Vegetable Additives in Food Packaging Polymeric Materials
Abstract
:1. Introduction
Classification of Natural Biodegradable Polymers and Additives
2. Lignocellulosic Materials and Plant Extracts in Polymeric Composites (As Reinforcements/Components/Additives)
2.1. Lignocellulosic Materials, Lignin and Nano-Cellulose As Reinforcements (Additives) in Polymer Matrices
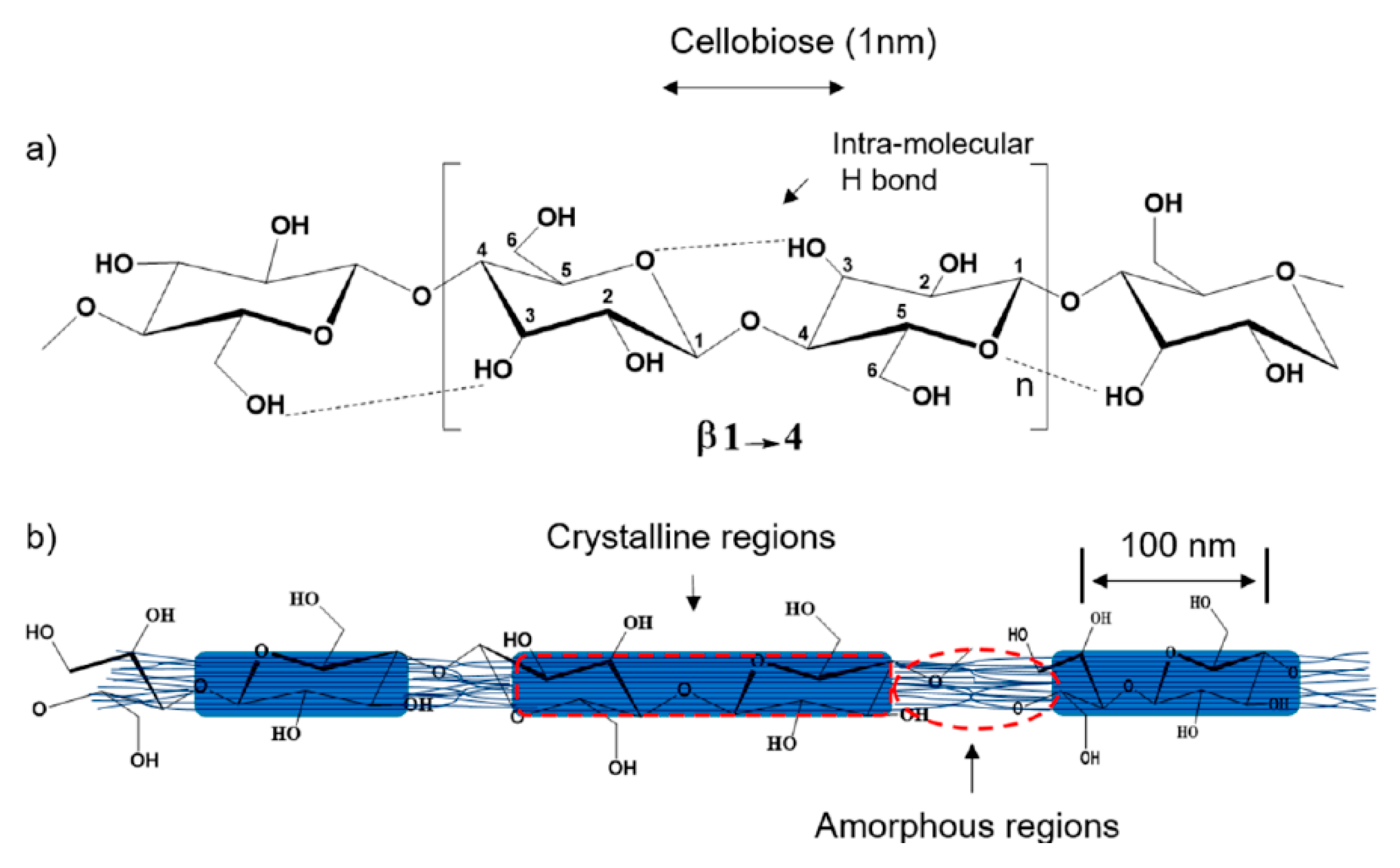
2.1.1. Lignocellulosic Materials
2.1.2. Lignin
2.1.3. Nanocellulose
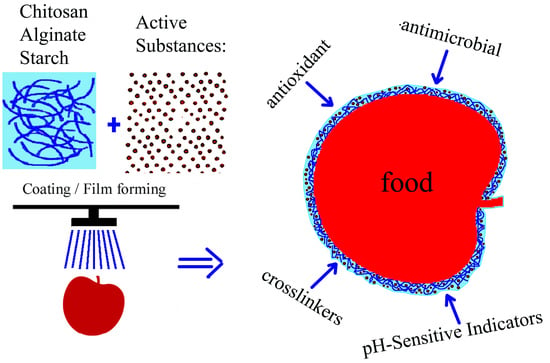
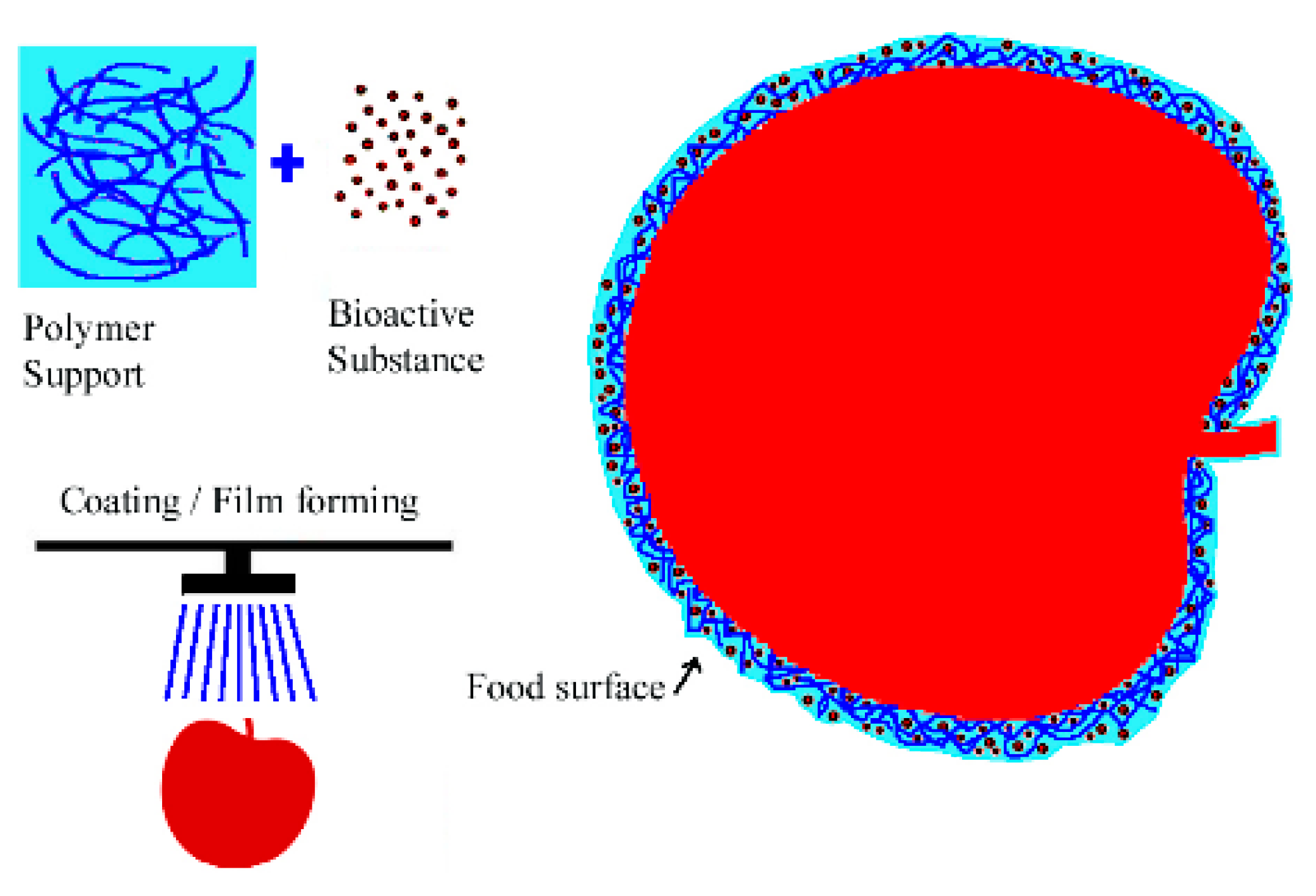
2.2. Plant Extracts as Active Ingredients in Food Packaging Materials Based on Polysaccharide Matrices (Chitosan/Starch/Alginate)
2.2.1. Chitosan/Starch/Alginate Containing Plant Extracts as Edible Food Packaging
2.2.2. Phenols from Plant Extracts as pH-Sensitive Indicators of Chitosan/Starch/Alginate Matrices
2.2.3. Plant Extracts Incorporated As Antioxidants in Chitosan/Starch/Alginate Matrices
2.2.4. Phenols from Plant Extracts as Crosslinkers for the Chitosan/Starch/Alginate Matrices
3. Conclusions
Funding
Conflicts of Interest
References
- Ambrogi, V.; Carfagna, C.; Cerruti, P.; Marturano, V. Chapter 4: Additives in polymers. In Modification of Polymer Properties; Jasso-Gastinel, C.F., Kenny, J.M., Eds.; Elsevier Inc: Amsterdam, The Netherlands, 2017; p. 87. [Google Scholar]
- Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and recycling of films based on biodegradable polymers: A short review. Polymers 2019, 11, 651. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Zhao, J.; Jiang, Z. Micromanufacturing of composite materials: A review. Int. J. Extrem. Manuf. 2019, 1, 012004. [Google Scholar] [CrossRef]
- Chung, D.D. Chapter 3-Polymer-Matrix Composites: Structure and Processing. In Carbon Composites: Composites with Carbon Fibers, Nanofibers, and Nanotubes, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 161–217. [Google Scholar]
- dos Santos Rosa, D.; Lenz, D.M. Chapter 16: Biocomposites: Influence of Matrix Nature and Additives on the Properties and Biodegradation Behaviour. In Biodegradation: Engineering and Technology; Chamy, R., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, T.Y.; Lim, Y.Y.; Yule, C.M. Distribution and characterisation of phenolic compounds in Macaranga pruinosa and associated soils in a tropical peat swamp forest. J. Trop. For. Sci. 2017, 29, 509–518. [Google Scholar]
- Lalit, R.; Mayank, P.; Ankur, K. Natural fibers and biopolymers characterization: A future potential composite material. Stroj. Cas. J. Mech. Eng. 2018, 68, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Jawaid, M.H.P.S.; Khalil, H.A. Cellulosic/synthetic fibre reinforced polymer hybrid composites: A review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Peças, P.; Carvalho, H.; Salman, H.; Leite, M. Natural fibre composites and their applications: A review. J. Compos. Sci. 2018, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Mochane, M.J.; Mokhena, T.C.; Mokhothu, T.H.; Mtibe, A.; Sadiku, E.R.; Ray, S.S.; Ibrahim, I.D.; Daramola, O.O. Recent progress on natural fiber hybrid composites for advanced applications: A review. Express Polym. Lett. 2019, 13, 159–198. [Google Scholar] [CrossRef]
- Ahuja, D.; Kaushik, A.; Singh, M. Simultaneous extraction of lignin and cellulose nanofibrils from waste jute bags using one pot pre-treatment. Int. J. Biol. Macromol. 2018, 107, 1294–1301. [Google Scholar] [CrossRef]
- Zander, N.E.; Park, J.H.; Boelter, Z.R.; Gillan, M.A. Recycled Cellulose Polypropylene Composite Feedstocks for Material Extrusion Additive Manufacturing. ACS Omega 2019, 4, 13879–13888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formela, K.; Zedler, Ł.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites-Current trends and future developments. Express Polym. Lett. 2018, 12, 24–57. [Google Scholar] [CrossRef]
- Kaynak, B.; Spoerk, M.; Shirole, A.; Ziegler, W.; Sapkota, J. Polypropylene/cellulose composites for material extrusion additive manufacturing. Macromol. Mater. Eng. 2018, 303, 1800037. [Google Scholar] [CrossRef] [Green Version]
- Tolinski, M.; Tolinski, M. Additives for Polyolefins: Getting the Most Out of Polypropylene, Polyethylene and TPO; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Dufresne, A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 376, 20170040. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High. Performance Tailored Materials; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2012; p. 411. [Google Scholar]
- Pérez-Pacheco, E.; Canto-Pinto, J.C.; Moo-Huchin, V.M.; Estrada-Mota, I.A.; Estrada-León, R.J.; Chel-Guerrero, L. Thermoplastic Starch (TPS)-Cellulosic Fibers Composites: Mechanical Properties and Water Vapor Barrier: A Review (Chapter 5). In Composites from Renewable and Sustainable Materials; Poletto, M., Ed.; IntechOpen: London, UK, 2016; p. 85. [Google Scholar]
- Ayoub, A.S.; Lucia, L.A. Fundamental Science and Applications for Biomaterials (Chapter 2) Starch-Based Plastics (& 2.4.3). In Introduction to Renewable Biomaterials: First Principles and Concepts; Ayoub, A.S., Lucia, L.A., Eds.; Wiley: Hoboken, NJ, USA, 2017; p. 57. [Google Scholar]
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.A.; Banaei, M. Application of Edible and Biodegradable Starch-Based Films in Food Packaging: A Systematic Review and Meta-Analysis. Curr. Res. Nutr. Food Sci. J. 2019, 7. [Google Scholar]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly (lactic) acid and starch for biodegradable food packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef]
- Kamilah, H.; Mahmood, K.; Poh, L.; Sulaiman, S.; Nafchi, A.; Fazilah, A.; Karim, A. Food Packaging: Starch and Non-Starch Blend Films (Chapter 61). In Encyclopedia of Polymer Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Glenn, G.M.; Orts, W.; Imam, S.; Chiou, B.S.; Wood, D.F. Starch plastic packaging and agriculture applications. In Starch Polymers; Halley, J., Avérous, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 421–452. [Google Scholar]
- Sandarani, M.D.J.C. A review: Different extraction techniques of Pectin. J. Pharmacogn. Nat. Prod. 2017, 3, 1–14. [Google Scholar] [CrossRef]
- Fishman, M.L.; Coffin, D.R.; Onwulata, C.I.; Willett, J.L. Two stage extrusion of plasticized pectin/poly (vinyl alcohol) blends. Carbohyd. Polym. 2006, 65, 421–429. [Google Scholar] [CrossRef]
- Liu, L.S.; Finkenstadt, V.L.; Liu, C.K.; Jin, T.; Fishman, M.L.; Hicks, K.B. Preparation of poly (lactic acid) and pectin composite films intended for applications in antimicrobial packaging. J. Appl. Polym. Sci. 2007, 106, 801–810. [Google Scholar] [CrossRef]
- Hadnađev, M.S.; Hadnađev-Dapčević, T.; Pojić, M.M.; Šarić, B.M.; Mišan, A.Č.; Jovanov, P.T.; Sakač, M.B. Progress in vegetable proteins isolation techniques: A review. Food Feed Res. 2017, 44, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Gavin, C.; Lay, M.C.; Verbeek, C.J. Extrusion foaming of protein-based thermoplastic and polyethylene blends. In Proceedings of the AIP Conference, 31st International Conference of the Polymer Processing Society, Jeju Island, Korea, 7–11 June 2015; AIP Publishing: Melville, NY, USA, 2016; Volume 1713, p. 100003. [Google Scholar]
- DeButts, B.L.; Hanzly, L.E.; Barone, J.R. Protein-polyisoprene rubber composites. J. Appl. Polym. Sci. 2018, 135, 46026. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Li, F.; Shi, S.Q.; Xia, C.; Zhou, W.; Zhang, D.; Gong, S.; Li, J. Tough, strong, and biodegradable composite film with excellent UV barrier performance comprising soy protein isolate, hyperbranched polyester, and cardanol derivative. Green Chem. 2019, 21, 3651–3665. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhang, J.; Li, G.; Liu, X.; Li, Z.; Liu, X.; Han, Y.; Zhao, Z. Nano polypeptide particles reinforced polymer composite fibers. ACS Appl. Mater. Interfaces 2015, 7, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Altan, A.; Aytac, Z.; Uyar, T. Carvacrol loaded electrospun fibrous films from zein and poly (lactic acid) for active food packaging. Food Hydrocoll. 2018, 81, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Busolo, M.A.; Torres-Giner, S.; Lagaron, J.M. Enhancing the gas barrier properties of polylactic acid by means of electrospun ultrathin zein fibers. In Proceedings of the 67th Annual Technical Conference, Chicago, IL, USA, 22–24 June 2009; Volume 5, pp. 2763–2768. [Google Scholar]
- Lin, D.; Lu, W.; Kelly, A.L.; Zhang, L.; Zheng, B.; Miao, S. Interactions of vegetable proteins with other polymers: Structure-function relationships and applications in the food industry. Trends Food Sci. Technol. 2017, 68, 130–144. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef] [Green Version]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefining 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Huang, J.; Fu, S.; Gan, L. Chapter 5-Lignin-Modified Thermoplastic Materials, Pages. In Lignin Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–161. [Google Scholar]
- Faruk, O.; Obaid, N.; Tjong, J.; Sain, M. 6-Lignin Reinforcement in Thermoplastic Composites. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; William Andrew Publishing: New York, NY, USA, 2016; pp. 95–118. [Google Scholar]
- Tanase, C.; Coşarcă, S.; Muntean, D.L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Abad, A.; Sanchez, G.; Ocio, M.J.; Lagaron, J.M. Polymeric Materials Containing Natural Compounds with Antibacterial and Virucide Properties (Chapter 11). In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; Muñoz-Bonilla, A., María, L., Fernández-García, M., Eds.; Royal Society of Chemistry: London, UK, 2013; pp. 310–326. [Google Scholar]
- Green Polymer Composites Technology: Properties and Applications; Inamuddin (Ed.) Imprint CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Burgos, N.; Jiménez, A.; Garrigós, M.D.C. Use of herbs, spices and their bioactive compounds in active food packaging. RSC Adv. 2015, 5, 40324–40335. [Google Scholar] [CrossRef] [Green Version]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the antimicrobial activity and cytotoxicity of different components of natural origin present in essential oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid.-Based Compl. Alt. Med. 2011, 2011, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, C.; Sivertsvik, M.; Miteluţ, A.; Brebu, M.; Stoleru, E.; Rosnes, J.; Tanase, E.E.; Khan, W.; Pamfil, D.; Cornea, P.C.; et al. Comparative analysis of the composition and active property evaluation of certain essential oils to assess their potential applications in active food packaging. Materials 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [Green Version]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly (Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [Green Version]
- Kawano, A.; Yamamoto, K.; Kadokawa, J.I. Preparation of self-assembled chitin nanofiber-natural rubber composite sheets and porous materials. Biomolecules 2017, 7, 47. [Google Scholar] [CrossRef]
- Torres-Hernández, Y.; Ortega-Díaz, G.; Téllez-Jurado, L.; Castrejón-Jiménez, N.; Altamirano-Torres, A.; García-Pérez, B.; Balmori-Ramírez, H. Biological compatibility of a polylactic acid composite reinforced with natural chitosan obtained from shrimp waste. Materials 2018, 11, 1465. [Google Scholar] [CrossRef] [Green Version]
- Petchsoongsakul, T.; Dittanet, P.; Loykulnant, S.; Kongkaew, C.; Prapainainar, P. Synthesis of Natural Composite of Natural Rubber Filling Chitosan Nanoparticles. Key Eng. Mater. 2019, 821, 96–102. [Google Scholar] [CrossRef]
- de Barros-Alexandrino, T.T.; Tosi, M.M.; Assis, O.B.G. Comparison Between Chitosan Nanoparticles and Cellulose Nanofibers as Reinforcement Fillers in Papaya Puree Films: Effects on Mechanical, Water Vapor Barrier, and Thermal Properties. Polym. Eng. Sci. 2019, 59, E287–E292. [Google Scholar] [CrossRef]
- van den Broek, L.A.; Knoop, R.J.; Kappen, F.H.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of chitosan—A challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Taurino, R.; Sciancalepore, C.; Collini, L.; Bondi, M.; Bondioli, F. Functionalization of PVC by chitosan addition: Compound stability and tensile properties. Compos. Part. B Eng. 2018, 149, 240–247. [Google Scholar] [CrossRef]
- Rihayat, T.; Riskina, S.; Syahputra, W. Formulation of Polyurethane with Bentonite-Chitosan as Filler Applied to Carbon Steel as an Antibacterial and Environmentally Friendly Paint. In Proceedings of the IOP Conference Series: Materials Science and Engineering 2019. International Conference on Science and Innovated Engineering (I-COSINE), Aceh, Indonesia, 21–22 October 2018; IOP Publishing: Bristol, UK, 2019; Volume 536, p. 012093. [Google Scholar]
- Onwulata, C.I.; Thomas, A.E.; Cooke, P.H. Effects of biomass in polyethylene or polylactic acid composites. J. Biobased Mater. Bioenergy 2009, 3, 172–180. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, B.; Muhammad, N.; Rahim, A.; Iqbal, F.; Sharif, F.; Safi, S.Z.; Khan, A.S.; Gonfa, G.; Uroos, M.; Rehman, I.U. Development of collagen/PVA composites patches for osteochondral defects using a green processing of ionic liquid. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 590–596. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Purification of natural clays (Chapter 7.1). In Developments in Clay Science, Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 213–221. [Google Scholar]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A review of the synthesis and applications of polymer—Nanoclay composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, F.; Nodehi, A.; Atai, M. PMMA/double-modified organoclay nanocomposites as fillers for denture base materials with improved mechanical properties. J. Mech. Behav. Biomed. Mater. 2019, 90, 11–19. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Sánchez, J.A.; Vidaltamayo, R. Nanoclays for biomedical applications. In Handbook of Ecomaterials; Torres-Martínez, L.M., Kharissova, O.V., Kharissov, B.I., Eds.; Springer International Publishing: New York, NY, USA, 2019; pp. 1–19. [Google Scholar]
- Berni, R.; Cai, G.; Hausman, J.F.; Guerriero, G. Plant Fibers and Phenolics: A Review on Their Synthesis, Analysis and Combined Use for Biomaterials with New Properties. Fibers 2019, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.; Apone, F.; Abdel-Salam, E.; Qahtan, A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyanarayana, K.G.; Arizaga, G.G.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Ahmed, B.; Aboudi, K.; Tyagi, V.K.; Álvarez-Gallego, C.J.; Fernández-Güelfo, L.A.; Romero-García, L.I.; Kazmi, A.A. Improvement of Anaerobic Digestion of Lignocellulosic Biomass by Hydrothermal Pretreatment. Appl. Sci. 2019, 9, 3853. [Google Scholar] [CrossRef] [Green Version]
- Pires, J.R.; de Souza, V.G.L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations. In Lecture Notes in Electrical Engineering, Innovation, Engineering and Entrepreneurship; Machado, J., Soares, F., Veiga, G., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of lignocellulosic biomass to nanocellulose: Structure and chemical process. Sci. World J. 2014, 2014, 20. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [Green Version]
- Ching, Y.C.; Ershad, A.; Luqman, C.A.; Choo, K.W.; Yong, C.K.; Sabariah, J.J.; Chuah, C.H.; Liou, N.S. Rheological properties of cellulose nanocrystal-embedded polymer composites: A review. Cellulose 2016, 23, 1011–1030. [Google Scholar] [CrossRef]
- Zarrinbakhsh, N.; Mohanty, A.K.; Misra, M. Formulation optimization of bioreinforced composites from polyolefins and dried distillers’ grains using statistical methods. Compos. Part. A Appl. Sci. Manuf. 2019, 119, 246–260. [Google Scholar] [CrossRef]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted pre-treatment of hemp bast fibres for optimal performance in biocomposite materials: A review. Ind. Crops Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef] [Green Version]
- Saradava, B.J.; Kathwadia, A.J.; Gorviyala, A.D.; Joshi, V.K. Mechanical characterization of hemp fiber reinforced polyester composites: A review. J. Polym. Compos. 2016, 4, 1–3. [Google Scholar]
- Catto, A.L.; Júnior, M.A.D.; Hansen, B.; Francisquetti, E.L.; Borsoi, C. Characterization of polypropylene composites using yerba mate fibers as reinforcing filler. Compos. Part. B Eng. 2019, 174, 106935. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Cai, H.; Lin, X.; Yi, W.; Zhang, J. Properties comparison of high density polyethylene composites filled with three kinds of shell fibers. Results Phys. 2019, 12, 1542–1546. [Google Scholar] [CrossRef]
- Pappu, A.; Pickering, K.L.; Thakur, V.K. Manufacturing and characterization of sustainable hybrid composites using sisal and hemp fibres as reinforcement of poly (lactic acid) via injection moulding. Ind. Crops Prod. 2019, 137, 260–269. [Google Scholar] [CrossRef]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part. B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Yong, M.; Zhang, Y.; Sun, S.; Liu, W. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling. J. Membr. Sci. 2019, 575, 50–59. [Google Scholar] [CrossRef]
- Dabral, S.; Turberg, M.; Wanninger, A.; Bolm, C.; Hernández, J. Mechanochemical Lignin-Mediated Strecker Reaction. Molecules 2017, 22, 146. [Google Scholar] [CrossRef] [Green Version]
- Pregi, E.; Kun, D.; Vu, V.; Pukánszky, B. Structure evolution in poly (ethylene-co-vinyl alcohol)/lignin blends: Effect of interactions and composition. Eur. Polym. J. 2019, 111, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.S.; Li, H.; Yuan, H.; Kuboki, T.; Xu, C.C. Effects of de-polymerized lignin content on thermo-oxidative and thermal stability of polyethylene. J. Anal. Appl. Pyrolysis 2019, 140, 413–422. [Google Scholar] [CrossRef]
- Nair, S.S.; Chen, H.; Peng, Y.; Huang, Y.; Yan, N. Polylactic acid biocomposites reinforced with nanocellulose fibrils with high lignin content for improved mechanical, thermal, and barrier properties. ACS Sustain. Chem. Eng. 2018, 6, 10058–10068. [Google Scholar] [CrossRef]
- Aqlil, M.; Moussemba Nzenguet, A.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene oxide filled lignin/starch polymer bionanocomposite: Structural, physical, and mechanical studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef] [PubMed]
- Souza de Miranda, C.; Ferreira, M.S.; Magalhães, M.T.; Gonçalves, A.P.B.; Carneiro de Oliveira, J.; Guimarães, D.H.; José, N.M. Effect of the glycerol and lignin extracted from Piassava fiber in cassava and corn starch films. Mater. Res. 2015, 18, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, E.M.; O’Keefe, S.F.; Kim, Y.T. Utilization of lignin in biopolymeric packaging films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polat, Y.; Stojanovska, E.; Negawo, T.A.; Doner, E.; Kilic, A. Lignin as an Additive for Advanced Composites. In Green Biocomposites. Green Energy and Technology; Jawaid, M., Sapuan, S., Alothman, O., Eds.; Springer: Cham, Switzerland, 2017; pp. 71–89. [Google Scholar]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasir, M.; Hashim, R.; Sulaiman, O.; Asim, M. Nanocellulose: Preparation methods and applications. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 261–276. [Google Scholar]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in advanced functional material applications of nanocellulose. Processes 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Vijay Kumar Thakur. Nanocellulose Polymer Nanocomposites: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yousri, O.M.; Abdellatif, M.H.; Bassioni, G. Effect of Al2O3 Nanoparticles on the Mechanical and Physical Properties of Epoxy Composite. Arab. J. Sci. Eng. 2018, 43, 1511–1517. [Google Scholar] [CrossRef]
- Karatrantos, A.; Clarke, N.; Kröger, M. Modeling of polymer structure and conformations in polymer nanocomposites from atomistic to mesoscale: A Review. Polym. Rev. 2016, 56, 385–428. [Google Scholar] [CrossRef]
- Bayer, I.S.; Fragouli, D.; Attanasio, A.; Sorce, B.; Bertoni, G.; Brescia, R.; Pompa, P.P. Water-repellent cellulose fiber networks with multifunctional properties. ACS Appl. Mater. Interfaces 2011, 3, 4024–4031. [Google Scholar] [CrossRef]
- Hirn, U.; Schennach, R. Comprehensive analysis of individual pulp fiber bonds quantifies the mechanisms of fiber bonding in paper. Sci. Rep. 2015, 5, 10503. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.K.; Ekielski, A. The self-assembly of lignin and its application in nanoparticle synthesis: A short review. Nanomaterials 2019, 9, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.Y.; Zhang, D.Z.; Lu, F.F.; Yao, J. New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein J. Nanotechnol. 2018, 9, 2479–2498. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Pinheiro, I.F.; de Souza, S.F.; Mei, L.H.; Lona, L.M. Polymer Composites Reinforced with Natural Fibers and Nanocellulose in the Automotive Industry: A Short Review. J. Compos. Sci. 2019, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Tayeb, A.H.; Amini, E.; Ghasemi, S.; Tajvidi, M. Cellulose nanomaterials—Binding properties and applications: A review. Molecules 2018, 23, 2684. [Google Scholar] [CrossRef] [Green Version]
- Mishra, R.K.; Ha, S.K.; Verma, K.; Tiwari, S.K. Recent progress in selected bio-nanomaterials and their engineering applications: An overview. J. Sci. Adv. Mater. Devices 2018, 3, 263–288. [Google Scholar] [CrossRef]
- Alila, S.; Besbes, I.; Vilar, M.R.; Mutjé, P.; Boufi, S. Non-woody plants as raw materials for production of microfibrillated cellulose (MFC): A comparative study. Ind. Crops Prod. 2013, 41, 250–259. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [Green Version]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. In Proceedings of the IOP conference series: Materials science and engineering 2016. 10th Joint Conference on Chemistry, Solo, Indonesia, 8–9 September 2015; IOP Publishing: Bristol, UK, 2016; Volume 107, p. 012045. [Google Scholar]
- Mokhena, T.; Sefadi, J.; Sadiku, E.; John, M.; Mochane, M.; Mtibe, A. Thermoplastic processing of PLA/cellulose nanomaterials composites. Polymers 2018, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wu, Q.; Ai, X.; Huang, M.; Lu, Q. Sono-chemical preparation of cellulose nanowhiskers from Luffa Cylindrica fibers optimized by response surface methodology. Cell. Chem. Technol. 2017, 51, 775–783. [Google Scholar]
- Santos, A.S.; Pereira-da-Silva, M.A.; Oliveira, J.E.; Mattoso, L.H.; Medeiros, E.S. Accelerated sonochemical extraction of cellulose nanowhiskers. J. Nanosci. Nanotechnol. 2016, 16, 6535–6539. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.S.; Pohlmann, B.C.; Calado, V.; Bojorge, N.; Pereira, N., Jr. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng. Life Sci. 2019, 19, 279–291. [Google Scholar] [CrossRef] [Green Version]
- El Achaby, M.; Kassab, Z.; Aboulkas, A.; Gaillard, C.; Barakat, A. Reuse of red algae waste for the production of cellulose nanocrystals and its application in polymer nanocomposites. Int. J. Biol. Macromol. 2018, 106, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Kawee, N.; Lam, N.T.; Sukyai, P. Homogenous isolation of individualized bacterial nanofibrillated cellulose by high pressure homogenization. Carbohyd. Polym. 2018, 179, 394–401. [Google Scholar] [CrossRef]
- Davoudpour, Y.; Hossain, S.; Khalil, H.A.; Haafiz, M.M.; Ishak, Z.M.; Hassan, A.; Sarker, Z.I. Optimization of high pressure homogenization parameters for the isolation of cellulosic nanofibers using response surface methodology. Ind. Crops Prod. 2015, 74, 381–387. [Google Scholar] [CrossRef]
- Jacob, J.; Peter, G.; Thomas, S.; Haponiuk, J.T.; Gopi, S. Chitosan and polyvinyl alcohol nanocomposites with cellulose nanofibers from ginger rhizomes and its antimicrobial activities. Int. J. Biol. Macromol. 2019, 129, 370–376. [Google Scholar] [CrossRef]
- Oh, J.W.; Chun, S.; Chandrasekaran, M. Preparation and In Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- de Araújo Braz, E.M.; e Silva, S.C.C.C.; da Silva, D.A.; de Amorim Carvalho, F.A.; Barreto, H.M.; Júnior, L.D.S.S.; da Silva Filho, E.C. Modified chitosan-based bioactive material for antimicrobial application: Synthesis and characterization. Int. J. Biol. Macromol. 2018, 117, 640–647. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, A.; Zhang, D. Improvement of polylactic acid film properties through the addition of cellulose nanocrystals isolated from waste cotton cloth. Int. J. Biol. Macromol. 2019, 129, 878–886. [Google Scholar] [CrossRef]
- Tekinalp, H.L.; Meng, X.; Lu, Y.; Kunc, V.; Love, L.J.; Peter, W.H.; Ozcan, S. High modulus biocomposites via additive manufacturing: Cellulose nanofibril networks as “microsponges”. Compos. Part. B Eng. 2019, 173, 106817. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A. Nanocellulose-based materials for water purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Coll. Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and How to incorporate them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, H.; Nair, S.A.; Neithalath, N. Insights into material design, extrusion rheology, and properties of 3D-printable alkali-activated fly ash-based binders. Mater. Design 2019, 167, 107634. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Beatrice, C.A.G. Polymer Nanocomposites with Different Types of Nanofiller. In Nanocomposites-Recent Evolutions; Sivasankaran, S., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Pelin, C.E.; Stefan, A.; Pelin, G.; Dinca, I.; Ficai, A.; Andronescu, E.; Voicu, G. Mechanical Properties of Nanofilled Polypropylene Composites. INCAS Bull. 2015, 7, 113. [Google Scholar]
- Irvin, C.W.; Satam, C.C.; Meredith, J.C.; Shofner, M.L. Mechanical reinforcement and thermal properties of PVA tricomponent nanocomposites with chitin nanofibers and cellulose nanocrystals. Compos. Part. A Appl. Sci. Manuf. 2019, 116, 147–157. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Muhamad, F.; Abu Osman, N.A.; Singh, R. Poly (vinyl alcohol)-α-chitin composites reinforced by oil palm empty fruit bunch fiber-derived nanocellulose. Int. J. Polym. Anal. Charact. 2017, 22, 294–304. [Google Scholar] [CrossRef]
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic Crosslinked Gelatin 3D Scaffolds for Bone Tissue Engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huerta, R.R.; Saldaña, M.D. Use of subcritical water technology to develop cassava starch/chitosan/gallic acid bioactive films reinforced with cellulose nanofibers from canola straw. J. Supercrit. Fluids 2019, 148, 55–65. [Google Scholar] [CrossRef]
- Lin, D.; Kuang, Y.; Chen, G.; Kuang, Q.; Wang, C.; Zhu, P.; Fang, Z. Enhancing moisture resistance of starch-coated paper by improving the film forming capability of starch film. Ind. Crops Prod. 2017, 100, 12–18. [Google Scholar] [CrossRef]
- Zhao, Y.; Saldaña, M.D. Hydrolysis of cassava starch, chitosan and their mixtures in pressurized hot water media. J. Supercrit. Fluids 2019, 147, 293–301. [Google Scholar] [CrossRef]
- Prochoń, M.; Marzec, A.; Szadkowski, B. Preparation and Characterization of New Environmentally Friendly Starch-Cellulose Materials Modified with Casein or Gelatin for Agricultural Applications. Materials 2019, 12, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Kwon, G.J.; Hwang, K.; Kim, D.Y. Cellulose–Chitosan Antibacterial Composite Films Prepared from LiBr Solution. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef] [Green Version]
- Hernández-López, M.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Zavaleta-Avejar, L.; Benítez-Jiménez, J.J.; Sabino-Gutiérrez, M.A.; Ortega-Gudiño, P. Bio-based composite fibers from pine essential oil and PLA/PBAT polymer blend. Morphological, physicochemical, thermal and mechanical characterization. Mater. Chem. Phys. 2019, 234, 345–353. [Google Scholar] [CrossRef]
- Sango, T.; Stoclet, G.; Joly, N.; Marin, A.; Yona, A.M.C.; Duchatel, L.; Ndikontar, K.M.; Lefebvre, J.M. Water–soluble extracts from banana pseudo–stem as functional additives for polylactic acid: Thermal and mechanical investigations. Eur. Polym. J. 2019, 112, 466–476. [Google Scholar] [CrossRef]
- Kostic, D.; Vukasinovic-Sekulic, M.; Armentano, I.; Torre, L.; Obradovic, B. Multifunctional ternary composite films based on PLA and Ag/alginate microbeads: Physical characterization and silver release kinetics. Mater. Sci. Eng. C 2019, 98, 1159–1168. [Google Scholar] [CrossRef]
- Ravichandran, S.; Radhakrishnan, J.; Jayabal, P.; Venkatasubbu, G.D. Antibacterial screening studies of electrospun Polycaprolactone nano fibrous mat containing Clerodendrum phlomidis leaves extract. Appl. Surf. Sci. 2019, 484, 676–687. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Oraby, A.H.; Asnag, G.M. Structural, thermal and electrical studies of polyethylene oxide/starch blend containing green synthesized gold nanoparticles. J. Mol. Struct. 2019, 1180, 15–25. [Google Scholar] [CrossRef]
- Greco, A.; Ferrari, F.; Maffezzoli, A. Mechanical properties of poly (lactid acid) plasticized by cardanol derivatives. Polym. Degrad. Stab. 2019, 159, 199–204. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. 3D printed cork/polyurethane composite foams. Mater. Des. 2019, 179, 107905. [Google Scholar] [CrossRef]
- Lizárraga-Laborín, L.L.; Quiroz-Castillo, J.M.; Encinas-Encinas, J.C.; Castillo-Ortega, M.M.; Burruel-Ibarra, S.E.; Romero-García, J.; Torres-Ochoa, J.A.; Cabrera-Germán, D.; Rodríguez-Félix, D.E. Accelerated weathering study of extruded polyethylene/poly (lactic acid)/chitosan films. Polym. Degrad. Stab. 2018, 155, 43–51. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest treatments of fresh produce. Philos. Trans. R. Soc. A Math. Phy. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef] [Green Version]
- Sandarani, M.D.J.C.; Dasanayaka, D.C.M.C.K.; Jayasinghe, C.V.L. Strategies Used to Prolong the Shelf Life of Fresh Commodities. J. Agric. Sci. Food Res. 2018, 9, 1–6. [Google Scholar]
- Zambrano-Zaragoza, M.L.; González-Reza, R.; Mendoza-Muñoz, N.; Miranda-Linares, V.; Bernal-Couoh, T.F.; Mendoza-Elvira, S.; Quintanar-Guerrero, D. Nanosystems in edible coatings: A novel strategy for food preservation. Int. J. Mol. Sci. 2018, 19, 705. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active packaging applications for food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Yang, F.; Wang, T.; Ni, M.; Chen, Y.; Yang, F.; Huang, D.; Fu, C.; Wang, S. Preparation and Characterization of Chitosan-Based Ternary Blend Edible Films with Efficient Antimicrobial Activities for Food Packaging Applications. J. Food Sci. 2019, 84, 1411. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Cai, H.; Liu, Y.; Xiao, L.; Song, J.; Liu, J. Development of active and intelligent films based on cassava starch and Chinese bayberry (Myrica rubra Sieb. et Zucc.) anthocyanins. RSC Adv. 2019, 9, 30905–30916. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Filho, J.G.; Rodrigues, J.M.; Valadares, A.C.F.; de Almeida, A.B.; de Lima, T.M.; Takeuchi, K.P.; Alves, C.C.F.; de Figueiredo Sousa, H.A.; da Silva, E.R.; Dyszy, F.B.; et al. Active food packaging: Alginate films with cottonseed protein hydrolysates. Food Hydrocoll. 2019, 92, 267–275. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Dumitriu, R.P.; Profire, L.; Sacarescu, L.; Hitruc, G.E.; Stoleru, E.; Dobromir, M.; Matricala, A.L.; Vasile, C. Hybrid nanostructures containing sulfadiazine modified chitosan as antimicrobial drug carriers. Nanomaterials 2016, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silve r-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 2643. [Google Scholar] [CrossRef] [Green Version]
- Macocinschi, D.; Filip, D.; Paslaru, E.; Munteanu, B.S.; Dumitriu, R.P.; Pricope, G.M.; Aflori, M.; Dobromir, M.; Nica, V.; Vasile, C. Polyurethane–extracellular matrix/silver bionanocomposites for urinary catheters. J. Bioact. Compat. Pol. 2015, 30, 99–113. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Paslaru, E.; Munteanu, B.S.; Dumitriu, R.P.; Lungu, M.; Vasile, C. Polyurethane biocompatible silver bionanocomposites for biomedical applications. J. Nanopart. Res. 2014, 16, 2710. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Sacarescu, L.; Vasiliu, A.L.; Hitruc, G.E.; Pricope, G.M.; Sivertsvik, M.; Rosnes, J.T.; Vasile, C. Antioxidant/antibacterial electrospun nanocoatings applied onto PLA films. Materials 2018, 11, 1973. [Google Scholar] [CrossRef] [Green Version]
- Vasile, C.; Darie, R.N.; Sdrobis, A.; Pâslaru, E.; Pricope, G.; Baklavaridis, A.; Munteanu, B.S.; Zuburtikudis, I. Effectiveness of chitosan as antimicrobial agent in LDPE/CS composite films as minced poultry meat packaging materials. Cell. Chem. Technol. 2014, 48, 325. [Google Scholar]
- Munteanu, B.S.; Paslaru, E.; Zemljic, L.F.; Sdrobis, A.; Pricope, G.M.; Vasile, C. Chitosan coatings applied to polyethylene surface to obtain food-packaging materials. Cell. Chem. Technol. 2014, 48, 565–575. [Google Scholar]
- Jeevahan, J.; Chandrasekaran, M.; Durairaj, R.B.; Mageshwaran, G.; Joseph, G.B. A Brief Review on Edible Food Packing Materials. J. Global Eng. Probl. Solut. 2017, 1, 9–19. [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.A.; Barbosa, S.E.; Alejandra García, M.A.; Castillo, L.A.; López, O.V. Starch-Based Materials in Food Packaging: Processing, Characterization and Applications; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Yusof, N.M.; Jai, J.; Hamzah, F. Effect of Coating Materials on the Properties of Chitosan-Starch-Based Edible Coatings. In Proceedings of the IOP Conference Series: Materials science and engineering, 5th International Conference on Advanced Engineering and Technology (5th ICAET), Incheon, South Korea, 14–16 December 2018; 2019; Volume 507, p. 012011. [Google Scholar]
- Escamilla-García, M.; Reyes-Basurto, A.; García-Almendárez, B.; Hernández-Hernández, E.; Calderón-Domínguez, G.; Rossi-Márquez, G.; Regalado-González, C. Modified starch-chitosan edible films: Physicochemical and mechanical characterization. Coatings 2017, 7, 224. [Google Scholar] [CrossRef] [Green Version]
- Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Chan, E.S. Preparation of Ca-alginate beads containing high oil content: Influence of process variables on encapsulation efficiency and bead properties. Carbohydr. Polym. 2011, 84, 1267–1275. [Google Scholar] [CrossRef]
- Chan, E.S.; Wong, S.L.; Lee, P.P.; Lee, J.S.; Ti, T.B.; Zhang, Z.; Poncelet, D.; Ravindra, P.; Phan, S.H.; Yim, Z.H. Effects of starch filler on the physical properties of lyophilized calcium–alginate beads and the viability of encapsulated cells. Carbohydr. Polym. 2011, 83, 225–232. [Google Scholar] [CrossRef]
- Fujiwara, G.M.; Campos, R.; Costa, C.K.; Dias, J.F.G.; Miguel, O.G.; Miguel, M.D.; Marques, F.A.; Zanin, S.M.W. Production and characterization of alginate-starch-chitosan microparticles containing stigmasterol through the external ionic gelation technique. Braz. J. Pharm. Sci. 2013, 49, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Homayouni, A.; Azizi, A.; Ehsani, M.R.; Yarmand, M.S.; Razavi, S.H. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008, 111, 50–55. [Google Scholar] [CrossRef]
- Xie, Y.L.; Jiang, W.; Li, F.; Zhang, Y.; Liang, X.Y.; Wang, M.; Zhou, X.; Wu, S.Y.; Zhang, C.H. Controlled Release of Spirotetramat Using Starch–Chitosan–Alginate-Encapsulation. Bull. Environ. Contam. Toxicol. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Etchepare, M.; Raddatz, G.C.; de Moraes Flores, E.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Ferreira Grosso, C.R.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT-Food Sci. Technol. 2016, 65, 511–517. [Google Scholar]
- Nnamonu, L.A.; Sha’Ato, R.; Onyido, I. Alginate Reinforced Chitosan and Starch Beads in Slow Release Formulation of Imazaquin Herbicide—Preparation and Characterization. Mater. Sci. Appl. 2012, 3, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the shelf life of fresh-cut ‘Royal Delicious’ apple with edible coatings and anti-browning agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M. State of the art of antimicrobial edible coatings for food packaging applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Meng, C.G.; Liu, S.; Kan, J.; Jin, C.H. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Valenzuela-Lopez, C.C.; Chaparro-Hernandez, S.; Ornelas-Paz, J.D.J.; Toro-Sanchez, C.L.D.; Marquez-Rios, E.; Lopez-Mata, M.A.; Odcanod-Hioguera, V.M.; Valdez-Hurtado, S. Effects of chitosan-tomato plant extract edible coatings on the quality and shelf life of chicken fillets during refrigerated storage. Food Sci. Technol. 2019, 39, 103–111. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.E.; Castillo-Yañez, F.J.; Montaño-Cota, E.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Canizales-Rodríguez, D.F.; Torres-Arreola, W.; Montoya-Camacho, N.; Ocaño-Higuera, V.M. Protective Effect of an Edible Tomato Plant Extract/Chitosan Coating on the Quality and Shelf Life of Sierra Fish Fillets. J. Chem. 2018, 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and antimicrobial edible chitosan films blended with stem, leaf and seed extracts of Pistacia terebinthus for active food packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Nie, Z.; Wan, C.; Chen, J. Preservation of Xinyu tangerines with an edible coating using Ficus hirta Vahl. fruits extract-incorporated chitosan. Biomolecules 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Karami, N.; Kamkar, A.; Sahbazi, Y.; Misaghi, A. Edible films based on chitosan-flaxseed mucilage: In vitro antimicrobial and antioxidant properties and their application on survival of food-borne pathogenic bacteria in raw minced trout fillets. Pharm. Biomed. Res. 2019, 5, 10–16. [Google Scholar] [CrossRef]
- Azimzadeh, B.; Jahadi, M. Effect of chitosan edible coating with Laurus nobilis extract on shelf life of cashew. Food Sci. Nutr. 2018, 6, 871–877. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.; Tylewicz, U.; Lotti, N. Characterization of Active Edible Films based on Citral Essential Oil, Alginate and Pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfay, S.Z.; Magwaza, L.S. Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 2017, 11, 40–48. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Castro-López, C.; Sánchez-Alejo, E.J.; Saucedo-Pompa, S.; Rojas, R.; Aranda-Ruiz, J.; Martínez-Avila, G.C.G. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon 2016, 2, e00152. [Google Scholar] [CrossRef] [Green Version]
- Baltacioglu, C.; Velioglu, S.; Karacabey, E. Changes in total phenolic and flavonoid contents of rowanberry fruit during postharvest storage. J. Food Qual. 2011, 34, 278–283. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess. Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Cofelice, M.; Lopez, F.; Cuomo, F. Quality Control of Fresh-Cut Apples after Coating Application. Foods 2019, 8, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; Berrios, J.D.J.; Sambo, P.; McHugh, T.H. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess. Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Davoodi, F.; Naji, M.H. Study of the effect of sodium alginate coating containing pomegranate peel extract on chemical, sensory and microbial quality of walnut kernel. Environ. Health Eng. Manag. J. 2018, 5, 249–257. [Google Scholar] [CrossRef]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food Bioprocess. Technol. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant. Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Nie, Z.; Wan, C.; Chen, C.; Chen, J. Comprehensive Evaluation of the Postharvest Antioxidant Capacity of Majiayou Pomelo Harvested at Different Maturities Based on PCA. Antioxidants 2019, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Peralta, J.; Bitencourt-Cervi, C.M.; Maciel, V.B.; Yoshida, C.M.; Carvalho, R.A. Aqueous hibiscus extract as a potential natural pH indicator incorporated in natural polymeric films. Food Packag. Shelf Life 2019, 19, 47–55. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, X.; Li, Z.; Zhang, J.; Shi, J.; Zou, X.; Huang, X.; Zhang, D.; Sun, Y.; Yang, Z.; Holmes, M.; et al. Natural biomaterial-based edible and pH-sensitive films combined with electrochemical writing for intelligent food packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef] [PubMed]
- Halász, K.; Csóka, L. Black chokeberry (Aronia melanocarpa) pomace extract immobilized in chitosan for colorimetric pH indicator film application. Food Packag. Shelf Life 2018, 16, 185–193. [Google Scholar] [CrossRef]
- Frond, A.D.; Iuhas, C.I.; Stirbu, I.; Leopold, L.; Socaci, S.; Stănilă, A.; Ayvaz, H.; Socaciu, A.; Socaciu, M.; Diaconeasa, Z.; et al. Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives. Molecules 2019, 24, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, J.; Kuskoski, E.M.; Navas, M.J.; Asuero, A.G. Antioxidant capacity of anthocyanin pigments. In Flavonoids: From Biosynthesis to Human Health; Justiono, J., Ed.; Intech: Rijeka, Croatia, 2017; pp. 205–255. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The Anti-inflammatory Effects of Dietary Anthocyanins against Ulcerative Colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.E.; Lim, S.M.; Azlan, A. Evidence-Based Therapeutic Effects of Anthocyanins from Foods. Pak. J. Nutr. 2019, 18, 1–11. [Google Scholar]
- Xiu-li, H.E.; Xue-li, L.I.; Yuan-ping, L.V.; Qiang, H.E. Composition and color stability of anthocyanin-based extract from purple sweet potato. Food Sci. Technol. (Campinas) 2015, 35, 468–473. [Google Scholar]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, T.; Cao, L.; Wang, L. Intelligent poly (vinyl alcohol)-chitosan nanoparticles-mulberry extracts films capable of monitoring pH variations. Int. J. Biol. Macromol. 2018, 108, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Vo, T.V.; Dang, T.H.; Chen, B.H. Synthesis of Intelligent pH Indicative Films from Chitosan/Poly (vinyl alcohol)/Anthocyanin Extracted from Red Cabbage. Polymers 2019, 11, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Ge, J.; Yue, P.; Yue, X.; Fu, R.; Liang, J.; Gao, X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Shafiee-Jood, M.; Cai, X. Reducing food loss and waste to enhance food security and environmental sustainability. Environ. Sci. Technol. 2016, 50, 8432–8443. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Vital, A.C.P.; Guerrero, A.; Kempinski, E.M.B.C.; de Oliveira Monteschio, J.; Sary, C.; Ramos, T.R.; Campo, M.D.M.; do Prado, I.N. Consumer profile and acceptability of cooked beef steaks with edible and active coating containing oregano and rosemary essential oils. Meat Sci. 2018, 143, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Yahaya, W.; Amnin, W.; Abu Yazid, N.; Azman, M.; Aini, N.; Almajano, M.P. Antioxidant Activities and Total Phenolic Content of Malaysian Herbs as Components of Active Packaging Film in Beef Patties. Antioxidants 2019, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Difonzo, G.; Squeo, G.; Calasso, M.; Pasqualone, A.; Caponio, F. Physico-Chemical, Microbiological and Sensory Evaluation of Ready-to-Use Vegetable Pâté Added with Olive Leaf Extract. Foods 2019, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Pabón-Baquero, L.C.; Otálvaro-Álvarez, Á.M.; Fernández, M.R.R.; Chaparro-González, M.P. Plant Extracts as Antioxidant Additives for Food Industry. In Antioxidants in Foods and Its Applications; Shalaby, E., Ed.; IntechOpen: London, UK, 2019; p. 87. [Google Scholar]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant migration studies in chitosan films incorporated with plant extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef] [PubMed]
- Benbettaïeb, N.; Chambin, O.; Assifaoui, A.; Al-Assaf, S.; Karbowiak, T.; Debeaufort, F. Release of coumarin incorporated into chitosan-gelatin irradiated films. Food Hydrocoll. 2016, 56, 266–276. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant. Foods Hum. Nutr. 2019, 74, 61–67. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.A. Health Benefits of Culinary Herbs and Spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic plants: Antioxidant capacity and polyphenol characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Ghandahari Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Optimization of the enzyme-assisted aqueous extraction of phenolic compounds from pistachio green hull. Food Sci. Nutr. 2019, 7, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Perazzo, K.K.N.C.L.; de Vasconcelos Conceição, A.C.; dos Santos, J.C.P.; de Jesus Assis, D.; Souza, C.O.; Druzian, J.I. Properties and antioxidant action of actives cassava starch films incorporated with green tea and palm oil extracts. PLoS ONE 2014, 9, e105199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohyd. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Chawda, P.J.; Shi, J.; Xue, S.; Young Quek, S. Co-encapsulation of bioactives for food applications. Food Qual. Safety 2017, 1, 302–309. [Google Scholar]
- Kanokpanont, S.; Yamdech, R.; Aramwit, P. Stability enhancement of mulberry-extracted anthocyanin using alginate/chitosan microencapsulation for food supplement application. Artif. Cell. Nanomedicine Biotechnol. 2018, 46, 773–782. [Google Scholar] [CrossRef] [Green Version]
- Aloui, H.; Khwaldia, K.; Sánchez-González, L.; Muneret, L.; Jeandel, C.; Hamdi, M.; Desobry, S. Alginate coatings containing grapefruit essential oil or grapefruit seed extract for grapes preservation. Int. J. Food Sci. Technol. 2014, 49, 952–959. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of grape pomace extract incorporation on chitosan films properties. Carbohyd. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of antibacterial and antioxidant properties of chitosan edible films incorporated with maqui berry (Aristotelia chilensis). LWT-Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and characterization of chitosan film incorporated with thinned young apple polyphenols as an active packaging material. Carbohyd. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Serrano-León, J.S.; Bergamaschi, K.B.; Yoshida, C.M.; Saldaña, E.; Selani, M.M.; Rios-Mera, J.D.; Alencar, S.M.; Contreras-Castillo, C.J. Chitosan active films containing agro-industrial residue extracts for shelf life extension of chicken restructured product. Food Res. Int. 2018, 108, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohyd. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Türk Baydır, A.; Aşçıoğlu, Ç. Effects of Antioxidant Capacity and Peroxide Value on Oxidation Stability of Sunflower Oil. Chem. Mater. 2018, 1, 5–7. [Google Scholar]
- Berizi, E.; Hosseinzadeh, S.; Shekarforoush, S.S.; Barbieri, G. Microbial, chemical, textural and sensory properties of coated rainbow trout by chitosan combined with pomegranate peel extract during frozen storage. Int. J. Biol. Macromol. 2018, 106, 1004–1013. [Google Scholar] [CrossRef]
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of Lipids in Foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Khan, S.; Ranjha, N.M. Effect of degree of cross-linking on swelling and on drug release of low viscous chitosan/poly (vinyl alcohol) hydrogels. Polym. Bull. 2014, 71, 2133–2158. [Google Scholar] [CrossRef]
- Kandav, G.; Bhatt, D.; Jindal, D.K. Formulation And Evaluation Of Allopurinol Loaded Chitosan Nanoparticles. Intern. J. Appl. Pharmaceutics 2019, 11, 49–52. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Chrzanowska, E. Characteristics of ascorbic acid release from TPP-crosslinked chitosan/alginate polyelectrolyte complex membranes. Prog. Chem. Appl. Chitin Deriv. 2018, 23, 76–87. [Google Scholar] [CrossRef]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of bioactive composite films from chitosan and carboxymethyl cellulose using glutaraldehyde, cinnamon essential oil and oleic acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Wang, F.; Peng, W.G.; He, Y.H. Preparation of chitosan and epichlorohydrin cross-linked adsorbents and adsorption property of dyes. In Proceedings of the Applied Mechanics and Materials 2013, Third International Conference on Applied Mechanics, Materials and Manufacturing (ICAMMM 2013), Dalian, China, 24–25 August 2013; Trans Tech Publications: Kapellweg 8, CH-8806 Baech, Switzerland, 2013; Volume 423, pp. 584–587. [Google Scholar]
- Kildeeva, N.R.; Kasatkina, M.A.; Mikhailov, S.N. Peculiarities of obtaining biocompatible films based on chitosan cross linked by genipin. Polym. Sci. Ser. D 2017, 10, 189–193. [Google Scholar] [CrossRef]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical cross-linking gelatin with natural phenolic compounds as studied by high-resolution NMR spectroscopy. Biomacromolecules 2010, 11, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Tegl, G.; Baumann, M.; Sommer, E.; Gorji, E.G.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Chitosan hydrogel formation using laccase activated phenolics as cross-linkers. Carbohyd. Polym. 2017, 157, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Lemes, B.M.; Novatski, A.; Ferrari, P.C.; Minozzo, B.R.; Justo, A.D.S.; Petry, V.E.K.; Vellosa, J.C.R.; Sabino, S.d.R.F.; Gunha, J.V.; Esmerino, L.A.; et al. Physicochemical, biological and release studies of chitosan membranes incorporated with Euphorbia umbellata fraction. Rev. Bras. De Farmacogn. 2018, 28, 433–443. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Smith, R.A.; Walker, R.C.; Levit, S.L.; Tang, C. Single-Step Self-Assembly and Physical Crosslinking of PEGylated Chitosan Nanoparticles by Tannic Acid. Polymers 2019, 11, 749. [Google Scholar] [CrossRef] [Green Version]
- Rivero, S.; García, M.A.; Pinotti, A. Crosslinking capacity of tannic acid in plasticized chitosan films. Carbohyd. Polym. 2010, 82, 270–276. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Yuan, M.L.; Fan, J.; Zhao, T.R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayachiew, P.; Devahastin, S. Effects of drying methods and conditions on release characteristics of edible chitosan films enriched with Indian gooseberry extract. Food Chem. 2010, 118, 594–601. [Google Scholar] [CrossRef]
- Hauser, C.; Peñaloza, A.; Guarda, A.; Galotto, M.J.; Bruna, J.E.; Rodríguez, F.J. Development of an Active Packaging Film Based on a Methylcellulose Coating Containing Murta (Ugni molinae Turcz) Leaf Extract. Food Bioprocess. Technol. 2016, 9, 298–307. [Google Scholar] [CrossRef]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and characterization of intelligent starch/PVA films for simultaneous colorimetric indication and antimicrobial activity for food packaging applications. Carbohyd. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Ghelejlu, S.B.; Esmaiili, M.; Almasi, H. Characterization of chitosan–nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016, 86, 613–621. [Google Scholar] [CrossRef]




| Source and Compound | Obtaining Method | Mixing Method | Application | |
|---|---|---|---|---|
| Plant Sources | ||||
| Polysaccharides | ||||
| Cellulose; used as pulp, nanocrystals, nanofibers and fibers | Cellulose can be isolated using a combination of chemical and mechanical treatments like ultrasonication combined with chemical pretreatments, high shear homogenization coupled with acid hydrolysis and steam explosion, etc. [13]. | Extrusion (for example in polypropylene composites [14]), reactive extrusion [15]. | Reinforcement in polymer composites [14,16,17,18]. | |
| Starch | Starch is extracted from seeds, roots and tubers, by wet grinding, washing, sieving and drying [19]. | Extrusion, injection molding, film casting [20], reactive extrusion [15]. For incorporating starch in plastics, commercialized technologies were developed to overcome the moisture sensitivity and inferior mechanical properties of starch [21]. | As a filler in biodegradable food packaging materials [22,23,24] or in plastic films can improve the biodegradability [25]. | |
| Pectin | Extracted using acids and enzymes [26]. | Extrusion (for example in polyvinyl alcohol composites) [27]. | Antimicrobial packaging materials [28]. | |
| Proteins | ||||
| Soy Protein, hydrolyzed proteins (wheat gluten, wheat gliadin), zein, polypeptides | - Alkaline extraction followed by protein precipitation at isoelectric pH; - protein extraction with salt solution, followed by precipitation from a salt extract by ultrafiltration, diafiltration membranes or dilution in cold water (micellization) [29]; and - novel techniques, such as ultrasound assisted extraction, enzyme-assisted extraction in the form of proteases and/or carbohydrolases [29]. | Extrusion foaming [30], reactive extrusion [15]. | Reinforcement in polymer composites [31,32]. Polypeptides: Reinforcement in polymer composites [33]. Food packaging applications [34] or incorporated as a reinforcement in films with enhanced barrier properties [35] (zein). Mixing different proteins with polysaccharides is an effective way to improve barrier and mechanical properties of protein- polysaccharides films [36]. | |
| Lignins | Industrially, lignin is isolated from cellulosic fibers by chemical treatment, which breaks down lignin–carbohydrate complexes. During this process, partial depolymerization of the complex lignin macromolecules occurs along with re-polymerization (condensation) which may alter the native lignin structure [37]. The paper pulping process (lignin extraction from lignocellulosic biomass) which produces industrial lignin as a byproduct [37] may include chemical methods [38], such as - Kraft process which uses a mixture of Na2S and NaOH (White Liquor) at high temperature (150–180 °C), - sulfite process which employs sulfite or bisulfite to digest biomass, - organosolv pretreatment of lignocellulose which involves a biomass extraction in a mixture of solvent (ethanol being the most common) and water under high pressure [39], - single pot soda cooking pre-treatment for extracting lignin and isolate cellulose nanofibrils simultaneously [13]. | The methods of blending lignin with thermoplastic polymers (natural or synthetic - as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polymethylmethacrylate (PMMA), polyvinyl alcohol (PVA), ethylene-vinyl acetate copolymer (EVA), polyester, starch, and protein) include melt-blending (extrusion, compression, injection, and blow-molding) and solution mixing [40]. | Lignin as reinforcer/fillers in thermoplastic polymers improved mechanical properties, decreased water absorption, antioxidant effect due to the phenols in the lignin structure [41], improved water resistance, and thermal stability of the natural polymers such as starch or proteins. [40]. | |
| Polyphenols Plant Extracts Essential Oils | The most commonly applied methods for the extraction of polyphenols uses water in combination with organic solvents (acetone, ethanol, methanol, ethyl acetate) as per the type of polyphenols present in the plant [42]. | Blending methods to circumvent the loss of the volatile compounds: - melt blending requires the addition of the active compound in a later stage of the mixing after the polymer is melted, low melting temperature and decreased mixing time [43]. - dispersion/dissolution of the polymer and all active components in a common solvent that is subsequently evaporated (solution casting technique)—method that can also be used as a coating technique by casting the dissolution onto the particular surface [43]. - novel method which involves electrospinning/electrospraying the polymer/active component solution—the advantage of faster solvent evaporation compared with the solution casting technique with the possibility to encapsulate volatile compounds into polymeric fibers/particles. | Plant extracts and essential oils [44,45] are mainly used as antioxidant and antibacterial agents due to the components present in essential oils (eugenol, eugenyl acetate, carvacrol, cinnamaldehyde, thymol, squalene, rosmarinic acid, tyrosol, β-caryophyllene [46]) and plant extracts (isoprenylflavones, flavonone phytoalexins, isoflavonoids, monomeric polyphenols, epicatechin, epicatechin gallate, epigallocatechin gallate, terpenes, alkaloids) [47]. The minimal inhibitory concentration of an antimicrobial agent is the lowest (i.e., minimal) concentration of the antimicrobial agent that inhibits a given bacterial isolate from multiplying and producing visible growth in the test system. For example, in ethanol, thyme, clove and tea tree essential oils had approximately 1, 12, 25 v/v % MIC against Staphylococcus aureus and 1, 3, 12 v/v % against Escherichia coli [48]. | |
| Animal Sources | ||||
| Polysaccharides | ||||
| Chitin | Isolation of chitin from crustaceans, such as crayfish, crab, shrimp, and other organisms such as fungi [49], by deproteinization with alkaline treatment at high temperatures, and demineralization with dilute hydrochloric acid [50]. | Chitin nanocrystals and nanofibers were added by melt-mixing as fillers into thermoplastic starch-based biocomposites [51]. Also, chitin nanofibers were added in molten PLA by extrusion [52]. | Reinforcement in polymer composites [52,53]. | |
| Chitosan | By chitin N-deacetylation [50,54]. | Solvent blending [55,56], extrusion blending and reactive extrusion blending [57] as chitosan may be heated up to temperatures below its glass transition temperature without affecting its physicochemical properties [58]. | Polymer composites (polyvinyl chloride, polyurethane) with antibacterial properties [59,60]. Reinforcement in polymer composites [54]. | |
| Proteins | ||||
| Silk/Wool | - In thermoplastics: melt mixing, single/twin screw extruder, and compression molding - In thermosets: vacuum assisted transfer molding, vacuum bag resin transfer molding and vacuum-assisted resin-infused repairing [12]. | Reinforcement in polymer composites [10,61]. | ||
| Collagen/hyaluronic acid | Hyaluronic acid it is mainly produced via streptococcal fermentation. Recently the production of hyaluronic acid via recombinant systems was studied due to the avoidance of potential toxins [49]. - Collagen can be basically obtained from the slaughter of pork and beef by chemical hydrolysis and enzymatic hydrolysis [62]. | Bioactive composite scaffolds for bone tissue engineering [63,64]. | ||
| Mineral Source - Clays/Nanoclays | ||||
| Natural clays: e.g., montmorillonite, hectorite, sepiolite, laponite, saponite, bentonite, kaolinite, | Relatively simple techniques are used in industrial processing for separation and purification of natural clays: decomposition of carbonates, dissolution of (hydr)oxides, oxidation of organic material, dissolution of silica, dialysis, and fractionation. [65]. | Polymer–nanoclay nanocomposites may be prepared by melt or solution blending, with partially exfoliated clays, in situ polymerization, and melt intercalation by conventional polymer extrusion process, microwave and ultrasound irradiation [66]. | Nanoclays used as fillers in various polymer matrices enhancing mechanical properties of the polymer matrix [67]. In biomedical field: - nanoclays as fillers in chitosan poli e-caprolactone poly-ethylene glycol poly(2-hydroxyethyl methacrylate) for drug delivery applications, as reinforcements for PMMA composites for bone cement applications or implants with improved bioactivity and mechanical properties or incorporated to polysaccharide hydrogels that can support cell proliferation (chitosan, gellan gum) [68]. | |
| Matrix | Additive (content) | Mixing/Preparation Method | Role, Change in Properties/Observations | Ref. |
|---|---|---|---|---|
| Antibacterial/Antioxidant Plastics | ||||
| PVC-based composites with self-sterilizing and antibacterial activity against S. aureus (functional antibacterial plastic). | Chitosan (wt % 0–40). | The mix was melt-compounded in an internal mixer at 150 °C. | Chitosan addition increased Young’s modulus evidencing a good CS–PVC interaction. Chitosan addition had no negative impact on thermal stability of the PVC composites which allows for possibility of producing composites by with thermo-mechanical processes, without risk of thermal decomposition. | [59] |
| Biodegradable polymer fiber nets of poly (lactic acid) (PLA)/poly (butylene adipateco-terephthalate) (PBAT) (60:40). Packaging material for fruit and vegetables preservation. | Pine essential oil (10%–20%). Some formulations were additionally coated with chitosan (1%). | Extruded biodegradable polymer. | With essential oil addition increased plasticity (at 10% Pine EO), elongation at break and decreased Young’s modulus. When chitosan was added as a coating, stiffening of the fiber was observed. | [142] |
| PLA-based composites for the packaging industry. | Water-soluble extracts (2%; 10%; 20%; 30%) from banana pseudo–stems. | Solution blending, casting and thermocompression. | Water-soluble extracts acted as a plasticizer on PLA (Tg decrease) and has slightly positive influence on its stiffness in the glassy state, whereas the drawability remained fairly acceptable when PLA-based materials where drawn at 75 °C above Tg. | [143] |
| Antimicrobial PLA films for food packaging with low silver release. | Alginate microbeads obtained by electrostatic extrusion (200 μm) with incorporated AgNPs (1.5 wt % Ag; 3 wt % alginate). | Solvent casting | PLA matrix acted as a diffusion barrier so that the released silver in water after 10 days was within the prescribed limit of 0.05 mg kg−1 while the films induced inhibitory effects against Staphylococcus aureus. | [144] |
| Poly caprolactone (PCL) nano fibrous mat with antioxidant activity for antimicrobial wound dressings. | Extract of medicinal plant Clerodendrum phlomidis. | Electrospinning | The plant extract conferred antibacterial activity and increased in wettability of the PCL fibers without affecting their mechanical properties. | [145] |
| Polyethylene oxide (PEO) These results will recommend these films a potential candidate in electrochemical and photoelectrical devices. | Starch (30 wt %) doped with various concentrations of gold nanoparticles (Au NPs) | Solvent casting | Differential scanning calorimetry (DSC) measurement indicated miscibility between the two polymers. Found electrical conductivity increased as Au NPs content increased. The miscibility between PEO and starch could be due to the oxygen atoms of PEO interacting through hydrogen H-bonds between the hydroxyl groups of starch. DSC revealed that the thermal stability of the blend polymer decreased after addition of the nanofiller. | [146] |
| Poly(lactic acid), PLA. The low cost and toxicological impact make cardanol a valid alternative to the plasticizer PEG. | Cardanol derived plasticizers (10%, 20% and 30%); three different plasticizers were used: neat cardanol, cardanol acetate (CA), and epoxidized cardanol acetate (ECA) were used, at contents ranging between 10% and 30%. | Mixing PLA, pre-dried at 70 °C for 24 h, and different amounts of plasticizers (10%, 20%, and 30%) for 15 min at 190 °C in a HAAKE RHEOMIX 600\610 mixer, with a rotor speed of 60 rpm. | PLA plasticized by cardanol derivatives showed lower modulus than PEG plasticized PLA. The tensile modulus of plasticized PLA was correlated to the evolution of glass transition temperature and degree of crystallinity. At low plasticizers content, the modulus of PLA decreased as the glass transition temperature decreased, due to a better miscibility of the plasticizer with PLA. The opposite occurred at high plasticizer content; in this case, the higher modulus found for more compatible plasticizers were attributed to an increased crystallization kinetic. | [147] |
| PU polyurethane 3D-printed foams as thermal insulation, sound absorption or as damping materials. | Cork powder (1%, 3%, and 5% wt/wt). | The TPU powder was mixed with cork powder (1%, 3%, and 5% wt/wt) in the Retsch cross beater mill SK1 without sieves. Afterwards, the mixtures were left over night in an oven at 105 °C to remove moisture. The mixtures were then extruded in a Felfil Evo Colours extruder using 4 rpm at 210 °C to produce the 3D printable filaments. | 3D-printed PU polyurethane composite foams for thermal applications with enhanced mechanical properties. Due to the presence of cork as well as to the presence of voids the resulting foams presented lower density, lower thermal conductivity and proved to be more flexible. The stiffness of the ensuing composites was also reduced but the elastomeric behavior of the 3D-printed foams produced may find applications that combine thermal insulation with damping properties. Yet, the use of cork did not affect the thermal stability of the composites. Cork is a well-known low thermal conductive material, which can further reduce the thermal conductivity of PU foams Besides their thermal insulation properties, their elastomeric behavior suggests that the 3D-printed foams produced may be used as thermal insulation, sound absorption or as damping materials. | [148] |
| Polyethylene/poly (lactic acid)/Degradable polymeric films | Chitosan (15 wt %) with and without poly (ethylene-g-maleic anhydride) (PEgMA) as compatibilizer. | Laboratory mixer-extruder. 145 °C and 155 °C for the screw barrel. | Polyethylene/poly (lactic acid)/chitosan films, with and without poly (ethylene-g-maleic anhydride) (PEgMA) as compatibilizer, were prepared by extrusion. It was demonstrated that blends of synthetic and natural polymers have a higher susceptibility to degradation in comparison to neat polyethylene and poly (lactic acid) films. Additionally, it is found that the incorporation of PEgMA into the extruded films apparently favored the polymer degradation, as it deduced from the fall of the mechanical properties when the films are exposed to accelerated weathering simulation. | [149] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, S.B.; Vasile, C. Vegetable Additives in Food Packaging Polymeric Materials. Polymers 2020, 12, 28. https://doi.org/10.3390/polym12010028
Munteanu SB, Vasile C. Vegetable Additives in Food Packaging Polymeric Materials. Polymers. 2020; 12(1):28. https://doi.org/10.3390/polym12010028
Chicago/Turabian StyleMunteanu, Silvestru Bogdănel, and Cornelia Vasile. 2020. "Vegetable Additives in Food Packaging Polymeric Materials" Polymers 12, no. 1: 28. https://doi.org/10.3390/polym12010028
APA StyleMunteanu, S. B., & Vasile, C. (2020). Vegetable Additives in Food Packaging Polymeric Materials. Polymers, 12(1), 28. https://doi.org/10.3390/polym12010028