Surface Engineering of Fluoropolymer Films via the Attachment of Crown Ether Derivatives Based on the Combination of Radiation-Induced Graft Polymerization and the Kabachnik–Fields Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Graft Polymerization of St-CHO
2.4. Surface KF-3CR of ETFE-g-PSt-CHO
2.5. Equations for the Determination of Grafting Degree (GD) Values
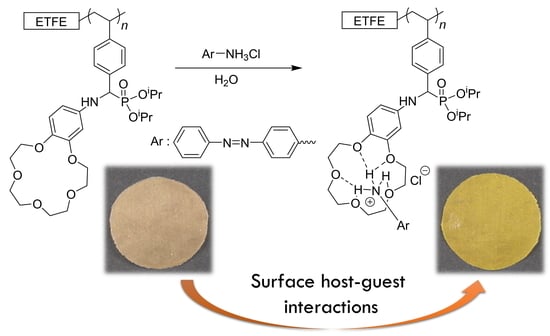
2.6. Surface Host–Guest Interaction of the Crown Ether-Tethered ETFE Films
3. Results and Discussion
3.1. Radiation-Induced Graft Polymerization of St-CHO
3.2. Attachment of Crown Ether Moieties on Materials Surfaces via the Surface KF-3CR on the ETFE Films Featuring PSt-CHO Grafts
3.3. Surface Host–Guest Interactions of ETFE-g-PAP-15-C-5-Ar via Hydrogen Bonding
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Benaglia, M.; Puglisi, A.; Cozzi, F. Polymer-Supported Organic Catalysts. Chem. Rev. 2003, 103, 3401–3430. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W.; Leevy, W.M.; Weber, M.E. Crown Ethers: Sensors for Ions and Molecular Scaffolds for Materials and Biological Models. Chem. Rev. 2004, 104, 2723–2750. [Google Scholar] [CrossRef] [PubMed]
- Krakowiak, K.E.; Bradshaw, J.S.; Zamecka-Krakowiak, D.J. Synthesis of aza-crown ethers. Chem. Rev. 1989, 89, 929–972. [Google Scholar] [CrossRef]
- Zhang, X.X.; Bradshaw, J.S.; Izatt, R.M. Enantiomeric Recognition of Amine Compounds by Chiral Macrocyclic Receptors. Chem. Rev. 1997, 97, 3313–3362. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.; Maguire, G.E.M.; Murillo, O.; Suzuki, I.; De Wall, S.L.; Gokel, G.W. Hydraphile Channels: Structural and Fluorescent Probes of Position and Function in a Phospholipid Bilayer. J. Am. Chem. Soc. 1999, 121, 9043–9052. [Google Scholar] [CrossRef]
- Sogah, G.D.Y.; Cram, D.J. Host-guest complexation. 14. Host covalently bound to polystyrene resin for chromatographic resolution of enantiomers of amino acid and ester salts. J. Am. Chem. Soc. 1979, 101, 3035–3042. [Google Scholar] [CrossRef]
- Sousa, L.R.; Sogah, G.D.Y.; Hoffman, D.H.; Cram, D.J. Host-guest complexation. 12. Total optical resolution of amine and amino ester salts by chromatography. J. Am. Chem. Soc. 1978, 100, 4569–4576. [Google Scholar] [CrossRef]
- Nonokawa, R.; Yashima, E. Detection and Amplification of a Small Enantiomeric Imbalance in α-Amino Acids by a Helical Poly(phenylacetylene) with Crown Ether Pendants. J. Am. Chem. Soc. 2003, 125, 1278–1283. [Google Scholar] [CrossRef]
- Nonokawa, R.; Oobo, M.; Yashima, E. Helicity Induction on a Poly(phenylacetylene) Derivative Bearing Aza-15-crown-5 Ether Pendants in Organic Solvents and Water. Macromolecules 2003, 36, 6599–6606. [Google Scholar] [CrossRef]
- Bradshaw, J.S.; Bruening, R.L.; Krakowiak, K.E.; Tarbet, B.J.; Bruening, M.L.; Izatt, R.M.; Christensen, J.J. Preparation of silica gel-bound macrocycles and their cation-binding properties. Chem. Commun. 1988, 812–814. [Google Scholar] [CrossRef]
- Favre-Réguillon, A.; Dumont, N.; Dunjic, B.; Lemaire, M. Polymeric and immobilized crown compounds, material for ion separation. Tetrahedron 1997, 53, 1343–1360. [Google Scholar] [CrossRef]
- Hamada, T.; Yamashita, S.; Omichi, M.; Yoshimura, K.; Ueki, Y.; Seko, N.; Kakuchi, R. Multicomponent-Reaction-Ready Biomass-Sourced Organic Hybrids Fabricated via the Surface Immobilization of Polymers with Lignin-Based Compounds. ACS Sustain. Chem. Eng. 2019, 7, 7795–7803. [Google Scholar] [CrossRef]
- Kakuchi, R. Multicomponent reactions in polymer synthesis. Angew. Chem. Int. Ed. 2014, 53, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Rudick, J.G. Innovative macromolecular syntheses via isocyanide multicomponent reactions. J. Polym. Sci. Part A 2013, 51, 3985–3991. [Google Scholar] [CrossRef]
- Theato, P. Multi-Component and Sequential Reactions in Polymer Synthesis; Springer International Publishing Switzerland: Basel, Switzerland, 2015; pp. V–VI. [Google Scholar]
- Yang, B.; Zhao, Y.; Wei, Y.; Fu, C.; Tao, L. The Ugi reaction in polymer chemistry: Syntheses, applications and perspectives. Polym. Chem 2015, 6, 8233–8239. [Google Scholar] [CrossRef]
- Kakuchi, R. The dawn of polymer chemistry based on multicomponent reactions. Polym. J. 2019. [Google Scholar] [CrossRef]
- Deng, X.X.; Li, L.; Li, Z.L.; Lv, A.; Du, F.S.; Li, Z.C. Sequence Regulated Poly(ester-amide)s Based on Passerini Reaction. ACS Macro Lett. 2012, 1, 1300–1303. [Google Scholar] [CrossRef]
- Kreye, O.; Türünç, O.; Sehlinger, A.; Rackwitz, J.; Meier, M.A.R. Structurally Diverse Polyamides Obtained from Monomers Derived via the Ugi Multicomponent Reaction. Chem. Eur. J. 2012, 18, 5767–5776. [Google Scholar] [CrossRef]
- Li, L.; Kan, X.W.; Deng, X.X.; Song, C.C.; Du, F.S.; Li, Z.C. Simultaneous dual end-functionalization of peg via the passerini three-component reaction for the synthesis of ABC miktoarm terpolymers. J. Polym. Sci. Part A 2013, 51, 865–873. [Google Scholar] [CrossRef]
- Kreye, O.; Tóth, T.; Meier, M.A.R. Introducing Multicomponent Reactions to Polymer Science: Passerini Reactions of Renewable Monomers. J. Am. Chem. Soc. 2011, 133, 1790–1792. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, H.; Choi, T.L. Cu-Catalyzed Multicomponent Polymerization To Synthesize a Library of Poly(N-sulfonylamidines). J. Am. Chem. Soc. 2013, 135, 3760–3763. [Google Scholar] [CrossRef]
- Jee, J.A.; Spagnuolo, L.A.; Rudick, J.G. Convergent Synthesis of Dendrimers via the Passerini Three-Component Reaction. Org. Lett. 2012, 14, 3292–3295. [Google Scholar] [CrossRef]
- Deng, X.X.; Cui, Y.; Du, F.S.; Li, Z.C. Functional highly branched polymers from multicomponent polymerization (MCP) based on the ABC type Passerini reaction. Polym. Chem. 2014, 5, 3316–3320. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Fu, C.K.; Zhu, C.Y.; Zhang, Y.L.; Wang, S.Q.; Wei, Y.; Tao, L. Introducing the Ugi reaction into polymer chemistry as a green click reaction to prepare middle-functional block copolymers. Polym. Chem. 2014, 5, 2704–2708. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Zhu, C.Y.; Zhang, Y.L.; Wang, S.Q.; Fu, C.K.; Wei, Y.; Tao, L. Introducing mercaptoacetic acid locking imine reaction into polymer chemistry as a green click reaction. Polym. Chem. 2014, 5, 2695–2699. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, B.; Zhao, Y.; Fu, C.; Tao, L.; Wei, Y. A new insight into the Biginelli reaction: The dawn of multicomponent click chemistry? Polym. Chem. 2013, 4, 5395–5400. [Google Scholar] [CrossRef]
- Kakuchi, R.; Theato, P. Efficient Multicomponent Postpolymerization Modification Based on Kabachnik-Fields Reaction. ACS Macro Lett. 2014, 3, 329–332. [Google Scholar] [CrossRef]
- Moldenhauer, F.; Kakuchi, R.; Theato, P. Synthesis of Polymers via Kabachnik-Fields Polycondensation. ACS Macro Lett. 2016, 5, 20–23. [Google Scholar] [CrossRef]
- Kakuchi, R.; Yoshida, S.; Sasaki, T.; Kanoh, S.; Maeda, K. Multi-component post-polymerization modification reactions of polymers featuring lignin-model compounds. Polym. Chem. 2018, 9, 2109–2115. [Google Scholar] [CrossRef]
- Sun, G.; Cheng, C.; Wooley, K.L. Reversible Addition Fragmentation Chain Transfer Polymerization of 4-Vinylbenzaldehyde. Macromolecules 2007, 40, 793–795. [Google Scholar] [CrossRef] [Green Version]
- Lanzinger, D.; Salzinger, S.; Soller, B.S.; Rieger, B. Poly(vinylphosphonate)s as Macromolecular Flame Retardants for Polycarbonate. Ind. Eng. Chem. Res. 2015, 54, 1703–1712. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omichi, M.; Yamashita, S.; Okura, Y.; Ikutomo, R.; Ueki, Y.; Seko, N.; Kakuchi, R. Surface Engineering of Fluoropolymer Films via the Attachment of Crown Ether Derivatives Based on the Combination of Radiation-Induced Graft Polymerization and the Kabachnik–Fields Reaction. Polymers 2019, 11, 1337. https://doi.org/10.3390/polym11081337
Omichi M, Yamashita S, Okura Y, Ikutomo R, Ueki Y, Seko N, Kakuchi R. Surface Engineering of Fluoropolymer Films via the Attachment of Crown Ether Derivatives Based on the Combination of Radiation-Induced Graft Polymerization and the Kabachnik–Fields Reaction. Polymers. 2019; 11(8):1337. https://doi.org/10.3390/polym11081337
Chicago/Turabian StyleOmichi, Masaaki, Shuhei Yamashita, Yamato Okura, Ryuta Ikutomo, Yuji Ueki, Noriaki Seko, and Ryohei Kakuchi. 2019. "Surface Engineering of Fluoropolymer Films via the Attachment of Crown Ether Derivatives Based on the Combination of Radiation-Induced Graft Polymerization and the Kabachnik–Fields Reaction" Polymers 11, no. 8: 1337. https://doi.org/10.3390/polym11081337
APA StyleOmichi, M., Yamashita, S., Okura, Y., Ikutomo, R., Ueki, Y., Seko, N., & Kakuchi, R. (2019). Surface Engineering of Fluoropolymer Films via the Attachment of Crown Ether Derivatives Based on the Combination of Radiation-Induced Graft Polymerization and the Kabachnik–Fields Reaction. Polymers, 11(8), 1337. https://doi.org/10.3390/polym11081337