Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biochar from Waste Brewed Coffee
2.3. Composite Preparation
2.4. Characterization
- -
- First, heating up from 30 to 180 °C at 10 °C/min.
- -
- Cooling down from 180 to 30 °C at 10 °C/min.
- -
- Second heating up from 30 to 180 °C at 10 °C/min.
3. Results and Discussion
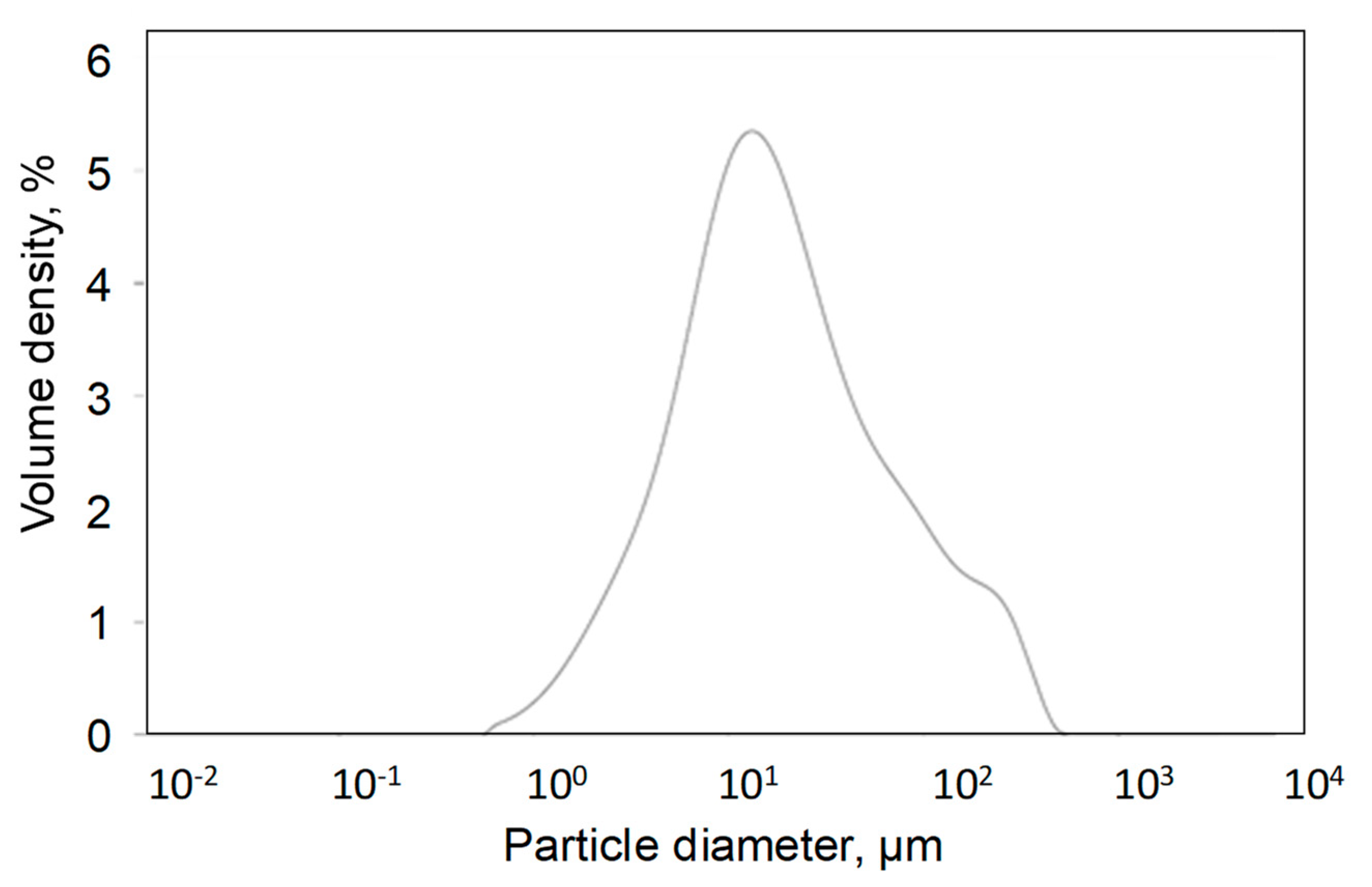
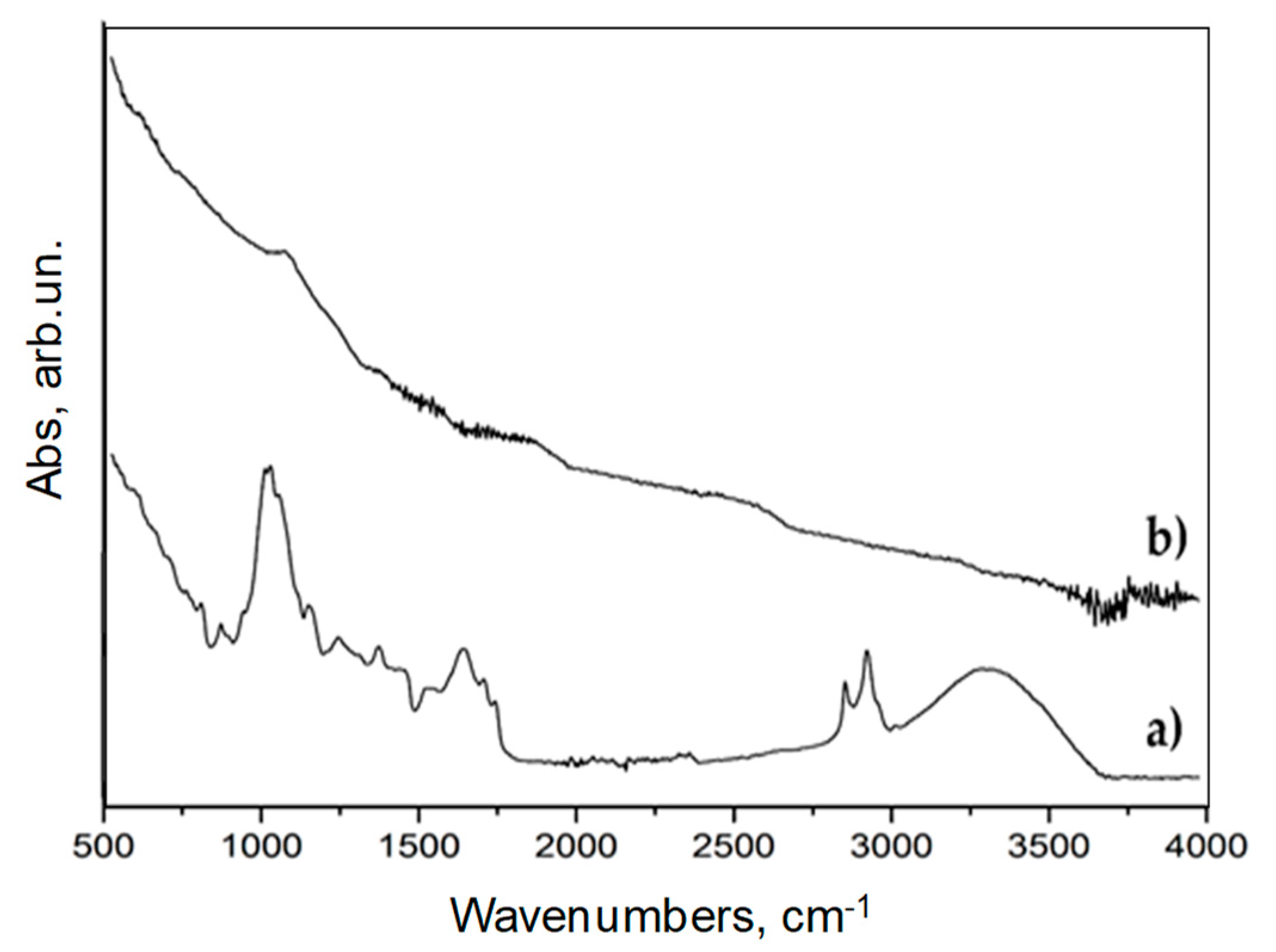
3.1. Characterization of Biochar
3.2. Characterization PE-Based Composites
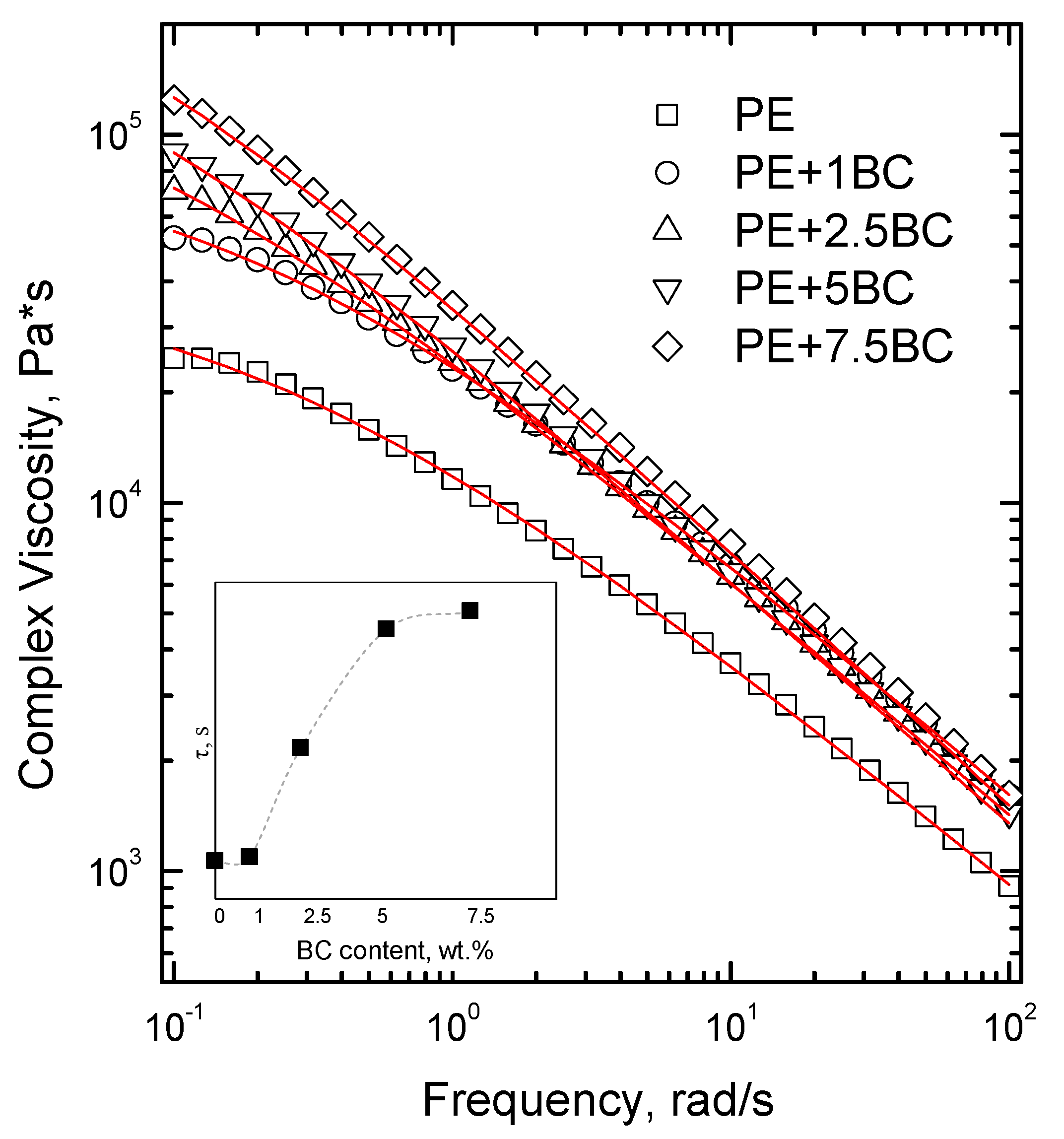
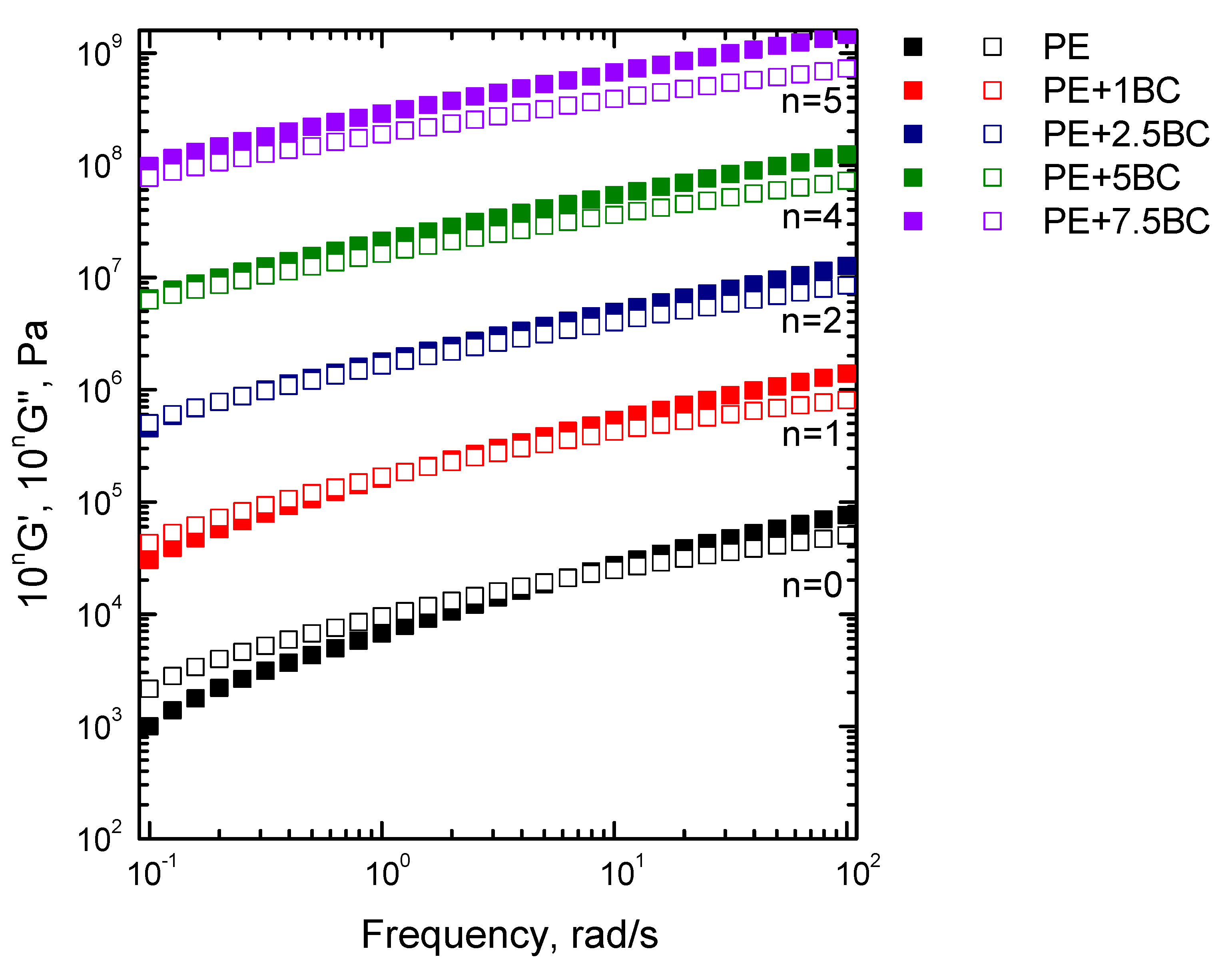
3.2.1. Linear Rheological Behavior
3.2.2. Non-Linear Rheological Behavior
3.2.3. Stress Relaxation Behavior
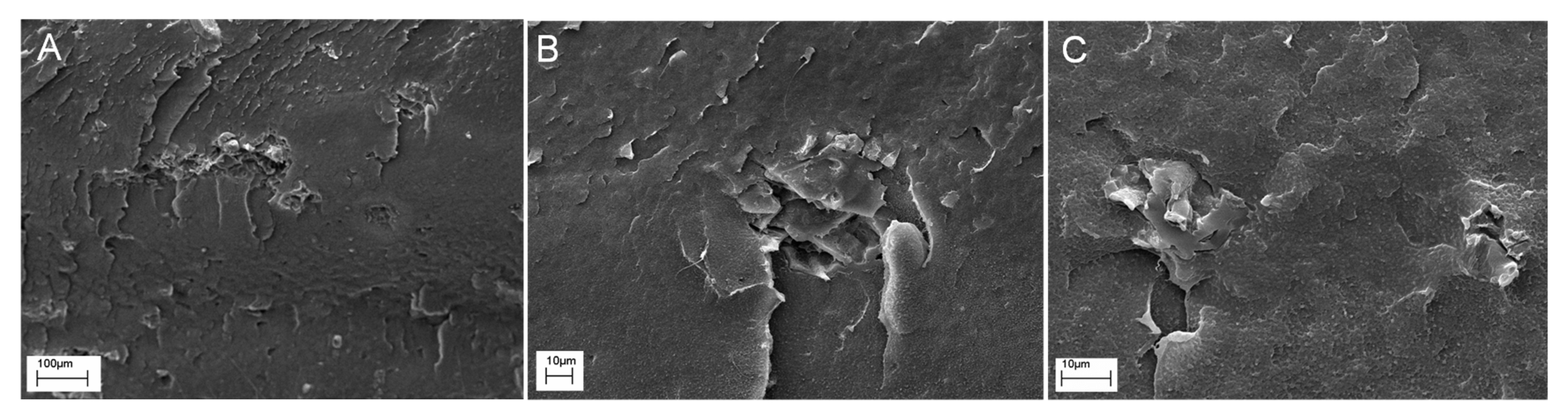
3.2.4. Morphology
3.2.5. Thermal Analyses
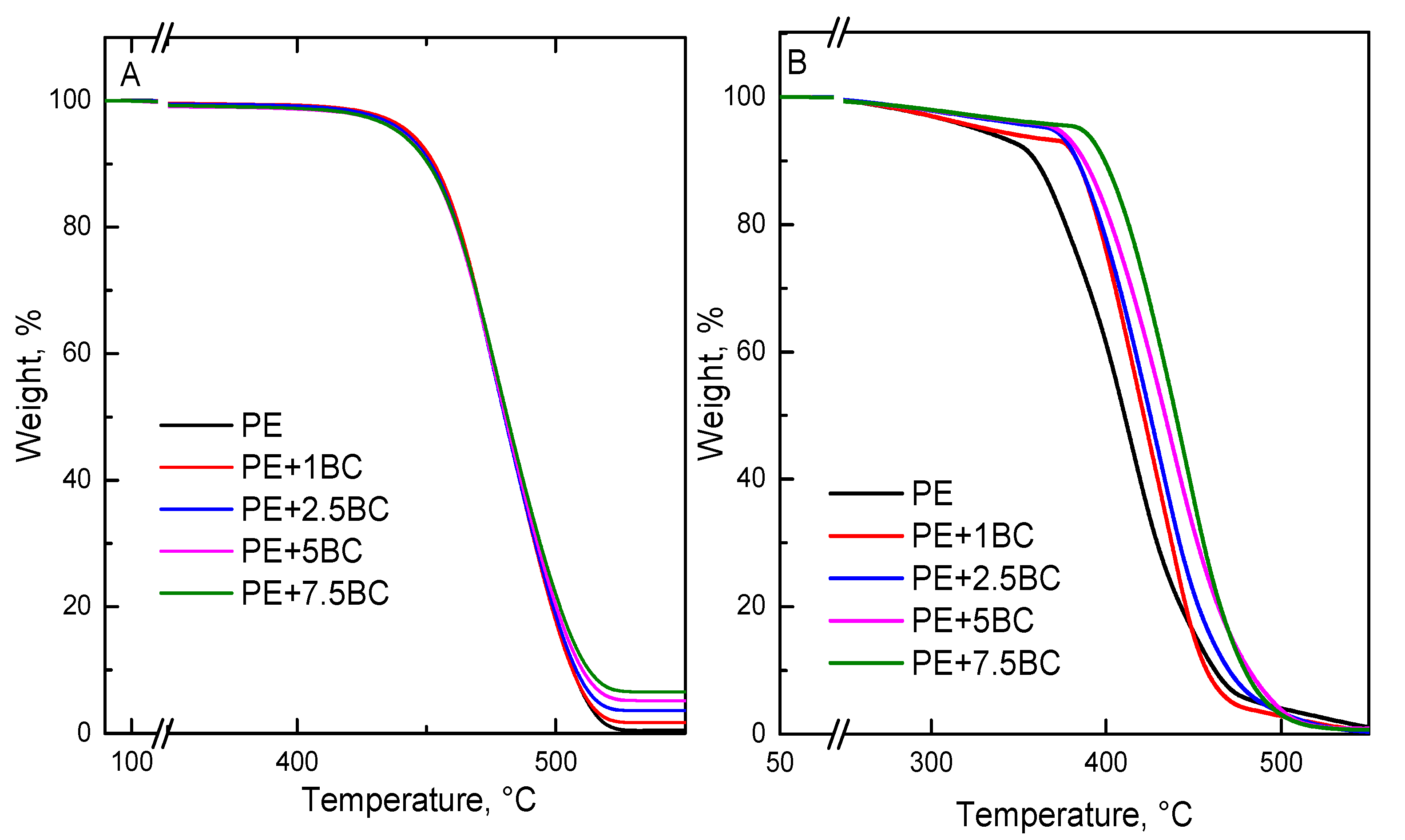
3.2.6. Thermal and Thermo-Oxidative Stability
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Yu, L.; Dean, K.; Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 2006, 31, 576–602. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct use of natural antioxidant-rich agro-wastes as thermal stabilizer for polymer: Processing and recycling. ACS Sustain. Chem. Eng. 2016, 4, 881–889. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; La Mantia, F.P.; Arrigo, R. Natural compounds as light stabilizer for a starch-based biodegradable polymer. J. Polym. Eng. 2014, 34, 441–449. [Google Scholar] [CrossRef]
- Ku, H.; Wang, H.; Pattarachaiyakoop, N.; Trada, M. A review on the tensile properties of natural fiber reinforced polymer composites. Compos. Part B Eng. 2011, 42, 856–873. [Google Scholar] [CrossRef] [Green Version]
- Wambua, P.; Ivens, J.; Verpoest, I. Natural fibres: Can they replace glass in fibre reinforced plastics. Compos. Sci. Technol. 2003, 63, 1259–1264. [Google Scholar] [CrossRef]
- Battegazzore, D.; Abt, T.; Maspoch, M.L.; Frache, A. Multilayer cotton fabric bio-composites based on PLA and PHB copolymer for industrial load carrying applications. Compos. Part B Eng. 2019, 163, 761–768. [Google Scholar] [CrossRef]
- Amenini, F.; Brocail, J.; Chauvin, M.; Thuillier, S. Dynamical properties of flax fibre reinforced PA11 over a large frequency range. Compos. Sci. Technol. 2019, 171, 234–243. [Google Scholar] [CrossRef]
- Wang, H.; Memon, H.; Hassan, E.A.M.; Miah, S.; Ali, M.A. Effect of Jute Fiber Modification on Mechanical Properties of Jute Fiber Composite. Materials 2019, 12, 1226. [Google Scholar] [CrossRef] [PubMed]
- Saheb, D.N.; Jog, J.P. Natural fiber polymer composites: A review. Adv. Polym. Technol. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Morreale, M.; Liga, A.; Mistretta, M.C.; Ascione, L.; La Mantia, F.P. Mechanical, thermomechanical and reprocessing behavior of green composites from biodegradable polymer and wood flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef]
- Kim, N.K.; Lin, R.J.T.; Bhattacharyya, D. Effects of wood fibres, ammonium polyphosphate and polymer viscosity on the flammability and mechanical performance of PP/wool composites. Polym. Degrad. Stab. 2015, 119, 167–177. [Google Scholar] [CrossRef]
- Kashani Rahimi, S.; Otaigbe, J.U. Natural cellulose fiber-reinforced polyamide 6 thermoplastic composites prepared via in situ anionic ring-opening polymerization. Polym. Compos. 2019, 40, 1104–1116. [Google Scholar] [CrossRef]
- Espinach, F.X.; Boufi, S.; Delgado-Aguilar, M.; Julián, F.; Mutjé, P.; Méndez, J.A. Composites from poly(lactic acid) and bleached chemical fibres: Thermal properties. Compos. Part B Eng. 2018, 134, 169–176. [Google Scholar] [CrossRef]
- Battegazzore, D.; Frache, A.; Abt, T.; Maspoch, M.L. Epoxy coupling agent for PLA and PHB copolymer-based cotton fabric bio-composites. Compos. Part B Eng. 2018, 148, 188–197. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M. Improving the properties of polypropylene-wood flour composites by utilization of maleated adhesion promoters. Compos. Interface 2007, 14, 685–698. [Google Scholar] [CrossRef]
- Wang, Q.; Xiao, S.; Shi, S.Q.; Cai, L. Mechanical property enhancement of self-bonded natural fiber material via controlling cell wall plasticity and structure. Mater. Des. 2019, 172, 107763. [Google Scholar] [CrossRef]
- Ok, Y.S.; Tsang, D.C.W.; Bolan, N.; Novak, J.M. Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ralebitso-Senior, T.K.; Orr, K.H. Biochar Application; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Behazin, E.; Ogunsona, E.; Rodriguezuribe, A.; Mohanty, A.; Misra, M.; Anyia, A. Mechanical, chemical, and physical properties of wood and perennial grass biochars for possible composite application. Bioresources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Özçimen, D.; Karaosmanoğlu, F. Production and characterization of bio-oil and biochar from rapeseed cake. Renew. Energy 2004, 29, 779–787. [Google Scholar] [CrossRef]
- George, J.; Azad, L.B.; Poulose, A.M.; An, Y.; Sarmah, A.K. Nano-mechanical behaviour of biochar-starch polymer composite: Investigation through advanced dynamic atomic force microscopy. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105486. [Google Scholar] [CrossRef]
- Nan, N.; De Valance, B.V.; Xie, X.; Wang, J. The effect of bio-carbon on the electrical, mechanical, and thermal properties of polyvinyl alcohol/biochar composites. J. Compos. Mater. 2016, 50, 1161–1168. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Biocomposites with size-fractionated biocarbon: Influence of the microstructure on macroscopic properties. ACS Omega 2016, 1, 636–647. [Google Scholar] [CrossRef]
- Behazina, E.; Misra, M.; Mohanty, A.K. Sustainable biocarbon from pyrolyzed perennial grasses and their effects on impact modified polypropylene biocomposites. Compos. Part B Eng. 2017, 118, 116–124. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Nanoindentation assisted analysis of biochar added biocomposites. Compos. Part B Eng. 2016, 91, 219–222. [Google Scholar] [CrossRef]
- Premarathna, K.S.D.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Gezahegn, S.; Lai, R.; Huang, L.; Chen, L.; Huang, F.; Blozowski, N.; Thomas, S.C.; Sain, M.; Tjong, J.; Jaffer, S.; et al. Porous graphitic biocarbon and reclaimed carbon fiber derived environmentally benign lightweight composites. Sci. Total Environ. 2019, 664, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ogunsona, E.O.; Codou, A.; Misra, M.; Mohanty, A.K. Thermally stable pyrolytic bioarbon as an effective and sustainable reinforcing filler for polyamide bio-composites fabrication. J. Polym. Environ. 2018, 26, 3574. [Google Scholar] [CrossRef]
- Idrees, M.; Jeelani, S.; Rangari, V. Three-dimensional-printed sustainable biochar-recycled PET composites. ACS Sustain. Chem. Eng. 2018, 6, 13940–13948. [Google Scholar] [CrossRef]
- She, D.; Dong, J.; Zhang, J.; Liu, L.; Sun, Q.; Geng, Z. Development of black and biodegradable biochar/gutta percha composite films with high stretchability and barrier properties. Compos. Sci. Technol. 2019, 175, 1–5. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, W.; Wang, L.; Cai, H. Mechanical properties of rice husk biochar reinforced high density polyethylene composites. Polymers 2018, 10, 286. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.T. Mechanical and flammability characteristics of biochar/polypropylene biocomposites. Comp. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Giorcelli, M.; Khan, A.; Pugno, N.M.; Rosso, C.; Tagliaferro, A. Biochar as a cheap and environmental friendly filler able to improve polymer mechanical properties. Biomass Bioenergy 2019, 120, 219–223. [Google Scholar] [CrossRef]
- Li, S.; Huang, A.; Chen, Y.J.; Li, D.; Turng, L.S. Highly filled biochar/ultra high molecular weight polyethylene/linear low density polyethylene composites for high performance electromagnetic interference shielding. Comp. Part B Eng. 2018, 153, 277–284. [Google Scholar] [CrossRef]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A. Waste coffee ground biochar: A material for humidity sensors. Sensors 2019, 19, 801. [Google Scholar] [CrossRef] [PubMed]
- Dintcheva, N.T.; Morici, E.; Arrigo, R.; La Mantia, F.P. Interaction in POSS-poly(ethylene-co-acrylic acid) nanocomposites. Polym. J. 2014, 46, 160–166. [Google Scholar] [CrossRef]
- Lee, J.C.W.; Porcar, L.; Rogers, S.A. Unveiling Temporal Nonlinear Structure–Rheology Relationships under Dynamic Shearing. Polymers 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lian, M.; Pan, Y.; Wang, X.; Zheng, G.; Liu, C.; Schubert, D.W.; Shen, C. An Alternating Skin–Core Structure in Melt Multi-Injection-Molded Polyethylene. Macromol. Mater. Eng. 2018, 303, 1700465. [Google Scholar] [CrossRef]
- Shi, S.; Wang, L.; Pan, Y.; Liu, C.; Liu, X.; Li, Y.; Zhang, J.; Zheng, G.; Guo, Z. Remarkably Strengthened microinjection molded linear low-density polyethylene (LLDPE) via multi-walled carbon nanotubes derived nanohybrid shish-kebab structure. Compos. Part B Eng. 2019, 167, 362–369. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Pierozzi, M.; Fingolo, A.C.; da Silva, W.P.; Strauss, M. Tuning Sugarcane Bagasse Biochar into a potential Carbon Black Substitute for Polyethylene Composites. J. Polym. Environ. 2019, 27, 1735–1745. [Google Scholar] [CrossRef]
- Kemp, K.C.; Baek, S.B.; Lee, W.-G.; Meyyappan, M.; Kim, K.S. Activated carbon derived from waste coffee grounds for stable methane storage. Nanotechnology 2015, 26, 385602. [Google Scholar] [CrossRef]
- Jagdale, P.; Sharon, M.; Kalita, G.; Mahmad Nabi Maldar, N.; Sharon, M. Carbon Nano Material Synthesis from Polyethylene by Chemical Vapor Deposition. Adv. Mater. Phys. Chem. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Kodjie, S.L.; Li, L.; Li, B.; Cai, W.; Li, C.Y.; Keating, M. Morphology and Crystallization Behavior of HDPE/CNT Nanocomposite. J. Macromol. Sci. B. 2006, 45, 231–245. [Google Scholar] [CrossRef]
- Magalhães da Silva, S.P.; Lima, P.S.; Oliveira, J.M. Rheological behaviour of cork-polymer composites for injection moulding. Comp. Part B Eng. 2016, 90, 172–178. [Google Scholar] [CrossRef]
- Arrigo, R.; Antonioli, D.; Lazzari, M.; Gianotti, V.; Laus, M.; Montanaro, L.; Malucelli, G. Relaxation Dynamics in Polyethylene Glycol/Modified Hydrotalcite Nanocomposites. Polymers 2018, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhao, C.; Zhang, S.; Yang, M.; Wang, Z. Low percolation thresholds of electrical conductivity and rheology in poly(ethylene terephthalate) through the networks of multi-walled carbon nanotubes. Polymer 2006, 47, 480–488. [Google Scholar] [CrossRef]
- Arrigo, R.; Dintcheva, N.T.; Tarantino, G.; Passaglia, E.; Coiai, S.; Cicogna, F.; Filippi, S.; Nasillo, G.; Chillura Martino, D. An insight into the interaction between functionalized thermoplastic elastomer and layered double hydroxides through rheological investigations. Comp. Part B Eng. 2018, 139, 47–54. [Google Scholar] [CrossRef]
- Hyun, K.; Kim, S.H.; Ahn, K.H.; Lee, S.J. Large amplitude oscillatory shear as a way to classify the complex fluids. J. Non-Newton. Fluid. Mech. 2002, 107, 51–65. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Progr. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Wu, D.; Xie, W.; Xie, W.; Wang, K.; Xia, Q.; Yang, H. Rheology of the cellulose nanocrystal filled poly(ε-caprolactone) biocomposites. Polymer 2018, 140, 167–178. [Google Scholar] [CrossRef]
- Arrigo, R.; Ronchetti, S.; Montanaro, L.; Malucelli, G. Effects of the nanofiller size and aspect ratio on the thermal and rheological behavior of PEG nanocomposites containing boehmites or hydrotalcites. J. Therm. Anal. Calorim. 2018, 134, 1167–1180. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Q.; Yao, F.; Xu, Y. Preparation and properties of recycled HDPE/natural fiber composites. Comp. Part A Appl. Sci. Manufact. 2007, 38, 1664–1674. [Google Scholar] [CrossRef]
- Grand, A.F.; Wilkie, C.A. Fire Retardancy of Polymeric Materials; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]





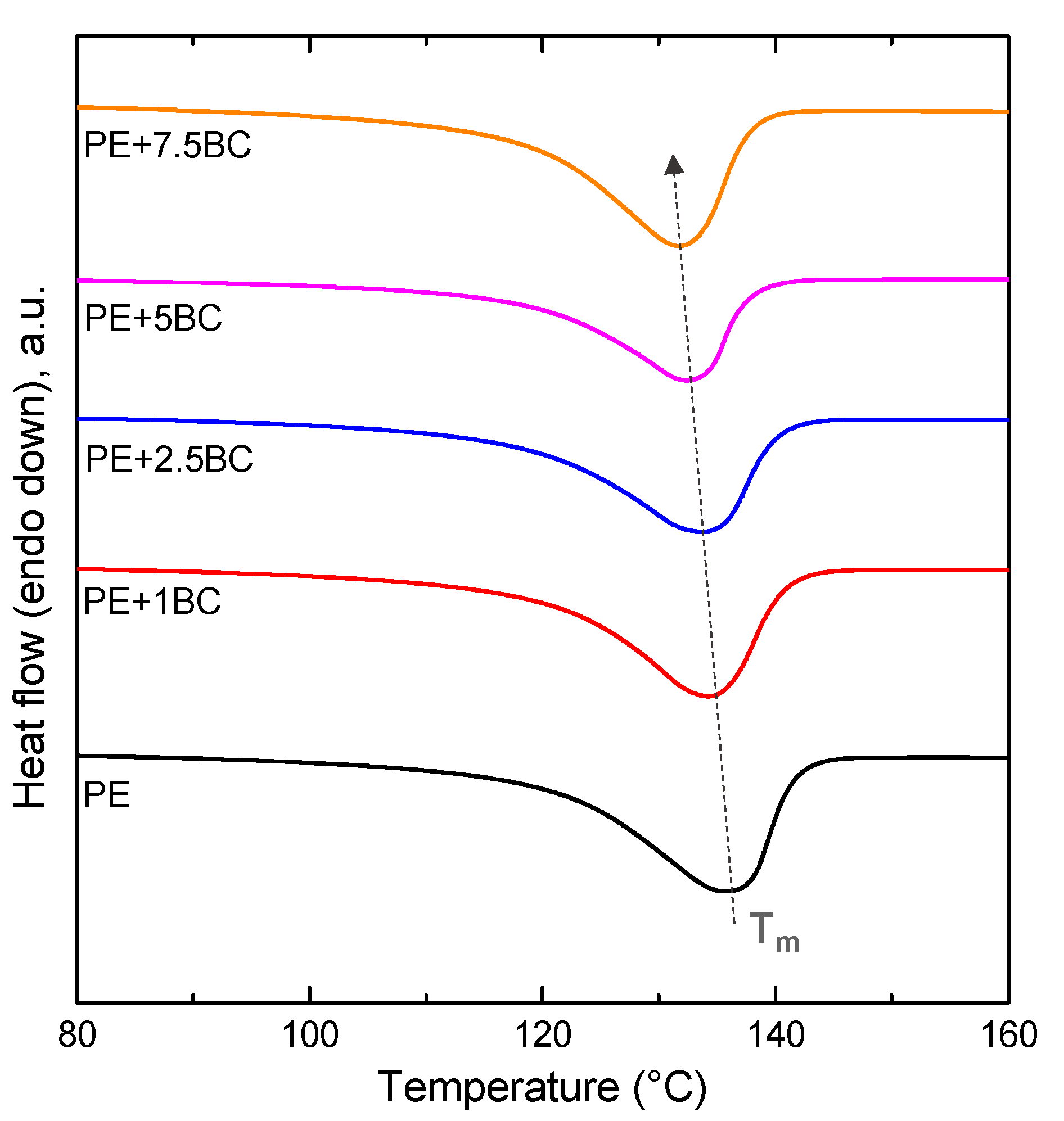
| Sample | Tm (°C) | ΔHm (J/g) | XC (%) |
|---|---|---|---|
| PE | 138.4 | 209.7 | 71 |
| PE+1BC | 134.2 | 179.5 | 61 |
| PE+2.5BC | 133.8 | 177.3 | 60 |
| PE+5BC | 132.5 | 171.1 | 58 |
| PE+7.5BC | 131.7 | 164.5 | 56 |
| Sample | T5% (°C) | T10% (°C) | TMax (°C) | Residue at 600 °C (%) | T5% (°C) | T10% (°C) | TMax (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|---|---|
| N2 | Air | |||||||
| PE | 439.9 | 450.9 | 483.2 | 0.56 | 327.9 | 365.2 | 411.8 | --- |
| PE+1BC | 443.0 | 452.5 | 482.1 | 1.65 | 330.4 | 383.3 | 422.3 | 0.06 |
| PE+2.5BC | 441.0 | 451.4 | 481.6 | 2.53 | 368.3 | 383.4 | 422.8 | 0.18 |
| PE+5BC | 439.1 | 450.8 | 481.7 | 5.08 | 371.7 | 387.4 | 426.5 | 0.63 |
| PE+7.5BC | 438.9 | 450.4 | 482.2 | 7.56 | 384.9 | 399.2 | 438.8 | 0.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrigo, R.; Jagdale, P.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers 2019, 11, 1336. https://doi.org/10.3390/polym11081336
Arrigo R, Jagdale P, Bartoli M, Tagliaferro A, Malucelli G. Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers. 2019; 11(8):1336. https://doi.org/10.3390/polym11081336
Chicago/Turabian StyleArrigo, Rossella, Pravin Jagdale, Mattia Bartoli, Alberto Tagliaferro, and Giulio Malucelli. 2019. "Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds" Polymers 11, no. 8: 1336. https://doi.org/10.3390/polym11081336
APA StyleArrigo, R., Jagdale, P., Bartoli, M., Tagliaferro, A., & Malucelli, G. (2019). Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers, 11(8), 1336. https://doi.org/10.3390/polym11081336










