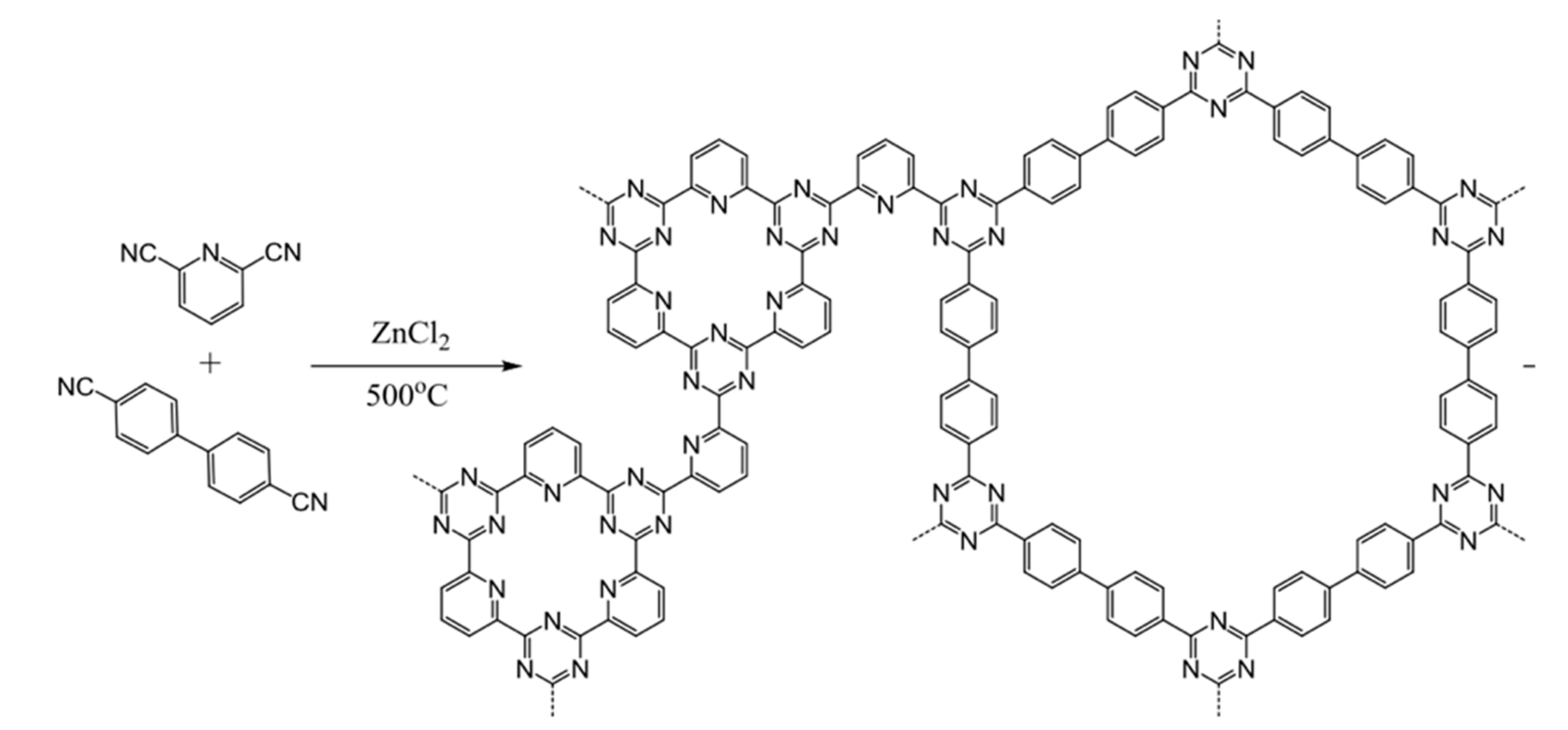
2.2. Polycondensation Synthesis Route
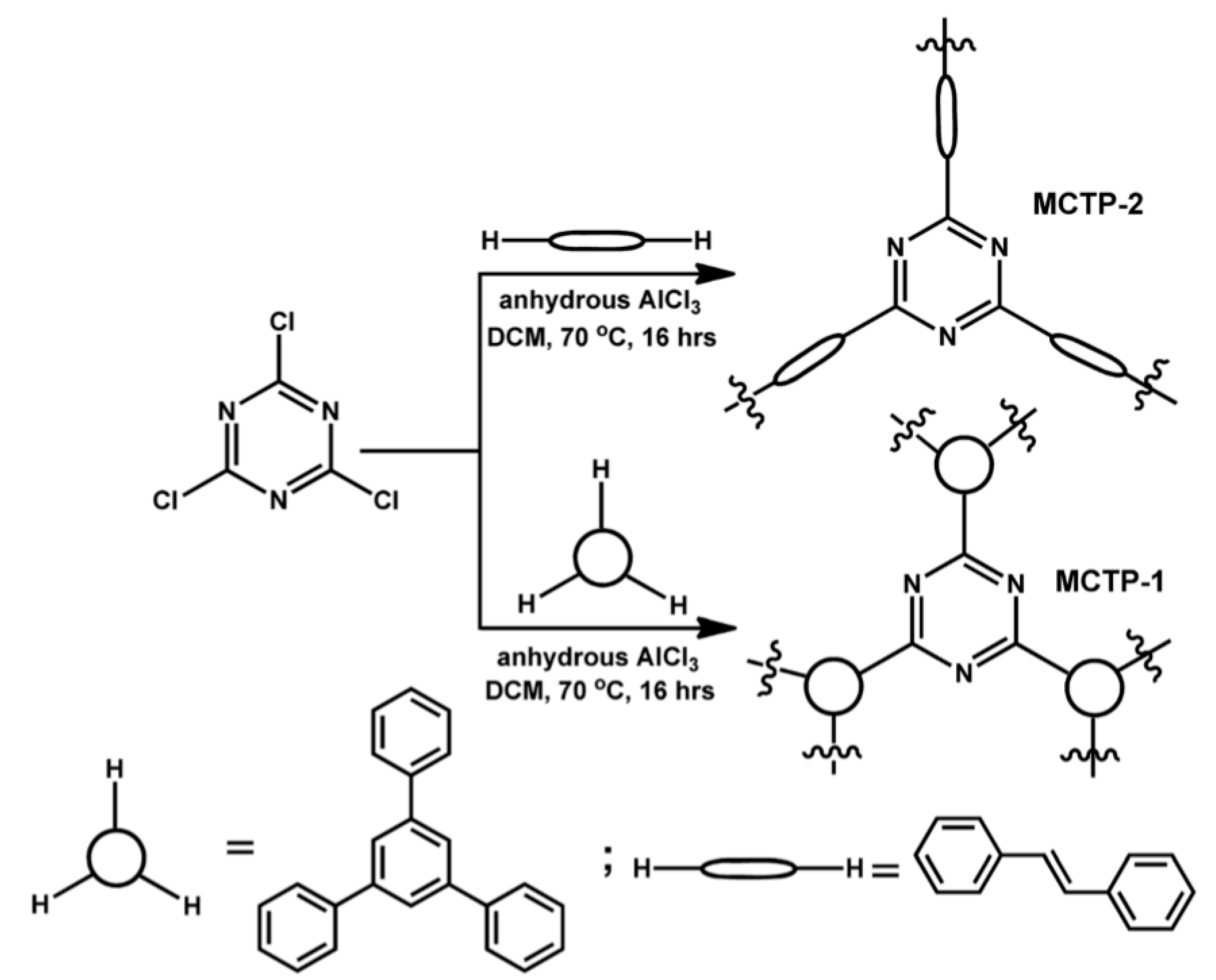
2.3. Cross-Coupling of Triazine-based Building Blocks
2.4. Characterization of CTFs
3. CTFs as Support for Heterogeneous Catalysis
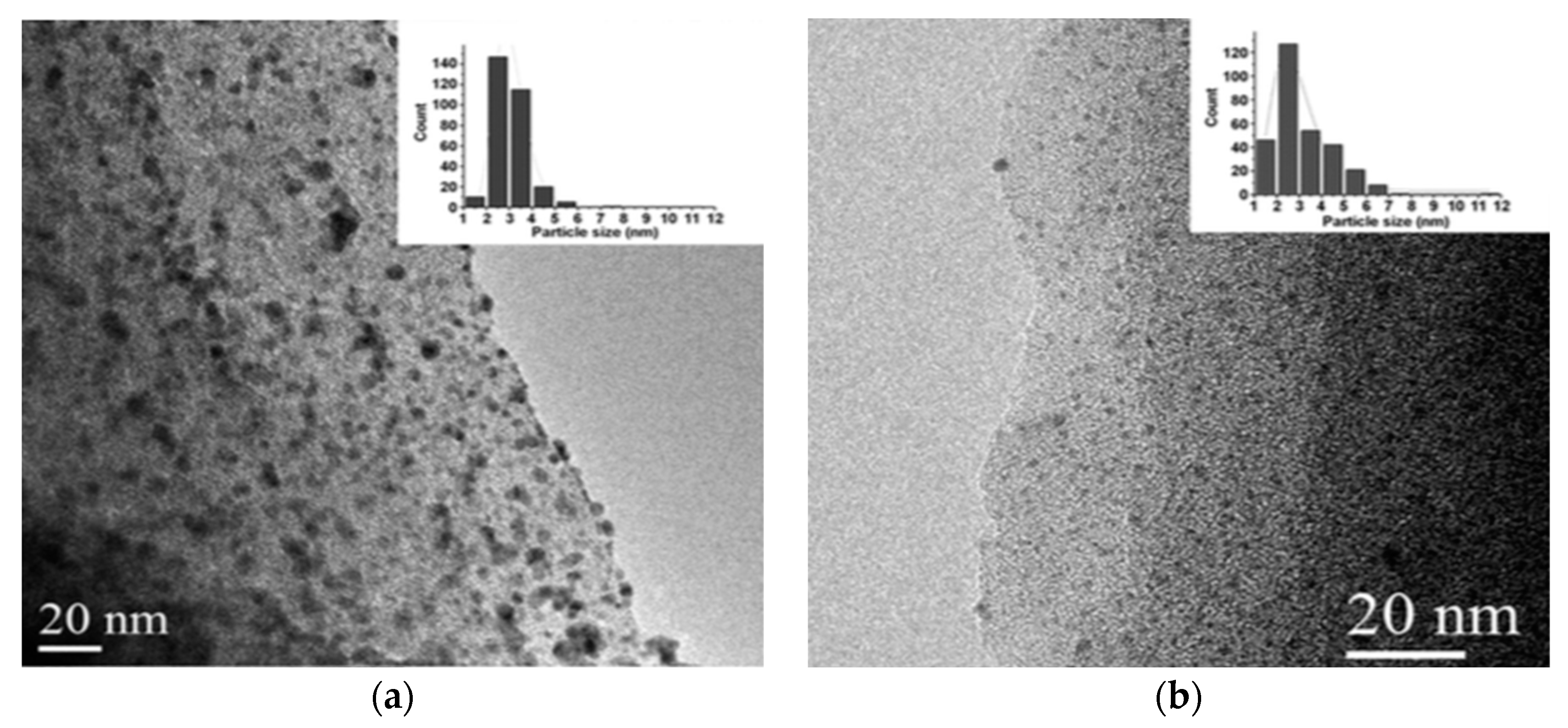
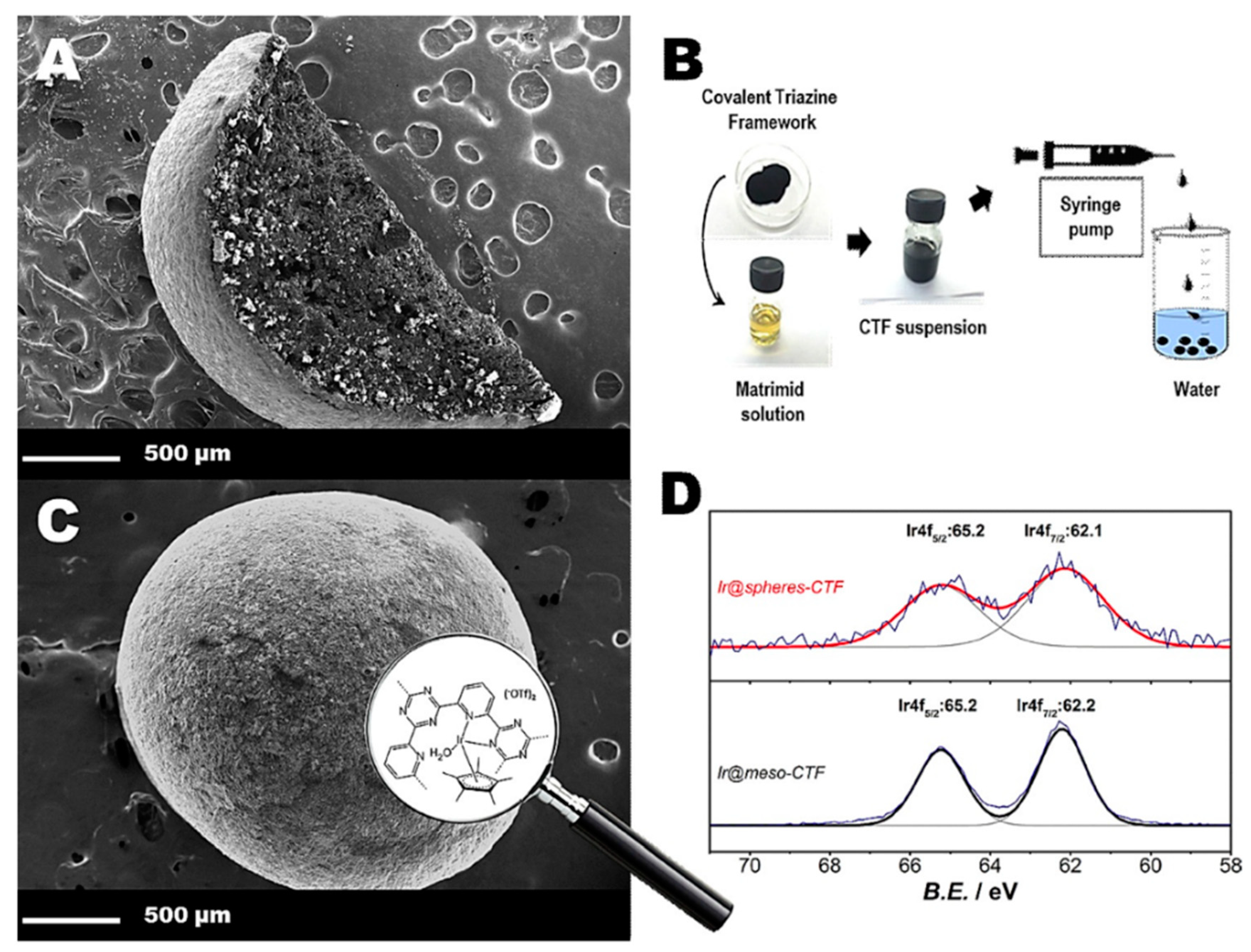
3.1. Support for Metal Nanoparticles
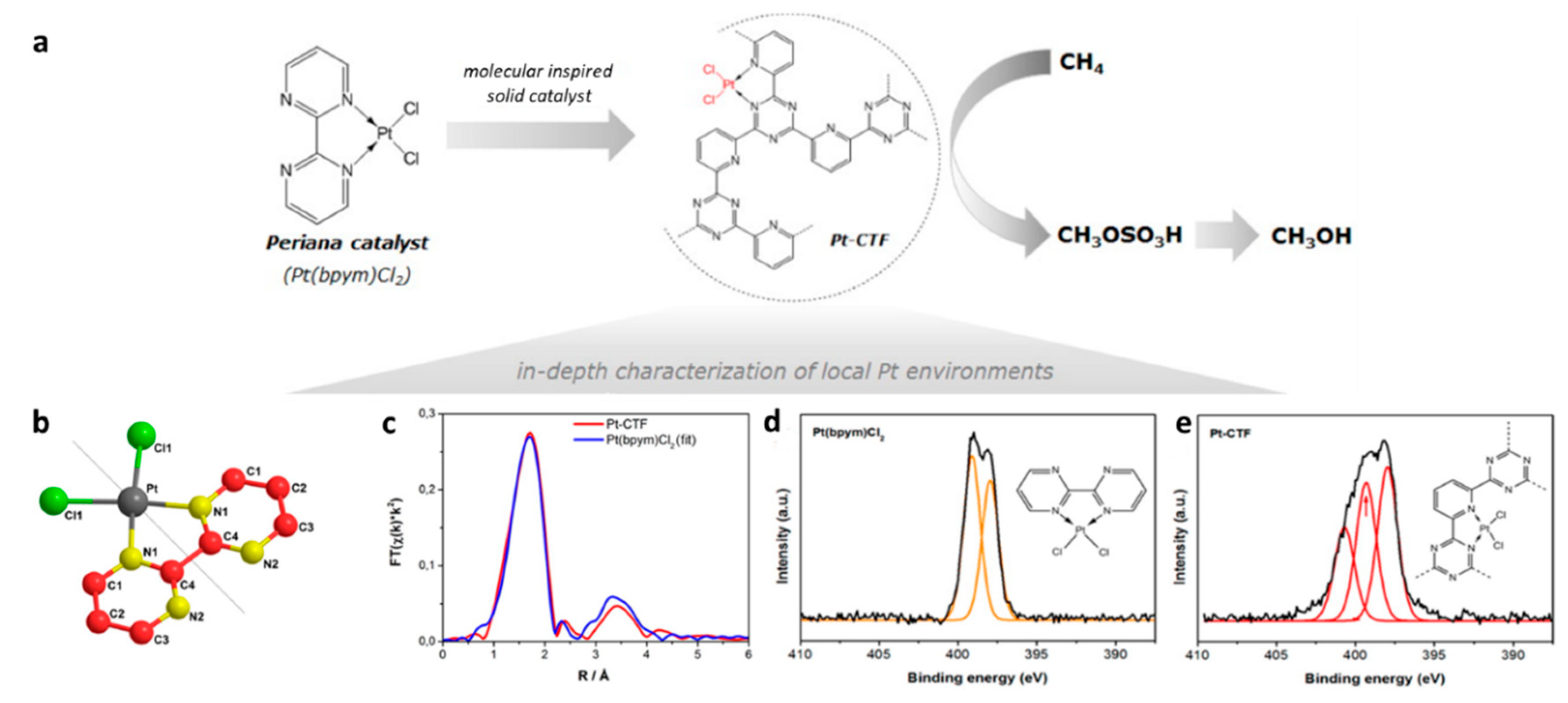
3.2. Immobilization of Molecular Metal Catalysts
4. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Toland, W.G. Polymerization of aromatic nitriles. U.S. Patent No.US3060179, 1962. [Google Scholar]
- Miller, G.H. Polymerization of aromatic nitriles. U.S. Patent No.3775380, 1973. [Google Scholar]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg. Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. Engl. 2008, 47, 3450–3453. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Forget, A.; Su, D.; Thomas, A.; Antonietti, M. From microporous regular frameworks to mesoporous materials with ultrahigh surface area: Dynamic reorganization of porous polymer networks. J. Am. Chem. Soc. 2008, 130, 13333–13337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, S.B. Recent advancements in the synthesis of covalent triazine frameworks for energy and environmental applications. Polymers 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Zhao, S.; Van Der Voort, P. Newly designed covalent triazine framework based on novel n-heteroaromatic building blocks for efficient co2 and h2 capture and storage. ACS Appl. Mater. Interfaces 2018, 10, 1244–1249. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Jena, H.S.; Krishnaraj, C.; Zhao, S.; Depauw, H.; Tahir, N.; Liu, Y.-Y.; Van Der Voort, P. A fluorine-containing hydrophobic covalent triazine framework with excellent selective co2 capture performance. J. Mater. Chem. A 2018, 6, 6370–6375. [Google Scholar] [CrossRef]
- Leus, K.; Folens, K.; Nicomel, N.R.; Perez, J.P.H.; Filippousi, M.; Meledina, M.; Dîrtu, M.M.; Turner, S.; Van Tendeloo, G.; Garcia, Y.; et al. Removal of arsenic and mercury species from water by covalent triazine framework encapsulated γ-fe2o3 nanoparticles. J. Hazard. Mater. 2018, 353, 312–319. [Google Scholar] [CrossRef]
- Osadchii, D.Y.; Olivos-Suarez, A.I.; Bavykina, A.V.; Gascon, J. Revisiting nitrogen species in covalent triazine frameworks. Langmuir 2017, 33, 14278–14285. [Google Scholar] [CrossRef]
- Puthiaraj, P.; Lee, Y.-R.; Zhang, S.; Ahn, W.-S. Triazine-based covalent organic polymers: Design, synthesis and applications in heterogeneous catalysis. J. Mater. Chem. A 2016, 4, 16288–16311. [Google Scholar] [CrossRef]
- Artz, J. Covalent triazine-based frameworks—Tailor-made catalysts and catalyst supports for molecular and nanoparticulate species. ChemCatChem 2018, 10, 1753–1771. [Google Scholar] [CrossRef]
- Ren, S.; Bojdys, M.J.; Dawson, R.; Laybourn, A.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 2012, 24, 2357–2361. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, L.-M.; Wang, X.; Guo, L.; Cheng, G.; Zhang, C.; Jin, S.; Tan, B.; Cooper, A. Covalent triazine frameworks via a low-temperature polycondensation approach. Angew. Chem. Int. Ed. 2017, 56, 14149–14153. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Mahmood, J.; Noh, H.J.; Seo, J.M.; Jung, S.M.; Shin, S.H.; Im, Y.K.; Jeon, I.Y.; Baek, J.B. Direct synthesis of a covalent triazine-based framework from aromatic amides. Angew. Chem. Int. Ed. 2018, 57, 8438–8442. [Google Scholar] [CrossRef] [PubMed]
- Puthiaraj, P.; Cho, S.-M.; Lee, Y.-R.; Ahn, W.-S. Microporous covalent triazine polymers: Efficient friedel–crafts synthesis and adsorption/storage of co2 and ch4. J. Mater. Chem. A 2015, 3, 6792–6797. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D. Synthesis of luminescent covalent–organic polymers for detecting nitroaromatic explosives and small organic molecules. Macromol. Rapid Commun. 2012, 33, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Thomas, A.; Antonietti, M. Toward tailorable porous organic polymer networks: A high-temperature dynamic polymerization scheme based on aromatic nitriles. Macromolecules 2009, 42, 319–326. [Google Scholar] [CrossRef]
- Katekomol, P.; Roeser, J.; Bojdys, M.; Weber, J.; Thomas, A. Covalent triazine frameworks prepared from 1,3,5-tricyanobenzene. Chem. Mater. 2013, 25, 1542–1548. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Jeromenok, J.; Thomas, A.; Antonietti, M. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity. Adv. Mater. 2010, 22, 2202–2205. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Hug, S.; Mesch, M.B.; Senker, J.; Lotsch, B.V. Phenyl-triazine oligomers for light-driven hydrogen evolution. Energy Environ. Sci. 2015, 8, 3345–3353. [Google Scholar] [CrossRef] [Green Version]
- Troschke, E.; Grätz, S.; Borchardt, L.; Haubold, D.; Senkovska, I.; Eychmueller, A.; Kaskel, S. Salt templated synthesis of hierarchical covalent triazine frameworks. Microporous Mesoporous Mater. 2017, 239, 190–194. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Yuan, Y.P.; Qiu, L.G.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Highly energy- and time-efficient synthesis of porous triazine-based framework: Microwave-enhanced ionothermal polymerization and hydrogen uptake. J. Mater. Chem. 2010, 20, 6413–6415. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Q.; Wang, S.; Li, Z.; Li, B.; Jin, S.; Tan, B. Crystalline covalent triazine frameworks by in situ oxidation of alcohols to aldehyde monomers. Angew. Chem. Int. Ed. 2018, 57, 11968–11972. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Bhunia, A.; Esquivel, D.; Janiak, C. Covalent triazine-based frameworks (ctfs) from triptycene and fluorene motifs for co2 adsorption. J. Mater. Chem. A 2016, 4, 6259–6263. [Google Scholar] [CrossRef]
- Rengaraj, A.; Puthiaraj, P.; Haldorai, Y.; Heo, N.S.; Hwang, S.-K.; Han, Y.-K.; Kwon, S.; Ahn, W.-S.; Huh, Y.S. Porous covalent triazine polymer as a potential nanocargo for cancer therapy and imaging. ACS Appl. Mater. Interfaces 2016, 8, 8947–8955. [Google Scholar] [CrossRef] [PubMed]
- Chan-Thaw, C.E.; Villa, A.; Katekomol, P.; Su, D.; Thomas, A.; Prati, L. Covalent triazine framework as catalytic support for liquid phase reaction. Nano Lett. 2010, 10, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Chan-Thaw, C.E.; Villa, A.; Wang, D.; Santo, V.D.; Orbelli Biroli, A.; Veith, G.M.; Thomas, A.; Prati, L. Pdhx entrapped in a covalent triazine framework modulates selectivity in glycerol oxidation. ChemCatChem 2015, 7, 2149–2154. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Villa, A.; Prati, L.; Thomas, A. Triazine-based polymers as nanostructured supports for the liquid-phase oxidation of alcohols. Chem. Eur. J. 2011, 17, 1052–1057. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Villa, A.; Veith, G.M.; Kailasam, K.; Adamczyk, L.A.; Unocic, R.R.; Prati, L.; Thomas, A. Influence of periodic nitrogen functionality on the selective oxidation of alcohols. Chem. Asian J. 2012, 7, 387–393. [Google Scholar] [CrossRef]
- He, T.; Liu, L.; Wu, G.; Chen, P. Covalent triazine framework-supported palladium nanoparticles for catalytic hydrogenation of n-heterocycles. J. Mater. Chem. A 2015, 3, 16235–16241. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Huang, Y.; Hu, Y.; Zhang, B. Covalent triazine framework-supported palladium as a ligand-free catalyst for the selective double carbonylation of aryl iodides under ambient pressure of co. Chem. Commun. 2016, 52, 2960–2963. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Mallmann, S.; Palkovits, R. Selective aerobic oxidation of hmf to 2,5-diformylfuran on covalent triazine frameworks-supported ru catalysts. ChemSusChem 2015, 8, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Artz, J.; Palkovits, R. Base-free aqueous-phase oxidation of 5-hydroxymethylfurfural over ruthenium catalysts supported on covalent triazine frameworks. ChemSusChem 2015, 8, 3832–3838. [Google Scholar] [CrossRef] [PubMed]
- Beine, A.K.; Krüger, A.J.D.; Artz, J.; Weidenthaler, C.; Glotzbach, C.; Hausoul, P.J.C.; Palkovits, R. Selective production of glycols from xylitol over ru on covalent triazine frameworks—Suppressing decarbonylation reactions. Green Chem. 2018, 20, 1316–1322. [Google Scholar] [CrossRef]
- Dang, Q.Q.; Liu, C.Y.; Wang, X.M.; Zhang, X.M. Novel covalent triazine framework for high-performance co2 capture and alkyne carboxylation reaction. ACS Appl. Mater. Interfaces 2018, 10, 27972–27978. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, R.; Antonietti, M.; Kuhn, P.; Thomas, A.; Schüth, F. Solid catalysts for the selective low-temperature oxidation of methane to methanol. Angew. Chem. Int. Ed. 2009, 48, 6909–6912. [Google Scholar] [CrossRef]
- Soorholtz, M.; Jones, L.C.; Samuelis, D.; Weidenthaler, C.; White, R.J.; Titirici, M.-M.; Cullen, D.A.; Zimmermann, T.; Antonietti, M.; Maier, J.; et al. Local platinum environments in a solid analogue of the molecular periana catalyst. ACS Catal. 2016, 6, 2332–2340. [Google Scholar] [CrossRef]
- Bavykina, A.V.; Mautscke, H.H.; Makkee, M.; Kapteijn, F.; Gascon, J.; Llabres i Xamena, F.X. Base free transfer hydrogenation using a covalent triazine framework based catalyst. CrystEngComm 2017, 19, 4166–4170. [Google Scholar] [CrossRef] [Green Version]
- Rozhko, E.; Bavykina, A.; Osadchii, D.; Makkee, M.; Gascon, J. Covalent organic frameworks as supports for a molecular ni based ethylene oligomerization catalyst for the synthesis of long chain olefins. J. Catal. 2017, 345, 270–280. [Google Scholar] [CrossRef]
- Pilaski, M.; Artz, J.; Islam, H.-U.; Beale, A.M.; Palkovits, R. N-containing covalent organic frameworks as supports for rhodium as transition-metal catalysts in hydroformylation reactions. Microporous Mesoporous Mater. 2016, 227, 219–227. [Google Scholar] [CrossRef]
- Bavykina, A.V.; Goesten, M.G.; Kapteijn, F.; Makkee, M.; Gascon, J. Efficient production of hydrogen from formic acid using a covalent triazine framework supported molecular catalyst. ChemSusChem 2015, 8, 809–812. [Google Scholar] [CrossRef]
- Bavykina, A.V.; Rozhko, E.; Goesten, M.G.; Wezendonk, T.; Seoane, B.; Kapteijn, F.; Makkee, M.; Gascon, J. Shaping covalent triazine frameworks for the hydrogenation of carbon dioxide to formic acid. ChemCatChem 2016, 8, 2217–2221. [Google Scholar] [CrossRef]
- Bavykina, A.V.; Olivos-Suarez, A.I.; Osadchii, D.; Valecha, R.; Franz, R.; Makkee, M.; Kapteijn, F.; Gascon, J. Facile method for the preparation of covalent triazine framework coated monoliths as catalyst support: Applications in c1 catalysis. ACS Appl. Mater. Interfaces 2017, 9, 26060–26065. [Google Scholar] [CrossRef]
- Hug, S.; Tauchert, M.E.; Li, S.; Pachmayr, U.E.; Lotsch, B.V. A functional triazine framework based on n-heterocyclic building blocks. J. Mater. Chem. 2012, 22, 13956–13964. [Google Scholar] [CrossRef]
- Park, K.; Gunasekar, G.H.; Prakash, N.; Jung, K.-D.; Yoon, S. A highly efficient heterogenized iridium complex for the catalytic hydrogenation of carbon dioxide to formate. ChemSusChem 2015, 8, 3410–3413. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Shin, J.; Jung, K.-D.; Park, K.; Yoon, S. Design strategy toward recyclable and highly efficient heterogeneous catalysts for the hydrogenation of co2 to formate. ACS Catal. 2018, 8, 4346–4353. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Jung, K.-D.; Yoon, S. Hydrogenation of co2 to formate using a simple, recyclable, and efficient heterogeneous catalyst. Inorg. Chem. 2019, 58, 3717–3723. [Google Scholar] [CrossRef]
- Sudakar, P.; Gunasekar, G.H.; Baek, I.H.; Yoon, S. Recyclable and efficient heterogenized rh and ir catalysts for the transfer hydrogenation of carbonyl compounds in aqueous medium. Green Chem. 2016, 18, 6456–6461. [Google Scholar] [CrossRef]
- Rajendiran, S.; Natarajan, P.; Yoon, S. A covalent triazine framework-based heterogenized al-co bimetallic catalyst for the ring-expansion carbonylation of epoxide to [small beta]-lactone. RSC Adv. 2017, 7, 4635–4638. [Google Scholar] [CrossRef]
- Tahir, N.; Muniz-Miranda, F.; Everaert, J.; Tack, P.; Heugebaert, T.; Leus, K.; Vincze, L.; Stevens, C.; Van Speybroeck, V.; Van Der Voort, P. Immobilization of ir(i) complex on covalent triazine frameworks for cah borylation reactions: A combined experimental and computational study. J. Catal. 2019, 371, 135–143. [Google Scholar] [CrossRef]
- Gunasekar, G.H.; Park, K.; Ganesan, V.; Lee, K.; Kim, N.-K.; Jung, K.-D.; Yoon, S. A covalent triazine framework, functionalized with ir/n-heterocyclic carbene sites, for the efficient hydrogenation of co2 to formate. Chem. Mater. 2017, 29, 6740–6748. [Google Scholar] [CrossRef]
- Park, K.; Lim, S.; Baik, J.H.; Kim, H.; Jung, K.-D.; Yoon, S. Exceptionally stable rh-based molecular catalyst heterogenized on a cationically charged covalent triazine framework support for efficient methanol carbonylation. Catal. Sci. Technol. 2018, 8, 2894–2900. [Google Scholar] [CrossRef]
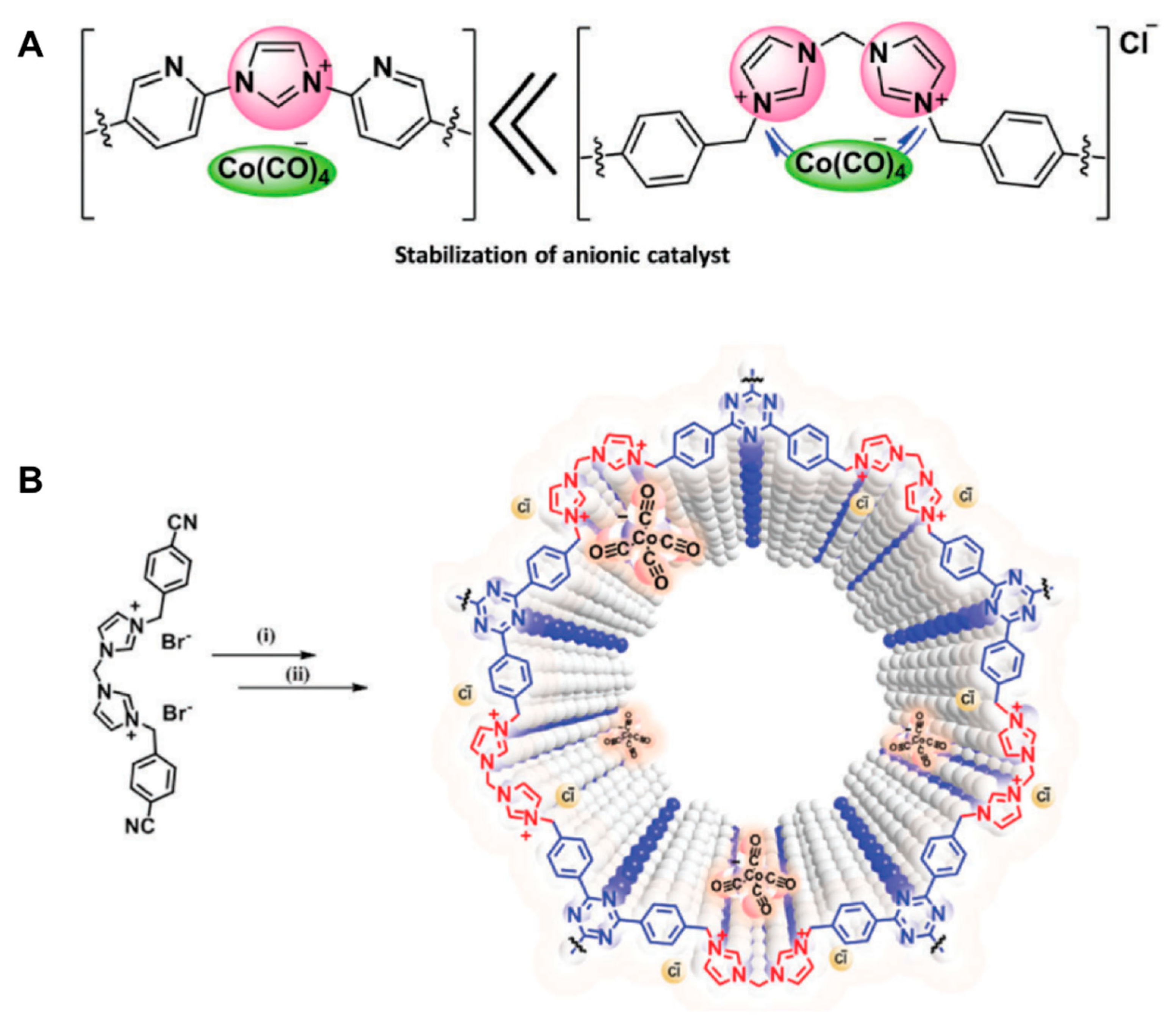
- Rajendiran, S.; Park, K.; Lee, K.; Yoon, S. Ionic-liquid-based heterogeneous covalent triazine framework cobalt catalyst for the direct synthesis of methyl 3-hydroxybutyrate from propylene oxide. Inorg. Chem. 2017, 56, 7270–7277. [Google Scholar] [CrossRef]
- Rajendiran, S.; Gunasekar, G.H.; Yoon, S. A heterogenized cobaltate catalyst on a bis-imidazolium-based covalent triazine framework for hydroesterification of epoxides. New J. Chem. 2018, 42, 12256–12262. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Wang, G.; Leus, K.; Schmidt, J.; Chaoui, N.; Van Der Voort, P. Acetylacetone covalent triazine framework: An efficient carbon capture and storage material and a highly stable heterogeneous catalyst. Chem. Mater. 2018, 30, 4102–4111. [Google Scholar] [CrossRef]





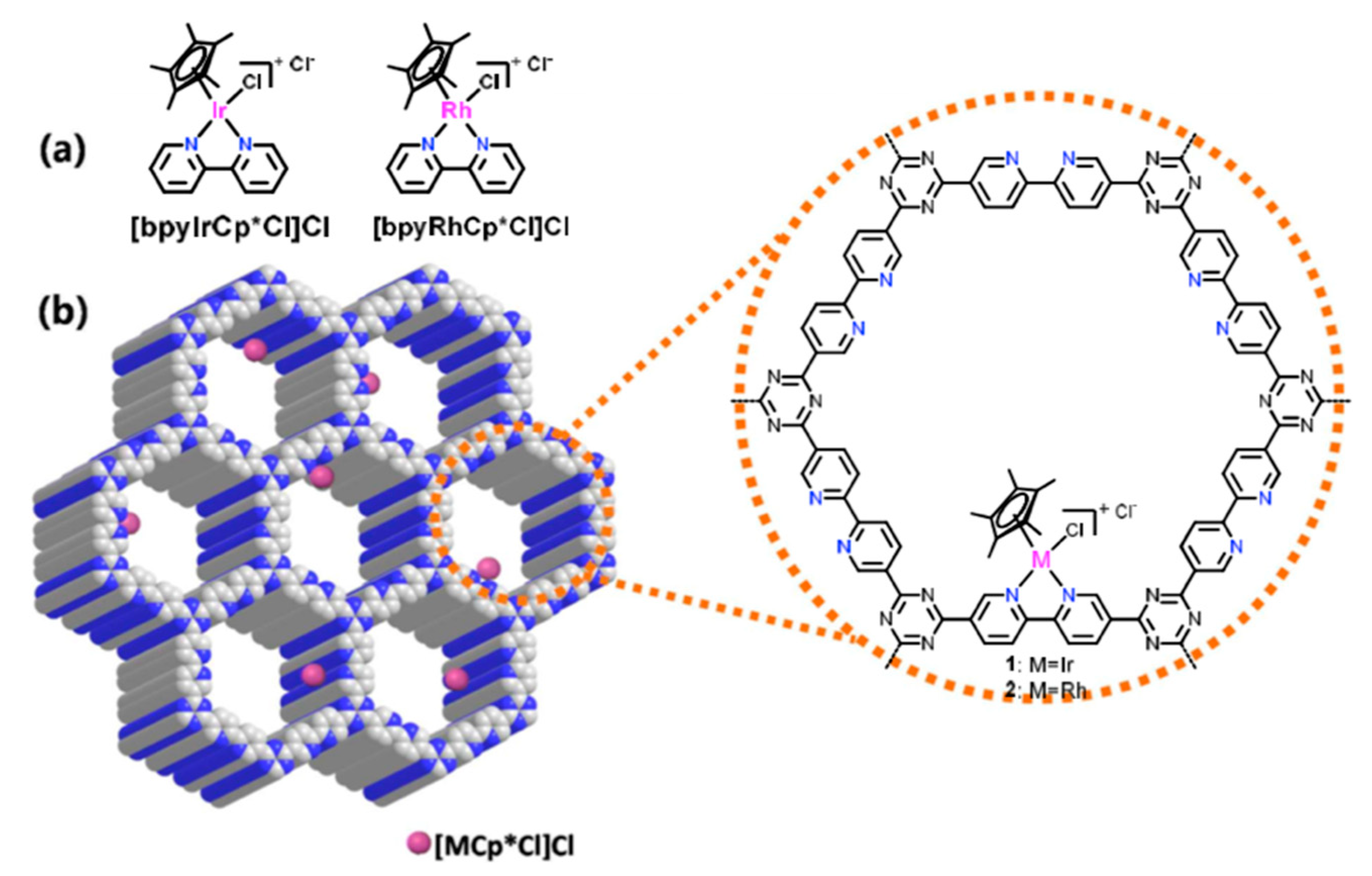
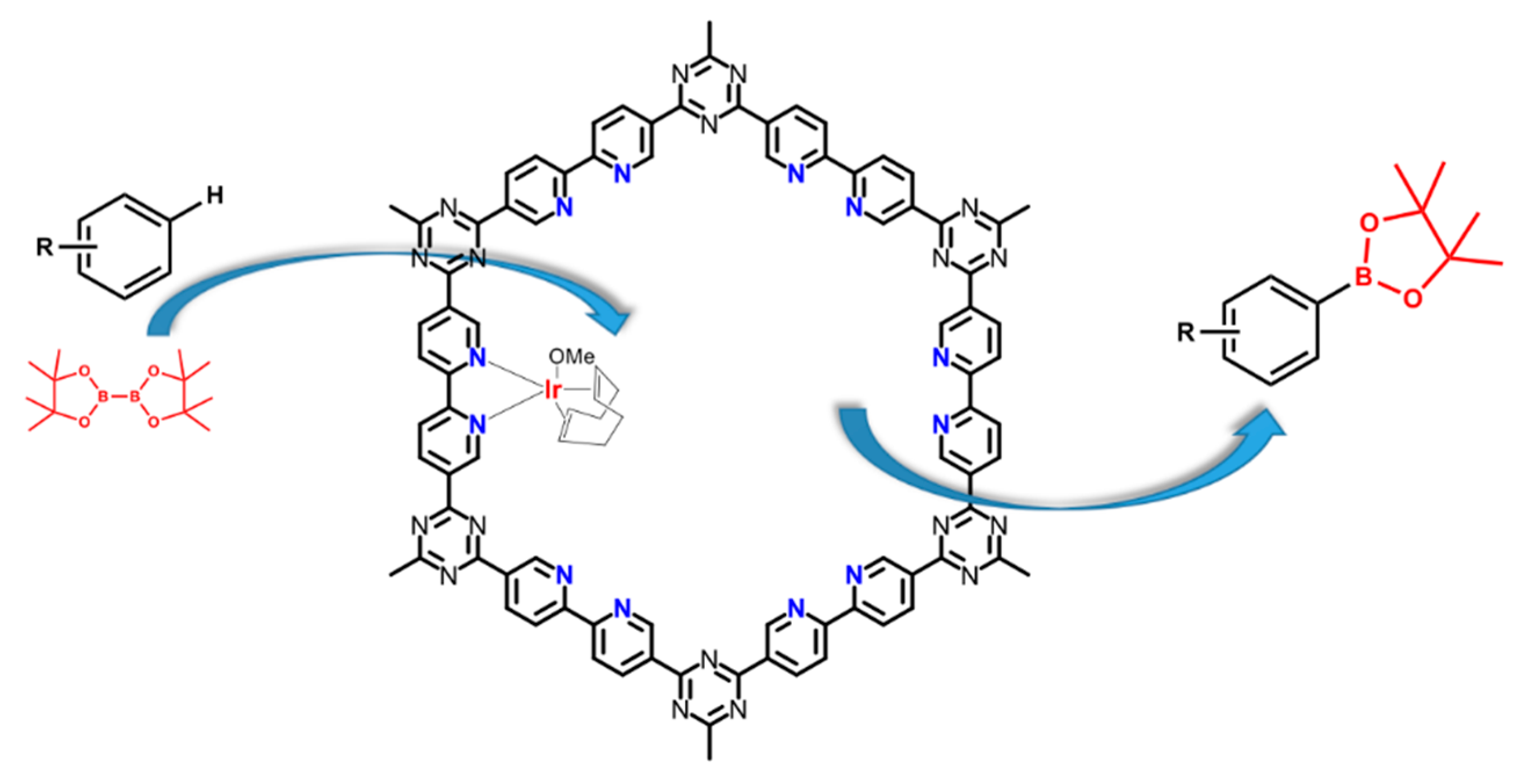
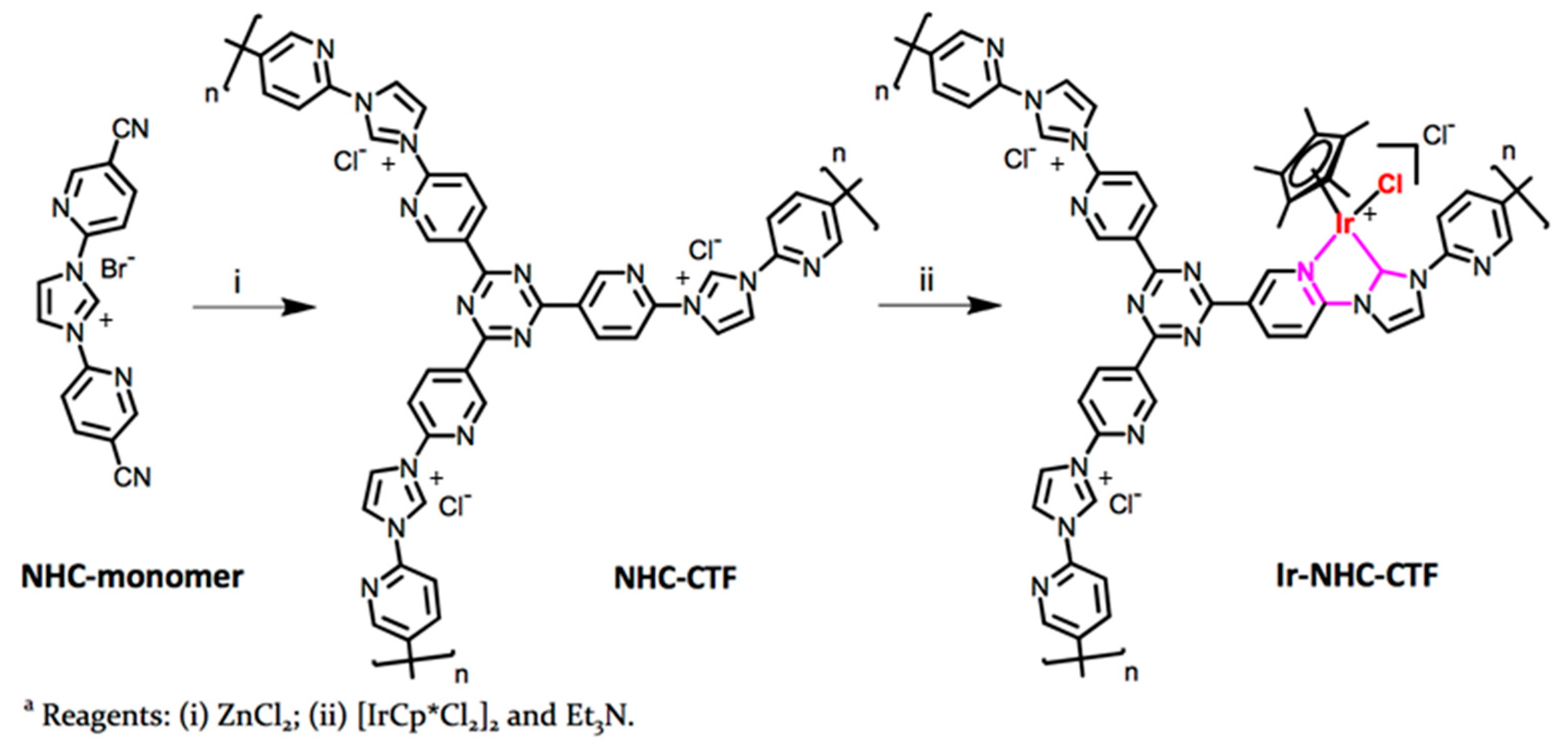
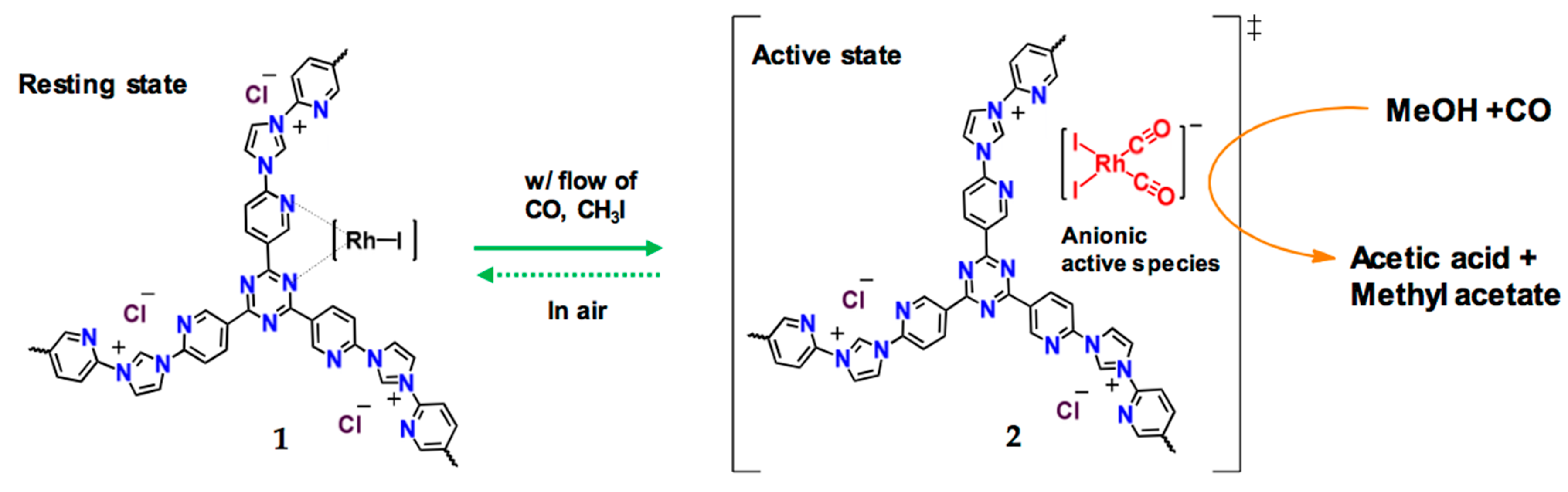

| Material | Monomer | Metal content | Type of Reaction | Activity | Ref |
|---|---|---|---|---|---|
| Pd/CTF | 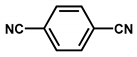 | 1 wt % Pd (nanoparticles) | Oxidation of glycerol into glyceric acid | Rate of glycerol mol·h−1·mol−1 consumption: 982 Selectivity = 81% | [28] |
| Pd/CTF |  | 1 wt % Pd (nanoparticles) | Oxidation of Benzyl alcohol | Turn-over frequency (TOF) = 1453 h−1 Selectivity = 98% | [30] |
| Pd/CTF | 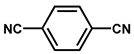 | 4 wt % Pd (nanoparticles) | Hydrogenation of N-heterocycles (1,10-phenanthroline) | Pressure/Selectivity = 30 bar/98.9%; 20 bar/97.9% for 8H-phen TOF = 47.6 h−1 (1st cycle) | [32] |
| Pd/CTF |  | 2.05 wt % Pd (nanoparticles) | Selective double carbonylation of aryl iodides | Several substrates tested Yield up to 90% with selectivity = 95% | [33] |
| Ru/CTF-c |  | 3.91 wt % Ru (nanoparticles) | Oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid | Conversion > 99% Yield = 41.4% | [35] |
| Ru/CTF-c | 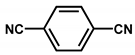 | 5 wt % Ru coordination using (RuCl2(p-cymene))2 | Hydrogenolysis of xylitol | Full conversion with 15% selectivity to propylene glycol | [36] |
| CTF-DCE-Ag |  | 4.3 wt % Ag (nanoparticles) | Carboxylation of terminal alkynes | TON: 247 Yield > 98.9 | [37] |
| Pt-CTF | 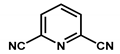 | - | Low temperature oxidation of methane to methanol | Selectivity > 75% TON = 246 | [38] |
| Ir@CTF | 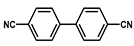 | 2.4 wt % Ir | Isomerization of 1-octen-3-ol to 3-octanone | TOF = 24 min−1 | [40] |
| Rh@CTF-c | 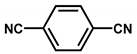 | 3.5 wt % Rh | Hydroformylation of crude 1-octene | 62% conversion TOF = 600h−1 TON = 12,000 | [42] |
| CTF-Ir |  | 16 wt % Ir | Dehydrogenation of formic acid | TOF = 27,000 h−1 TON = 1,060,000 | [43] |
| Ir@meso-CTF | 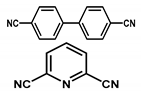 | 2 wt % Ir | Hydrogenation of CO2 | TON = 358 | [44] |
| Ir@meso-CTF@monolith | 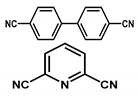 | 0.23 μmol/0.045 mg Ir | Dehydrogenation of formic acid | TOF = 207,200 h−1/TON = 2230 | [45] |
| Bpy-CTF-(IrCp*Cl)Cl | 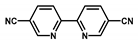 | 4.7 wt % Ir | Hydrogenation of CO2 to formate | TOF = 5300 h−1 TON = 5000 | [47] |
| (bpy-CTF-Ru(acac)2)Cl |  | 1.68 wt % Ru | Hydrogenation of CO2 to formate | TOF = 22,700 h−1 TON = 21,200 | [48] |
| (bpy-CTF-RuCl3) |  | 2.1 wt % Ru | Hydrogenation of CO2 to formate | TOF = 38,800 h−1 TON = 20,000 | [49] |
| (bpy-CTF(RhCp*Cl)Cl) &(bpy-CTF(IrCp*Cl)Cl) |  | 1.78 wt % Rh & 4.7 wt % Ir | Transfer hydrogenation of carbonyl compounds | Conversion = 99% Rh catalyst is more active than Ir catalyst. But, only Ir catalyst maintained activity during recycling. | [50] |
| (bpy-CTF-Al(OTf)2) (Co(CO)4) |  | 3.76 wt % Al & 2.67 wt % Co | Carbonylation of epoxides into β-lactones | Conversion > 99% Selectivity = 90% | [51] |
| Ir(I)@bipyCTF |  | 8.26 wt % Ir | C-H borylation of 1,2-dichlorobenzene | TON = 64 Yield = 95% | [52] |
| Ir0.68-NHC-CTF | 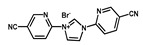 | 0.68 wt % Ir | Hydrogenation of CO2 to formate | TOF = 1600 h−1 TON = 24,300 | [53] |
| Rh-bpim-CTF | 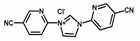 | 0.61 wt % Rh | Carbonylation of methanol | Conversion = 93% TOF = 2100 h−1 | [54] |
| (imidazolium-CTF)(Co(CO)4) |  | 3.62 wt % Co | Direct Synthesis of Methyl 3-Hydroxybutyrate from Propylene Oxide | Conversion > 99% Selectivity = 86% | [55] |
| V@acacCTF | 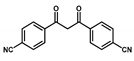 | 1.6 wt % V | Mannich reaction between 2-naphthol and N-methylmorpholine N-oxide | TON = 213 Conversion = 100% Yield = 95% | [57] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahir, N.; Krishnaraj, C.; Leus, K.; Van Der Voort, P. Development of Covalent Triazine Frameworks as Heterogeneous Catalytic Supports. Polymers 2019, 11, 1326. https://doi.org/10.3390/polym11081326
Tahir N, Krishnaraj C, Leus K, Van Der Voort P. Development of Covalent Triazine Frameworks as Heterogeneous Catalytic Supports. Polymers. 2019; 11(8):1326. https://doi.org/10.3390/polym11081326
Chicago/Turabian StyleTahir, Norini, Chidharth Krishnaraj, Karen Leus, and Pascal Van Der Voort. 2019. "Development of Covalent Triazine Frameworks as Heterogeneous Catalytic Supports" Polymers 11, no. 8: 1326. https://doi.org/10.3390/polym11081326
APA StyleTahir, N., Krishnaraj, C., Leus, K., & Van Der Voort, P. (2019). Development of Covalent Triazine Frameworks as Heterogeneous Catalytic Supports. Polymers, 11(8), 1326. https://doi.org/10.3390/polym11081326







