Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Size Exclusion Chromatography (SEC)
2.3. Potentiometric Titration
2.4. Dynamic Light Scattering (DLS) of the Polycarboxylate/BMP-2 Complexes
2.5. Cell Culture
2.6. Cytotoxicity of Polycarboxylates
2.7. Anticoagulant Activity of Polycarboxylates
2.8. Osteogenic Differentiation and Alkaline Phosphatase (ALP) Activity in MC3T3-E1 Cells
2.9. Mineralization of MC3T3-E1 Cells
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterizations of Polycarboxylates
3.2. Biological Characterizations of Polycarboxylates
3.3. ALP Activity in MC3T3-E1 Cells Treated with the Polycarboxylate/BMP-2 Complexes
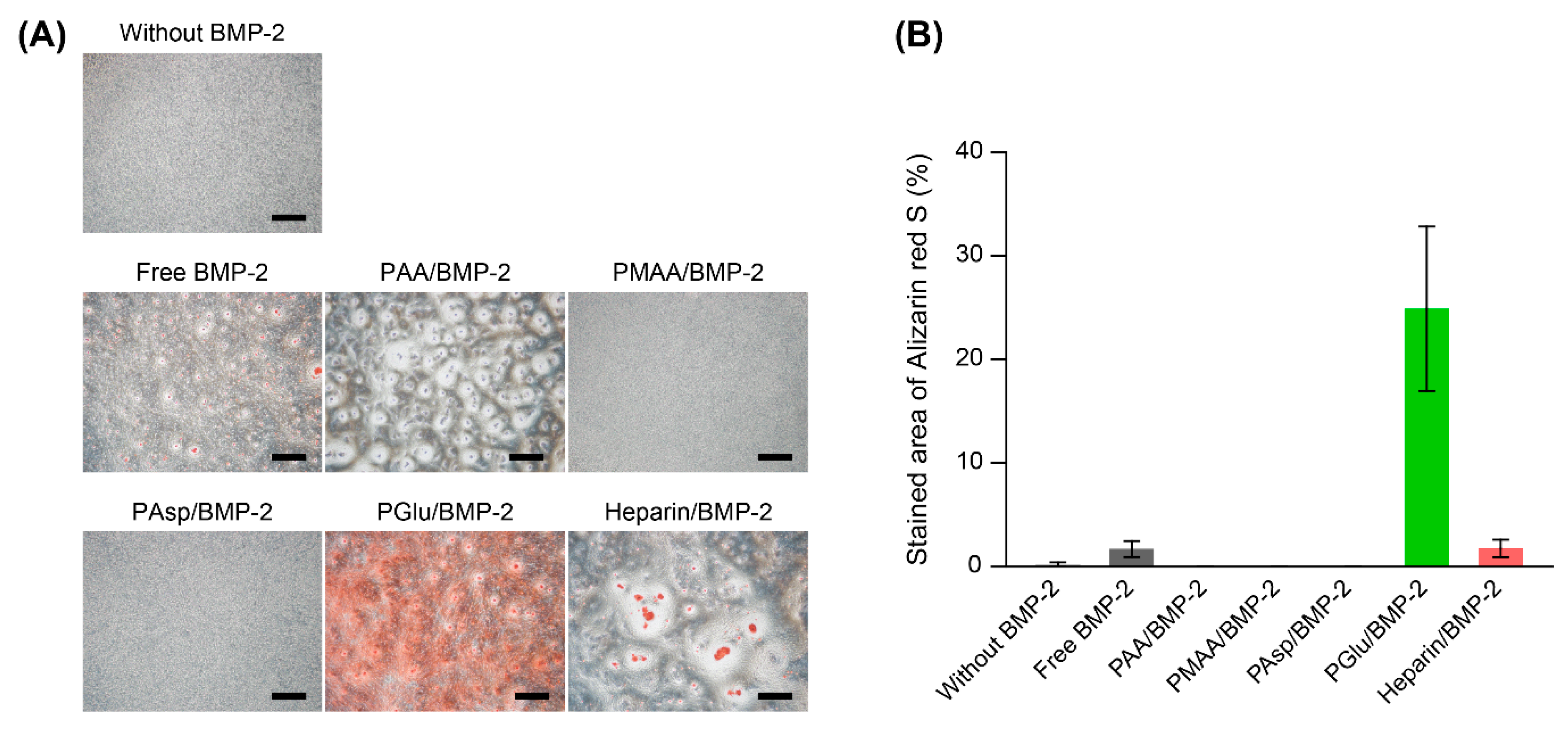
3.4. Mineralization of MC3T3-E1 Cells Treated with Polycarboxylate/BMP-2 Complexes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Block, M.S.; Kent, J.N. Sinus Augmentation for Dental Implants: The Use of Autogenous Bone. J. Oral Maxillofac Surg. 1997, 55, 1281–1286. [Google Scholar] [CrossRef]
- Ahlmann, E.; Patzakis, M.; Roidis, N.; Shepherd, L.; Holtom, P. Comparison of Anterior and Posterior Iliac Crest Bone Grafts in Terms of Harvest-Site Morbidity and Functional Outcomes. J. Bone Joint Surg. Am. 2002, 84, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J.; Kaban, L.B.; Murray, J.E.; Folkman, J.; Mulliken, J.B. Application of the Biological Principle of Induced Osteogenesis for Craniofacial Defects. Lancet 1981, 1, 959–962. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- LeGeros, R.Z. PROPERTIES of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Ikeda, G.; Nishida, K.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular Polyelectrolyte Complexes of Bone Morphogenetic Protein-2 with Sulfonated Polyrotaxanes to Induce Enhanced Osteogenic Differentiation. Macromol. Biosci. 2015, 15, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Inada, T.; Kanemaru, T.; Ikeda, G.; Tonegawa, A.; Nishida, K.; Arisaka, Y.; Tamura, A.; Yamaguchi, S.; Yui, N. Potentiating Bioactivity of BMP-2 by Polyelectrolyte Complexation with Sulfonated Polyrotaxanes to Induce Rapid Bone Regeneration in A Mouse Calvarial Defect. J. Biomed. Mater. Res. A 2017, 105, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Tamura, A.; Terauchi, M.; Yamaguchi, S.; Yui, N. A Silencing-Mediated Enhancement of Osteogenic Differentiation by Supramolecular Ternary siRNA Polyplexes Comprising Biocleavable Cationic Polyrotaxanes and Anionic Fusogenic Peptides. Biomater. Sci. 2018, 6, 440–450. [Google Scholar] [CrossRef]
- Terauchi, M.; Inada, T.; Tonegawa, A.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular Inclusion Complexation of Simvastatin with Methylated β-cyclodextrins for Promoting Osteogenic Differentiation. Int. J. Biol. Macromol. 2016, 93, 1492–1498. [Google Scholar] [CrossRef]
- Terauchi, M.; Tamura, A.; Yamaguchi, S.; Yui, N. Enhanced Cellular Uptake and Osteogenic Differentiation Efficiency of Melatonin by Inclusion Complexation with 2-Hydroxypropyl β-Cyclodextrin. Int. J. Pharm. 2018, 547, 53–60. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The Role of Growth Factors in the Repair of Bone. Biology and Clinical Applications. J. Bone Joint Surg. Am. 2002, 84, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the Distinct Orthotopic Bone-Forming Activity of 14 BMPs Using Recombinant Adenovirus-Mediated Gene Delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Boden, S.D.; Kang, J.; Sandhu, H.; Heller, J.G. Use of Recombinant Human Bone Morphogenetic Protein-2 to Achieve Posterolateral Lumbar Spine Fusion in Humans: A Prospective, Randomized Clinical Pilot Trial: 2002 Volvo Award in Clinical Studies. Spine 2002, 27, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G.; et al. Recombinant Human Bone Morphogenetic Protein-2 for Treatment of Open Tibial Fractures: A Prospective, Controlled, Randomized Study of Four Hundred and Fifty Patients. J. Bone Joint Surg. Am. 2002, 84, 2123–2134. [Google Scholar]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of Large Bone Defects with the Use of Autologous Bone Marrow Stromal Cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Kaji, H.; Kobayashi, T.; Nishiyama, K.; Fukase, M.; Kumegawa, M.; Chihara, K. Stimulatory Effect of Bone Morphogenetic Protein-2 on Osteoclast-Like Cell Formation and Bone-Resorbing Activity. J. Bone Miner. Res. 1995, 10, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Feldman, G.J.; Billings, P.C.; Patel, R.V.; Caron, R.J.; Guenther, C.; Kingsley, D.M.; Kaplan, F.S.; Shore, E.M. Over-expression of BMP4 and BMP5 in a Child with Axial Skeletal Malformations and Heterotopic Ossification: A New Syndrome. Am. J. Med. Genet. A 2007, 143A, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation in Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Takada, T.; Katagiri, T.; Ifuku, M.; Morimura, N.; Kobayashi, M.; Hasegawa, K.; Ogamo, A.; Kamijo, R. Sulfated Polysaccharides Enhance the Biological Activities of Bone Morphogenetic Proteins. J. Biol. Chem. 2003, 278, 43229–43235. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin Potentiates the in Vivo Ectopic Bone Formation Induced by Bone Morphogenetic protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef]
- Kanzaki, S.; Ariyoshi, W.; Takahashi, T.; Okinaga, T.; Kaneuji, T.; Mitsugi, S.; Nakashima, K.; Tsujisawa, T.; Nishihara, T. Dual Effects of Heparin on BMP-2-Induced Osteogenic Activity in MC3T3-E1 Cells. Pharmacol. Rep. 2011, 63, 1222–12230. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyauchi, S.; Tawada, A.; Anada, T.; Matsuzaka, S.; Suzuki, O. Oversulfated Chondroitin Sulfate-E Binds to BMP-4 and Enhances Osteoblast Differentiation. J. Cell Physiol. 2008, 217, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Bramono, D.S.; Murali, S.; Rai, B.; Ling, L.; Poh, W.T.; Lim, Z.X.; Stein, G.S.; Nurcombe, V.; van Wijnen, A.J.; Cool, S.M. Bone Marrow-Derived Heparan Sulfate Potentiates the Osteogenic Activity of Bone Morphogenetic Protein-2 (BMP-2). Bone 2012, 50, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue Integration of Growth Factor-eluting Layer-by-Layer Polyelectrolyte Multilayer Coated Implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Damus, P.S.; Hicks, M.; Rosenberg, R.D. Anticoagulant Action of Heparin. Nature 1973, 246, 355–357. [Google Scholar] [CrossRef]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The Anticoagulant Activation of Antithrombin by Heparin. Proc. Natl. Acad. Sci. USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef]
- Cao, L.; Werkmeister, J.A.; Wang, J.; Glattauer, V.; McLean, M.M.; Liu, C. Bone Regeneration Using Photocrosslinked Hydrogel Incorporating rhBMP-2 Loaded 2-N, 6-O-Sulfated Chitosan Nanoparticles. Biomaterials 2014, 35, 2730–2742. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, J.; Wang, J.; Yao, W.; Liu, C.; Chen, J.; Cao, X. Enhanced Bioactivity of Bone Morphogenetic Protein-2 with Low Dose of 2-N, 6-O-Sulfated Chitosan in Vitro and in Vivo. Biomaterials 2009, 30, 1715–1724. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Paluck, S.J.; McGahran, A.J.; Heather, D.; Maynard, H.D. Poly(vinyl sulfonate) Facilitates bFGF-Induced Cell Proliferation. Biomacromolecules 2015, 16, 2684–2692. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.K.; Moon, S.W.; Lee, D. Surface Modification of Titanium with BMP-2/GDF-5 by a Heparin Linker and Its Efficacy as A Dental Implant. Int. J. Mol. Sci. 2017, 18, 229. [Google Scholar] [CrossRef]
- Tan, H.; Wang, H.; Chai, Y.; Yu, Y.; Hong, H.; Yang, F.; Qu, X.; Liu, C. Engineering A Favourable Osteogenic Microenvironment by Heparin Mediated Hybrid Coating Assembly and rhBMP-2 Loading. RSC Adv. 2017, 7, 11439–11447. [Google Scholar] [CrossRef]
- Hettiaratchi, M.H.; Rouse, T.; Chou, C.; Krishnan, L.; Stevens, H.Y.; Li, M.A.; McDevitt, T.C.; Guldberg, R.E. Enhanced in Vivo Retention of Low Dose BMP-2 via Heparin Microparticle Delivery Does Not Accelerate Bone Healing in A Critically Sized Femoral Defect. Acta Biomater. 2017, 59, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.D.; Damus, P.S. The Purification and Mechanism of Action of Human Antithrombin-Heparin Cofactor. J. Biol. Chem. 1973, 248, 6490–6505. [Google Scholar] [PubMed]
- Hogg, P.J.; Jackson, C.M. Fibrin Monomer Protects Thrombin from Inactivation by Heparin-Antithrombin III: Implications for Heparin Efficacy. Proc. Natl. Acad. Sci. 1989, 86, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Raschke, R.; Warkentin, T.E.; Dalen, J.E.; Deykin, D.; Poller, L. Heparin: Mechanism of Action, Pharmacokinetics, Dosing Considerations, Monitoring, Efficacy, and Safety. Chest 1995, 108, 258S–275S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monien, B.H.; Cheang, K.I.; Desai, U.R. Mechanism of Poly(acrylic acid) Acceleration of Antithrombin Inhibition of Thrombin: Implications for the Design of Novel Heparin Mimics. J. Med. Chem. 2005, 48, 5360–5368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 Controls Alkaline Phosphatase Expression and Osteoblast Mineralization by A Wnt Autocrine Loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships During Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef] [PubMed]





| Sample | Mn,SEC1 | Mw/Mn1 | pKa 2 | α at pH 7.4 2 |
|---|---|---|---|---|
| PAA | 10,100 | 1.56 | 6.65 | 0.71 |
| PMAA | 5100 | 1.36 | 6.84 | 0.64 |
| PAsp | 13,500 | 1.37 | 5.74 | 0.94 |
| PGlu | 12,700 | 1.16 | 6.05 | 0.90 |
| Heparin | 25,600 | 1.23 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terauchi, M.; Tamura, A.; Tonegawa, A.; Yamaguchi, S.; Yoda, T.; Yui, N. Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers 2019, 11, 1327. https://doi.org/10.3390/polym11081327
Terauchi M, Tamura A, Tonegawa A, Yamaguchi S, Yoda T, Yui N. Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers. 2019; 11(8):1327. https://doi.org/10.3390/polym11081327
Chicago/Turabian StyleTerauchi, Masahiko, Atsushi Tamura, Asato Tonegawa, Satoshi Yamaguchi, Tetsuya Yoda, and Nobuhiko Yui. 2019. "Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates" Polymers 11, no. 8: 1327. https://doi.org/10.3390/polym11081327
APA StyleTerauchi, M., Tamura, A., Tonegawa, A., Yamaguchi, S., Yoda, T., & Yui, N. (2019). Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers, 11(8), 1327. https://doi.org/10.3390/polym11081327





