Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Preparation of PCL-g-PLA Copolymers during Reaction in Solution
2.2.2. Preparation of PCL/PLA Blends via Reactive Blending
2.3. Characterization of PCL/PLA Blends
3. Results
3.1. Grafting/Branching of PCL and PLA Polymers via Peroxide Initiators
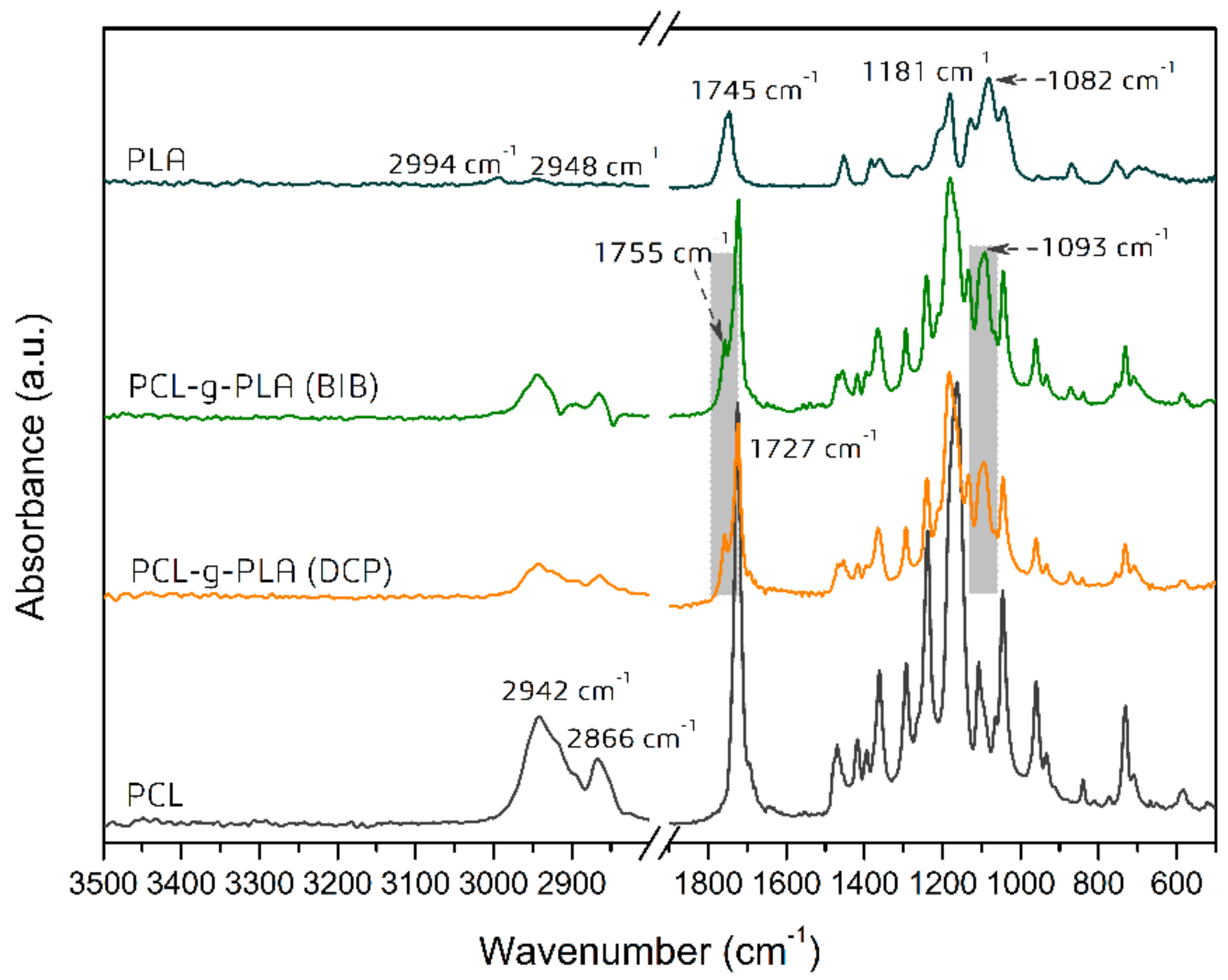
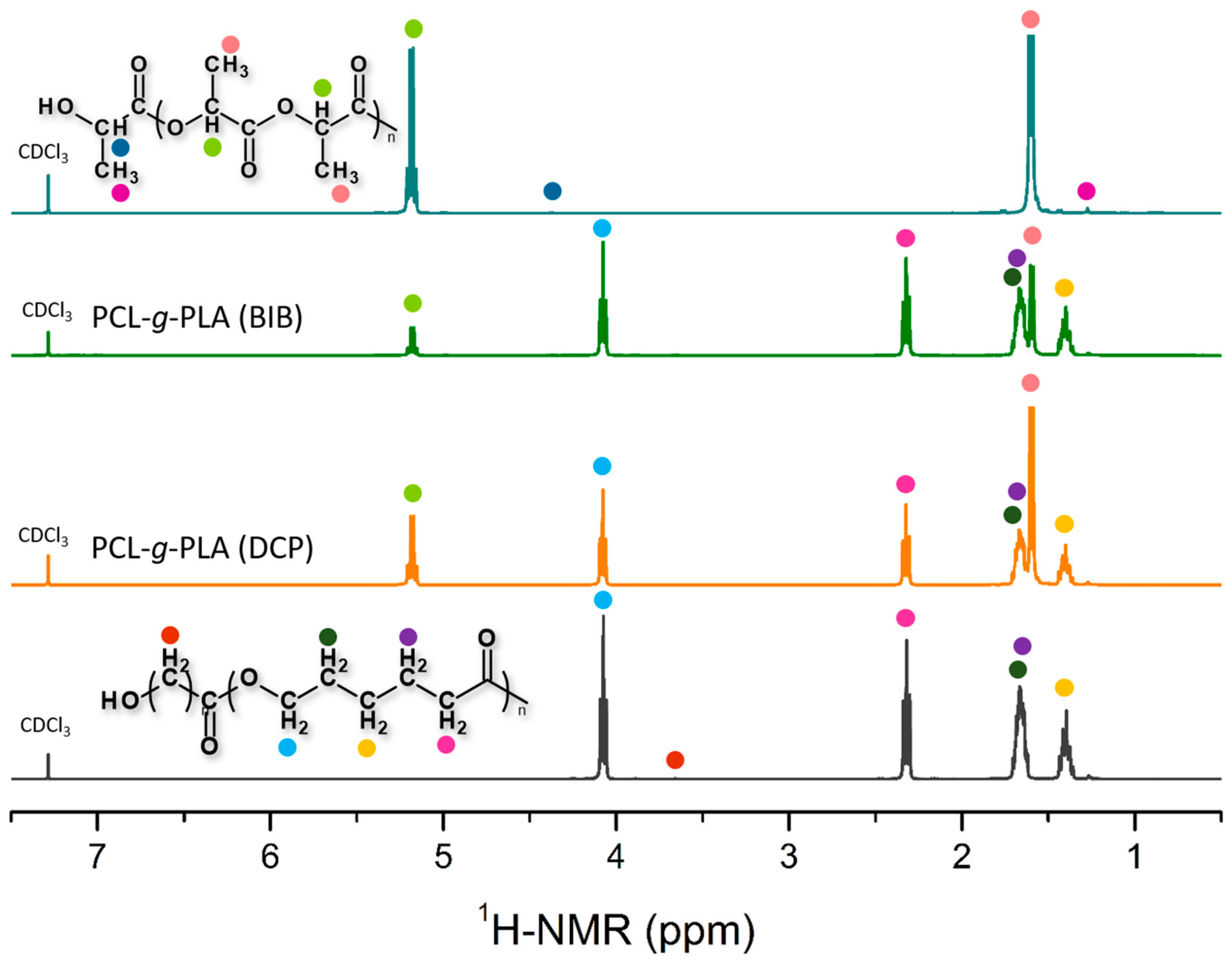
3.2. Chemical Structure of PCL-g-PLA Copolymers Prepared in Solution
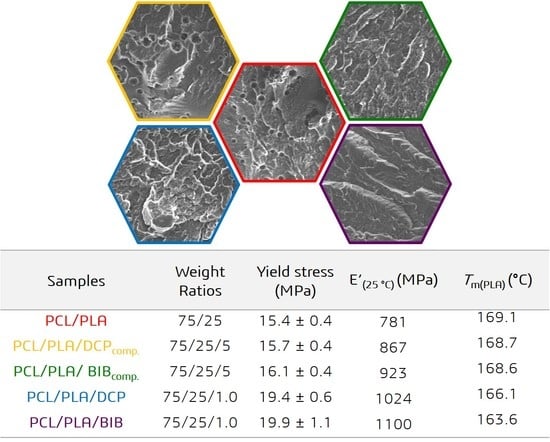
3.3. Morphology of PCL/PLA Blends
3.4. Thermal Stability
3.5. Thermal and Crystallization Properties
3.6. Dynamic Mechanical Analysis (DMA)
3.7. Physico-Mechanical and Rheological Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2015, 49, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Broz, M.E.; Vanderhart, D.L.; Washburn, N.R. Structure and mechanical properties of poly(d, l-lactic acid)/poly(ε-caprolactone) blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites—Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly (lactic acid)-Mass production, processing, industrial applications, and end of life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.-B.; Li, K.-A.; Du, A.-K. Compatibilization strategies in poly (lactic acid)-based blends. RSC Adv. 2015, 41, 32546–32565. [Google Scholar] [CrossRef]
- Akos, N.I.; Wahit, M.U.; Mohamed, R.; Yussuf, A.A. Preparation, characterization, and mechanical properties of poly (ε-caprolactone)/polylactic acid blend composites. Polym. Compos. 2013, 34, 763–768. [Google Scholar] [CrossRef]
- Liu, H.; Feng, C.; Bo, L.; Estep, G.; Zhang, J. Super toughened poly (lactic acid) ternary blends by simultaneous dynamic vulcanization and interfacial compatibilization. Macromolecules 2010, 43, 6058–6066. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Z.; Dong, W.; Li, Y.; Li, J. Formation of interfacial janus nanomicelles by reactive blending and their compatibilization effects on immiscible polymer blends. J. Phys. Chem. B 2016, 120, 9240–9252. [Google Scholar] [CrossRef]
- Wei, L.; Mcdonald, A.G. Peroxide induced cross-linking by reactive melt processing of two biopolyesters: Poly (3-hydroxybutyrate) and poly (L-lactic acid) to improve their melting processability. J. Appl. Polym. Sci. 2015, 132, 1–15. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS blends via in situ compatibilization using dicumyl peroxide as a free-radical grafting initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Coltelli, M.; Bronco, S.; Chinea, C. The effect of free radical reactions on structure and properties of poly (lactic acid) (PLA) based blends. Polym. Degrad. Stab. 2010, 95, 332–341. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Sustainable biobased blends of poly (lactic acid) (PLA) and poly (glycerol succinate-co-maleate) (PGSMA) with balanced performance prepared by dynamic vulcanization. RSC Adv. 2017, 7, 38594–38603. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Ran, X.; Su, X.; Zhang, K.; Liu, N. Effect of peroxide crosslinking on thermal and mechanical properties of poly (ε-caprolactone). Polym. Int. 2007, 56, 593–600. [Google Scholar] [CrossRef]
- Takamura, M.; Nakamura, T.; Takahashi, T.; Koyama, K. Effect of type of peroxide on crosslinking of poly (L-lactide). Polym. Degrad. Stab. 2008, 93, 1909–1916. [Google Scholar] [CrossRef]
- Dean, K.M.; Petinakis, E.; Meure, S.; Yu, L.; Chryss, A. Melt strength and rheological properties of biodegradable poly (lactic acid) modified via alkyl radical-based reactive extrusion processes. J. Polym. Environ. 2012, 20, 741–747. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Kotaki, M.; Hamada, H. Effect of compounding procedure on mechanical properties and dispersed phase morphology of poly (lactic acid)/polycaprolactone blends containing peroxide. J. Appl. Polym. Sci. 2006, 103, 1066–1074. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Rayón, E.; Carbonell-Verdu, A.; Balart, R. Improvement of the compatibility between poly (3-hydroxybutyrate) and poly (ε-caprolactone) by reactive extrusion with dicumyl peroxide. Eur. Polym. J. 2017, 86, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-situ compatibilization of poly (lactic acid) and poly (butylene adipate-co-terephthalate) blends by using dicumyl peroxide as a free-radical initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Nerkar, M.; Ramsay, J.A.; Ramsay, B.A.; Vasileiou, A.A.; Kontopoulou, M. Improvements in the melt and solid-state properties of poly (lactic acid), poly-3-hydroxyoctanoate and their blends through reactive modification. Polymer 2015, 64, 51–61. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic effects in mechanical properties of PLA/PCL blends with optimized composition, processing, and morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Frone, A.N.; Batalu, D.; Chiulan, I.; Oprea, M.; Gabor, A.R.; Nicolae, C.-A.; Raditoiu, V.; Trusca, R.; Panaitescu, D.M. Morpho-Structural, thermal and mechanical properties of PLA/PHB/Cellulose biodegradable nanocomposites obtained by compression molding, extrusion, and 3D printing. Nanomaterials 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brostow, W.; Hagg Lobland, H.E.; Khoja, S. Brittleness and toughness of polymers and other materials. Mater. Lett. 2015, 159, 478–480. [Google Scholar] [CrossRef]


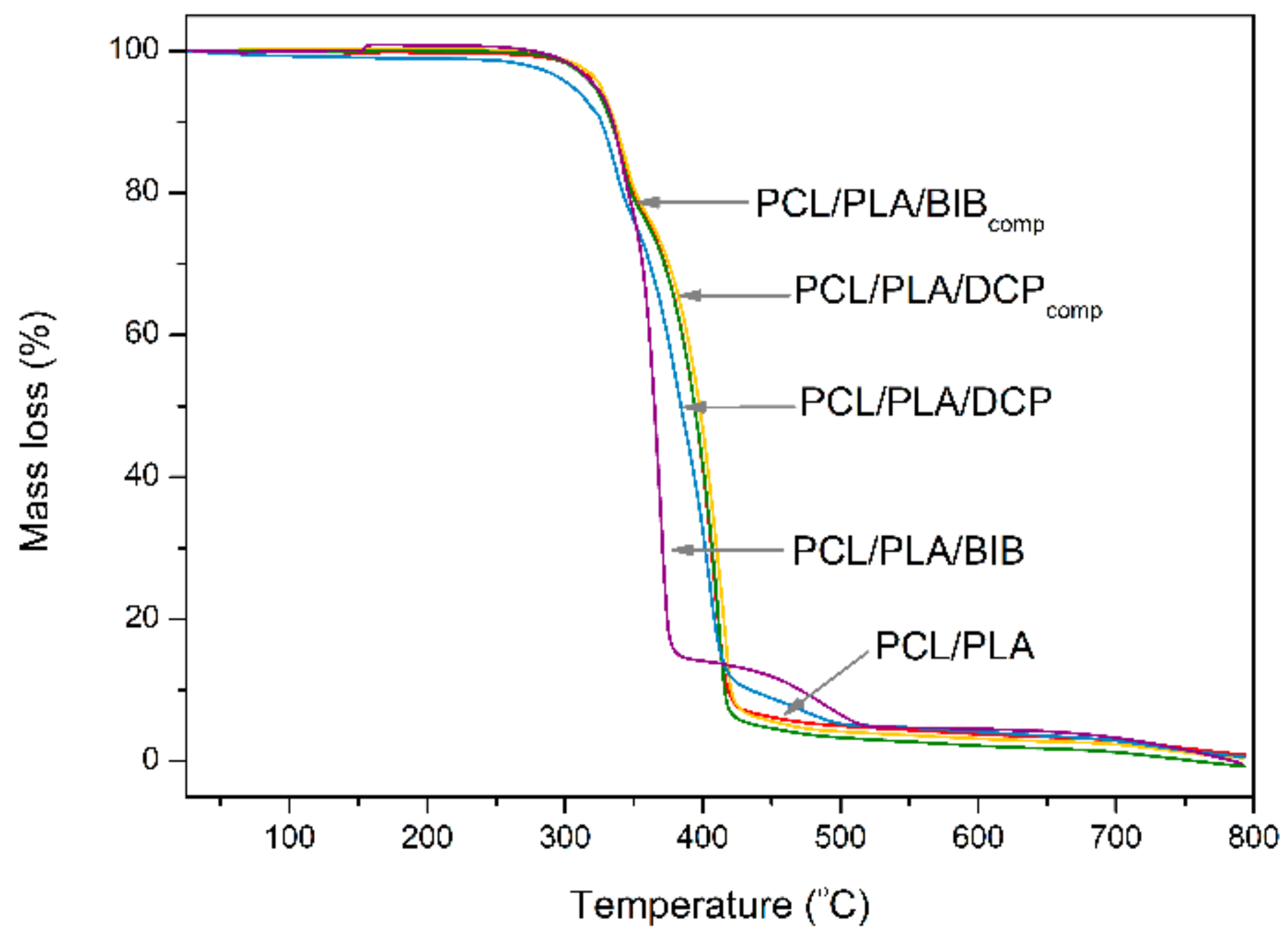



| Samples | Weight Ratios | T−2% (°C) | T−5% (°C) | T−10% (°C) | T−50% (°C) | R700 °C (%) |
|---|---|---|---|---|---|---|
| PCL/PLA | 75/25 | 304 | 322 | 335 | 394 | 2.9 |
| PCL/PLA/DCPcomp | 75/25/5 | 309 | 326 | 336 | 398 | 2.4 |
| PCL/PLA/BIBcomp | 75/25/5 | 303 | 321 | 333 | 394 | 1.3 |
| PCL/PLA/DCP | 75/25/1 | 272 | 305 | 326 | 384 | 2.9 |
| PCL/PLA/BIB | 75/25/1 | 305 | 322 | 335 | 365 | 3.3 |
| Samples | Weight Ratios | Tm(PCL) (°C) | ΔHm(PCL) (J/g) | Tc(PCL) (°C) | ΔHc(PCL) (J/g) | Tm(PLA) (°C) | ΔHm(PLA) (J/g) | Tcc(PLA) (°C) | Xc(PCL) (%) | Xc(PLA) (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PCL/PLA | 75/25 | 58.2 | 30.7 | 31.2 | 35.8 | 169.1 | 10.3 | 99.6 | 30.1 | 44.3 |
| PCL/PLA/DCPcomp | 75/25/5 | 57.2 | 28.6 | 32.5 | 39.9 | 168.7 | 11.7 | 102.1 | 28.0 | 50.3 |
| PCL/PLA/BIBcomp | 75/25/5 | 57.3 | 30.7 | 32.3 | 39.9 | 168.6 | 9.4 | 101.0 | 30.1 | 40.4 |
| PCL/PLA/DCP | 75/25/1 | 58.0 | 36.8 | 36.1 | 40.6 | 166.1 | 8.0 | 99.2 | 36.1 | 34.4 |
| PCL/PLA/BIB | 75/25/1 | 59.1 | 36.6 | 32.5 | 43.6 | 163.6 | 7.0 | - | 35.9 | 30.0 |
| Samples | Weight Ratios | E’(25 °C) (MPa) | E”(PCL) (°C) | E”(PLA) (°C) |
|---|---|---|---|---|
| PCL | 100 | 546 | −44.0 | - |
| PCL/PLA | 75/25 | 781 | −42.0 | 70.0 |
| PCL/PLA/DCPcomp | 75/25/5 | 867 | −41.0 | 70.0 |
| PCL/PLA/BIBcomp | 75/25/5 | 923 | −42.0 | 70.0 |
| PCL/PLA/DCP | 75/25/1 | 1024 | −35.0 | 68.0 |
| PCL/PLA/BIB | 75/25/1 | 1100 | −40.0 | 65.0 |
| PLA | 100 | 3562 | - | 78.0 |
| Samples | Weight Ratios | Yield Stress (MPa) | Elongation at Break (%) | Hardness (ShD) | Gel Fraction (%) | MFI170 °C/2.16 kg (g/10 min) | B 1010 %·Pa |
|---|---|---|---|---|---|---|---|
| PCL/PLA | 75/25 | 15.4 ± 0.4 | 160 ± 65 | 56.8 ± 0.4 | soluble | 3.6 ± 0.3 | 0.080 |
| PCL/PLA/DCPcomp | 75/25/5 | 15.7 ± 0.4 | 221 ± 59 | 58.0 ± 0.4 | soluble | 4.1 ± 0.3 | 0.052 |
| PCL/PLA/BIBcomp | 75/25/5 | 16.1 ± 0.4 | 238 ± 35 | 58.3 ± 0.6 | soluble | 3.6 ± 0.3 | 0.046 |
| PCL/PLA/DCP | 75/25/1 | 19.4 ± 0.6 | 288 ± 68 | 59.7 ± 0.4 | not measurable ** | 0.9 ± 0.2 * | 0.034 |
| PCL/PLA/BIB | 75/25/1 | 19.9 ± 1.1 | 10.4 ± 0.4 | 59.9 ± 0.5 | 72.0 ± 4.0 | - | 0.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies. Polymers 2020, 12, 228. https://doi.org/10.3390/polym12010228
Przybysz-Romatowska M, Haponiuk J, Formela K. Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies. Polymers. 2020; 12(1):228. https://doi.org/10.3390/polym12010228
Chicago/Turabian StylePrzybysz-Romatowska, Marta, Józef Haponiuk, and Krzysztof Formela. 2020. "Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies" Polymers 12, no. 1: 228. https://doi.org/10.3390/polym12010228
APA StylePrzybysz-Romatowska, M., Haponiuk, J., & Formela, K. (2020). Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies. Polymers, 12(1), 228. https://doi.org/10.3390/polym12010228